The Research Progress on the Interaction between Mammalian Gut Microbiota and the Host’s Metabolism Homeostasis during Hibernation
Abstract
1. Introduction
2. Effects of Hibernation on the Gut Microbes of Mammalian Animals
2.1. The Effects of Hibernation on the Host’s Gut Microbiota Diversity
2.2. The Effects of Hibernation on the Structure of the Host’s Gut Microbiota
2.3. The Effects of Hibernation on the Metabolites of the Host’s Gut Microbiota
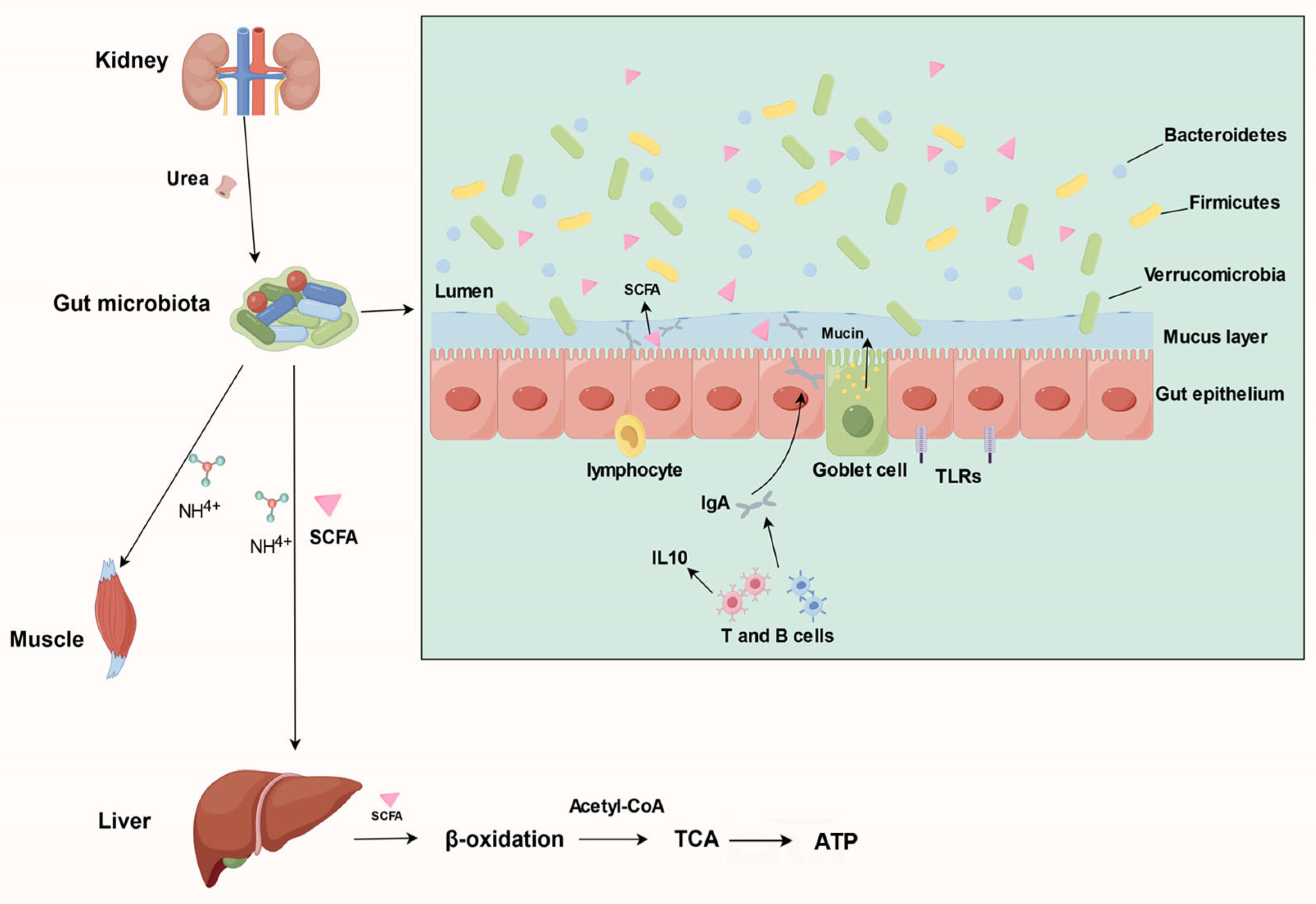
3. Effects of Gut Microbiota on the Host during Hibernation
3.1. Regulating the Host’s Glucose and Lipid Metabolism
3.2. Mediating the Host’s Nitrogen Cycling
3.3. Remodeling the Host’s Intestinal Immune System
4. The Effect of External Factors on the Host’s Gut Microbiota
4.1. The Effect of Dietary on the Host’s Gut Microbiota
4.2. The Effect of Temperature on the Host’s Gut Microbiota
4.3. The Effect of Photoperiod on the Host’s Gut Microbiota
5. Conclusions
6. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTUs | Operational Taxonomic Units |
| SCFAs | Short-chain fatty acids |
| GC-MS | Gas chromatography–mass spectrometry |
| Trp | Tryptophan |
| BA | Bile acids |
| NAFLD | Non-alcoholic fatty liver disease |
| UFAs | Unsaturated fatty acids |
| DEGs | Differential expression of genes |
| UT-B | Urea transporter protein |
| TLR | Toll-like receptor |
| GSPEs | Grape seed proanthocyanidins |
References
- Geiser, F. Hibernation. Curr. Biol. 2013, 23, R188–R193. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Guo, C.; Chen, Y.; Li, C.; Lei, T.; Zhou, S.; Qi, D.; Xiang, Z. Fatty acid metabolization and insulin regulation prevent liver injury from lipid accumulation in Himalayan marmots. Cell Rep. 2023, 42, 112718. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.G.; Grabek, K.R.; Epperson, L.E.; Karimpour-Fard, A.; Martin, S.L. Metabolic changes associated with the long winter fast dominate the liver proteome in 13-lined ground squirrels. Physiol. Genom. 2014, 46, 348–361. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Carey, H.V.; Assadi-Porter, F.M. The Hibernator Microbiome: Host-Bacterial Interactions in an Extreme Nutritional Symbiosis. Annu. Rev. Nutr. 2017, 37, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Sonoyama, K.; Fujiwara, R.; Takemura, N.; Ogasawara, T.; Watanabe, J.; Ito, H.; Morita, T. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl. Environ. Microbiol. 2009, 75, 6451–6456. [Google Scholar] [CrossRef]
- Tong, Q.; Dong, W.J.; Xu, M.D.; Hu, Z.F.; Guo, P.; Han, X.Y.; Cui, L.Y. Characteristics and a comparison of the gut microbiota in two frog species at the beginning and end of hibernation. Front. Microbiol. 2023, 14, 1057398. [Google Scholar] [CrossRef]
- Regan, M.D.; Chiang, E.; Liu, Y.; Tonelli, M.; Verdoorn, K.M.; Gugel, S.R.; Suen, G.; Carey, H.V.; Assadi-Porter, F.M. Nitrogen recycling via gut symbionts increases in ground squirrels over the hibernation season. Science 2022, 375, 460–463. [Google Scholar] [CrossRef]
- Sisa, C.; Turroni, S.; Amici, R.; Brigidi, P.; Candela, M.; Cerri, M. Potential role of the gut microbiota in synthetic torpor and therapeutic hypothermia. World J. Gastroenterol. 2017, 23, 406–413. [Google Scholar] [CrossRef]
