Influence of Citrus sunki and Poncirus trifoliata Root Extracts on Metabolome of Phytophthora parasitica
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
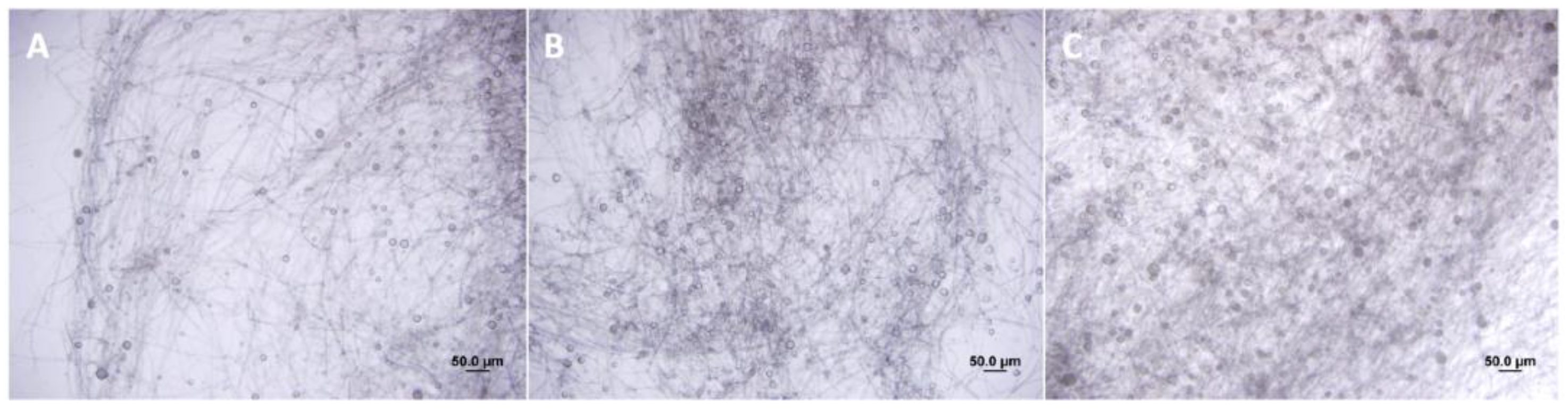
3.1. Growth Rate, Hyphae Morphology, Sporangia Development, and Zoospore Behavior
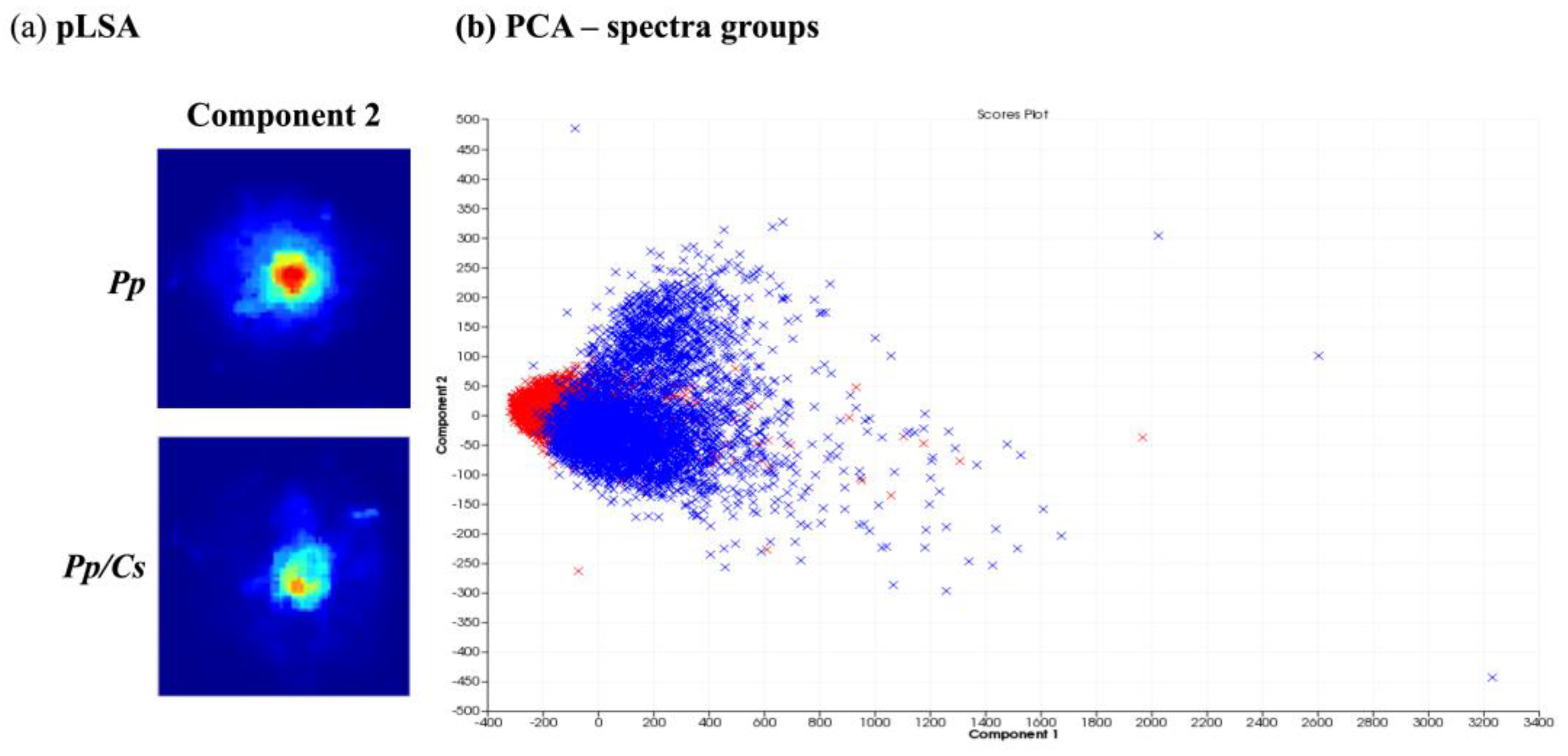
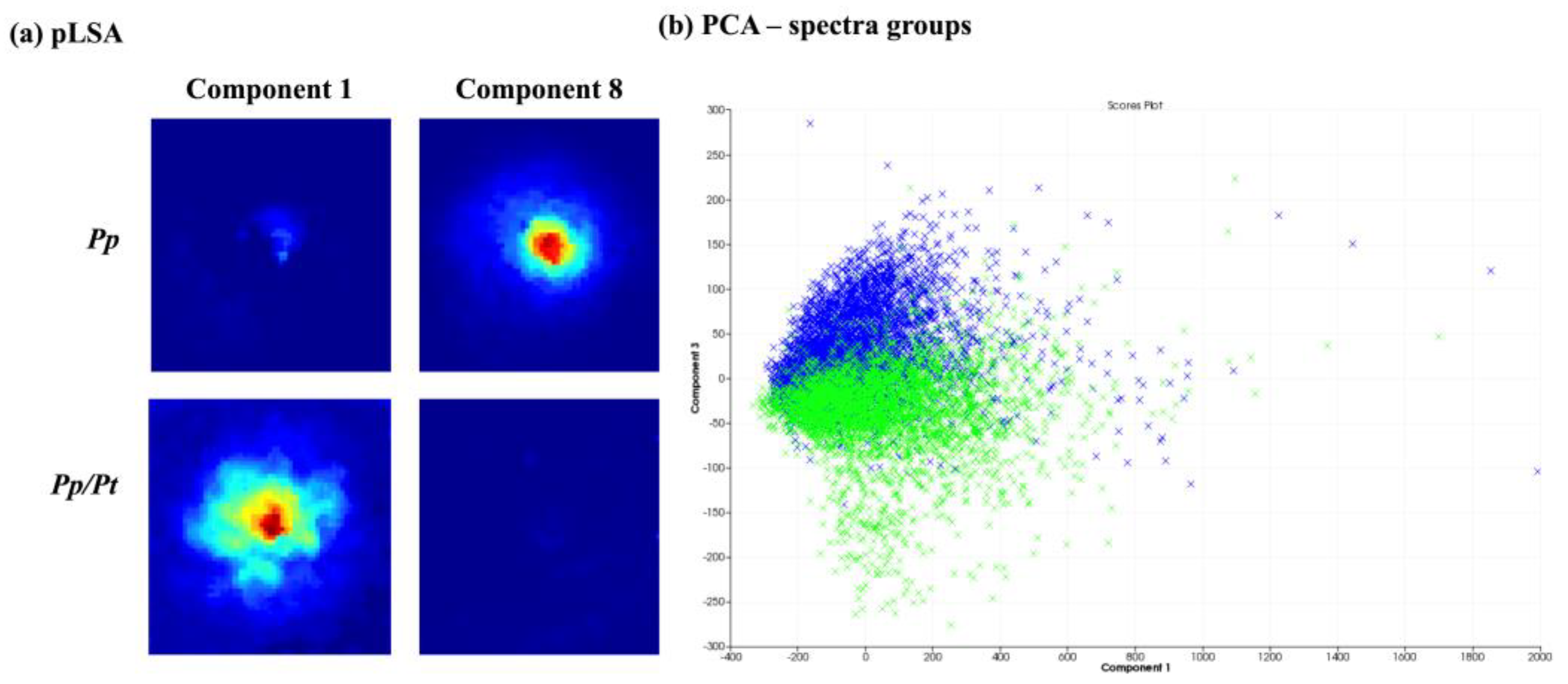
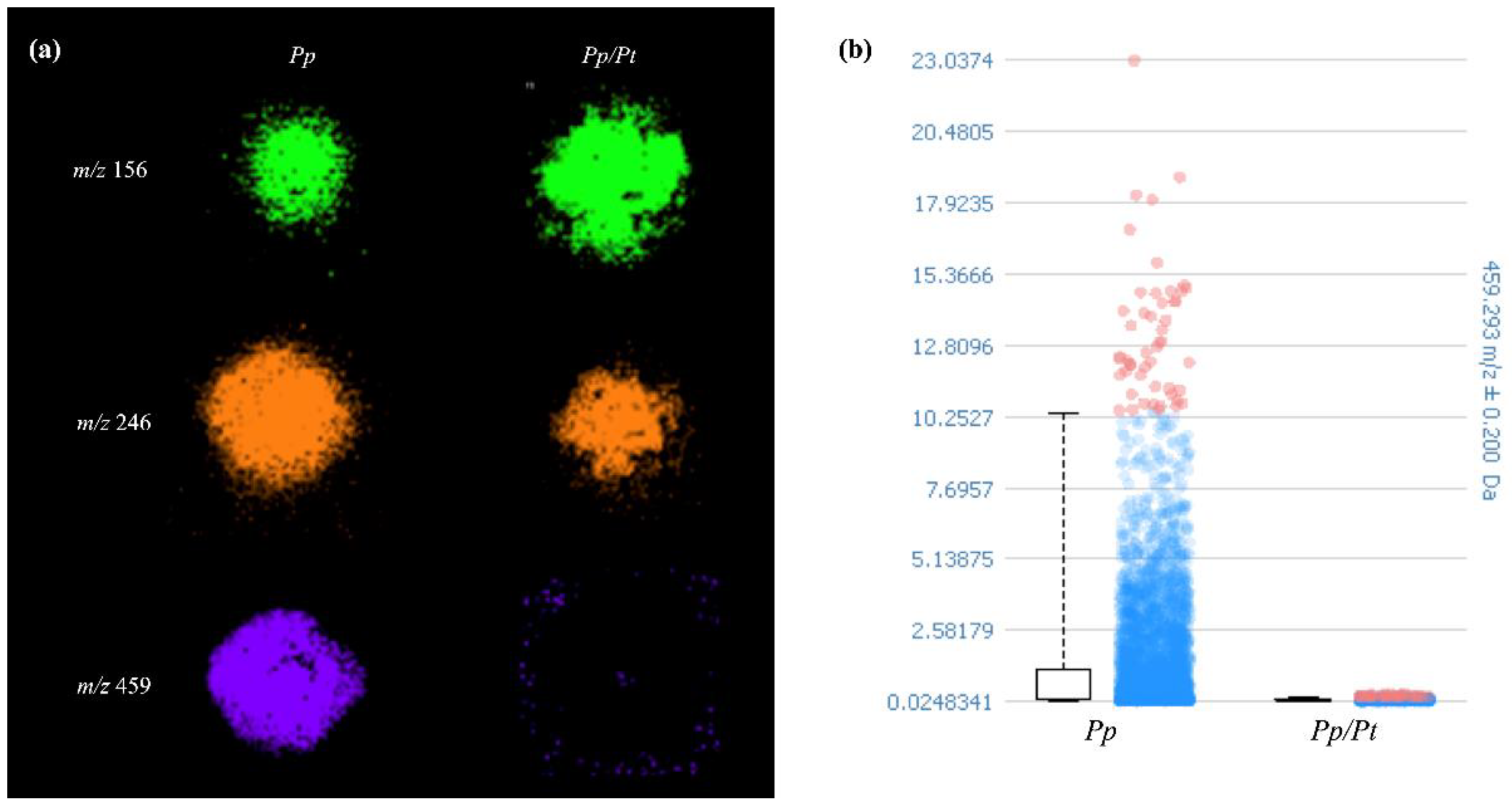
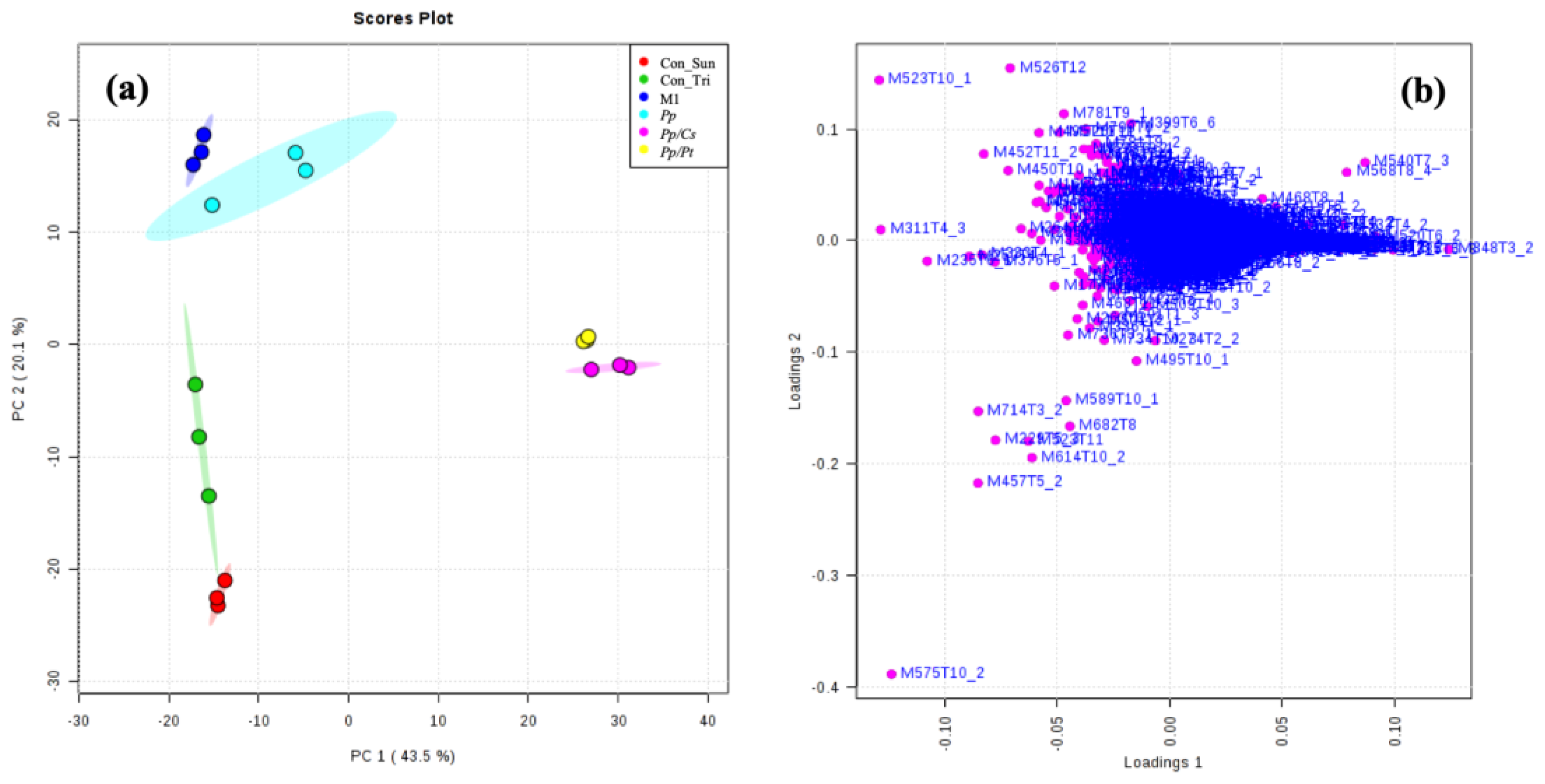
3.2. MALDI-MSI
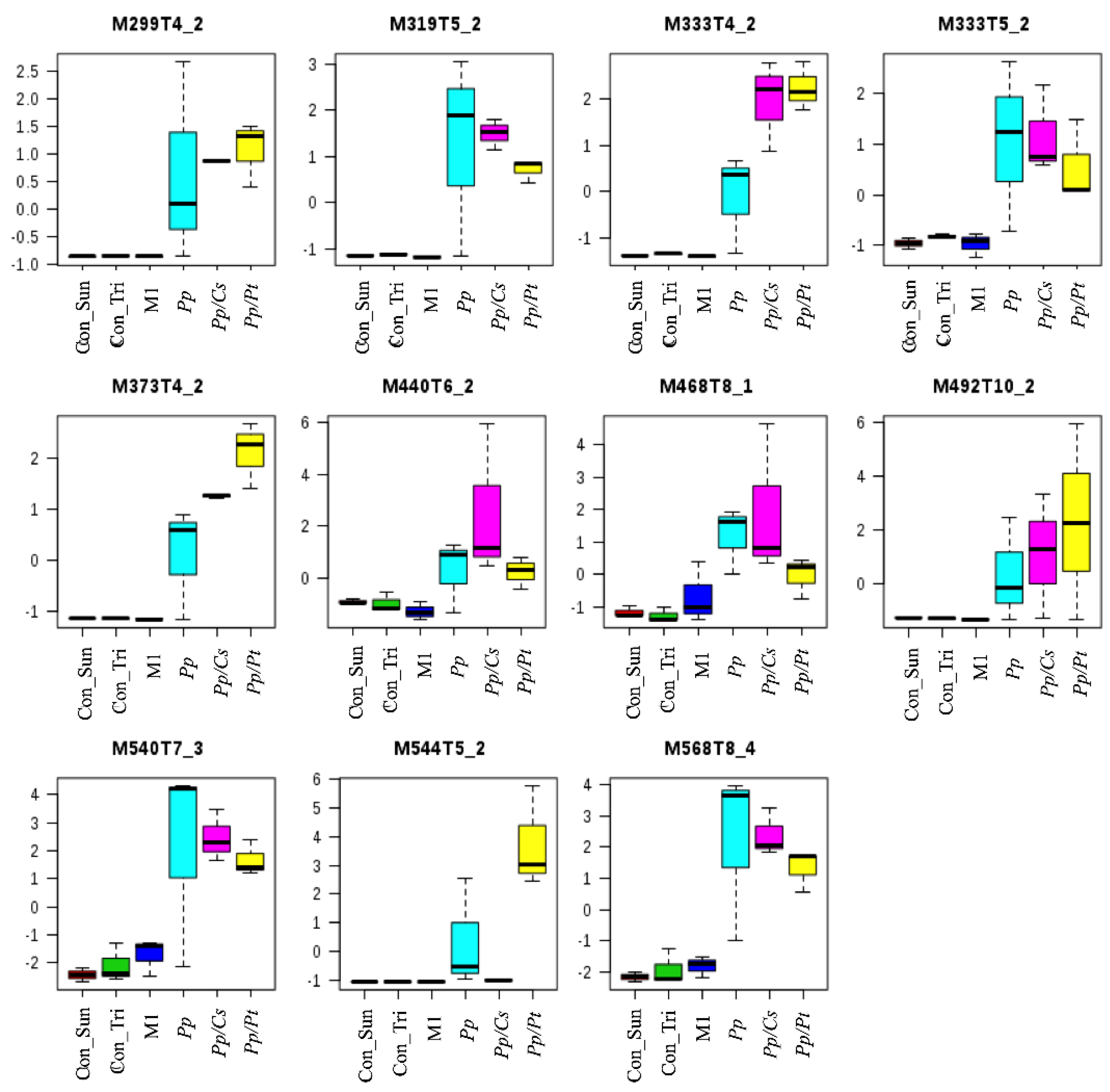
3.3. UHPLC-ESI-Q-TOF-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strullu-Derrien, C.; Kenrick, P.; Rioult, J.P.; Strullu, D.G. Evidence of parasitic Oomycetes (Peronosporomycetes) infecting the stem cortex of the Carboniferous seed fern Lyginopteris oldhamia. Proc. Biol. Sci. 2011, 278, 675–680. [Google Scholar] [PubMed]
- Kamoun, S.; Zipfel, C. Fungal pathogenesis: Host modulation every which way. Nat. Microbiol. 2016, 1, 16075. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Ann. