New Implications of Metabolites and Free Fatty Acids in Quality Control of Crossbred Wagyu Beef during Wet Aging Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Cold Storage
2.2. Sensory Evaluation
2.3. Metabolomics Analysis by GC/MS
2.4. Analysis of the Triacylglycerides, Free Amino Acids, and Nucleic Acid-Related Components
2.5. Analysis of Total Fatty Acid and Free Fatty Acids
2.6. Statistical Analyses
3. Results
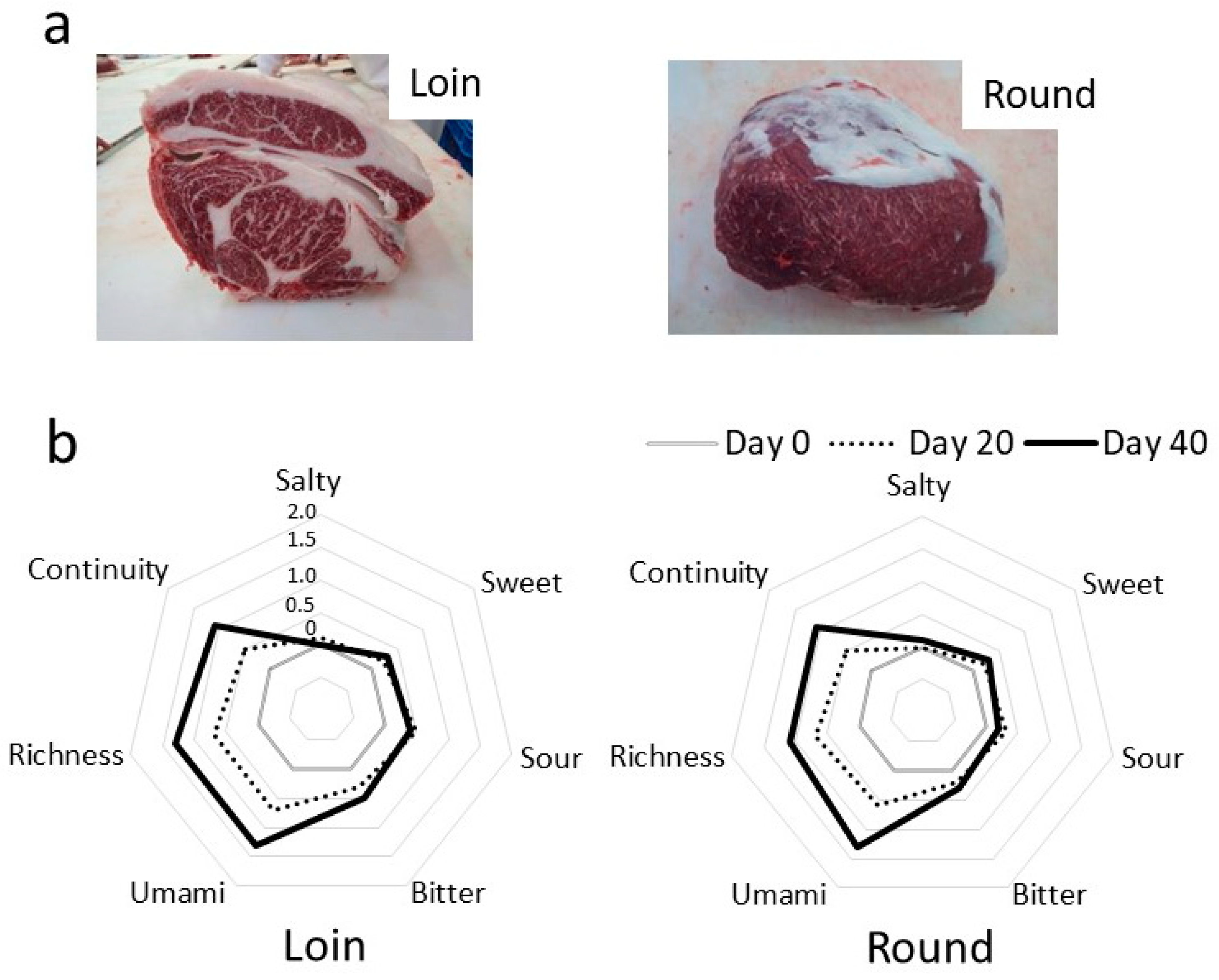
3.1. Changes in the Taste of Hybrid Wagyu Beef during Wet Aging
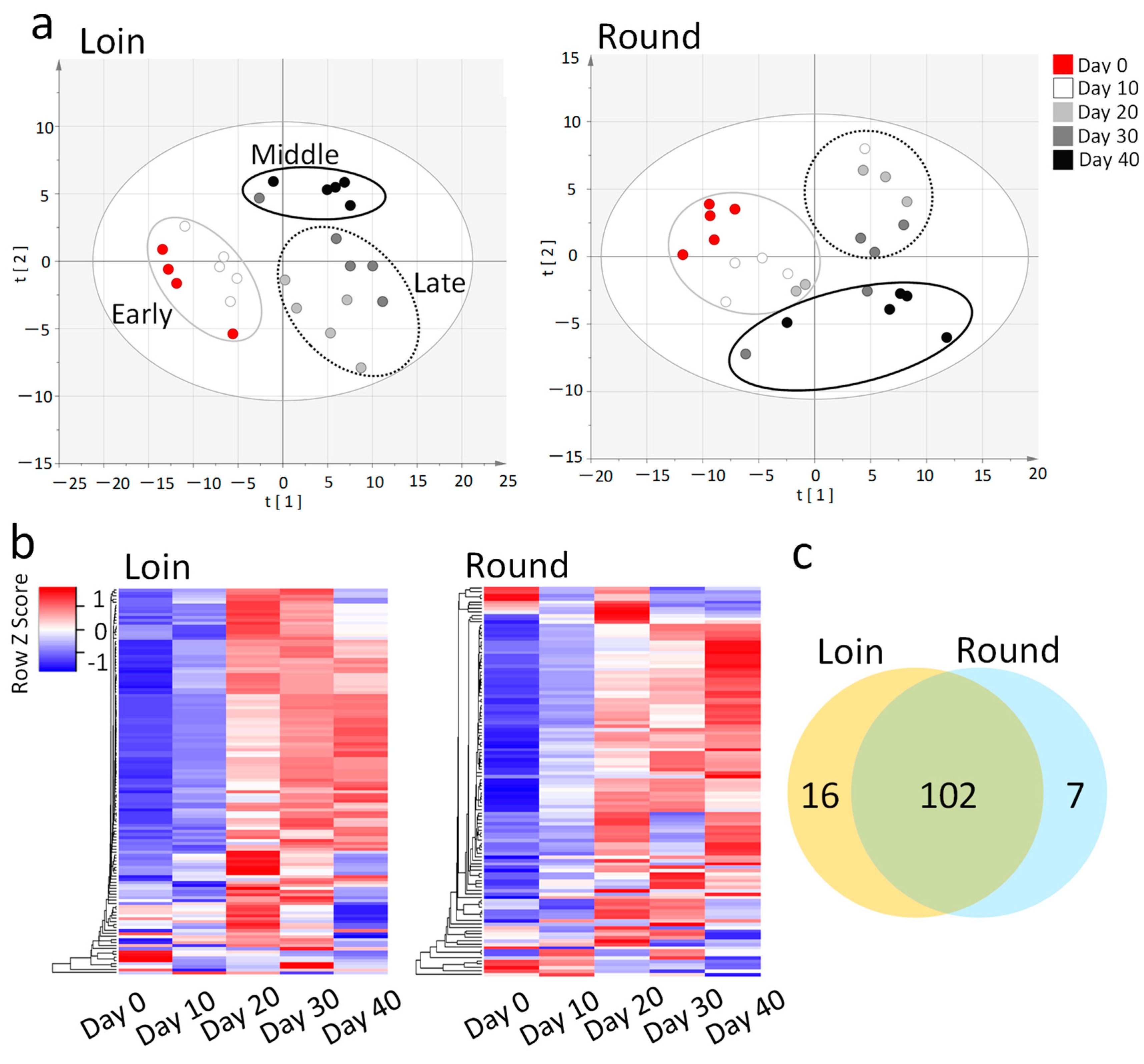
