Evaluating the Metabolomic Profile and Anti-Pathogenic Properties of Cannabis Species
Abstract
1. Introduction
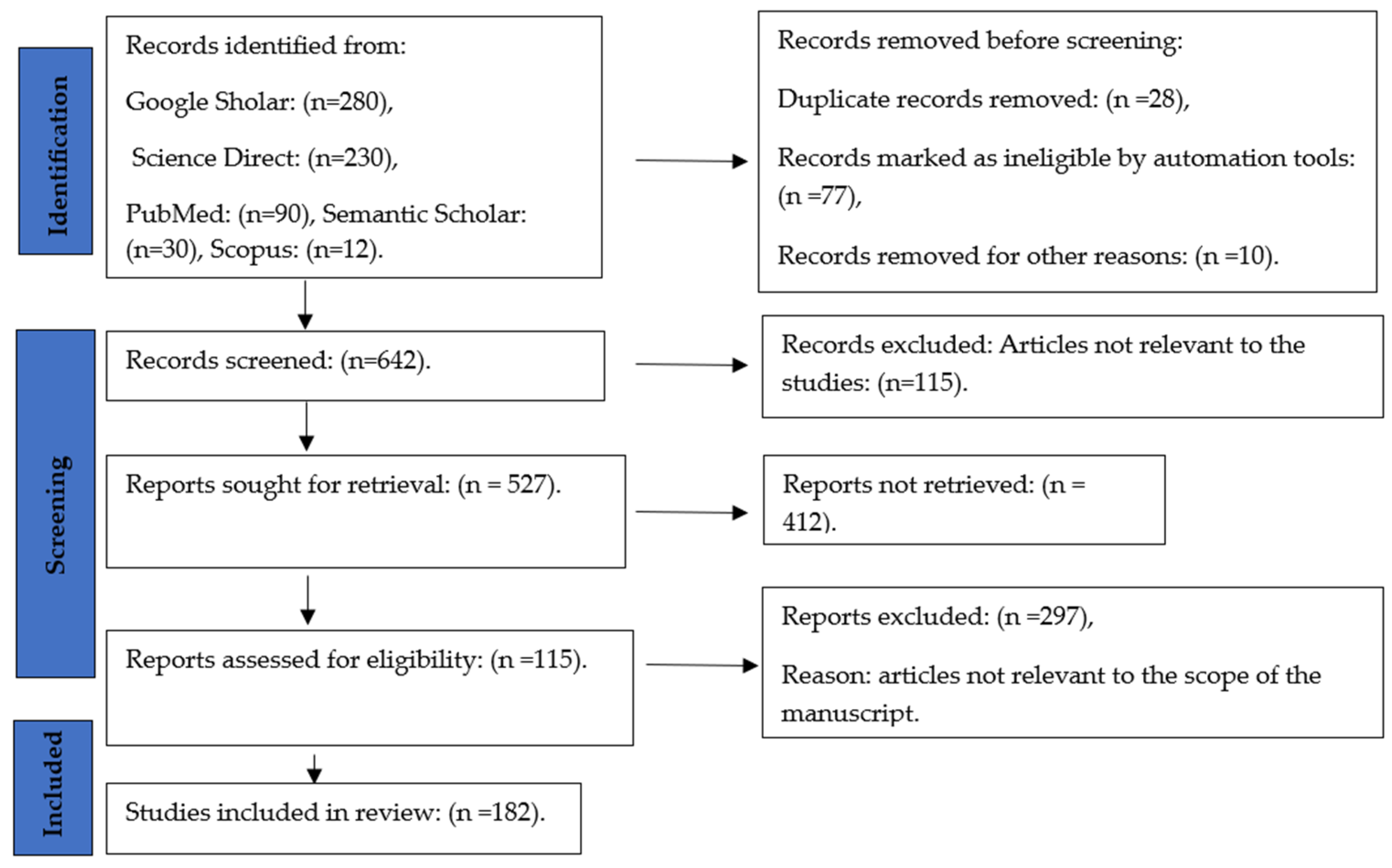
2. Materials and Methods
2.1. Search Strategies
2.2. Selection Criteria
2.3. Risk of Bias Assessment
3. Results and Discussion
3.1. The Use of Advanced Metabolomics Tools in Research Studies
3.2. Application of Metabolomics in Cannabis (Cannabinomics)
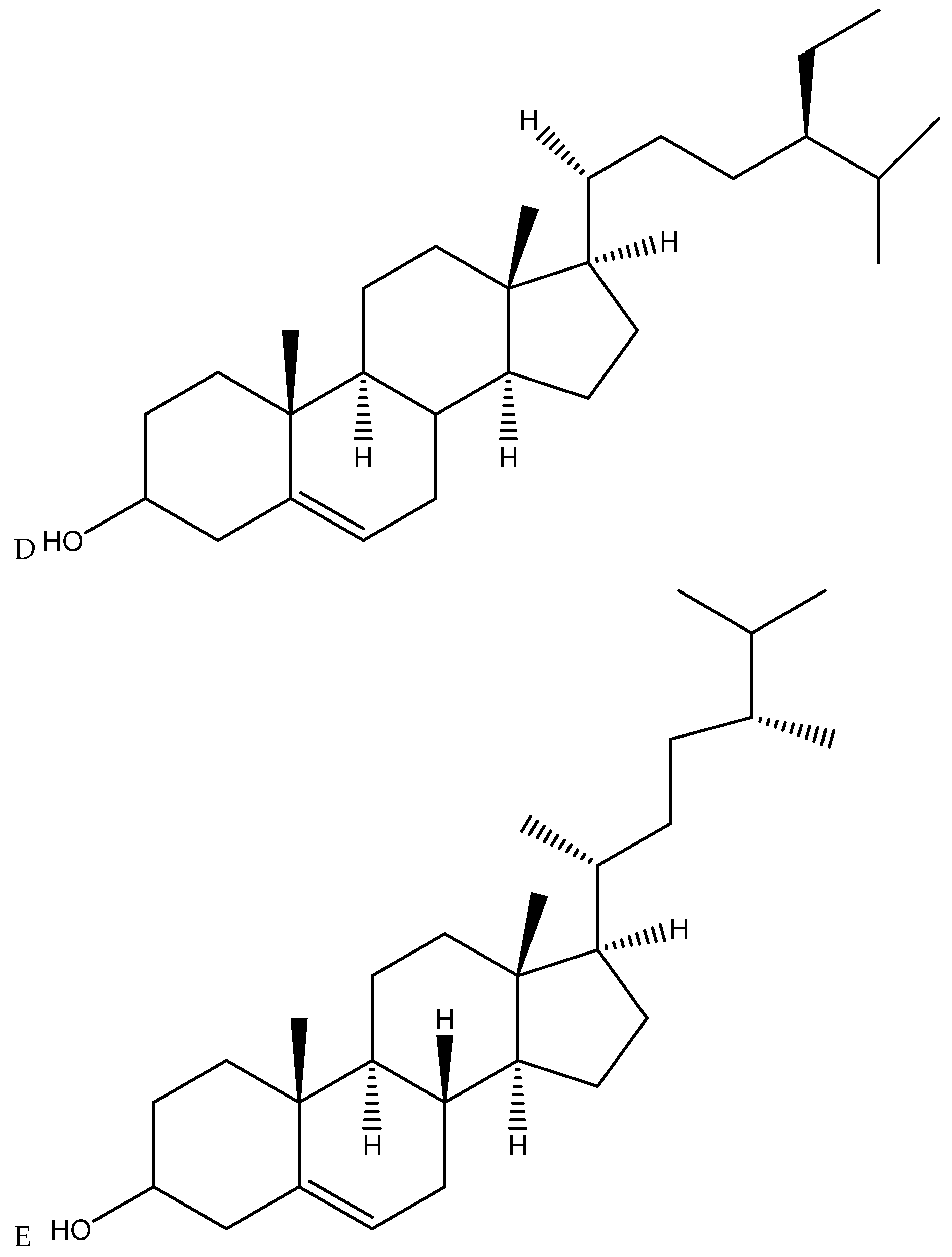
3.3. Cannabis Active Compounds
3.4. Metabolomics Pathways of Cannabinoid
3.5. Antibacterial Activity of Cannabis
3.6. Antifungal Activity of Cannabis
3.7. Antiviral Activity of Cannabis
3.8. Anti-Nematicidal Activities of Cannabis
3.9. Acaricidal Activities of Cannabis
3.10. Insecticidal Activities of Cannabis
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacula, R.L.; Smart, R. Medical Marijuana and Marijuana Legalization. Annu. Rev. Clin. Psychol. 2017, 13, 397–419. [Google Scholar] [CrossRef]
- Cox, C. The Canadian Cannabis Act legalizes and regulates recreational Cannabis use in 2018. Health Policy 2018, 122, 205–209. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Cascini, F.; Aiello, C.; Di Tanna, G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal Cannabis over time: Systematic review and meta-analysis. Curr. Drug Abus. Rev. 2012, 5, 32–40. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Duggan, P.J. The chemistry of cannabis and cannabinoids. Aust. J. Chem. 2021, 74, 369–387. [Google Scholar] [CrossRef]
- Gloss, D. An overview of products and Bias in Research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef]
- Zirpel, B.; Stehle, F.; Kayser, O. Production of tetrahydrocannabinolic acid from cannabigerolic acid by whole cells of Pichia (Komagataella) pastoris expressing D9-tetrahydrocannabinolic acid synthase from Cannabis sativa L. Biotechnol. Lett. 2015, 37, 1869–1875. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, I.; Stefanini, S.; Grandi, B.; Sgorbati, B.; Biavati; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Cannabis and Cannabis edibles: A review. J. Agric. Food Chem. 2021, 69, 1751–1774. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Maqsood, A.; Qin, P.; Zumin, G.; Yuyang, L.; Aatika, S.; Dilbar, H.; Ansar, J.; Jamil, S.; Mazher, F.I.; Ran, A.; et al. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Nat. Res. 2020, 10, 522. [Google Scholar]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Veter. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Mashela, P.W.; Dube, Z.P.; Pofu, K.M. Managing the Phytotoxicity and Inconsistent Nematode Suppression in Soil Amended with Phytonematicides. In Organic 47 Amendments and Soil Suppressiveness in Plant Disease Management, Soil Biology; Meghvansi, M.K., Vorma, A., Eds.; Springe International Publishers: Cham, Switzerland, 2015; Volume 46. [Google Scholar]
- Shricharan, S.; Mahalakshmi, S.; Kaviraj, S. In vitro evaluation of efficacy of botanicals against Aspergillus niger causing collar rot in groundnut. J. Pharmacogn. Phytochem. 2020, 9, 3177–3179. [Google Scholar]
- Isahq, M.S.; Afridi, M.S.; Ali, J.; Hussain, M.M.; Ahmad, S.; Kanwal, F. Proximate composition, phytochemical screening, GC-MS studies of biologically active cannabinoids and antimicrobial activities of Cannabis indica. Asian Pac. J. Trop. Dis. 2015, 5, 897–902. [Google Scholar] [CrossRef]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plants extracts as antimicrobials in food products: Types. In Handbook of Natural Antimicrobials for Food Safety and Quality; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–47. [Google Scholar]
- Ali, E.M.; Almagboul, A.Z.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Fuentes, G.; Azucena, I.; Dalila, O.; Fangio, F.; Ramos, F.; Mitton, G.; Fuselli, S.; Matias, M.; Ramirez, C.L. Antibacterial activity of Cannabis (Cannabis sativa L.) female inflorescence and root extract against Paenibacillus larvae, causal agent of American foulbrood. Biocatal. Agric. Biotechnol. 2023, 47, 102575. [Google Scholar]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The antimicrobial activity of cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef]
- Riaz, Z.; Khalid, S.; Ali, Q.; Ashfaq, N.; Hayat, S.; Ahmad, F.; Haider, M.S. Anti-microbial potential of cannabis plant extract against bacteria isolated from gut and mucus of Oreochromis mossambicus. J. Biosci. 2022, 2022. [Google Scholar] [CrossRef]
- Abdalhamed, A.M.; Zeedan, G.S.G.; Abou Zeina, H.A.A. Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Vet. World 2018, 11, 355. [Google Scholar] [CrossRef]
