Abstract
The quality of crops is closely associated with their geographical location and yield, which is reflected in the composition of their metabolites. Hence, we employed GC–MS pseudotargeted metabolomics to investigate the metabolic characteristics of high-, medium-, and low-yield Nicotiana tabacum (tobacco) leaves from the Bozhou (sweet honey flavour) and Shuicheng (light flavour) regions of Guizhou Province. A total of 124 metabolites were identified and classified into 22 chemical categories. Principal component analysis revealed that the geographical location exerted a greater influence on the metabolic profiling than the yield. Light-flavoured tobacco exhibited increased levels of sugar metabolism- and glycolysis-related intermediate products (trehalose, glucose-6-phosphate, and fructose-6-phosphate) and a few amino acids (proline and leucine), while sweet honey-flavoured tobacco exhibited increases in the tricarboxylic acid cycle (TCA cycle) and the phenylpropane metabolic pathway (p-hydroxybenzoic acid, caffeic acid, and maleic acid). Additionally, metabolite pathway enrichment analysis conducted at different yields and showed that both Shuicheng and Bozhou exhibited changes in six pathways and four of them were the same, mainly C/N metabolism. Metabolic pathway analysis revealed higher levels of intermediates related to glycolysis and sugar, amino acid, and alkaloid metabolism in the high-yield samples, while higher levels of phenylpropane in the low-yield samples. This study demonstrated that GC–MS pseudotargeted metabolomics-based metabolic profiling can be used to effectively discriminate tobacco leaves from different geographical locations and yields, thus facilitating a better understanding of the relationship between metabolites, yield, and geographical location. Consequently, metabolic profiles can serve as valuable indicators for characterizing tobacco yield and geographical location.
1. Introduction
Tobacco (Nicotiana tabacum L.) is widely distributed in China’s growing regions and serves as an important model plant for studying plant genetics, breeding, and biochemistry [1]. Tobacco leaves contain abundant metabolites, including saccharides, organic acids, alkaloids, and free amino acids, which play important roles in determining the quality and flavour of tobacco [2,3]. These chemical compositions are strongly influenced by environmental conditions and geographical location. Therefore, investigating the geographical location of metabolites will offer novel perspectives on the formation of regional style characteristics [4].
Metabolomics has been extensively used to trace and analyse the quality of agricultural products from various geographical locations; for instance, previous studies have investigated the bioactive components present in Glycyrrhiza uralensis taproots from different locations. Glycycoumarin and licoricone were found predominantly in Jiuquan, while neoliquiritin, isolicoflavonol, isoisoflavone alcohol, and glycerol were mainly detected in Lanzhou [5]. Similarly, Zhao et al. identified 43 differentially expressed metabolites, such as fructose, glycine, and serine, between tobacco leaves originating from Guizhou Province and those from Yunnan Province. These metabolites exert a substantial impact on the tobacco leaf flavour [6]. Guizhou tobacco exhibits distinct characteristics in different geographical regions, such as sweet honey, light, and burnt sweet flavours [7], and distinct metabolic profiles may be observed for different flavour types. Therefore, it is imperative to investigate the relationship between biochemical components and flavour types via metabolomics.
Yield, as an important evaluation index of crops, is also closely related to metabolites. During the formation process, crop yield is affected by the synthesis and degradation of metabolites, such as carbohydrates, proteins, and fats [8]. Many studies have proposed improving plant productivity and yield by increasing the photosynthetic rate and capacity [9,10]. The enhancement of rice productivity and stress resistance under favourable moisture conditions has been demonstrated through the regulation of sugar transport and metabolism, as well as the improvement in photosynthetic capacity associated with high-yield rice gene expression, resulting in a remarkable 30% increase in grain yield [11]. Previous research has found a close correlation between carbon, nitrogen metabolism systems and growth, yield [12]. Therefore, C/N metabolic pathways are intricately associated with plant yield. Significant variations were observed in the phenotypes of tobacco leaves with different yields within the same geographical region. Compared with those of low-yield tobacco plants, the leaves of high-yield tobacco plants are broader and thicker [13]. Consequently, differences in yield inevitably lead to the redistribution of metabolites, causing changes in metabolic pathways [14]. However, studies on metabolic alterations in varying yields are limited. By investigating the metabolic disparities among high-, medium-, and low-yield tobacco leaves, we can identify distinct profiles, as well as biomarkers, that influence metabolic pathways and unravel the correlation between yields and metabolic networks.
In recent years, pseudotargeted metabolomics has emerged as a pivotal tool for investigating plant disease resistance and cultivating superior varieties [15,16]. This technology is designed to rapidly, reliably, and sensitively conduct systematic and comprehensive analyses of characteristic metabolites produced in organisms, tissues, cells, and other systems by monitoring the dynamic changes in plant metabolites and their metabolic pathways [17]. The primary analytical platforms for pseudotargeted metabolomics include gas chromatography–mass spectrometry (GC–MS), liquid chromatography–mass spectrometry (LC–MS), and capillary electrophoresis–mass spectrometry (CE–MS) [18]. Among them, GC–MS is the most widely employed owing to its excellent reproducibility, high precision, extensive dynamic range, and mature metabolite database [19,20]. In 2012, Li et al. first proposed the retention time-locking GC–SIM–MS pseudotargeted metabolomics method and applied it to characteristic metabolites in tobacco leaves from different geographical locations [21]. Cai et al. utilized GC–MS pseudotargeted metabolomics to accurately analyse metabolites in Oryza sativa soil, and they demonstrated that this approach enhances the specificity, sample throughput, and coverage of the detected metabolites [22]. This method combines the benefits of both targeted and untargeted approaches, providing high sensitivity, precise quantification, and a broad linear range, and represents a promising technique that has been successfully employed for studying metabolic profiling across various tissue samples [23,24]. Therefore, the utilization of pseudotargeted metabolomics enables more accurate and sensitive monitoring of tobacco metabolites from different geographical locations and yields, with better discerning metabolic characteristics.
