Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation
Abstract
1. Introduction
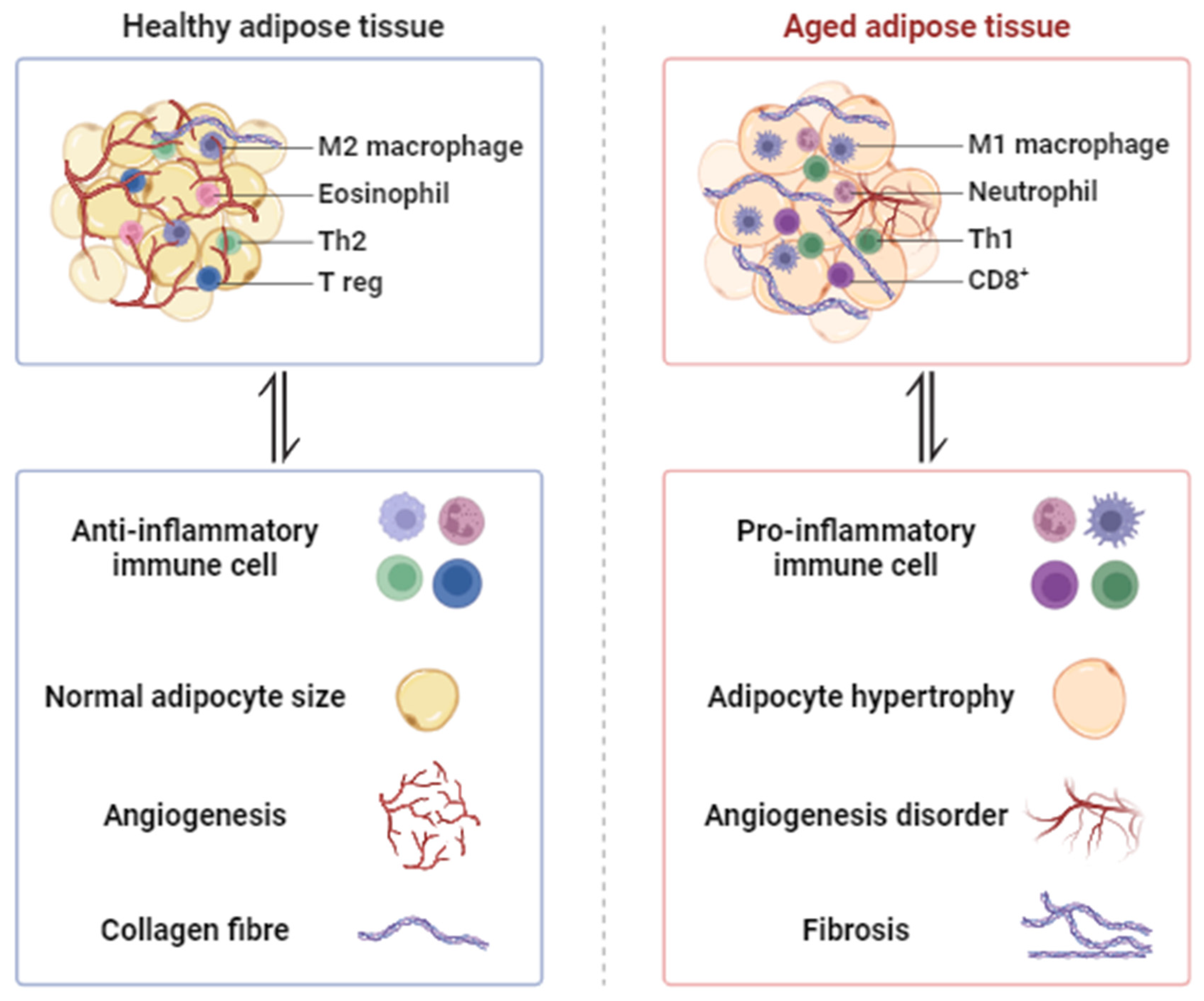
2. Morphological Changes in Aged Adipose Tissue
2.1. Hypertrophy and Adipogenesis Declines
2.2. Hypoxia and Angiogenesis Disorder
2.3. Fibrosis
2.4. Inflammation
3. Therapeutic Approaches to Enhance Aging Adipose Tissue
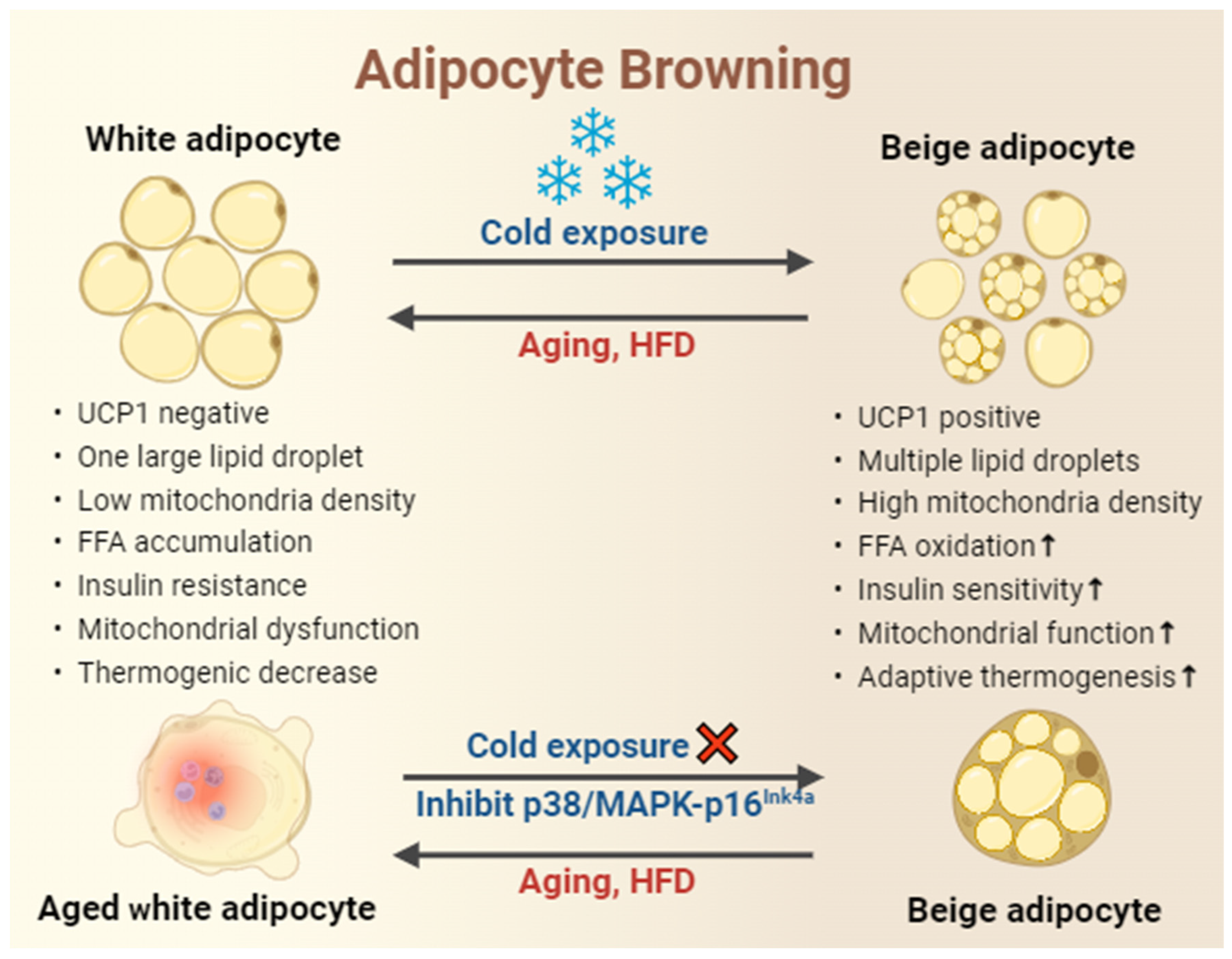
3.1. Cold Exposure
3.2. Local Hyperthermia Therapy
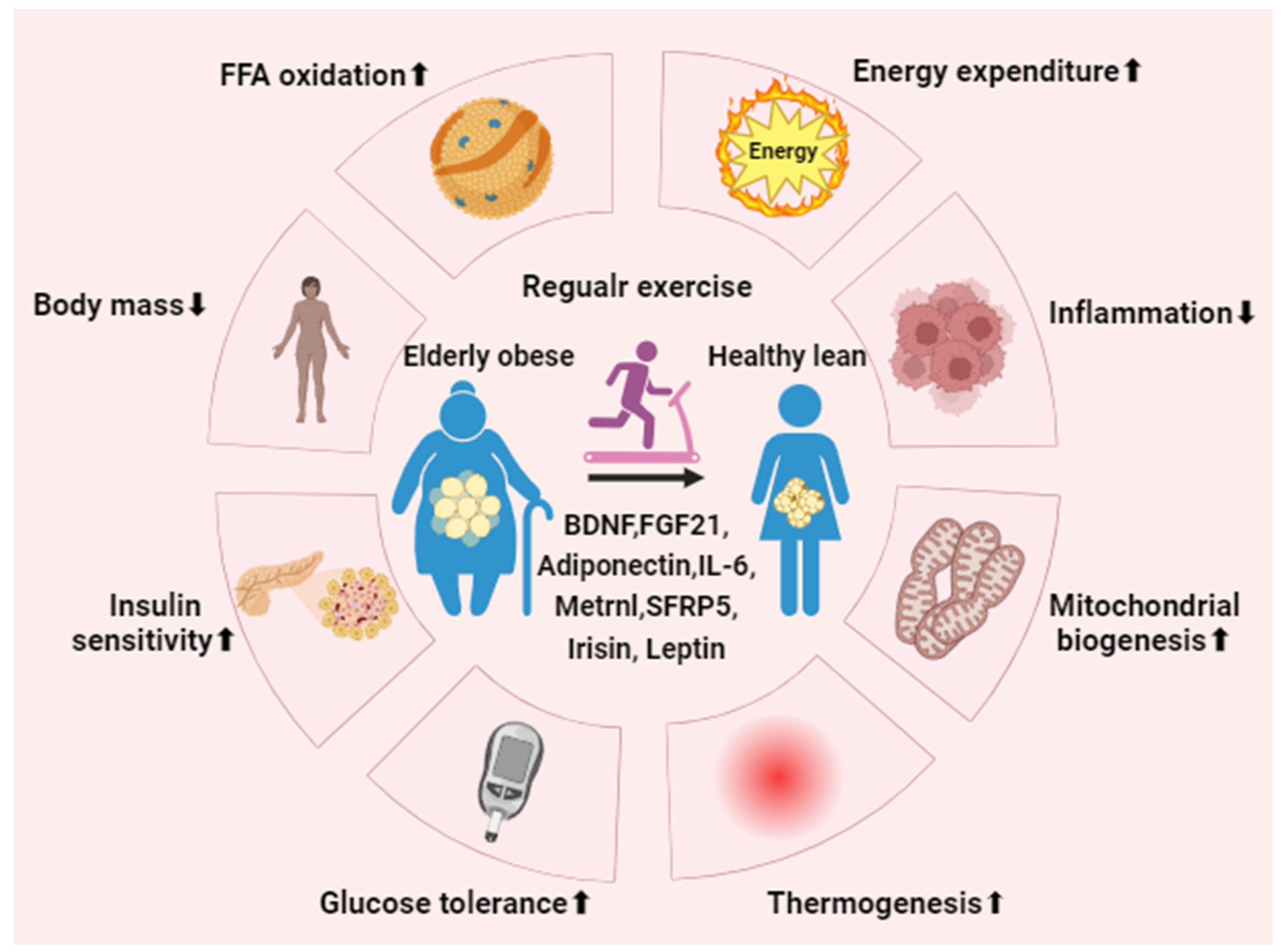
3.3. Regular Exercise
4. The Potential Role of Regular Exercise in Aged Adipose Tissue
4.1. White Adipose Tissue
4.2. Brown Adipose Tissue
4.3. Beige Adipose Tissue
5. Effect of Exercise-Induced Adipokine in Aged Adipose Tissue
| Adipokines | Main Mechanism | Main Biological Action | Target | Refs |
|---|---|---|---|---|
| Leptin | Srebp-1c/FGF21/ PGC-1α | Regulates FA biosynthesis and mitochondrial biogenesis | AT | Kobayashi, M., et al. [149] |
| Resistin | CRP/IL-6/TNF-α | Associates with aging-related cardiovascular disease | Heart | Gencer, B., et al. [135] |
| Chemerin | PRDM16/CPT1/ DIO2 | Regulates formation and function of BAT | BAT | Zhang, Y., et al. [136] |
| RBP4 | JNK/TNF/IL-1β | Causes insulin resistance and inflammation by activating innate immunity | AT | Moraes-Vieira, P. M., et al. [137] |
| LCN2 | mTORC1/ERK | Regulates mitochondrial bioenergetics | BAT | Su, H., et al. [138] |
| IL-6 | IL-1β/TNF-α | Impact age-associated inflammatory diseases | AT | Starr, M. E., et al. [145] |
| Adiponectin | ARG1/TNF | Mediates the anti-inflammatory effects of niacin | AT | Graff, E. C., et al. [150] |
| Vaspin | ANGPTL4/DNA methylation | Reduces inflammation and activists BAT | BAT | Weiner, J., et al. [140] |
| SFRP5 | JNK/Wnt | Regulates inflammation and obesity-related complication | AT | Koutaki, D., et al. [142] |
| CTRPs | AMPK/Akt, ERK | Mitigates heart failure by improving inflammation | Heart | Shanaki, M., et al. [151] |
| Omentin-1 | AMPK/Akt | Improves cardiovascular disease by mitigating inflammation | Heart | Xu, F., et al. [152] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Shilian, H.; Jing, W.; Cui, C.; Xinchun, W. Analysis of epidemiological trends in chronic diseases of Chinese residents. Aging Med. 2020, 3, 226–233. [Google Scholar] [CrossRef]
- Tabula Muris, C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 2020, 583, 590–595. [Google Scholar] [CrossRef]
- May, F.J.; Baer, L.A.; Lehnig, A.C.; So, K.; Chen, E.Y.; Gao, F.; Narain, N.R.; Gushchina, L.; Rose, A.; Doseff, A.I.; et al. Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep. 2017, 18, 1558–1572. [Google Scholar] [CrossRef]
- Lehnig, A.C.; Dewal, R.S.; Baer, L.A.; Kitching, K.M.; Munoz, V.R.; Arts, P.J.; Sindeldecker, D.A.; May, F.J.; Lauritzen, H.; Goodyear, L.J.; et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. iScience 2019, 11, 425–439. [Google Scholar] [CrossRef]
- Sepa-Kishi, D.M.; Ceddia, R.B. Exercise-Mediated Effects on White and Brown Adipose Tissue Plasticity and Metabolism. Exerc. Sport Sci. Rev. 2016, 44, 37–44. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef]
- Martinez-Tellez, B.; Sanchez-Delgado, G.; Acosta, F.M.; Alcantara, J.M.A.; Amaro-Gahete, F.J.; Martinez-Avila, W.D.; Merchan-Ramirez, E.; Munoz-Hernandez, V.; Osuna-Prieto, F.J.; Jurado-Fasoli, L.; et al. No evidence of brown adipose tissue activation after 24 weeks of supervised exercise training in young sedentary adults in the ACTIBATE randomized controlled trial. Nat. Commun. 2022, 13, 5259. [Google Scholar] [CrossRef]