- Chauhan, S.; Sahu, R.; Upadhyay, L.S. Medicinal plants: A potent antimicrobial source and an alternative to combat antibiotic resistance. In Ethnopharmacology and Biodiversity of Medicinal Plants; Apple Academic Press: Williston, VT, USA, 2019; pp. 239–264. [Google Scholar]
- Khodadadi, H.; Salles, E.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.V.; Dhandapani, K.; Baban, B. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1462. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids alleviate the LPS-induced cytokine storm via attenuating NLRP3 inflammasome signaling and TYK2-mediated STAT3 signaling pathways in vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Afrose, S.; Hasan, M.R.; Saleh, M.A. Antiviral peptides against the main protease of SARS-CoV-2: A molecular docking and dynamics study. Arab. J. Chem. 2021, 14, 103–315. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Tebani, A.; Afonso, C.; Bekri, S. Advances in metabolome information retrieval: Turning chemistry into biology part II: Biological information recovery. J. Inherit. Metab. 2018, 41, 393–406. [Google Scholar] [CrossRef]
- Jadhav, P.D.; Shim, Y.Y.; Paek, O.J.; Jeon, J.-T.; Park, H.-J.; Park, I.; Park, E.-S.; Kim, Y.J.; Reaney, M.J.T. A Metabolomics and Big Data Approach to Cannabis Authenticity (Authentomics). Int. J. Mol. Sci. 2023, 24, 8202. [Google Scholar] [CrossRef]
- Maia, M.; Monteiro, F.; Sebastiana, M.; Marques, A.P.; Ferreira, A.E.; Freire, A.P.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Metabolite extraction for high-throughput FTICR-MS-based metabolomics of grapevine leaves. EuPA Open Proteom. 2016, 12, 4–9. [Google Scholar] [CrossRef]
- Muntendam, R.; Happyana, N.; Erkelens, C.; Bruining, F.; Kayser, O. Time dependent metabolomics and transcriptional analysis of cannabinoid biosynthesis in Cannabis sativa var. Bedrobinol and Bediol grown under standardized condition and with genetic homogeneity. J. Med. Plant Res. 2012, 1, 31–40. [Google Scholar]
- Tugizimana, F.; Engel, J.; Salek, R.; Dubery, I.; Piater, L.; Burgess, K. The disruptive 4IR in the Life Sciences: Metabolomic. Lect. Notes Electr. Eng. 2000, 674, 227–256. [Google Scholar]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K. Modern plant metabolomics: Advanced natural product gene discoveries, improved technologies, and future prospects. Nat. Prod. Rep. 2015, 32, 212–229. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic analysis in food science: A review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass. Spectrom. Rev. 2012, 31, 49–69. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M. Metabolomics approaches based on mass spectrometry for food safety, quality and traceability. Trends Anal. Chem. 2013, 52, 74–87. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Bonvallot, N.; David, A.; Chalmel, F.; Chevrier, C.; Cordier, S.; Cravedi, J.P.; Zalko, D. Metabolomics as a powerful tool to decipher the biological effects of environmental contaminants in humans. Curr. Opin. Food Sci. Toxicol. 2018, 8, 48–56. [Google Scholar] [CrossRef]
- Viant, M.R.; Ebbels, T.M.; Beger, R.D.; Ekman, D.R.; Epps, D.J.; Kamp, H.; Leonards, P.E.; Loizou, G.D.; MacRae, J.I.; Van Ravenzwaay, B.; et al. Use cases, best practice and reporting standards for metabolomics in regulatory toxicology. Nat. Commun. 2019, 10, 3041. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbalides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Jabaji, S. Metabolomics—A robust bioanalytical approach for the discovery of the modes-of-action of pesticides: A review. Pestic. Biochem. Physiol. 2011, 100, 105–117. [Google Scholar] [CrossRef]
- Nemadodzi, L.E.; Vervoort, J.; Prinsloo, G. NMR-based metabolomic analysis and microbial composition of soil supporting burkea africana growth. Metabolites 2020, 10, 402. [Google Scholar] [CrossRef]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta. 2012, 750, 82–97. [Google Scholar] [CrossRef]