In this study, pseudotargeted GC–MS metabolomics was used to investigate the effects of the geographical location and yield factors on the metabolic characteristics, aiming to resolve the following issues: 1. interaction effects of geographical location and yield on metabolites in tobacco leaves; 2. influence of different geographical regions on tobacco flavour; and 3. changes in metabolic profiles under different yields.
2. Materials and Methods
2.1. Chemicals and Reagents
The metabolite standards were purchased from Sigma Aldrich (MO, USA), Tokyo Chemical Industry (Tokyo, Japan), Aladdin (Shanghai, China), J&K Chemicals (Beijing, China), and Toronto Research Chemicals (Toronto, Canada). The extraction solvents methanol and chloroform were obtained from Sinopharm Chemical Reagent (Beijing, China). Methoxyamine hydrochloride (MEOX, ≥98%), N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA, ≥98%), and anhydrous pyridine (≥99.5%) were used as derivatization reagents and were obtained from Sigma. Internal standards (ISs) of phenyl beta-D-glucopyranoside (TCI, ≥99%), hexanedioic acid (Aladdin, ≥99%), and L-norvaline (Aladdin, ≥99%) were used.
2.2. Sample Preparation
Guizhou Province is situated in the southwestern region of China and is characterized by a gradual decrease in elevation from west to east and an increase in average annual rainfall from north to south. Bozhou and Shuicheng are the primary tobacco-cultivation areas for sweet honey and light flavours in Guizhou Province, respectively, and exhibit distinct differences in geographical and climatic conditions. Bozhou is located in the middle of Guizhou Province and has a higher temperature, abundant rainfall, and shorter daylight hours, whereas Shuicheng, located in western Guizhou Province, has lower temperatures, less rainfall, and more intense sunlight. The annual ecological factor data for 2021 were provided by the Guizhou Meteorological Bureau (Table S1). Thirty-six fresh flue-cured tobacco samples (cultivar: Yunyan 87) were collected from Bozhou and Shuicheng at three yield levels in 2021. Samples of low-yield (90–110 kg/mu), medium-yield (120–140 kg/mu), and high-yield (150–170 kg/mu) flue-cured tobacco were collected from more than 500 mu of contiguous tobacco fields with six biological duplicates per treatment. During sampling, the middle leaf was identified as the tenth leaf when counting from top to bottom. The base and tip of each leaf were removed, and the middle portion was retained. Subsequently, each leaf was divided into two halves along the main vein boundary, wrapped in tin foil, and flash-frozen in liquid nitrogen. Then, the samples were freeze-dried and ground into a powder at a low temperature. After passing through a 40-mesh sieve, the samples were stored at −80 °C in an ultralow temperature refrigerator. In addition, quality control (QC) samples were obtained by thoroughly blending with the same amount of each sample.
2.3. Metabolite Extraction and Derivatization
The leaf powder (50 mg) was added to a 10 mL centrifuge tube, followed by the addition of 40 µL of internal standard solution (hexanedioic acid at a concentration of 10 mg/mL, phenylglucoside at a concentration of 8.04 mg/mL, and L-norvaline at a concentration of 4.9 mg/mL in a methanol–water ratio of 1:1, v/v). Subsequently, 3 mL of the extract solution (methanol–chloroform–water 2.5:1:1, v/v/v) was added. After vortexing for 1 min, ultrasound extraction was performed at 4–10 °C for 40 min, after which the mixture was centrifuged at 3000–5000 rpm for 5 min. Three-hundred microlitres of supernatant were dried under N2 flow at room temperature and then further dried completely by adding three-hundred microlitres of dichloromethane.
Following this step, the derivatization reaction was carried out by reacting with a solution containing MEOX/pyridine (40 µL of 25 mg/mL) as an oximation agent (40 °C, 120 min), which protected carbonyl groups and reduced the ring reactions of sugars to minimize isomer formation. Trimethylsilylation was subsequently performed by adding BSTFA reagent containing TMCS (1%) (81 °C, 90 min), after which 90 µL of acetonitrile was added (81 °C, 90 min) to improve the derivatization efficiency of the amino group. Then, the samples were centrifuged at 10,000 rpm for 3 min, and the supernatant was subjected to GC–MS analysis.
2.4. GC–MS Pseudotargeted Metabolomics
GC–MS analysis was performed on an Agilent 7890A-5975C instrument (Palo Alto, CA, USA) equipped with a CTC PAL autoinjection system. Separation was achieved utilizing an HP-5 MS (60 m × 250 µm × 0.25 µm film thickness) capillary column. The injector port temperature was maintained at 280 °C, and a sample volume of 1 µL was injected through the autosampler at a split ratio of 1:10. The flow rate of the helium carrier gas remained constant at 1.0 mL/min. A temperature gradient program was employed for the oven, starting at 60 °C for 2 min, followed by an increase of 5 °C/min until reaching and holding at 230 °C for another 5 min; then, it was further increased by 8 °C/min to reach and hold at 290 °C for 21.5 min, for a total run time of 70 min. The ion source and quadrupole temperatures were set to 230 °C and 150 °C, respectively, while the transfer line temperature was maintained at 280 °C. The mass spectrometer was operated in the electron ionization mode (EI) at 70 eV. The full-scan acquisition mode was adopted for identification within the mass range of 45–600 m/z with a solvent delay time of 11.90 min. Pseudotargeted metabolomics incorporates an algorithm designed to choose ions for selected ion monitoring (SIM) from identified metabolites. The SIM data were acquired based on the published literature [21], and AMDIS software version 2.73 (Automated Mass Spectral Deconvolution and Identification System) was used for the selection of characteristic ions. The detailed peak table is shown in Table S2. The metabolites in the QC sample were identified using a standard mass spectrometry database (NIST14 and Willy08 library), the literature, and the linear retention index (LRI). Hexanedioic acid (10.00 mg/mL), phenyl beta-D-glucopyranoside (8.04 mg/mL), and L-norvaline (4.90 mg/mL) were used as ISs for quantification, and the correction factor was F = 1 for relative quantification.