- Peres Valgas da Silva, C.; Hernandez-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Montanari, T.; Poscic, N.; Colitti, M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: A review. Obes. Rev. 2017, 18, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lu, B.; Fu, Y. Autophagic Clearance of Lipid Droplets Alters Metabolic Phenotypes in a Genetic Obesity-Diabetes Mouse Model. Phenomics 2023, 3, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Scambi, I.; Peroni, D.; Nodari, A.; Merigo, F.; Benati, D.; Boschi, F.; Mannucci, S.; Frontini, A.; Visona, S.; Sbarbati, A.; et al. The transcriptional profile of adipose-derived stromal cells (ASC) mirrors the whitening of adipose tissue with age. Eur. J. Cell Biol. 2022, 101, 151206. [Google Scholar] [CrossRef]
- Rogers, N.H.; Landa, A.; Park, S.; Smith, R.G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 2012, 11, 1074–1083. [Google Scholar] [CrossRef]
- Von Bank, H.; Kirsh, C.; Simcox, J. Aging adipose: Depot location dictates age-associated expansion and dysfunction. Ageing Res. Rev. 2021, 67, 101259. [Google Scholar] [CrossRef] [PubMed]
- Guillermier, C.; Fazeli, P.K.; Kim, S.; Lun, M.; Zuflacht, J.P.; Milian, J.; Lee, H.; Francois-Saint-Cyr, H.; Horreard, F.; Larson, D.; et al. Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight 2017, 2, e90349. [Google Scholar] [CrossRef]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.E.; Guillermier, C.; Patwari, P.; Steinhauser, M.L. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef]
- Shen, H.; Huang, X.; Zhao, Y.; Wu, D.; Xue, K.; Yao, J.; Wang, Y.; Tang, N.; Qiu, Y. The Hippo pathway links adipocyte plasticity to adipose tissue fibrosis. Nat. Commun. 2022, 13, 6030. [Google Scholar] [CrossRef]
- Yuan, F.; Jiang, H.; Yin, H.; Jiang, X.; Jiao, F.; Chen, S.; Ying, H.; Chen, Y.; Zhai, Q.; Guo, F. Activation of GCN2/ATF4 signals in amygdalar PKC-delta neurons promotes WAT browning under leucine deprivation. Nat. Commun. 2020, 11, 2847. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Miki, Y.; Murase, R.; Yamamoto, K.; Murakami, M. Secreted Phospholipase PLA2G2D Contributes to Metabolic Health by Mobilizing omega3 Polyunsaturated Fatty Acids in WAT. Cell Rep. 2020, 31, 107579. [Google Scholar] [CrossRef]
- Ma, Q.X.; Zhu, W.Y.; Lu, X.C.; Jiang, D.; Xu, F.; Li, J.T.; Zhang, L.; Wu, Y.L.; Chen, Z.J.; Yin, M.; et al. BCAA-BCKA axis regulates WAT browning through acetylation of PRDM16. Nat. Metab. 2022, 4, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, V.; Toussaint, C.; Schafer, A.; Rissmann, M.; Dietrich, O.; Mettenleiter, T.C.; Pei, G.; Balkema-Buschmann, A.; Saliba, A.E.; Dorhoi, A. Landscape and age dynamics of immune cells in the Egyptian rousette bat. Cell Rep. 2022, 40, 111305. [Google Scholar] [CrossRef]
- He, Y.; Zhang, R.; Yu, L.; Zahr, T.; Li, X.; Kim, T.W.; Qiang, L. PPARgamma Acetylation in Adipocytes Exacerbates BAT Whitening and Worsens Age-Associated Metabolic Dysfunction. Cells 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, K.K.L.; Jiang, X.; Xu, A.; Cheng, K.K.Y. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin. Sci. 2020, 134, 315–330. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sharma, D.; Dinabandhu, A.; Sanchez, J.; Applewhite, B.; Jee, K.; Deshpande, M.; Flores-Bellver, M.; Hu, M.W.; Guo, C.; et al. Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice. J. Clin. Investig. 2023, 133, e163290. [Google Scholar] [CrossRef]
- Liang, Y.; Ruan, W.; Jiang, Y.; Smalling, R.; Yuan, X.; Eltzschig, H.K. Interplay of hypoxia-inducible factors and oxygen therapy in cardiovascular medicine. Nat. Rev. Cardiol. 2023, 20, 723–737. [Google Scholar] [CrossRef]
- Soro-Arnaiz, I.; Li, Q.O.Y.; Torres-Capelli, M.; Melendez-Rodriguez, F.; Veiga, S.; Veys, K.; Sebastian, D.; Elorza, A.; Tello, D.; Hernansanz-Agustin, P.; et al. Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep. 2016, 16, 2991–3002. [Google Scholar] [CrossRef]