- Nagana Gowda, G.; Raftery, D. Recent advances in NMR-based metabolomics. Anal. Chem. 2016, 89, 490–510. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Hu, Q.; Noll, R.J.; Li, H.; Makarov, A.; Hardman, M.; Graham Cooks, R. The Orbitrap: A new mass spectrometer. J. Mass Spectrom. 2005, 40, 430–443. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass. Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Ramautar, R.; Somsen, G.W.; De Jong, G.J. CE-MS in metabolomics. Electrophoresis 2009, 30, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, T.; Zamboni, N. High-throughput discovery metabolomics. Curr. Opin. Biotechnol. 2015, 31, 73–78. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A review of methods for the chemical characterization of Cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction methods, analysis, and physicochemical characterization. Stud. Nat. Prod. Chem. 2019, 61, 143–173. [Google Scholar] [CrossRef]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From cultivar to chemovar II-a metabolomics approach to Cannabis classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- Lewis, M.A.; Russo, E.B.; Smith, K.M. Pharmacological foundations of cannabis chemovars. Planta Med. 2018, 84, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of metabolomics in cannabis (Cannabis sativa L.) research and development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, R. Microextraction techniques for analysis of cannabinoids. Trends Anal. Chem. 2016, 80, 156–166. [Google Scholar] [CrossRef]
- Meng, Q.; Buchanan, B.; Zuccolo, J.; Poulin, M.M.; Gabriele, J.; Baranowski, D.C. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS ONE 2018, 13, e0196396. [Google Scholar] [CrossRef] [PubMed]
- McGeeney, B.E. Cannabinoids and hallucinogens for headache. J. Headache Pain 2013, 53, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Williamson, E.M.; Evans, F.J. Cannabinoids in clinical practice. Drugs 2000, 60, 1303–1314. [Google Scholar] [CrossRef]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil-Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Borhade, S.S. Chemical composition and characterization of hemp (Cannabis sativa) seed oil and essential fatty acids by HPLC method. Arch. Appl. Sci. Res. 2013, 5, 5–8. [Google Scholar]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Ruiu, S.; Anzani, N.; Orrù, A.; Floris, C.; Caboni, P.; Maccioni, E.; Distinto, S.; Alcaro, S.; Cottiglia, F. N-Alkyl dien-and trienamides from the roots of Otanthus maritimus with binding affinity for opioid and cannabinoid receptors. Bioorg. Med. Chem. 2013, 21, 7074–7082. [Google Scholar] [CrossRef]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Kannan, R.; Babu, U.V. Identity and pharmacognosy of Ruta graveolens Linn. Anc. Sci. Life 2012, 32, 16–19. [Google Scholar] [CrossRef]
- Parray, S.A.; Bhat, J.U.; Ahmad, G.; Jahan, N.; Sofi, G.; Ifs, M. Ruta graveolens: From traditional system of medicine to modern pharmacology: An overview. Am. J. Pharm. Tech. Res. 2012, 2, 239–252. [Google Scholar]
- Wu, T.S.; Shi, L.S.; Wang, J.J.; Iou, S.C.; Chang, H.C.; Chen, Y.P.; Kuo, Y.H.; Chang, Y.L.; Tenge, C.M. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J. Chin. Chem. Soc. 2003, 50, 171–178. [Google Scholar] [CrossRef]
- Dimmito, M.P.; Stefanucci, A.; Della Valle, A.; Scioli, G.; Cichelli, A.; Mollica, A. An overview on plants cannabinoids endorsed with cardiovascular effects. Biomed. Pharmacother. 2021, 142, 111963. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Korte, G.; Dreiseitel, A.; Schreier, P.; Oehme, A.; Locher, S.; Geiger, S.; Heilmann, J.; Sand, P.G. Tea catechins’ affinity for human cannabinoid receptors. Phytomedicine 2010, 17, 19–22. [Google Scholar] [CrossRef]
- Cairney, S.; Maruff, P.; Clough, A.R. The neurobehavioural effects of kava. Aust. N. Z. J. Psychiatry 2002, 36, 657–662. [Google Scholar] [CrossRef]
- Ligresti, A.; Villano, R.; Allarà, M.; Ujváry, I.; Di Marzo, V. Kavalactones and the endocannabinoid system: The plant-derived yangonin is a novel CB1 receptor ligand. Pharmacol. Res. 2012, 66, 163–169. [Google Scholar] [CrossRef]
- Chicca, A.; Schafroth, M.A.; Reynoso-Moreno, I.; Erni, R.; Petrucci, V.; Carreira, E.M.; Gertsch, J. Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high. Sci. Adv. 2018, 4, eaat2166. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, J.X.; Wang, Q.; Li, H.L.; Tao, M.; Luo, Q.; Liu, H. New chromane and chromene meroterpenoids from flowers of Rhododendron rubiginosum Franch. var. rubiginosum. Fitoterapia 2018, 127, 396–401. [Google Scholar] [CrossRef]
- Fuhr, L.; Rousseau, M.; Plauth, A.; Schroeder, F.C.; Sauer, S. Amorfrutins are natural PPARγ agonists with potent anti-inflammatory properties. J. Nat. Prod. 2015, 78, 1160–1164. [Google Scholar] [CrossRef]
- Pollastro, F.; De Petrocellis, L.; Schiano-Moriello, A.; Chianese, G.; Heyman, H.; Appendino, G.; Taglialatela-Scafati, O. Amorfrutin-type phytocannabinoids from Helichrysum umbraculigerum. Fitoterapia 2017, 123, 13–17. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Muñoz, R.; De la Peña, F.; Julio, L.F.; Burillo, J. Nematicidal potential of hydrolates from the semi-industrial vapor-pressure extraction of Spanish aromatic plants. Environ. Sci. Pollut. Res. 2018, 25, 29834–29840. [Google Scholar] [CrossRef]