2.5. Statistical Analysis
Chemometric analysis included different multivariate data analysis methods, such as principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA). Simca software 13.0 (Sartorius, Umeå, Sweden) was utilized to construct these models. Metabolic pathway analysis, a heatmap, and a volcano map analysis were carried out using metware cloud (https://cloud.metware.cn/) accessed on 16 August 2023. To normalize the data, log transformation and Pareto scaling were performed. The screening of highly characteristic metabolites among the samples was conducted according to the standard of Cai et al. [22]. Chromatograms of the QC samples were generated using Origin 2021 software version SR1 (OriginLab Corp., Northampton, MA, USA).
3. Results
3.1. Metabolite Identification in Tobacco Leaves
A total of thirty-six tobacco leaf samples from different geographical locations and yields were comprehensively analysed. Figure 1A shows the typical chromatogram of the QC sample, which represented a ‘mean’ sample containing all possible metabolites. A total of 124 metabolites, mainly amino acids, saccharides, sugar acids, and sugar alcohols, were identified (Table S2). These metabolites were identified with standards and LRIs or mass spectral libraries and were classified into 22 chemical categories (Figure 1B; Table S2). The top ten chemical classifications were amino acids (25 accounting for 20.2%), saccharides (17, 13.7%), sugar acids (9, 7.3%), phosphate esters or phosphate compounds (9, 7.3%), sugar alcohols (8, 6.5%), dicarboxylic acids (7, 5.6%), polyhydroxy carboxylic acids (6, 4.8%), and phenolic acids (6, 4.8%). Short-chain fatty acids, long-chain fatty acids, polyamines, and saccharolactones contributed 3.2% individually. The amino acid group comprised twenty-one proteinogenic amino acids, as well as four nonproteinogenic amino acids or derivatives such as gamma-aminobutyric acid, pyroglutamic acid, 5-hydroxytryptophan, and pipecolinic acid. The saccharide group consisted of thirteen monosaccharides, including hexoses, pentoses, tetrose, and triose, along with four disaccharides. The tobacco pseudotargeted metabolomics approach facilitated the detection of a wider range of metabolites from various chemical classes representing key metabolic pathways for tobacco metabolic profiling.
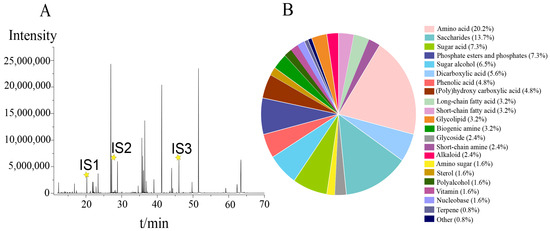
Figure 1.
(A) Pseudotargeted-mode chromatogram of the QC sample. IS1, IS2, and IS3 represent the chromatographic peaks of the internal standards L-norvaline, hexanedioic acid, and phenylglucoside, respectively.  is retention time of IS 1 (20.241 min), IS2 (27.219 min) and IS3 (46.081 min); (B) numerical distribution and ratio of 21 chemical classifications.
is retention time of IS 1 (20.241 min), IS2 (27.219 min) and IS3 (46.081 min); (B) numerical distribution and ratio of 21 chemical classifications.
 is retention time of IS 1 (20.241 min), IS2 (27.219 min) and IS3 (46.081 min); (B) numerical distribution and ratio of 21 chemical classifications.
is retention time of IS 1 (20.241 min), IS2 (27.219 min) and IS3 (46.081 min); (B) numerical distribution and ratio of 21 chemical classifications.
3.2. Interaction Effect of Geographical Location and Yield on Metabolites
PCA was employed to discriminate between the geographical locations of the tobacco leaves from Bozhou and Shuicheng (Figure 2A). The first two principal components (PCs) explained 50.1% of the total variance, with PC1 and PC2 explaining 31.0% and 19.1% of the variance, respectively. PC1 distinguished the geographical location, while PC2 distinguished the yield. The QC samples were tightly clustered in the centre of the score plot, indicating that the sample analysis results were precise. Based on distinct separation, thirty-six samples were categorized into two groups. Significant differences were observed between the Shuicheng and Bozhou regions in PC1. However, samples from the same region, but with different yields, could not be completely distinguished in PC2, such as medium versus low yields in Bozhou and medium versus high yields in Shuicheng. This observation was further supported by the conversion of the PCA data into the corresponding metabolic trajectories (Figure 2B), suggesting that the influence of the geographical location on the metabolite levels may outweigh that of the yield variation.
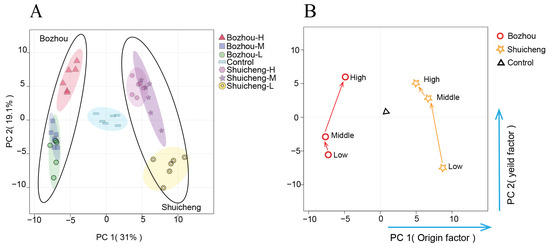
Figure 2.
Interaction effect of geographical location and yield on metabolites. (A) PCA score plot; (B) metabolic trajectory diagram.
Based on the loading factor of PCA, the contribution rates of tobacco metabolites to geographical location and yield differentiation were analysed (Table 1). The metabolites that contributed significantly to discriminating the geographical location (absolute value > 0.12) included saccharides, sugar acids, sugar alcohols, and phosphorylated sugars, which indicated that regional factors mainly affected carbohydrate metabolism and phosphorylation. On the other hand, the metabolites that contributed significantly to discriminating the yield factors (absolute value > 0.12) were mainly nitrogenous metabolites, such as amino acids and polyamines, which showed that nitrogen metabolism was a key determinant for achieving the desired crop yields. In brief, the geographical location had a greater influence than yield on metabolic changes in tobacco leaves, and these critical metabolites play crucial roles in plant development and growth regulation.

Table 1.
Metabolite contributions to geographical location and yield factors.