- Kurtz, C.C.; Otis, J.P.; Regan, M.D.; Carey, H.V. How the gut and liver hibernate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 253, 110875. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Li, J.M.; Lv, W.L. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front. Immunol. 2022, 13, 923599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hao, C.; Yao, W.; Zhu, D.; Lu, H.; Li, L.; Ma, B.; Sun, B.; Xue, D.; Zhang, W. Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 2020, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Montenegro Junior, R.M.; Ponte, C.M.M.; Castelo, M.; de Oliveira Silveira, A.C.; Fernandes, V.O.; D’Alva, C.B.; Oliveira, L.F.V.; Hristov, A.D.; Bandeira, S.P.; da Cruz Paiva, G.E.; et al. Reduced gut microbiota diversity in patients with congenital generalized lipodystrophy. Diabetol. Metab. Syndr. 2022, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yao, Y.; Zhang, X.; Zhong, J.; Gao, F.; Zhang, H.; Han, Y.; Weng, Q.; Yuan, Z. Seasonal Changes in the Distinct Taxonomy and Function of the Gut Microbiota in the Wild Ground Squirrel (Spermophilus dauricus). Animals 2021, 11, 2685. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Ståhlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Fröbert, O.; Bäckhed, F. The Gut Microbiota Modulates Energy Metabolism in the Hibernating Brown Bear Ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Liu, S.; Xiao, Y.; Zhu, Y.; Zhao, H.; Li, A.; Li, Z.; Feng, J. Seasonal Changes in Gut Microbiota Diversity and Composition in the Greater Horseshoe Bat. Front. Microbiol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Roshanravan, N.; Mesri Alamdari, N.; Safaiyan, A.; Mosharkesh, E.; Hadi, A.; Barati, M.; Ostadrahimi, A. The interplay between fasting, gut microbiota, and lipid profile. Int. J. Clin. Pract. 2021, 75, e14591. [Google Scholar] [CrossRef]
- Carey, H.V.; Walters, W.A.; Knight, R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R33–R42. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Zhang, H.; Han, Y.; Gao, F.; Weng, Q.; Yuan, Z. Adaptive Changes in Structure and Function of Gut Microbiota in Wild Ground Squirrel (Spermophilus dauricus) during Induced Hibernation. Chin. J. Anim. Nutr. 2022, 34, 671–680. [Google Scholar] [CrossRef]
- Chiang, E.; Deblois, C.L.; Carey, H.V.; Suen, G. Characterization of captive and wild 13-lined ground squirrel cecal microbiotas using Illumina-based sequencing. Anim. Microbiome 2022, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Han, Y.; Yang, Y.; Hao, Z.; An, N.; Chen, J.; Zhang, Z.; Gao, X.; Storey, K.B.; Chang, H.; et al. Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor-Arousal Cycles of the Daurian Ground Squirrel. Int. J. Mol. Sci. 2022, 23, 9026. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, J.P.; de Vos, W.M.; Belzer, C. Glycobiome: Bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.J.; Duddleston, K.N.; Buck, C.L. Effects of season and host physiological state on the diversity, density, and activity of the arctic ground squirrel cecal microbiota. Appl. Environ. Microbiol. 