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, M.; Maqbool, A.; Jiménez-Dalmaroni, M.J.; Pennington, H.G.; Kamoun, S.; Banfield, M.J. Effectors of Filamentous Plant Pathogens: Commonalities amid Diversity. Microbiol. Mol. Biol. Rev. 2017, 81, e00066-16. [Google Scholar] [CrossRef] [PubMed]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Horta Jung, M.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant–Phytophthora interactions. Forest Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Panabieres, F.; Ali, G.S.; Allagui, M.B.; Dalio, R.J.D.; Gudmestad, N.C.; Kuhn, M.; Roy, S.G.; Schena, L.; Zampounis, A. Phytophthora nicotianae diseases worldwide: New knowledge of a long-recognised pathogen. Phytopatologia Mediterrânea 2016, 55, 20–40. [Google Scholar]
- Tyler, B.M.; Wu, M.; Wang, J.; Cheung, W.; Morris, P.F. Chemotactic preferences and strain variation in the response of Phytophthora sojae zoospores to host isoflavones. Appl. Environ. Microbiol. 1996, 62, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Boava, L.P.; Cristofani-Yaly, M.; Mafra, V.S.; Kubo, K.; Kishi, L.T.; Takita, M.A.; Ribeiro-Alves, M.; Machado, M.A. Global gene expression of Poncirus trifoliata, Citrus sunki and their hybrids under infection of Phytophthora parasitica. BMC Genom. 