3.3. Metabolic Profiling in Different Geographical Locations
To further visualize the differences between the two geographical locations (flavour types) of the metabolites, we applied PLS-DA for discrimination (Figure 3A). The score plots of PC1 and PC2 clearly demonstrated the distinct separation between the Shuicheng and Bozhou samples, accounting for 32.4% and 13.1% of the total variance, respectively. A volcanic map with variable importance in projection (VIP) was drawn according to the screening standards of p value < 0.05, FC > 1.5, and VIP > 1.2; thus, 31 characteristic biomarker metabolites were found (Figure S1). These biomarkers included primary metabolites, such as maleic acid, threonic acid, proline, and phenylalanine, as well as secondary metabolites, such as caffeic acid and quinic acid. Subsequently, heatmap analysis was performed on these characteristic metabolites (Figure 3B), which revealed two distinct groups. Group A mainly consisted of the Bozhou samples with greater abundances of phenylpropane metabolism (salicylic acid, VIP = 1.72; p-hydroxybenzoic acid, VIP = 1.7; caffeic acid, VIP = 1.69) and the TCA cycle (fumaric acid, VIP = 1.64; maleic acid, VIP = 1.68), along with sugar acid (6C sugar acid, VIP = 1.58; 5C sugar acid, VIP = 1.32) and saccharolactones (L-arabonic acid-1,4-lactone, VIP = 1.66). Group B predominantly comprised the Shuicheng samples exhibiting relatively high levels of sugar metabolism- and glycolysis-related intermediate products (trehalose, VIP = 1.36; glucose-6-phosphate, VIP = 1.3; fructose-6-phosphate, VIP = 1.38) and a few amino acids (proline, VIP = 1.33; leucine, VIP = 1.39), which are all crucial factors influencing the location (flavour type).
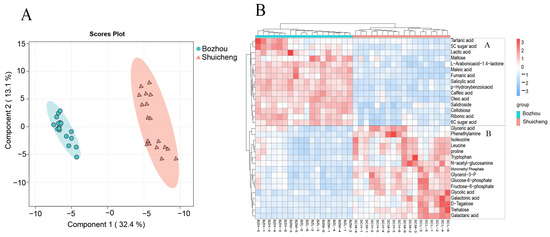
Figure 3.
Analysis of metabolites in different geographical locations. (A) PLS-DA analysis. (B) Heatmap analysis of characteristic metabolites.
3.4. Characteristic Metabolites and Their Metabolic Pathways at Different Yields
The characteristic metabolites in tobacco leaves with different yields were analysed. According to Figure S2, the three yields in the Shuicheng area were effectively distinguished, whereas distinguishing between the middle and low yields in Bozhou was challenging. To further investigate the differences among these three yields in Bozhou and Shuicheng, the PLS-DA of VIP > 1.2, FC > 1.5, and p < 0.05 was used to conduct characteristic metabolite screening (Figures S3 and S4), and metabolite pathway enrichment analysis was subsequently conducted at different yields (Figure 4). The main enrichment pathways of the Bozhou samples were phenylalanine metabolism; phenylalanine, tyrosine, and tryptophan biosynthesis; starch and sucrose metabolism; glycine, serine, and threonine metabolism; alanine, aspartate, and glutamate metabolism; and isoquinoline alkaloid biosynthesis (Figure 4A). The main enrichment pathways of the Shuicheng samples were phenylalanine metabolism; arginine biosynthesis; starch and sucrose metabolism; glycine, serine, and threonine metabolism; alanine, aspartic acid, and glutamate metabolism; and linoleic acid metabolism (Figure 4B). These findings suggest that samples from both Shuicheng and Bozhou exhibited changes in six pathways, four of which were the same and mainly involved C/N metabolism.
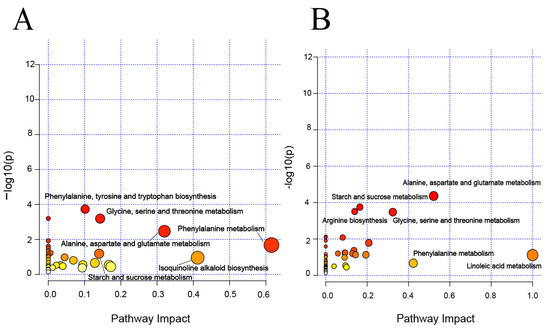
Figure 4.
Metabolite pathway enrichment analysis for tobacco leaves with different yields. (A) Bozhou. (B) Shuicheng. The larger the circle and the darker the colour, the more significantly enriched the metabolites are in this pathway.
Further analysis of these metabolic pathways (Figure 5 and Figure S5) revealed higher levels of intermediates related to glycolysis and sugar metabolism (e.g., glucose, sucrose, fructose, fructose-6-phosphate, and glucose-6-phosphate) in the high-yield tobacco samples than in the other samples. However, sugar alcohols exhibited a significant increase in both the middle-yield and low-yield tobacco samples in the two regions. The content of amino acid metabolism intermediates, such as serine, threonine, and valine, increased in high-yield tobacco leaves. Moreover, the levels of phenylalanine, tryptophan, and shikimic acid were also elevated, indicating that the metabolic pathway of phenylpropane in the high-yield tobacco leaves of the two regions improved. High-yield tobacco leaves also improved the urea cycle in both regions, thereby increasing the content of polyamines and nicotine alkaloids.