2014, 80, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.T.; Martens, E.C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ma, S.; Zhang, Y.; Yang, X.; Zhang, H.; Han, Y.; Liu, Y.; Gao, F.; Yuan, Z. Seasonal Variation in Gut Microbiota of the Wild Daurian Ground Squirrel (Spermophilus dauricus): Metagenomic Insights into Seasonal Breeding. Animals 2023, 13, 2235. [Google Scholar] [CrossRef] [PubMed]
- Seth, E.C.; Taga, M.E. Nutrient cross-feeding in the microbial world. Front. Microbiol. 2014, 5, 350. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Jankovic, A.; Kalezic, A.; Korac, A.; Buzadzic, B.; Storey, K.B.; Korac, B. Integrated Redox-Metabolic Orchestration Sustains Life in Hibernating Ground Squirrels. Antioxid. Redox Signal. 2023, 40, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.Y.; Shi, H.T.; Tan, Y.R.; Shen, S.Y.; Yi, P.F.; Shen, H.Q.; Fu, B.D. Baicalin inhibits APEC-induced lung injury by regulating gut microbiota and SCFA production. Food Funct. 2021, 12, 12621–12633. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.L.; Bramstång, L.; Singh, A.; Jayarathna, S.; Rasmusson, A.J.; Moazzami, A.; Müller, B. Impact of time and temperature on gut microbiota and SCFA composition in stool samples. PLoS ONE 2020, 15, e0236944. [Google Scholar] [CrossRef] [PubMed]
- Tøien, Ø.; Blake, J.; Edgar, D.M.; Grahn, D.A.; Heller, H.C.; Barnes, B.M. Hibernation in black bears: Independence of metabolic suppression from body temperature. Science 2011, 331, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, X.; Du, H.; Zhao, M.; Zhang, Z.; Zhu, R.; He, B.; Zhang, Y.; Li, X.; Li, J.; et al. Foodborne Carbon Dot Exposure Induces Insulin Resistance through Gut Microbiota Dysbiosis and Damaged Intestinal Mucus Layer. ACS Nano 2023, 17, 6081–6094. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Chen, J.; Li, Y.; Kuang, Z.; Dende, C.; Raj, P.; Quinn, G.; Hu, Z.; Srinivasan, T.; et al. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. Science 2023, 381, 851–857. [Google Scholar] [CrossRef]
- Anhê, F.F.; Zlitni, S.; Zhang, S.Y.; Choi, B.S.; Chen, C.Y.; Foley, K.P.; Barra, N.G.; Surette, M.G.; Biertho, L.; Richard, D.; et al. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut 2023, 72, 460–471. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H.; et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375.e369. [Google Scholar] [CrossRef] [PubMed]
- Young, S.G.; Fong, L.G.; Beigneux, A.P.; Allan, C.M.; He, C.; Jiang, H.; Nakajima, K.; Meiyappan, M.; Birrane, G.; Ploug, M. GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism. Cell Metab. 2019, 30, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Santos, I.; Castellano-Castillo, D.; Lara, M.F.; Fernandez-Garcia, J.C.; Tinahones, F.J.; Macias-Gonzalez, M. IGFBP-3 Interacts with the Vitamin D Receptor in Insulin Signaling Associated with Obesity in Visceral Adipose Tissue. Int. J. Mol. Sci. 2017, 18, 2349. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, D.; Wei, C.; Holland-Fischer, P.; Kristensen, K.; Rittig, S.; Lange, A.; Hørlyck, A.; Solvig, J.; Grønbæk, H.; Birkebæk, N.H.; et al. Effects of lifestyle intervention on IGF-1, IGFBP-3, and insulin resistance in children with obesity with or without metabolic-associated fatty liver disease. Eur. J. Pediatr. 2023, 182, 855–865. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B.; Mølbak, L.; de Bruijn, A.; Schierbeek, H.; Boye, M.; Boehm, G.; Renes, I.; van Goudoever, J.; Burrin, D. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G300–G310. [Google Scholar] [CrossRef]
- Du Toit, A. Busy symbionts during hibernation. Nat. Rev. Microbiol. 2022, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Krone, J.E.C.; Agyekum, A.K.; Ter Borgh, M.; Hamonic, K.; Penner, G.B.; Columbus, D.A. Characterization of urea transport mechanisms in the intestinal tract of growing pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G839–G844. [Google Scholar] [CrossRef]
- Grond, K.; Buck, C.L.; Duddleston, K.N. Microbial gene expression during hibernation in arctic ground squirrels: Greater differences across gut sections than in response to pre-hibernation dietary protein content. Front. Genet. 2023, 14, 1210143. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Smits, S.A.; Sonnenburg, E.D.; Costello, E.K.; Higginbottom, S.K.; Domino, S.E.; Holmes, S.P.; Relman, D.A.; et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. USA 2013, 110, 17059–17064. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B(12) Production by Intestinal Symbionts. mBio 2017, 8, e00770-17. [Google Scholar] [CrossRef]
- Liu, M.J.; Yang, J.Y.; Yan, Z.H.; Hu, S.; Li, J.Q.; Xu, Z.X.; Jian, Y.P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [PubMed]
- Dill-McFarland, K.A.; Neil, K.L.; Zeng, A.; Sprenger, R.J.; Kurtz, C.C.; Suen, G.; Carey, H.V. Hibernation alters the diversity and composition of mucosa-associated bacteria while enhancing antimicrobial defence in the gut of 13-lined ground squirrels. Mol. Ecol. 2014, 23, 4658–4669. [Google Scholar] [CrossRef] [PubMed]
- Pachathundikandi, S.K.; Tegtmeyer, N.; Backert, S. Masking of typical TLR4 and TLR5 ligands modulates inflammation and resolution by Helicobacter pylori. Trends Microbiol. 2023, 31, 903–915. [Google Scholar] [CrossRef]
- Moor, K.; Diard, M.; Sellin, M.E.; Felmy, B.; Wotzka, S.Y.; Toska, A.; Bakkeren, E.; Arnoldini, M.; Bansept, F.; Co, A.D.; et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 2017, 544, 498–502. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Hatton, J.J.; Stevenson, T.J.; Buck, C.L.; Duddleston, K.N. Diet affects arctic ground squirrel gut microbial metatranscriptome independent of community structure. Environ. Microbiol. 2017, 19, 1518–1535. [Google Scholar] [CrossRef]
- Siutz, C.; Nemeth, M.; Quint, R.; Wagner, K.H.; Millesi, E. PUFA Changes in White Adipose Tissue during Hibernation in Common Hamsters. Physiol. Biochem. Zool. 2022, 95, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Weitten, M.; Tissier, M.L.; Robin, J.P.; Habold, C. Dietary proteins improve hibernation and subsequent reproduction in the European hamster, Cricetus cricetus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R848–R855. [Google Scholar] [CrossRef] [PubMed]