- Marques, F.M.; Figueira, M.M.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. In vitro anti-inflammatory activity of terpenes via suppression of superoxide and nitric oxide generation and the NF-κB signalling pathway. Inflammopharmacology 2019, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis Roots: A Traditional Therapy with Future Potential for Treating Inflammation and Pain. Cannabis Cannabinoid Res. 2017, 210–216. [Google Scholar] [CrossRef]
- Vázquez, L.H.; Palazon, J.; Navarro-Ocaña, A. The Pentacyclic Triterpenes α, β-amyrins: A Review of Sources and Biological Activities. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Venketeshwer, R., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Upadhyay, K.; Gupta, N.K.; Dixit, V.K. Development and characterization of phyto-vesicles of β-sitosterol for the treatment of androgenetic alopecia. Arch. Dermatol. Res. 2012, 304, 511–519. [Google Scholar] [CrossRef]
- Kwon, Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci. Biotechnol. 2019, 28, 1599–1606. [Google Scholar] [CrossRef]
- Lone, T.A.; Lone, R.A. Extraction of cannabinoids from Cannabis sativa L. J. Med. Dent. 2012, 1, 51–55. [Google Scholar]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Morita, H.; Wong, C.P.; Abe, I. How structural subtleties lead to molecular diversity for the type III polyketide synthases. J. Biol. Chem. 2019, 294, 15121–15136. [Google Scholar] [CrossRef]
- Carvalho, Â.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Author Correction: Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2020, 580, E2. [Google Scholar] [CrossRef]
- Small, E.; Antle, T. A Preliminary Study of Pollen Dispersal in Cannabis sativa in Relation to Wind Direction. J. Ind. Hemp 2003, 8, 37–50. [Google Scholar] [CrossRef]
- Eržen, M.; Košir, I.J.; Ocvirk, M.; Kreft, S.; Čerenak, A. Metabolomic analysis of cannabinoid and essential oil profiles in different hemp (Cannabis sativa L.) phenotypes. Plants 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.B.; Malik, K.M.; Balakrishnan, N.; Suresh, B. Evaluation of larvicidal activity of the different extracts against important species of mosquito: Anopheles stephensi. J. Parasitol. Vector Biol. 2013, 6, 11–15. [Google Scholar]
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.H.; Deng, G. Evaluation of Hemp (Cannabis sativa L.) as an Industrial Crop: A Review. Environ. Sci. Pollut. Res. 2021, 28, 52832–52843. [Google Scholar] [CrossRef] [PubMed]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.; Ayariga, J.A.; Ajayi, O.S.; Xu, J.; Villafane, R.; Samuel-Foo, M. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against Salmonella typhimurium and S. newington. Molecules 2022, 27, 2669. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extract as potential mosquito larvicides. Indian. J. Med. Res. 2012, 135, 581–598. [Google Scholar]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; NacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 2020, 100, 154073. [Google Scholar] [CrossRef]
- Sarmadyan, H.; Solhi, H.; Hajimir, T.; Najarian-Araghi, N.; Ghaznavi-Rad, E. Determination of the antimicrobial effects of hydro-alcoholic extract of Cannabis sativa on multiple drug resistant bacteria isolated from nosocomial infections. Iran. J. Toxicol. 2014, 7, 967–972. [Google Scholar]
- Pavela, R.; Maggi, F.; Lupidi, G.; Mbuntcha, H.; Woguem, V.; Womeni, H.M.; Barboni, L.; Tapondjou, L.A.; Benelli, G. Clausena anisate and Dysphania ambrosioides essential oils: From ethno-medicine to modern uses as effective insecticides. Environ. Sci. Pollut. Res. 2017, 25, 10493–10503. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Panglong, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. essential oils. Front. Cell. Infect. 2018, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Gioa, D.D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT Food Sci. Technol. 2020, 124, 109–149. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: A preliminary observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, S.; Rahman, K.; Ikram, M.; Nisar, M.; Khan, I. Screening of antibacterial activity of medicinal plants. Int. J. Pharm. Sci. Res. 2012, 14, 25–29. [Google Scholar]
- Das, B.; Mishra, P.C. Antibacterial analysis of crude extracts from the leaves of Tageteserecta and Cannabis sativa. Int. J. Environ. Sci. Technol. 2011, 2, 1605–1609. [Google Scholar]