2011, 12, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Máximo, H.J.; Dalio, R.J.D.; Rodrigues, C.M.; Breton, M.C.; Machado, M.A. Reference genes for RT-qPCR analysis in Citrus and Poncirus infected by zoospores of Phytophthora parasitica. Trop. Plant Pathol. 2017, 42, 76–85. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics reviewed: A new “omics” platform technology for systems biology and implications for natural products research. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef]
- Katajamaa, M.; Orešič, M.J. Data processing for mass spectrometry-based metabolomics. Chromatog. A 2007, 1158, 318–328. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.O. MALDI-TOF mass spectrometry of bacteria. Mass Spectrom. Rev. 2001, 20, 172–194. [Google Scholar] [CrossRef] [PubMed]
- Wieser, A.; Schneider, L.; Jung, J.; Schubert, S. MALDI-TOF MS in microbiological diagnostics—Identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 2012, 93, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hoefler, B.C.; Straight, P.D. Imaging mass spectrometry, metabolism, and new views of the microbial world. In Natural Products Analysis: Instrumentation, Methods, and Applications; Havlíček, V., Spížek, J., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2014; pp. 349–396. [Google Scholar]
- Gonzalez, D.J.; Xu, Y.; Yang, Y.L.; Esquenazi, E.; Liu, W.T.; Edlund, A.; Duong, T.; Du, L.; Molnár, I.; Gerwick, W.H.; et al. Observing the invisible through imaging mass spectrometry, a window into the metabolic exchange patterns of microbes. J. Proteom. 2012, 75, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Moree, W.J.; Phelan, V.V.; Wu, C.H.; Bandeira, N.; Cornett, D.S.; Duggan, B.M.; Dorrestein, P.C. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc. Nat. Acad. Sci. USA 2012, 109, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.D.S.; Araujo, W.L.; Eberlin, M.N. Potential of Burkholderia seminalis TC3.4.2R3 as Biocontrol Agent Against Fusarium oxysporum Evaluated by Mass Spectrometry Imaging. J. Am. Soc. Mass Spec. 2017, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Lam, C.W.; Curreem, S.O.T.; Lee, K.C.; Chow, W.N.; Lau, C.C.Y.; Sridhar, S.; Wong, S.C.Y.; Martelli, P.; Hui, S.W.; et