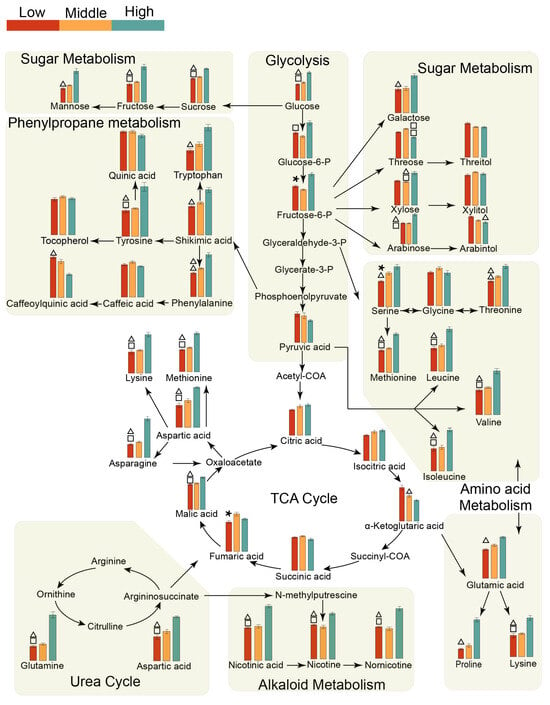
Figure 5.
Metabolic pathway plot of the differentially abundant metabolites among the three yields in Bozhou. Red, yellow, and green indicate the relative concentrations of metabolites at low yield, intermediate yield, and high yield, respectively. △, *, and □ represent p values of metabolites using nonparametric tests that were less than 0.05 for the low-yield vs. high-yield, low-yield vs. middle-yield, and middle-yield vs. high-yield comparisons, respectively.
4. Discussion
4.1. Metabolite Identification and Method Evaluation
In general, methanol–chloroform–water is an effective metabolite extraction system for water-soluble and hydrophobic metabolites in plant matrices [23]. The accurate identification of 124 metabolites belonging to 22 chemical categories was successfully achieved in tobacco leaves. These metabolites play crucial roles as significant contributors to the C/N metabolic cycle. In addition, the reproducibility of the results is also a crucial aspect when evaluating the quality of an analytical method [25,26]. All metabolites were subjected to normalization using an internal standard for relative quantification. As indicated in Table S2, 97.6% and 87.9% of all metabolites had a relative standard deviation (RSD) below 20% for repeatability and reproducibility, respectively. The repeatability and reproducibility were considered acceptable and in line with the values commonly found for plant metabolomics (ca. 25–35%) [27]. All the results indicated that tobacco pseudotargeted GC–MS analysis is a dependable approach for metabolic profiling.
Although the precision met the requirements for relative quantification, it is recommended that an appropriate internal standard system with each chemical classification is needed to effectively improve the accuracy and precision. The isotopic or homologous internal standard is the best choice for further research [22]. Furthermore, pseudotargeted analysis cannot detect metabolites that have not been identified. Untargeted metabolomics is a complementary approach for discovering crucial signals of unknown metabolites in tobacco.
4.2. Characteristic Metabolites of Different Geographical Locations and Their Effects on Flavour Type
Previous studies have shown that light-flavoured tobacco is characterized by freshness, floral notes, and acidity, while fully flavoured tobacco predominantly possesses a high aroma profile with a rich and pure fragrance [28,29]. Carbohydrates constitute the most significant precursors of aroma in tobacco, accounting for 40–50% of its weight [30,31]. These compounds generate flavour components and acidic substances in mainstream smoke that mitigate the harsh taste during smoking while enhancing the overall flavour characteristics and aroma perception [32]. By screening the characteristic metabolites of the two geographical locations, the abundances of saccharides and phosphorylated sugars in the Shuicheng sample were greater than those in the Bozhou samples. Notably, trehalose, fructose-6-phosphate, and glucose-6-phosphate were identified, two of which are intermediate products of glycolysis (the oxidation process from glucose to pyruvate). Additionally, proline and leucine were more abundant in the Shuicheng samples than in the Bozhou samples; proline contributes to freshness and floral attributes, while leucine significantly enhances acidic notes [33]. Therefore, these metabolic characteristics may lead to the formation of light-flavoured tobacco leaves. It is widely recognized that a decreased nitrogen nutrition level promotes the formation of a delicate aroma profile in flue-cured tobacco, whereas an increased nitrogen nutrition level enhances the expression of a strong and abundant aroma style in tobacco [34]. The abundance of organic acids in the Bozhou samples was much greater than that in the Shuicheng samples. Organic acids play a crucial role in smoke equilibrium and tobacco pH regulation, ultimately influencing the aroma quality indirectly [35]. For instance, Bozhou has a higher concentration of oleic acid than Shuicheng, while high levels of unsaturated fatty acids can enhance the flavour of acidic wax and fat [34]. Moreover, as intermediate products of the phenylpropane metabolic pathway (caffeic acid), the abundance of the Bozhou samples was also greater than that of the Shuicheng samples. Phenylpropanoid biosynthesis in most plants initiates the conversion of phenylalanine to cinnamic acid, resulting in diverse aromatic compounds and affecting the aroma of tobacco leaves [36]. Furthermore, a high concentration of ester compounds leads to a stronger irritant taste, and the L-arabonic acid-1,4-lactone in Bozhou samples may be one of the reasons for the abundant aroma [37]. Therefore, these metabolic characteristics may lead to the sweet honey and light flavour types in Guizhou.
4.3. Characteristic Metabolites and Metabolic Pathways of Different Yields
The metabolic pathways involved in C metabolism, such as sugar metabolism, glycolysis, the TCA cycle, and shikimate–phenylpropanoid metabolism, play crucial roles in generating energy that can be utilized by plants for growth and development, simultaneously improving the resistance of plants and providing the carbon skeletons necessary for various biosynthetic processes [38]. It is widely acknowledged that the production of carbohydrates in source organs and their utilization in sink organs are tightly coordinated processes that ultimately determine the yield [39]. Starch and sucrose metabolism comprised the main enrichment metabolic pathway, and there was an increase in the abundance of glucose, fructose, sucrose, fructose-6-phosphate, glucose-6-phosphate, and other saccharides in the high-yield tobacco samples from the two regions. The metabolic pathways of starch and sucrose metabolism are complex biochemical processes that rely on the synergistic action of multiple enzymes [40]. Phenylpropane metabolism intermediates are crucial for plant growth and the long-distance transport of water and nutrients while also aiding plant defence against abiotic and biotic stresses [41,42]. For instance, quinic acid plays a vital role as an antioxidant by protecting enzyme structures within plants [43]. Notably, low-yield tobacco leaves exhibited increases in caffeic acid, caffeoquinic acid, and quinic acid levels, indicating an higher expression in the phenylpropane metabolic pathway. Due to cultivation stress, this pathway can produce abundant antioxidants and protect tobacco plants from the stress effects. Conversely, high-yield tobacco showed a significant increase in phenylalanine but a decrease in phenylpropane metabolites due to potential inhibition of phenylalanine ammonia lyase enzyme activity [44].