- Zoico, E.; Policastro, G.; Rizzatti, V.; Nori, N.; Darra, E.; Rossi, A.P.; Fantin, F.; Zamboni, M. Mechanisms of adipose tissue extracellular matrix alterations in an in vitro model of adipocytes hypoxia and aging. Mech. Ageing Dev. 2020, 192, 111374. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef]
- Starling, S. Unravelling adipose tissue fibrosis in obesity. Nat. Rev. Endocrinol. 2022, 18, 393. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef] [PubMed]
- Bel Lassen, P.; Charlotte, F.; Liu, Y.; Bedossa, P.; Le Naour, G.; Tordjman, J.; Poitou, C.; Bouillot, J.L.; Genser, L.; Zucker, J.D.; et al. The FAT Score, a Fibrosis Score of Adipose Tissue: Predicting Weight-Loss Outcome After Gastric Bypass. J. Clin. Endocrinol. Metab. 2017, 102, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liang, Z.; Zhang, Y.; Liu, J.; Liao, Y.; Lu, F.; Gao, J.; Cai, J. Brown adipose tissue transplantation improves skin fibrosis in localized scleroderma. FASEB J. 2023, 37, e23315. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.U.; Cohen, J.L.; Vangala, P.; Tencerova, M.; Nicoloro, S.M.; Yawe, J.C.; Shen, Y.; Czech, M.P.; Aouadi, M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014, 19, 162–171. [Google Scholar] [CrossRef]
- Camell, C.D.; Gunther, P.; Lee, A.; Goldberg, E.L.; Spadaro, O.; Youm, Y.H.; Bartke, A.; Hubbard, G.B.; Ikeno, Y.; Ruddle, N.H.; et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019, 30, 1024–1039.e6. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.P.; Myoung Suh, J.; Fang, S.; Liu, S.; Zhang, Y.; Cheng, A.; Zhou, C.; Liang, Y.; LeBlanc, M.; Liddle, C.; et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 2015, 528, 137–141. [Google Scholar] [CrossRef]
- Blondin, D.P.; Tingelstad, H.C.; Noll, C.; Frisch, F.; Phoenix, S.; Guerin, B.; Turcotte, E.E.; Richard, D.; Haman, F.; Carpentier, A.C. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat. Commun. 2017, 8, 14146. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun. 2016, 7, 10184. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. New powers of brown fat: Fighting the metabolic syndrome. Cell Metab. 2011, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- u Din, M.; Raiko, J.; Saari, T.; Kudomi, N.; Tolvanen, T.; Oikonen, V.; Teuho, J.; Sipila, H.T.; Savisto, N.; Parkkola, R.; et al. Human brown adipose tissue [(15)O]O2 PET imaging in the presence and absence of cold stimulus. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1878–1886. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Berry, D.C.; Jiang, Y.; Arpke, R.W.; Close, E.L.; Uchida, A.; Reading, D.; Berglund, E.D.; Kyba, M.; Graff, J.M. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metab. 2017, 25, 166–181. [Google Scholar] [CrossRef]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Bhadada, S.V.; Patel, B.M.; Mehta, A.A.; Goyal, R.K. beta(3) Receptors: Role in Cardiometabolic Disorders. Ther. Adv. Endocrinol. Metab. 2011, 2, 65–79. [Google Scholar] [CrossRef]
- Larsen, T.M.; Toubro, S.; van Baak, M.A.; Gottesdiener, K.M.; Larson, P.; Saris, W.H.; Astrup, A. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am. J. Clin. Nutr. 2002, 76, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; de Jonge, L.; Fang, X.; Gamlin, B.; Recker, D.; Greenway, F.L.; Smith, S.R.; Ravussin, E. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: A double-blind, placebo-controlled randomized study. J. Clin. Endocrinol. Metab. 