- Khan, I.H.; Javaid, A. Antifungal Activity of Leaf Extract of Cannabis sativa against Aspergillus flavipes. Pak. J. Weed Sci. Res. 2020, 26, 447–453. [Google Scholar] [CrossRef]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-biofilm activity of cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Wanas, A.S.; Mohammed, M.; Radwan-Mehmedic, Z.; Jacob, M.; Khan, I.A.; Elsohly, M.A. Antifungal Activity of the Volatiles of High Potency Cannabis Sativa L. against Cryptococcus neoformans. Rec. Nat. Prod. 2016, 10, 214–220. [Google Scholar]
- Vinod, K.; Tripathi, M.K.; Shikha, K. Antibacterial activity of Cannabis sativa against some pathogens isolated from burns of patient. Med. Plants Int. J. Phytomed. 2011, 3, 243–247. [Google Scholar]
- Khan, I.H.; Javaid, A.; Shad, A. Comparative efficacy of organic solvent fractions of leaf extract of hemp against Aspergillus versicolor. Pak. J. Weed Sci. Res. 2021, 27, 101–108. [Google Scholar] [CrossRef]
- Pal, G.K.; Kumar, B.; Shahi, S.K. Antifungal activity of some common weed extracts against phytopathogenic fungi Alternaria spp. Int. J. Univers. Pharm. Bio Sci. 2013, 24, 6–14. [Google Scholar]
- Schofs, L.; Sparo, M.D.; Sánchez Bruni, S.F. The antimicrobial effect behind Cannabis sativa. Pharmacol. Res. Perspect. 2021, 9, e00761. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Metin, S.; Didinen, B.I.; Mercimek, E.B.; Ersoy, A.T. Antibacterial activity of some essential plant oils against some bacterial fish pathogens. Aquac. Res. 2017, 17, 59–69. [Google Scholar]
- Khosravi, A.R.; Shokri, H.; Sharifrohani, M.; Ebrahimzadeh Mousavi, H.; Moosavi, Z. Evaluation of the antifungal activity of Zataria multiflora, Geranium herbarium and Eucalyptus camaldolensis essential oils on Saprolegnia parasitica infected rainbow trout (Oncorhynchus mykiss) eggs. Foodborne Pathog. Dis. 2012, 9, 674–679. [Google Scholar] [CrossRef]
- Gormez, O.; Diler, O. In vitro antifungal activity of essential oils from Tymbra, Origanum, Satureja species and some pure compounds on the fish pathogenic fungus, Saprolegnia parasitica. Aquac. Res. 2012, 45, 1196–1201. [Google Scholar] [CrossRef]
- Kanchan, C.; Imjai, P.; Kanchan, N.; Panchai, K.; Hatai, K. Virulence of Aeromonas hydrophila in Siamese fighting fish (Betta splendens) and the bacterium susceptibility to some herbal plants. Iran. J. Fish. Sci. 2019, 18, 349–354. [Google Scholar]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
- DeMarino, C.; Cowen, M.; Khatkar, P.; Cotto, B.; Branscome, H.; Kim, Y.; Sharif, S.A.; Agbottah, E.T.; Zhou, W.; Costiniuk, C.T.; et al. Cannabinoids Reduce Extracellular Vesicle Release from HIV-1 Infected Myeloid Cells and Inhibit Viral Transcription. Cells 2022, 11, 723. [Google Scholar] [CrossRef]
- Lowe, H.I.C.; Toyang, N.J.; McLaughlin, W. Potential of cannabidiol for the treatment of viral hepatitis. Pharmacogn. Res. 2017, 9, 116–118. [Google Scholar]
- Romme, J.; Christensen, M.; Komori, M.R.; von Essen, F. CSF Inflammatory biomarkers responsive to treatment in progressive multiple sclerosis capture residual inflammation associated with axonal damage. Mult. Scler. Int. 2019, 25, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.A.; Cesarman, E.; Boshoff, C. Kaposi’s sarcoma and its associated herpesvirus. Nat. Rev. Cancer 2010, 10, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.C.; Adegbala, O.M.; Adejumo, K.L.; Bukong, T.N. Reduced incidence and better liver disease outcomes among chronic HCV infected patients who consume Cannabis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9430953. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in-vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z.; et al. In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- van-Breeman, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids block cellular entry of SARS-CoV-2 and the emerging variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Puopolo, T.; Li, H.; Cai, A.; Seeram, N.P.; Ma, H. Identification of SARS-CoV-2 Main protease inhibitors from a library of minor cannabinoids by biochemical inhibition assay and surface plasmon resonance characterized binding affinity. Molecules 2022, 27, 6127. [Google Scholar] [CrossRef] [PubMed]
- El Ouafi, Z.; Rhalem, W.; Habib, N.; Idrissi Azami, A.; Sehli, S.; Al Idrissi, N.; Bakkali, F.; Abderrazak, R.; Imifanekiso, M. Molecular modeling targeting the ACE2 receptor with Cannabis sativa’s active ingredients for antiviral drug discovery against SARS-CoV-2 infections. Bioinform. Biol. Insights 2022, 16, 1–9. [Google Scholar] [CrossRef]