The metabolic pathways involved in N metabolism, such as amino acid metabolism, polyamine metabolism, and the urea cycle, serve as crucial physiological mechanisms that regulate the synthesis and decomposition of nitrogen-containing compounds in plants [45]. Amino acids serve as precursors for numerous nitrogen-containing compounds [46]. The content of most amino acids in the Bozhou samples decreased from high yield to middle yield to low yield. Notably, the altered metabolic pathways included alanine, aspartate, and glutamate metabolism and glycine, serine, and threonine metabolism in the two regions. Glycine and serine, which are essential components of photorespiration, contribute to the provision of one-carbon (1-C) units that actively engage in diverse metabolic pathways, such as polyamine metabolism and nucleic acid metabolism [47]. Furthermore, high-yield tobacco in Bozhou significantly enhanced the urea cycle, leading to increased contents of polyamines and nicotine while altering the isoquinoline alkaloid biosynthesis pathway. However, the breeding objective of tobacco has always been to reduce the levels of nicotine and related alkaloids [48]. Furthermore, a previous study indicated that treatment of tobacco plants with polyamine biosynthesis inhibitors can reduce the polyamine content and ameliorate the phenotype [49]. Hence, polyamine and nicotine biosynthesis in tobacco involves complex interactions that affect the quality of tobacco leaves.
To summarize, the variation in plant metabolites is primarily influenced by the geographical location and yield [50]. Xu et al. reveal that metabolic differences of E. purpurea were related to geographical location (latitude and longitude) and environmental variables (climate and soil) with NMR [51], while, Benmahieddine et al. used HPLC-DA to identify metabolic characteristics of Pistacia atlantica Desf. with gender, organ type (roots, buds, and fruits), geographical location, and stage of ripening [52]. The factors influencing metabolites are highly complex. Therefore, Further research needs to consider the effect of more environmental factors and different harvest time on metabolic characteristics and tobacco flavour types.
5. Conclusions
A total of 124 metabolites were identified in Guizhou tobacco leaves of different geographical locations and yields by GC–MS pseudotargeted metabolomics and were divided into 22 chemical categories. Multifactor analysis revealed that the geographical location had a greater influence on metabolites than the yield factors. A screening of the characteristic metabolites in tobacco leaves from different regions revealed that the levels of sugar metabolism- and glycolysis-related intermediate products and amino acids were greater in the Shuicheng samples (light flavour), and the contents of organic acid, sugar acid, and glycolactone involved in phenylpropane metabolism and the TCA cycle were greater in the Bozhou samples (sweet honey flavour). Metabolic pathway analysis revealed that glycolysis and sugar, amino acid, and alkaloid metabolism were maintained at higher levels in the high-yield samples, while higher expression of phenylpropane metabolism was maintained in the low-yield samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo14040176/s1. Table S1. Sample information; Table S2. List of 124 identified metabolites from 22 classifications detected with pseudotargeted GC-MS. Figure S1. Characteristic metabolite volcano map; Figure S2. (A) PCA analysis of low-, middle-, and high-yields in Bozhou. (B) PCA analysis of low-, middle-, and high-yields in Shuicheng; Figure S3. Volcanic maps and VIP of characteristic metabolites in Bozhou. (A) High- versus low-yield, (B) middle- versus low-yield, and (C) high- versus middle-yield tobacco leaves; Figure S4. Volcanic maps and VIP of characteristic metabolites in Shuicheng. (A) High- versus low-yield, (B) middle- versus low-yield, and (C) high- versus middle-yield tobacco; Figure S5. Metabolic pathway plot of the differentially abundant metabolites among the three yields in Shuicheng.