2007, 92, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.; Freire, E.; Almendra, R.; Silva, G.L.; Santana, P. The impact of winter cold weather on acute myocardial infarctions in Portugal. Environ. Pollut. 2013, 183, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Eymann, T.M.; Francisco, M.A.; Howard, M.J.; Minson, C.T. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J. Appl. Physiol. 2016, 121, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Khan, H.; Zaccardi, F.; Laukkanen, J.A. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern. Med. 2015, 175, 542–548. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 2022, 185, 949–966.e19. [Google Scholar] [CrossRef]
- Labbadia, J.; Brielmann, R.M.; Neto, M.F.; Lin, Y.F.; Haynes, C.M.; Morimoto, R.I. Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging. Cell Rep. 2017, 21, 1481–1494. [Google Scholar] [CrossRef]
- Tharp, K.M.; Higuchi-Sanabria, R.; Timblin, G.A.; Ford, B.; Garzon-Coral, C.; Schneider, C.; Muncie, J.M.; Stashko, C.; Daniele, J.R.; Moore, A.S.; et al. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 2021, 33, 1322–1341.e13. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Ge, R.; She, Y.; Zhao, J.; Yan, J.; Yu, X.; Jin, Y.; Shang, W.; Zhang, Z. Adipose tissue spexin in physical exercise and age-associated diseases. Ageing Res. Rev. 2022, 73, 101509. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; She, Y.; Yu, M.; Min, W.; Shang, W.; Zhang, Z. Adipose-Muscle crosstalk in age-related metabolic disorders: The emerging roles of adipo-myokines. Ageing Res. Rev. 2023, 84, 101829. [Google Scholar] [CrossRef]
- Sahl, R.E.; Patsi, I.; Hansen, M.T.; Romer, T.; Frandsen, J.; Rasmusen, H.K.; Ingersen, A.; Poulsen, S.S.; Dela, F.; Larsen, S.; et al. Prolonged endurance exercise increases macrophage content and mitochondrial respiration in adipose tissue in trained men. J. Clin. Endocrinol. Metab. 2023, 109, e799–e808. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, H.; Byrkjeland, R.; Njerve, I.U.; Akra, S.; Solheim, S.; Arnesen, H.; Seljeflot, I.; Opstad, T.B. Effects of exercise training on markers of adipose tissue remodeling in patients with coronary artery disease and type 2 diabetes mellitus: Sub study of the randomized controlled EXCADI trial. Diabetol. Metab. Syndr. 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Joseph, L.C.; Morrow, J.P. Paracardial fat and vitamin A: A mechanism for regulating exercise performance. J. Clin. Investig. 2021, 131, e145969. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Ohde, D.; Walz, C.; Langhammer, M.; Schultz, J.; Hoeflich, A. Analysis of Activity-Dependent Energy Metabolism in Mice Reveals Regulation of Mitochondrial Fission and Fusion mRNA by Voluntary Physical Exercise in Subcutaneous Fat from Male Marathon Mice (DUhTP). Cells 2020, 9, 2697. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, P.; Jiang, Q.; Cai, X.; Wang, T.; Peng, W.; Sun, J.; Zhu, C.; Zhang, C.; Yue, D.; et al. Exercise-induced alpha-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO J. 2020, 39, e103304. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, Z.; Zhang, B.; Wang, C.; Mao, F.; Liu, X.; Hu, K.; Sun, X.; Jin, W.; Kuang, S. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 2019, 33, 5876–5886. [Google Scholar] [CrossRef]
- Nigro, P.; Middelbeek, R.J.W.; Alves, C.R.R.; Rovira-Llopis, S.; Ramachandran, K.; Rowland, L.A.; Moller, A.B.; Takahashi, H.; Alves-Wagner, A.B.; Vamvini, M.; et al. Exercise Training Promotes Sex-Specific Adaptations in Mouse Inguinal White Adipose Tissue. Diabetes 2021, 70, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Gupta, R.K. The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol. Cell. Endocrinol. 2017, 445, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, E. Exercise and heart disease. Science 1977, 195, 822. [Google Scholar] [CrossRef]