- Goerl, B.; Watkins, S.; Metcalf, C.; Smith, M.; Beenhakker, M. Cannabidiolic acid exhibits entourage-like improvements of anti-convulsant activity in an acute rat model of seizures. Epilepsy Res. 2021, 169, 106525. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of full-spectrum Cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165771. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Kamboh, A.; Syed, S.; Babazadeh, D.; Suheryani, I.; Shah, Q.; Umar, M.; Kakar, I.; Naveed, M.; Abd El-Hack, M.E.; et al. Phytochemistry and beneficial impacts of cinnamon (Cinnamomum zeylanicum) as a dietary supplement in poultry diets. Worlds Poult. Sci. J. 2018, 74, 331–346. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewinska, E.; Sembratowicz, I.; Grela, E.; Czech, A. The potential of using plant antioxidants to stimulate antioxidant mechanisms in poultry. Worlds Poult. Sci. J. 2016, 72, 291–298. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Azarfar, A.; Chegini, S.; Farahvand, P.; Moatameni, R. Effect of Different Levels of Cannabis Diets for Broiler Chickens on Performance, Weigh of Internal Organs and Blood Cholesterol. In 5th Iranian Congress on Animal Science; Isfahan Technology University, Department of Poultry Nutrition: Isfahan, Iran, 2011. [Google Scholar]
- Khan, S.A.; Abid, M.; Hussain, F. Nematicidal activity of seaweeds against Meloidogyne javanica. Pak. J. Nematol. 2015, 33, 195–203. [Google Scholar]
- Farzana; Abid, M.; Hussain, F. Root-knot disease complex ofvegetables in Karachi region and their non-chemical control. Int. J. Biotechnol. Res. 2016, 13, 143–151. [Google Scholar]
- Zaidat, S.A.E.; Mouhouche, F.; Babaali, D.; Abdessemed, N.; De Cara, M.; Hammache, M. Nematicidal activity of aqueous and organic extracts of local plants against Meloidogyne incognita (Kofoid and White) Chitwood in Algeria under laboratory and greenhouse conditions. Egypt. J. Biol. Pest. Control. 2020, 30, 46. [Google Scholar] [CrossRef]
- Leela, N.K.; Pervez, R.; Ramana, K.V.; Rosana, O.B.; Eapen, S.J. Nematicidal activity of Strychnos nuxvomica leaf and its con-stituents against root-knot nematodes, Meloidogyne incognita. J. Nematol. 2012, 40, 157–162. [Google Scholar]
- Khalil, M.S.; Darwesh, D.M. Soil amendments the alternative approach in modern agriculture. J. Agric. Sci. Technol. 2017, 4, 555646. [Google Scholar] [CrossRef]
- Hussain, J.; Khan, F.U.; Ullah, R.; Muhammad, Z.; Rehman, N.U.; Shinwari, Z.K.; Khan, I.U.; Zohaib, M.; Imad-ud-din; Hussain, A.M. Nutrient evaluation and elemental analysis of four selected medicinal plants of Khyber Pakhtoon Khwa. Pak. J. Bot. 2011, 43, 427–434. [Google Scholar]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.G.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Kayani, M.Z.; Mukhtar, T.; Hussain, M.A. Evaluation of nematicidal effects of Cannabis sativa L. and Zanthoxylum alatum Roxb. against root-knot nematodes, Meloidogyne incognita. Crop Prot. 2012, 39, 52–56. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, Z.L.; Han, B.J.; Zhou, K.Q.; Hashemi, M.; Liu, X.B. Immediate responses of cyst nematode, soil-borne pathogens and soybean yield to one-season crop disturbance after continuous soybean in Northeast China. Int. J. Plant Prod. 2013, 2, 341–353. [Google Scholar]
- Faria, J.M.S.; Sena, I.; Vieira da Silva, I.; Ribeiro, B.; Barbosa, P.; Ascensão, L.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. In Vitro Co-Cultures of Pinus Pinaster with Bursaphelenchus Xylophilus: A Biotechnological Approach to Study Pine Wilt Disease. Planta 2015, 241, 325–1336. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Argentier, M.P.; Radicci, V.; Grassi, F.; Avato, P. Artemisia annua compounds have potential to manage root-knot and potato Cyst nematodes. Ind. Crops Prod. 2017, 108, 195–200. [Google Scholar] [CrossRef]
- Bawa, J.A.; Mohammed, I.; Liadh, S. Nematicidal effect of some plants extracts on root-knot nematode (Meloidogyne incognita) of tomato (Lycopersicon esculentum). World J. Life Sci. Med. Res. 2014, 3, 81–87. [Google Scholar]
- Taniwiryono, D.; Berg, H.; Riksen, J.A.G.; Rietjens, I.M.C.M.; Djiwantia, S.R.; Kammenga, J.E.; Murk, A.J. Nematicidal activity of plant extracts against the root-knot nematode, Meloidogyne incognita. J. Nat. Prod. 2009, 2, 77–85. [Google Scholar]
- Tabari, M.A.; Khodashenas, A.; Jafari, M.; Petrelli, R.; Cappellacci, L.; Nabissi, M.; Maggi, F.; Pavela, R.; Youssefi, M.R. Acaricidal properties of hemp (Cannabis sativa L.) essential oil against Dermanyssus gallinae and Hyalomma dromedarii. Ind. Crops Prod. 2020, 147, 112–238. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- McPartland, J.M.; Sheikh, Z. A review of Cannabis sativa-based insecticides, Miticides, and repellents. J. Entomol. Zool. 2018, 6, 1288–1299. [Google Scholar]
- Betoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- McPartland, J.M.; MacDonald, C.; Young, M.; Grant, P.S.; Furkert, D.P.; Glass, M. Affinity and efficacy studies of Tetrahydrocannabinol acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Med. 2017, 2, 87–95. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Nasreen, N.; Niaz, S.; Khan, A.; Zaman, M.A.; Ayaz, S.; Naeem, H.; Khan, N.; Elgorban, A.M. The potential of Allium sativum and Cannabis sativa extracts for anti-tick activities against Rhipicephalus (Boophilus) microplus. Exp. Appl. Acarol. 2020, 82, 281–294. [Google Scholar] [CrossRef]
- Marčić, D.; Međo, I. Sublethal effects of azadirachtin-A (NeemAzal-T/S) on Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol. 2015, 20, 25–38. [Google Scholar]
- Sonia Olmeda, A.; Mar Blanco, M.; Perez Sanchez, J.L.; Luzon, M.; Villarroel, M.; Gibello, A. Occurrence of the Oribatid Mite Trhypochthoniellus longisetus (Acari: Trhypochthoniidae) on Tilapia (Oreochromis niloticus). Dis. Aquat. Org. 2011, 94, 77–81. [Google Scholar] [CrossRef]
- Petrelli, R.; Ranjbarian, F.; Dall’Acqua, S.; Papa, F.; Iannarelli, R.; Kamte, S.L.N.; Vittori, S.; Benelli, G.; Maggi, F.; Hofer, A.; et al. An overlooked horticultural crop, Smyrnium olusatrum, as a potential source of compounds effective against African trypanosomiasis. Parasitol. Int. 2017, 66, 146–151. [Google Scholar] [CrossRef]
- Roy, D.N.; Goswami, R.; Pal, A. The insect repellents: A silent environmental chemical toxicant to the health. Environ. Toxicol. Pharmacol. 2017, 50, 91–102. [Google Scholar] [CrossRef]
- Freitas, J.P.; de Jesus, I.L.R.; Chaves, J.K.O.; Gijsen, I.S.; Campos, D.R.; Baptista, D.P.; Chaves, D.S.A. Efficacy and residual effect of Illicium verum (staranise) and Pelargonium graveolens (rose geranium) essential oil on cat fleas Ctenocephalides felis felis. Rev. Bras. Parasitol. Vet. 2021, 30, e002821. [Google Scholar] [CrossRef]
- Minh Chau, D.T.; Chung, N.T.; Huong, L.T.; Hung, N.H.; Ogunwande, I.A.; Dai, N.D.; Setzer, W.N. Chemical Compositions, Mosquito Larvicidal and Antimicrobial Activities of Leaf Essential Oils of Eleven Species of Lauraceae from Vietnam. Plants 2020, 9, 606. [Google Scholar] [CrossRef]
- Abé, H.; Foko, D.L.; Nkondjio, C.A.; Awono-Ambene, P.H.; Tamesse, J.L. Insecticidal activity of Cannabis sativa L. leaf essential oil on the malaria vector Anopheles gambiae s.l (Giles). Int. J. Mosq. 2018, 5, 65–74. [Google Scholar]
- Rossi, P.; Cappelli, A.; Marinelli, O.; Valzano, M.; Pavoni, L.; Bonacucina, G.; Petrelli, R.; Pompei, P.; Mazzara, E.; Ricci, I. Mosquitocidal and anti-inflammatory properties of the essential oils obtained from monoecious, male, and female inflorescences of hemp (Cannabis sativa L.) and their encapsulation in nano-emulsions. Molecules 2020, 25, 3451. [Google Scholar] [CrossRef]
- Rossie, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Del Giudice, E.M. Role of cannabinoids in obesity. Int. J. Mol. Sci. 2018, 19, E2690. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Egunjobi, F.B.; Okoye, I.C. Ovicidal and larvicidal activities of ethanolic leaf extracts of three botanicals against the malaria vector–Anopheles gambiae. Int. Ann. Sci. 2020, 9, 111–121. [Google Scholar] [CrossRef]
- Dawet, A.; Ikani, A.G.; Yakubu, D.P. Larvicidal effect of Hyptis suaveolens and Chenopodium ambrosoides on Anopheles mosquito larvae. Int. J. Eng. Res. 2016, 7, 456–470. [Google Scholar]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Oswald, I.W.H.; Ojeda, M.A.; Pobanz, R.J.; Koby, K.A.; Buchanan, A.J.; Del Rosso, J. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6, 31667–31676. [Google Scholar] [CrossRef] [PubMed]
- Deletre, E.; Chandre, F.; Barkman, B.; Menut, C.; Martin, T. Naturally occurring bioactive compounds from four repellent essential oils against Bemisia tabaci whiteflies. Pest. Manag. Sci. 2016, 72, 179–189. [Google Scholar] [CrossRef]
- Ahmad, F.; Iqbal, N.; Zaka, S.M.; Qureshi, M.K.; Saeed, Q.; Khan, K.A. Comparative insecticidal activity of different plant materials from six common plant species against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 1804–1808. [Google Scholar] [CrossRef]
- Shivakumar, M.S.; Srinivasan, R.; Natarajan, D. larvicidal potential of some indian medicinal plant extracts against Aedes aegypti (L.). Asian Int. J. Pharm. Clin. Res. 2023, 6, 77–80. [Google Scholar]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Ind. Crops Prod. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Sha, W.; Shulaev, V.; Stins, M.F.; Sullivan, D.J. Plasmodium falciparum– infected erythrocytes induce NF-κB regulated inflammatory pathways in human cerebral endothelium. Blood 2009, 114, 4243–4252. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 2, 913–920. [Google Scholar] [CrossRef]