- Dai Pra, R.; Mohr, S.M.; Merriman, D.K.; Bagriantsev, S.N.; Gracheva, E.O. Ground squirrels initiate sexual maturation during hibernation. Curr. Biol. 2022, 32, 1822–1828.e4. [Google Scholar] [CrossRef] [PubMed]
- Trefna, M.; Goris, M.; Thissen, C.M.C.; Reitsema, V.A.; Bruintjes, J.J.; de Vrij, E.L.; Bouma, H.R.; Boerema, A.S.; Henning, R.H. The influence of sex and diet on the characteristics of hibernation in Syrian hamsters. J. Comp. Physiol. B 2017, 187, 725–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Xiao, Y.; Wang, X.; Guo, D.; Wang, Y.; Wang, Y. Effects of Microhabitat Temperature Variations on the Gut Microbiotas of Free-Living Hibernating Animals. Microbiol. Spectr. 2023, 11, e0043323. [Google Scholar] [CrossRef]
- Williams, C.E.; Williams, C.L.; Logan, M.L. Climate change is not just global warming: Multidimensional impacts on animal gut microbiota. Microb. Biotechnol. 2023, 16, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.M.; Duncan, M.J.; Bartke, A.; Bona-Gallo, A.; Goldman, B.D. Influence of photoperiod and gonadal steroids on hibernation in the European hamster. J. Comp. Physiol. A 1988, 163, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Mulero, M.; Muguerza, B.; Suárez, M.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Microbiota Influences the Photoperiod Effects on Proanthocyanidins Bioavailability in Diet-Induced Obese Rats. Mol. Nutr. Food Res. 2023, 67, e2200600. [Google Scholar] [CrossRef]
- Zhu, H.; Li, G.; Liu, J.; Xu, X.; Zhang, Z. Gut microbiota is associated with the effect of photoperiod on seasonal breeding in male Brandt’s voles (Lasiopodomys brandtii). Microbiome 2022, 10, 194. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yuan, Y.; Wang, J.; Zhang, S.; Zhu, R.; Wang, Y.; Wu, Y.; Liao, X.; Mi, J. Reducing light exposure enhances the circadian rhythm of the biological clock through interactions with the gut microbiota. Sci. Total Environ. 2023, 858, 160041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Song, F.; Wang, L.; Yuan, Z. The Research Progress on the Interaction between Mammalian Gut Microbiota and the Host’s Metabolism Homeostasis during Hibernation. Metabolites 2024, 14, 134. https://doi.org/10.3390/metabo14030134
Zhang Z, Song F, Wang L, Yuan Z. The Research Progress on the Interaction between Mammalian Gut Microbiota and the Host’s Metabolism Homeostasis during Hibernation. Metabolites. 2024; 14(3):134. https://doi.org/10.3390/metabo14030134
Chicago/Turabian StyleZhang, Zhepei, Fengcheng Song, Linjuan Wang, and Zhengrong Yuan. 2024. "The Research Progress on the Interaction between Mammalian Gut Microbiota and the Host’s Metabolism Homeostasis during Hibernation" Metabolites 14, no. 3: 134. https://doi.org/10.3390/metabo14030134
APA StyleZhang, Z., Song, F., Wang, L., & Yuan, Z. (2024). The Research Progress on the Interaction between Mammalian Gut Microbiota and the Host’s Metabolism Homeostasis during Hibernation. Metabolites, 14(3), 134. https://doi.org/10.3390/metabo14030134