- Sarma, S.; MacNamara, J.P.; Balmain, B.N.; Hearon, C.M., Jr.; Wakeham, D.J.; Tomlinson, A.R.; Hynan, L.S.; Babb, T.G.; Levine, B.D. Challenging the Hemodynamic Hypothesis in Heart Failure With Preserved Ejection Fraction: Is Exercise Capacity Limited by Elevated Pulmonary Capillary Wedge Pressure? Circulation 2023, 147, 378–387. [Google Scholar] [CrossRef]
- Sachdev, V.; Sharma, K.; Keteyian, S.J.; Alcain, C.F.; Desvigne-Nickens, P.; Fleg, J.L.; Florea, V.G.; Franklin, B.A.; Guglin, M.; Halle, M.; et al. Supervised Exercise Training for Chronic Heart Failure With Preserved Ejection Fraction: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2023, 147, e699–e715. [Google Scholar] [CrossRef]
- Fudim, M.; Sobotka, P.A.; Dunlap, M.E. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ. Heart Fail. 2021, 14, e007308. [Google Scholar] [CrossRef]
- Tucker, W.J.; Kitzman, D.W. Defining the Specific Skeletal Muscle Adaptations Responsible for Exercise Training Improvements in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e010003. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.B.; Jeffress, R.N.; Lamb, D.R. Exercise: Effects on hexokinase activity in red and white skeletal muscle. Science 1968, 160, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Nigro, P.; Vamvini, M.; Yang, J.; Caputo, T.; Ho, L.L.; Carbone, N.P.; Papadopoulos, D.; Conlin, R.; He, J.; Hirshman, M.F.; et al. Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix. Cell Rep. 2023, 42, 112392. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.E.; Oydanich, M.; Zhang, J.; Campbell, S.C.; Vatner, S.F. Exercise enhancement by RGS14 disruption is mediated by brown adipose tissue. Aging Cell 2023, 22, e13791. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Vamvini, M.; Nigro, P.; Ho, L.L.; Galani, K.; Alvarez, M.; Tanigawa, Y.; Renfro, A.; Carbone, N.P.; Laakso, M.; et al. Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells. Cell Metab. 2022, 34, 1578–1593.e6. [Google Scholar] [CrossRef] [PubMed]
- Engin, B.; Willis, S.A.; Malaikah, S.; Sargeant, J.A.; Yates, T.; Gray, L.J.; Aithal, G.P.; Stensel, D.J.; King, J.A. The effect of exercise training on adipose tissue insulin sensitivity: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13445. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221, jeb161570. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Scheele, C.; Pedersen, B.K. Exercise and browning of white adipose tissue—A translational perspective. Curr. Opin. Pharmacol. 2020, 52, 18–24. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef] [PubMed]
- Trevellin, E.; Scorzeto, M.; Olivieri, M.; Granzotto, M.; Valerio, A.; Tedesco, L.; Fabris, R.; Serra, R.; Quarta, M.; Reggiani, C.; et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014, 63, 2800–2811. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Fenicchia, L.M.; Miller, C.S.; Ploutz-Synder, L.L.; Weinstock, R.S.; Carhart, R.; Azevedo, J.L., Jr. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Zachwieja, J.J.; Hendry, S.L.; Smith, S.R.; Harris, R.B. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes 1997, 46, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Felix-Soriano, E.; Sainz, N.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Celay, J.; Fernandez-Galilea, M.; Pejenaute, A.; Lostao, M.P.; Martinez-Climent, J.A.; Moreno-Aliaga, M.J. Differential remodeling of subcutaneous white and interscapular brown adipose tissue by long-term exercise training in aged obese female mice. J. Physiol. Biochem. 2023, 79, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown adipose tissue: Physiological function and evolutionary significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.J.; Seong, J.K. AMP-activated protein kinase activation in skeletal muscle modulates exercise-induced uncoupled protein 1 expression in brown adipocyte in mouse model. J. Physiol. 2022, 600, 2359–2376. [Google Scholar] [CrossRef]