al. Metabolomic profiling of Burkholderia pseudomallei using UHPLC-ESI-Q-TOF-MS reveals specific biomarkers including 4-methyl-5-thiazoleethanol and unique thiamine degradation pathway. Cell Biosci. 2015, 5, 26–40. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Kell, D.B.; Goodacre, R. Flow-injection electrospray ionization mass spectrometry of crude cell extracts for high-throughput bacterial identification. J. Am. Soc. Mass. Spectrom. 2002, 13, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications, 3rd ed.; John Wiley & Sons Ltd: Chichester, UK, 2009. [Google Scholar]
- Milne, S.; Ivanova, P.; Forrester, J.; Brown, H.A. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods 2006, 39, 92–103. [Google Scholar] [CrossRef]
- Yang, J.Y.; Phelan, V.V.; Simkovsky, R.; Watrous, J.D.; Trial, R.M.; Fleming, T.C.; Wenter, R.; Moore, B.S.; Golden, S.S.; Pogliano, K.; et al. Primer on agar-based microbial imaging mass spectrometry. J. Bacteriol. 2012, 194, 6023–6028. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. LC/MS Preprocessing and Analysis with xcms. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Cho, K.; Uritboonthai, W.; Zhu, Z.; Patti, G.J.; Siuzdak, G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012, 30, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.M. Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu. Rev. Phytopathol. 2002, 40, 137–167. [Google Scholar] [CrossRef]
- Judelson, H.S.; Blanco, F.A. The spores of Phytophthora: Weapons of the plant destroyer. Nat. Rev. Micro-Biol. 2005, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Maille, G.O.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass. Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, C.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucl. Acids Res. 2007, 35, 606–612. [Google Scholar] [CrossRef]


| Sunki | Trifoliata | |||
|---|---|---|---|---|
| Encystment Rate | Zoospores Mobility | Encystment Rate | Zoospores Mobility | |
| Water | None | Active | None | Active |
| Root extract | None | Active | Increased | Spinning |
| m/z | VIP Scores | p Value | Retention Time (Min) | MS/MS Fragment Masses | Type of Ion | Putative Assignments | D (ppm) |