- Carreño Otero, A.L.; Vargas Méndez, L.Y.; Duque, J.E.; Kouznetsov, V.V. Design, synthesis, acetylcholinesterase inhibition and larvicidal activity of girgensohnine analogs on Aedes aegypti, vector of dengue fever. Eur. J. Med. Chem. 2014, 78, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Morales, R.M.; Stashenko, E.; Duque, J.E. Mitochondrial affectation, DNA damage and AChE inhibition induced by Salvia officinalis essential oil on Aedes aegypti larvae. Comp. Biochem. Physiol. 2019, 221, 29–37. [Google Scholar] [CrossRef] [PubMed]




| Cannabis | Target/Mechanism of Action | References |
|---|---|---|
| Cannabis indica (leaves, stems, and seeds extracts) | Possess strong anti-bacterial activity against multidrug-resistant bacterial strains such as S. aureus, Bacillus cereus, E. coli and Klebsiella pneumoniae, and Pseudomonas aeruginosa. | [16] |
| seed oil | Exerted strong antibacterial activity against Bacillus subtili and S. aureus. | [18] |
| CBV and THV | Exerts membrane disruption on bacterial species. | [111] |
| Cannabis leaf extract | Exerted the greatest antimicrobial effects on S. aureus 25923, with an inhibition zone of 14 mm. | [116] |
| Essential oils | Reduce the virulence of the food contaminant of Listeria monocytogenes. | [118] |
| CBD | Effective in reducing the bacterial colony count in dental plaque. | [120] |
| Crude alkaloid extracted from Cannabis leaf | Effectiveness against β strain of E. coli bacterial strains and the representative of skin, mouth, and ear microflora. | [123] |
| Cannabis | Target/Mechanism of Action | References |
|---|---|---|
| n-hexane fraction of C. sativa | Inhibit the growth and development of Cryptococcus neoformans known to be responsible for lung infection in humans with an IC50 value of 33.15 µg/mL. | [125] |
| Cannabis petroleum ether extract | Possess strong antifungal activity against Candida albicans. | [126] |
| n-butanol leaf extract of C. sativa | Inhibit the growth of Aspergillus versicolor | [127] |
| Cannabis inflorescence | Possess antifungal activity against Alternaria species | [131] |
| C. sativa leaf extracts | Significant reduction in number of galls, egg masses, nematode fecundity, and build. | [159] |
| Dried flowers and leaves of Cannabis | Effective in killing or repelling plant pathogenic nematodes. | [160] |
| Cannabinoids/Compounds | Virus | Target/Mechanism of Action | References |
|---|---|---|---|
| CBD | HIV | Reduce extracellular vesicle release from HIV-infected monocytic cells and their viral cargo. | [137] |
| CBD | Hepatitis | Exert cytotoxicity effect on the liver cell line against hepatitis B and hepatitis C. | [138] |
| CBD | Kaposi sarcoma | Affect the proliferation and viability of Kaposi sarcoma through the VEGFR-3 signaling pathway | [140] |
| CBDA and CBGA | SARS-CoV-2 | Interact with the SARS-CoV-2 spike protein S1 subunit and prevent the entry of several live viral variants into human epithelial cells | [144] |
| ∆9-THC | SARS-CoV-2 | Inhibits viral 3CLpro (IC50 3.62 μM) | [144] |
| CBV and ∆9-THCA-A | SARS-CoV-2 | Molecular modeling predicts the inhibition of human ACE2 | [149] |
| CBGA | SARS-CoV-2 | Binds orthosterically and allosterically to the SARS-CoV-2 spike protein S1 subunit (Kd = 5.6 μM) and prevents cell entry of human epithelial cells and Vero cells by SARS-CoV-2 and early variants. | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monyela, S.; Kayoka, P.N.; Ngezimana, W.; Nemadodzi, L.E. Evaluating the Metabolomic Profile and Anti-Pathogenic Properties of Cannabis Species. Metabolites 2024, 14, 253. https://doi.org/10.3390/metabo14050253
Monyela S, Kayoka PN, Ngezimana W, Nemadodzi LE. Evaluating the Metabolomic Profile and Anti-Pathogenic Properties of Cannabis Species. Metabolites. 2024; 14(5):253. https://doi.org/10.3390/metabo14050253
Chicago/Turabian StyleMonyela, Shadrack, Prudence Ngalula Kayoka, Wonder Ngezimana, and Lufuno Ethel Nemadodzi. 2024. "Evaluating the Metabolomic Profile and Anti-Pathogenic Properties of Cannabis Species" Metabolites 14, no. 5: 253. https://doi.org/10.3390/metabo14050253
APA StyleMonyela, S., Kayoka, P. N., Ngezimana, W., & Nemadodzi, L. E. (2024). Evaluating the Metabolomic Profile and Anti-Pathogenic Properties of Cannabis Species. Metabolites, 14(5), 253. https://doi.org/10.3390/metabo14050253