- Khalagi, K.; Ansarifar, A.; Fahimfar, N.; Sanjari, M.; Gharibzdeh, S.; Sharifi, F.; Shafiee, G.; Heshmat, R.; Nabipour, I.; Larijani, B.; et al. Cardio-metabolic and socio-demographic risk factors associated with dependency in basic and instrumental activities of daily living among older Iranian adults: Bushehr elderly health program. BMC Geriatr. 2021, 21, 172. [Google Scholar] [CrossRef]
- Rossato, M. Aging and brown adipose tissue activity decline in human: Does the brain extinguish the fire? Aging Clin. Exp. Res. 2016, 28, 579–581. [Google Scholar] [CrossRef]
- Enerback, S. Human brown adipose tissue. Cell Metab. 2010, 11, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Cannon, B. The changed metabolic world with human brown adipose tissue: Therapeutic visions. Cell Metab. 2010, 11, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Harb, E.; Kheder, O.; Poopalasingam, G.; Rashid, R.; Srinivasan, A.; Izzi-Engbeaya, C. Brown adipose tissue and regulation of human body weight. Diabetes Metab. Res. Rev. 2023, 39, e3594. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qi, Z.; Ding, S. Exercise-Induced Adipose Tissue Thermogenesis and Browning: How to Explain the Conflicting Findings? Int. J. Mol. Sci. 2022, 23, 13142. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Olza, J.; Aguilera, C.M.; Gil, A.; Ruiz, J.R. Role of Exercise in the Activation of Brown Adipose Tissue. Ann. Nutr. Metab. 2015, 67, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, G.E.; El Khoury, D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019, 2019, 6561726. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Shi, Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J. Biomed. Res. 2010, 24, 6–15. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Nie, J.; Andersen, J.P.; Rendon, S.; Zheng, Y.; Liu, X.; Tian, Z.; Shi, Y. Cardiolipin Remodeling by ALCAT1 Links Hypoxia to Coronary Artery Disease by Promoting Mitochondrial Dysfunction. Mol. Ther. 2021, 29, 3498–3511. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Qi, S.; Liu, Z.; Zhang, X.; Zheng, Y.; Andersen, J.P.; Zhang, W.; Strong, R.; Martinez, P.A.; et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell 2019, 18, e12941. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Wang, H.; Zhang, W.; Chan, D.C.; Shi, Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc. Natl. Acad. Sci. USA 2012, 109, 6975–6980. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Cheng, H.; Yang, K.; Abendschein, D.R.; Gross, R.W. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 2005, 44, 16684–16694. [Google Scholar] [CrossRef]
- Sustarsic, E.G.; Ma, T.; Lynes, M.D.; Larsen, M.; Karavaeva, I.; Havelund, J.F.; Nielsen, C.H.; Jedrychowski, M.P.; Moreno-Torres, M.; Lundh, M.; et al. Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab. 2018, 28, 159–174.e11. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.; Seale, P. Medicine. Beige can be slimming. Science 2010, 328, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Mooren, F.C.; Keller, J.; Couturier, A.; Wen, G.; Hirche, F.; Stangl, G.I.; Eder, K.; Kruger, K. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol. Nutr. Food Res. 2011, 55 (Suppl. S2), S193–S202. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huang, C.; Liu, H.; Zhang, Q.; Sun, Q.; Jia, Y.; Liu, S.; Dong, M.; Hou, M.; et al. FGF2 disruption enhances thermogenesis in brown and beige fat to protect against adiposity and hepatic steatosis. Mol. Metab. 2021, 54, 101358. [Google Scholar] [CrossRef]