|---|---|---|---|---|---|---|---|
| 175.1191 | 0.85 | 0.008 | 0.37 | 175, 158, 130, 116, 70, 60 | [M + H]+ | L-Arginine | 1 |
| 296.1431 | 0.56 | 0.020 | 5.65 | - | - | - | No match |
| 303.1775 | 0.48 | 0.011 | 0.42 | 303, 286, 175, 130, 112, 70, 60 | [M + H]+ | Arg-Gln | 0 |
| 303.2320 | 1.66 | 0.011 | 6.81 | 303, 233, 208, 191, 175, 167, 135, 118, 102, 93, 71 | - | - | No match |
| 313.2722 | 0.42 | 0.083 | 5.00 | - | - | - | No match |
| 392.3133 | 0.24 | 0.034 | 7.08 | 392, 371, 336, 311, 276, 242, 224, 194, 182, 130, 118, 102, 89, 59 | - | - | No match |
| 399.1792 | 1.03 | 0.020 | 5.69 | - | - | - | No match |
| 401.2298 | 1.02 | 0.011 | 4.70 | 401, 340, 264, 194, 181, 158, 131, 118, 101 | - | - | No match |
| 489.2270 | 0.85 | 0.015 | 6.78 | 489, 240, 119, 105, 91 | - | - | No match |
| 721.6454 | 0.08 | 0.018 | 9.03 | - | - | - | No match |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximo, H.J.; Araújo, F.D.d.S.; Pagotto, C.C.; Boava, L.P.; Dalio, R.J.D.; Duarte, G.H.B.; Eberlin, M.N.; Machado, M.A. Influence of Citrus sunki and Poncirus trifoliata Root Extracts on Metabolome of Phytophthora parasitica. Metabolites 2024, 14, 206. https://doi.org/10.3390/metabo14040206