Author Contributions
Y.J.: Methodology, conceptualization, data curation, investigation, formal analysis, writing—original draft preparation; W.C. and X.Q.: Visualization, validation; S.Q. and W.G.: Investigation, data curation; C.L.: Reviewing and editing; W.Q.: Investigation, reviewing and editing, funding acquisition; K.C.: Writing, conceptualization, writing—reviewing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the Guizhou Provincial Basic Research Program (Natural Science) (No. QianKeHe-ZK [2024] General 645), the Science and Technology Program of Guizhou Provincial Branch of the CNTC (No. 2023XM16), the Key Program for Science and Technology of CNTC (No. 110202202030), the Young Elite Scientists Sponsorship Program of CNTC, the Talent Project of Science and Technology Department of Guizhou Province (No. 20206020), and the Guizhou Provincial Characteristic Key Laboratory (No. QJHKY [2021]002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request. Data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.T.; Zhang, Y.; Du, Y.Y.; Chen, S.Y.; Tang, H.R. Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.Y.; Guo, J.Z.; Xia, Q.L.; Zhao, G.; Zhou, H.N.; Xie, F.W. Metabolic profiling of Chinese tobacco leaf of different geographical origins by GC-MS. J. Agric. Food Chem. 2013, 61, 2597–2605. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Li, L.L.; Zhao, Y.N.; Zhao, C.X.; Chen, X.; Liu, P.P.; Zhou, H.N.; Zhang, J.J.; Hu, C.X.; Chen, A.G.; et al. Metabolic changes in primary, secondary, and lipid metabolism in tobacco leaf in response to topping. Anal. Bioanal. Chem. 2018, 410, 839–851. [Google Scholar] [CrossRef]
- Chang, W.; Zhao, H.N.; Yu, S.Z.; Yu, J.; Cai, K.; Sun, W.; Liu, X.M.; Li, X.D.; Yu, M.N.; Ali, S.; et al. Comparative transcriptome and metabolomic profiling reveal the complex mechanisms underlying the developmental dynamics of tobacco leaves. Genomics 2020, 112, 4009–4022. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.Y.; Bao, F.; Fan, X.R.; Han, S.; Zheng, W.H.; Sun, L.L.; Yan, N.; Du, H.; Zhao, H.Y.; Yang, Z.G. Metabolomics study of different parts of licorice from different geographical origins and their anti-inflammatory activities. J. Sep. Sci. 2020, 43, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Hu, C.X.; Zeng, J.; Zhao, Y.N.; Zhang, J.J.; Chang, Y.W.; Li, L.L.; Zhao, C.X.; Lu, X.; Xu, G.W. Study of polar metabolites in tobacco from different geographical origins by using capillary electrophoresis–mass spectrometry. Metabolomics 2014, 10, 805–815. [Google Scholar] [CrossRef]
- Yang, C.; Wu, W.; Wu, S.C.; Liu, H.B.; Peng, Q. Aroma types of flue-cured tobacco in China: Spatial distribution and association with climatic factors. Theor. Appl. Clim. 2014, 115, 541–549. [Google Scholar] [CrossRef]
- Aguirre, M.; Kiegle, E.; Leo, G.; Ezquer, I. Carbohydrate reserves and seed development: An overview. Plant Reprod. 2018, 31, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.; Park, J.S.; Nagai, Y.; Hwang, S.K.; Cho, Y.C.; Roh, K.H.; Lee, S.M.; Kim, D.H.; Choi, S.B.; Ito, H.; et al. Exploiting leaf starch synthesis as a transient sink to elevate photosynthesis, plant productivity and yields. Plant Sci. 2011, 181, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Ambavaram, M.M.R.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Gao, X.; Peng, J.J. The allometry and dynamics of carbohydrate and nitrogen in vegetative organs and the relationship to cotton yield. J. Plant Nutr. 2021, 45, 3080–3093. [Google Scholar] [CrossRef]
- Djajadi, D. Tobacco diversity in Indonesia. J. Biol. Res. 2015, 20, 27–32. [Google Scholar] [CrossRef]
- Reichert, J.M.; Pellegrini, A.; Rodrigues, M.F. Tobacco growth, yield and quality affected by soil constraints on steeplands. Ind. Crops Prod. 2019, 128, 512–526. [Google Scholar] [CrossRef]
- Shen, S.Q.; Zhan, C.S.; Yang, C.K.; Fernie, A.R.; Luo, J. Metabolomics-centered mining of plant metabolic diversity and function: Past decade and future perspectives. Mol. Plant 2023, 16, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Wang, L.C.; Xuan, Q.H.; Zhao, X.J.; Liu, X.Y.; Shi, X.Z.; Xu, G.W. Pseudotargeted method based on parallel column two-dimensional liquid chromatography-mass spectrometry for broad coverage of metabolome and lipidome. Anal. Chem. 2020, 92, 6043–6050. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhou, L.N.; Shi, X.Z.; Xu, G.W. New advances in analytical methods for mass spectrometry-based large-scale metabolomics study. Trends Anal. Chem. 2019, 121, 115665. [Google Scholar] [CrossRef]
- Zheng, F.J.; Zhao, X.J.; Zeng, Z.D.; Wang, L.C.; Lv, W.J.; Wang, Q.Q.; Xu, G.W. Development of a plasma pseudotargeted metabolomics method based on ultra-high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2020, 15, 2519–2537. [Google Scholar] [CrossRef]
- Koh, Y.; Pasikanti, K.K.; Yap, C.; Eric, C. Comparative evaluation of software for retention time alignment of gas chromatography/time-of-flight mass spectrometry-based metabonomic data. J. Chromatogr. A 2010, 1217, 8308–8316. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, Q.; Li, Y.L.; Ye, G.Z.; Lu, X.; Lin, X.H.; Xu, G.W. A novel approach to transforming a non-targeted metabolic profiling method to a pseudo-targeted method using the retention time locking gas chromatography/mass spectrometry-selected ions monitoring. J. Chromatogr. A 2012, 1255, 228–236. [Google Scholar] [CrossRef]