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C.; et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 2013, 19, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, N.Z.; Larsen, T.J.; Peijs, L.; Daugaard, S.; Homoe, P.; Loft, A.; de Jong, J.; Mathur, N.; Cannon, B.; Nedergaard, J.; et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013, 17, 798–805. [Google Scholar] [CrossRef]
- Shinoda, K.; Luijten, I.H.; Hasegawa, Y.; Hong, H.; Sonne, S.B.; Kim, M.; Xue, R.; Chondronikola, M.; Cypess, A.M.; Tseng, Y.H.; et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 2015, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Talib, N.F.; Zhu, J.; Yang, H.I.; Kim, K.S. Effects of aging-induced obesity on the transcriptional expression of adipogenesis and thermogenic activity in the gonadal white adipose, brown adipose, and skeletal muscle tissues. Phys. Act. Nutr. 2023, 27, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Y.; Sun, C.; Yin, H. Transient p53 inhibition sensitizes aged white adipose tissue for beige adipocyte recruitment by blocking mitophagy. FASEB J. 2019, 33, 844–856. [Google Scholar] [CrossRef]
- Khanh, V.C.; Zulkifli, A.F.; Tokunaga, C.; Yamashita, T.; Hiramatsu, Y.; Ohneda, O. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 500, 682–690. [Google Scholar] [CrossRef]
- Duteil, D.; Tosic, M.; Willmann, D.; Georgiadi, A.; Kanouni, T.; Schule, R. Lsd1 prevents age-programed loss of beige adipocytes. Proc. Natl. Acad. Sci. USA 2017, 114, 5265–5270. [Google Scholar] [CrossRef]
- Jiang, Y.; Berry, D.C.; Graff, J.M. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. Elife 2017, 6, e30329. [Google Scholar] [CrossRef]
- Tarantini, S.; Subramanian, M.; Butcher, J.T.; Yabluchanskiy, A.; Li, X.; Miller, R.A.; Balasubramanian, P. Revisiting adipose thermogenesis for delaying aging and age-related diseases: Opportunities and challenges. Ageing Res. Rev. 2023, 87, 101912. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Beltran-Velasco, A.I.; Martin-Rodriguez, A.; Martinez-Guardado, I.; Navarro-Jimenez, E.; Laborde-Cardenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Wijetunge, S.; Ratnayake, R.; Kotakadeniya, H.; Rosairo, S.; Albracht-Schulte, K.; Ramalingam, L.; Moustaid-Moussa, N.; Kalupahana, N.S. Association between serum and adipose tissue resistin with dysglycemia in South Asian women. Nutr. Diabetes 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska-Gancarz, M.; Jonas, M.; Owczarz, M.; Kurylowicz, A.; Polosak, J.; Franek, E.; Slusarczyk, P.; Mossakowska, M.; Puzianowska-Kuznicka, M. Age-related changes of leptin and leptin receptor variants in healthy elderly and long-lived adults. Geriatr. Gerontol. Int. 2015, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Auer, R.; de Rekeneire, N.; Butler, J.; Kalogeropoulos, A.; Bauer, D.C.; Kritchevsky, S.B.; Miljkovic, I.; Vittinghoff, E.; Harris, T.; et al. Association between resistin levels and cardiovascular disease events in older adults: The health, aging and body composition study. Atherosclerosis 2016, 245, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, W.J.; Qiu, S.; Yang, P.; Dempsey, G.; Zhao, L.; Zhou, Q.; Hao, X.; Dong, D.; Stahl, A.; et al. Chemerin regulates formation and function of brown adipose tissue: Ablation results in increased insulin resistance with high fat challenge and aging. FASEB J. 2021, 35, e21687. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Guo, H.; Qiu, X.; Lin, T.Y.; Qin, C.; Celio, G.; Yong, P.; Senders, M.; Han, X.; Bernlohr, D.A.; et al. Lipocalin 2 regulates mitochondrial phospholipidome remodeling, dynamics, and function in brown adipose tissue in male mice. Nat. Commun. 2023, 14, 6729. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef]