Maximo HJ, Araújo FDdS, Pagotto CC, Boava LP, Dalio RJD, Duarte GHB, Eberlin MN, Machado MA. Influence of Citrus sunki and Poncirus trifoliata Root Extracts on Metabolome of Phytophthora parasitica. Metabolites. 2024; 14(4):206. https://doi.org/10.3390/metabo14040206
Chicago/Turabian StyleMaximo, Héros José, Francisca Diana da Silva Araújo, Carolina Clepf Pagotto, Leonardo Pires Boava, Ronaldo José Durigan Dalio, Gustavo Henrique Bueno Duarte, Marcos Nogueira Eberlin, and Marcos Antonio Machado. 2024. "Influence of Citrus sunki and Poncirus trifoliata Root Extracts on Metabolome of Phytophthora parasitica" Metabolites 14, no. 4: 206. https://doi.org/10.3390/metabo14040206
APA StyleMaximo, H. J., Araújo, F. D. d. S., Pagotto, C. C., Boava, L. P., Dalio, R. J. D., Duarte, G. H. B., Eberlin, M. N., & Machado, M. A. (2024). Influence of Citrus sunki and Poncirus trifoliata Root Extracts on Metabolome of Phytophthora parasitica. Metabolites, 14(4), 206. https://doi.org/10.3390/metabo14040206