- Cai, K.; Zhao, Y.P.; Kang, Z.J.; Wang, S.L.; Wright, A.L.; Jiang, X.J. Environmental pseudotargeted metabolomics: A high throughput and wide coverage method for metabolic profiling of 1000-year paddy soil chronosequences. Sci. Total Environ. 2023, 858, 159978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Zhao, C.X.; Lu, X.; Zhou, H.N.; Li, Y.L.; Zhou, J.; Chang, Y.W.; Zhang, J.J.; Jin, L.F.; Lin, F.C.; et al. Investigation of the relationship between the metabolic profile of tobacco leaves in different planting regions and climate factors using a pseudotargeted method based on gas chromatography/mass spectrometry. J. Proteome Res. 2013, 12, 5072–5083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Zhao, C.X.; Li, Y.L.; Chang, Y.W.; Zhang, J.J.; Zeng, Z.D.; Lu, X.; Xu, G.W. Study of metabolite differences of flue-cured tobacco from different regions using a pseudotargeted gas chromatography with mass spectrometry selected-ion monitoring method. J. Sep. Sci. 2014, 37, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.C.M.; Caldana, C.; Wolf, L.D.; de Abreu, L.G.F. The importance of experimental design, quality assurance, and control in plant metabolomics experiments. In Plant Metabolomics; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1778, pp. 3–17. [Google Scholar]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Phélippé, M.; Coat, R.; Bras, C.L.; Perrochaud, L.; Peyretaillade, E.; Kucma, D.; Arhaliass, A.; Thouand, G.; Cogne, G.; Gonçalves, O. Characterization of an easy-to-use method for the routine analysis of thecentral metabolism using an affordable low-resolution GC–MS system: Application to Arthrospira platensis. Anal. Bioanal. Chem. 2018, 410, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, X.M.; Song, S.Q.; Han, T.; Karangwa, E. Identification of aroma types and their characteristic volatile compounds of Chinese faint-scent cigarettes based on descriptive sensory analysis and GC–MS and partial least squares regression. Eur. Food Res. Technol. 2016, 242, 869–880. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Z.Y.; Shi, J.X. Quality characteristics of tobacco leaves with different aromatic styles from Guizhou Province, China. Agric. Sci. China 2007, 6, 220–226. [Google Scholar]
- Banožić, M.; Jokić, S.; Ačkar, Đ.; Blažić, M.; Subaric, D. Carbohydrates—Key players in tobacco aroma formation and quality determination. Molecules 2020, 25, 1734. [Google Scholar] [CrossRef]
- Liu, A.; Yuan, K.L.; Xu, H.Q.; Zhang, Y.G.; Tian, J.K.; Li, Q.; Zhu, W.; Ye, H. Proteomic and metabolomic revealed differences in the distribution and synthesis mechanism of aroma precursors in yunyan 87 tobacco leaf, stem, and root at the seedling stage. ACS Omega 2022, 7, 33295–33306. [Google Scholar] [CrossRef]
- Purkis, S.W.; Mueller, C.; Intorp, M. The fate of ingredients in and impact on cigarette smoke. Food Chem. Toxicol. 2011, 49, 3238–3248. [Google Scholar] [CrossRef]
- Yin, F.; Karangwa, E.; Song, S.; Duhoranimana, E.; Lin, S.; Cui, H.; Zhang, X.M. Contribution of tobacco composition compounds to characteristic aroma of Chinese faint-scent cigarettes through chromatography analysis and partial least squares regression. J. Chromatogr. B 2019, 1105, 217–227. [Google Scholar] [CrossRef]
- Yun, F.; Liu, G.S.; Shi, H.Z.; Yang, X.W. Interactive effects of light intensity and nitrogen supply on the neutral volatile aroma components and organic acids of flue-cured tobacco. J. Food Agric. Environ. 2013, 11, 1187–1194. [Google Scholar]
- Xiang, G.; Yang, H.Y.; Yang, L.; Zhang, X.; Cao, Q.E.; Miao, M.M. Multivariate statistical analysis of tobacco of different origin, grade and variety according to polyphenols and organic acids. Microchem. J. 2010, 95, 198–206. [Google Scholar] [CrossRef]
- Tsaballa, A.; Sarrou, E.; Xanthopoulou, A.; Tsaliki, E.; Kissoudis, C.; Karagianni, E.; Michailidis, M.; Martens, S.; Sperdouli, E.; Hilioti, Z.; et al. Comprehensive approaches reveal key transcripts and metabolites highlighting metabolic diversity among three oriental tobacco varieties. Ind. Crops Prod. 2020, 143, 111933. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Zhang, X.Y.; Chen, Y.; Zhao, L.; Su, J.E.; Qu, S.B.; Ji, X.W.; Wang, T.; Li, Z.J.; et al. The applicability of different tobacco types to heated tobacco products. Ind. Crops Prod. 2021, 168, 113579. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Rossi, M.; Bermudez, L.; Carrari, F. Crop yield: Challenges from a metabolic perspective. Curr. Opin. Plant Biol. 2015, 25, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, R.; Wang, X.; Liang, W.; Liao, J.; Huang, X.; Cai, Z.; Liu, D.; Huang, L.; Wei, X. The starch-sugar interconversion mechanism during bulb development of Cardiocrinum giganteum (wall.) makino revealed by transcriptome and metabolite analysis. Ind. Crops Prod. 2022, 187, 1–11. [Google Scholar] [CrossRef]
- Kim, J.I.; Hidalgo-Shrestha, C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Zhang, X.B.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Zhao, J.Y.; Zhao, C.X.; Zhou, H.N.; Li, Y.L.; Zhang, J.J.; Li, L.L.; Hu, C.X.; Li, W.Z.; Peng, X.J.; et al. A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci. Rep. 2015, 5, 16346. [Google Scholar] [CrossRef] [PubMed]
- Nölke, G.; Chudobova, I.; Houdelet, M.; Volke, D.; Lusso, M.; Frederick, J.; Kudithipudi, C.; Shen, Y.X.; Warek, U.; Strickland, J.A.; et al. Impact of nicotine pathway downregulation on polyamine biosynthesis and leaf ripening in tobacco. Plant Direct 2021, 5, e00329. [Google Scholar] [CrossRef]
- Nölke, G.; Volke, D.; Chudobová, I.; Houdelet, M.; Lusso, M.; Frederick, J.; Adams, A.; Kudithipudi, C.; Warek, U.; Strickland, J.A.; et al. Polyamines delay leaf maturation in low-alkaloid tobacco varieties. Plant Direct 2018, 2, e00077. [Google Scholar] [CrossRef]
- Bayona, L.M.; Leeuwen, G.V.; Erol, O.; Swierts, T.; Ent, E.V.D.; de Voogd, N.J.; Choi, Y.H. Influence of geographical location on the metabolic production of giant barrel sponges (Xestospongia spp.) revealed by metabolomics tools. ACS Omega 2020, 5, 12398–12408. [Google Scholar] [CrossRef]
- Xu, W.Q.; Cheng, Y.L.; Guo, Y.H.; Yao, W.R.; Qian, H. Effects of geographical location and environmental factors on metabolite content and immune activity of Echinacea purpurea in China based on metabolomics analysis. Ind. Crops Prod. 2022, 189, 115782. [Google Scholar] [CrossRef]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Zerey-Belaskri, A.E.; Gismondi, A.; Marco, G.D.; Canini, A.; Bechlaghem, N.; Bekkara, F.A.; Djebli, N. Influence of plant and environment parameters on phytochemical composition and biological properties of Pistacia atlantica Desf. Biochem. Syst. Ecol. 2021, 95, 104231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).