- Weiner, J.; Rohde, K.; Krause, K.; Zieger, K.; Kloting, N.; Kralisch, S.; Kovacs, P.; Stumvoll, M.; Bluher, M.; Bottcher, Y.; et al. Brown adipose tissue (BAT) specific vaspin expression is increased after obesogenic diets and cold exposure and linked to acute changes in DNA-methylation. Mol. Metab. 2017, 6, 482–493. [Google Scholar] [CrossRef]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef]
- Koutaki, D.; Michos, A.; Bacopoulou, F.; Charmandari, E. The Emerging Role of Sfrp5 and Wnt5a in the Pathogenesis of Obesity: Implications for a Healthy Diet and Lifestyle. Nutrients 2021, 13, 2459. [Google Scholar] [CrossRef]
- Fang, P.; Guo, W.; Ju, M.; Huang, Y.; Zeng, H.; Wang, Y.; Yu, M.; Zhang, Z. Exercise training rescues adipose tissue spexin expression and secretion in diet-induced obese mice. Physiol. Behav. 2022, 256, 113958. [Google Scholar] [CrossRef]
- Miller, K.N.; Burhans, M.S.; Clark, J.P.; Howell, P.R.; Polewski, M.A.; DeMuth, T.M.; Eliceiri, K.W.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 2017, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.E.; Saito, M.; Evers, B.M.; Saito, H. Age-Associated Increase in Cytokine Production During Systemic Inflammation-II: The Role of IL-1beta in Age-Dependent IL-6 Upregulation in Adipose Tissue. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zhuo, Y.; Jiang, D.; Li, J.; Huang, X.; Zhu, Y.; Li, Z.; Yan, L.; Jin, C.; Jiang, X.; et al. Identification of hepatic fibroblast growth factor 21 as a mediator in 17beta-estradiol-induced white adipose tissue browning. FASEB J. 2018, 32, 5602–5611. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef]
- Kobayashi, M.; Uta, S.; Otsubo, M.; Deguchi, Y.; Tagawa, R.; Mizunoe, Y.; Nakagawa, Y.; Shimano, H.; Higami, Y. Srebp-1c/Fgf21/Pgc-1alpha Axis Regulated by Leptin Signaling in Adipocytes-Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients 2020, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Graff, E.C.; Fang, H.; Wanders, D.; Judd, R.L. The Absence of Adiponectin Alters Niacin’s Effects on Adipose Tissue Inflammation in Mice. Nutrients 2020, 12, 2427. [Google Scholar] [CrossRef]
- Shanaki, M.; Shabani, P.; Goudarzi, A.; Omidifar, A.; Bashash, D.; Emamgholipour, S. The C1q/TNF-related proteins (CTRPs) in pathogenesis of obesity-related metabolic disorders: Focus on type 2 diabetes and cardiovascular diseases. Life Sci. 2020, 256, 117913. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, F.X.; Lin, X.; Zhong, J.Y.; Wu, F.; Shan, S.K.; Tan, C.M.; Yuan, L.Q.; Liao, X.B. Adipose tissue-derived omentin-1 attenuates arterial calcification via AMPK/Akt signaling pathway. Aging 2019, 11, 8760–8776. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, D.; Zhang, H.; Liu, T.; Wang, R. Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites 2024, 14, 135. https://doi.org/10.3390/metabo14030135
Jia D, Zhang H, Liu T, Wang R. Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites. 2024; 14(3):135. https://doi.org/10.3390/metabo14030135
Chicago/Turabian StyleJia, Dandan, Huijie Zhang, Tiemin Liu, and Ru Wang. 2024. "Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation" Metabolites 14, no. 3: 135. https://doi.org/10.3390/metabo14030135
APA StyleJia, D., Zhang, H., Liu, T., & Wang, R. (2024). Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites, 14(3), 135. https://doi.org/10.3390/metabo14030135