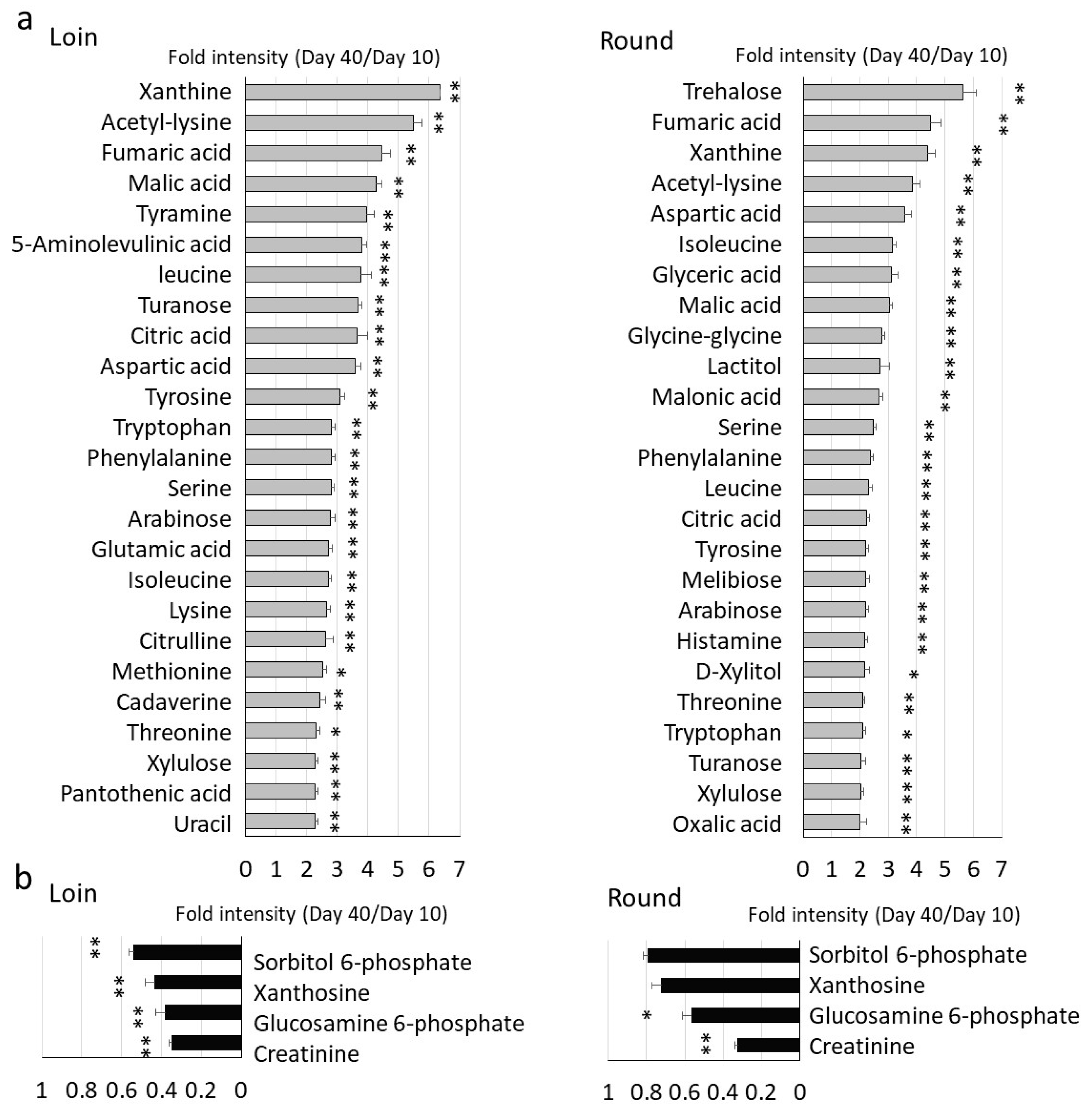
3.2. Comprehensive GC/MS Analysis of Metabolite Changes during Wet Aging
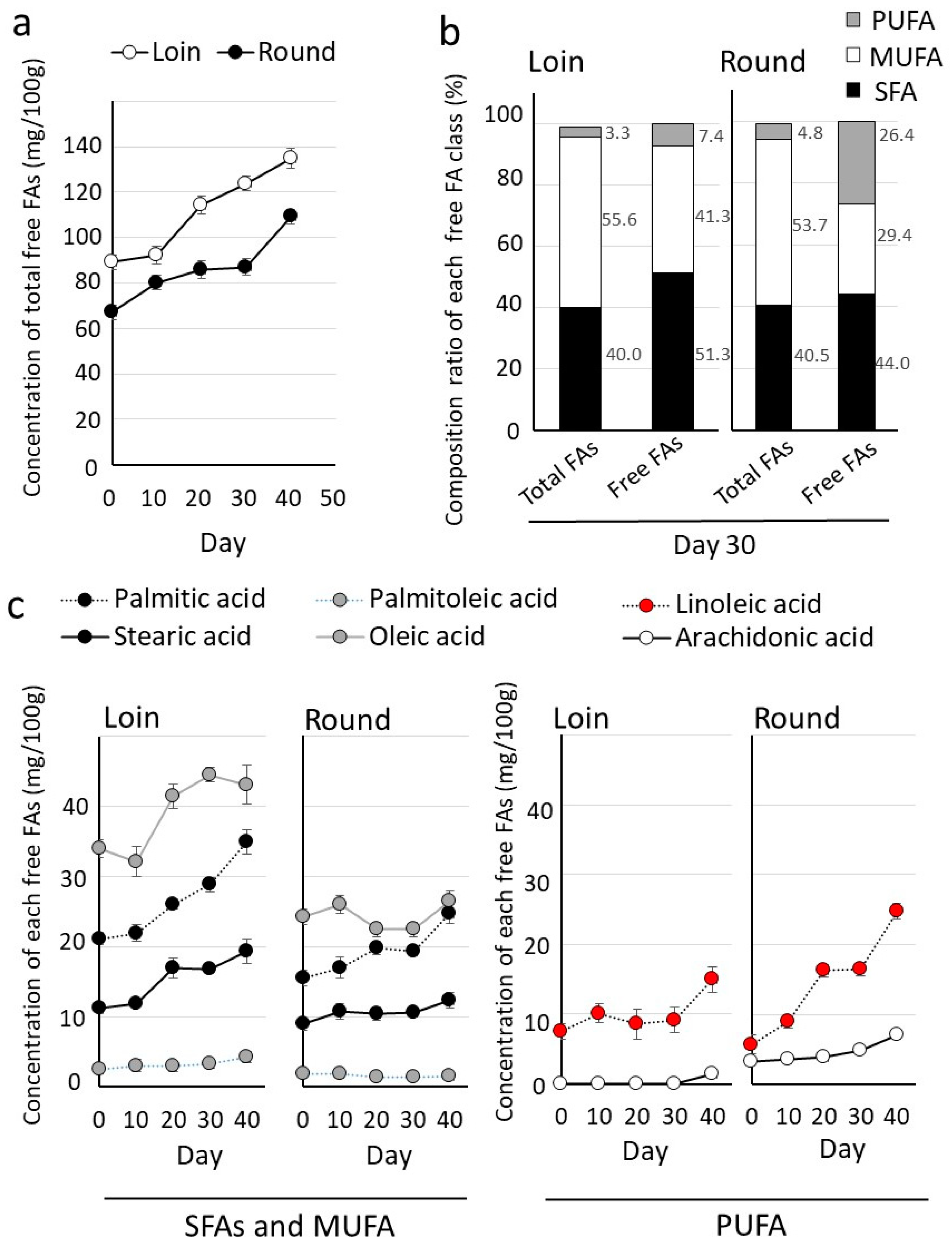
3.3. Comparison of Lipid Composition in Hybrid Wagyu Beef
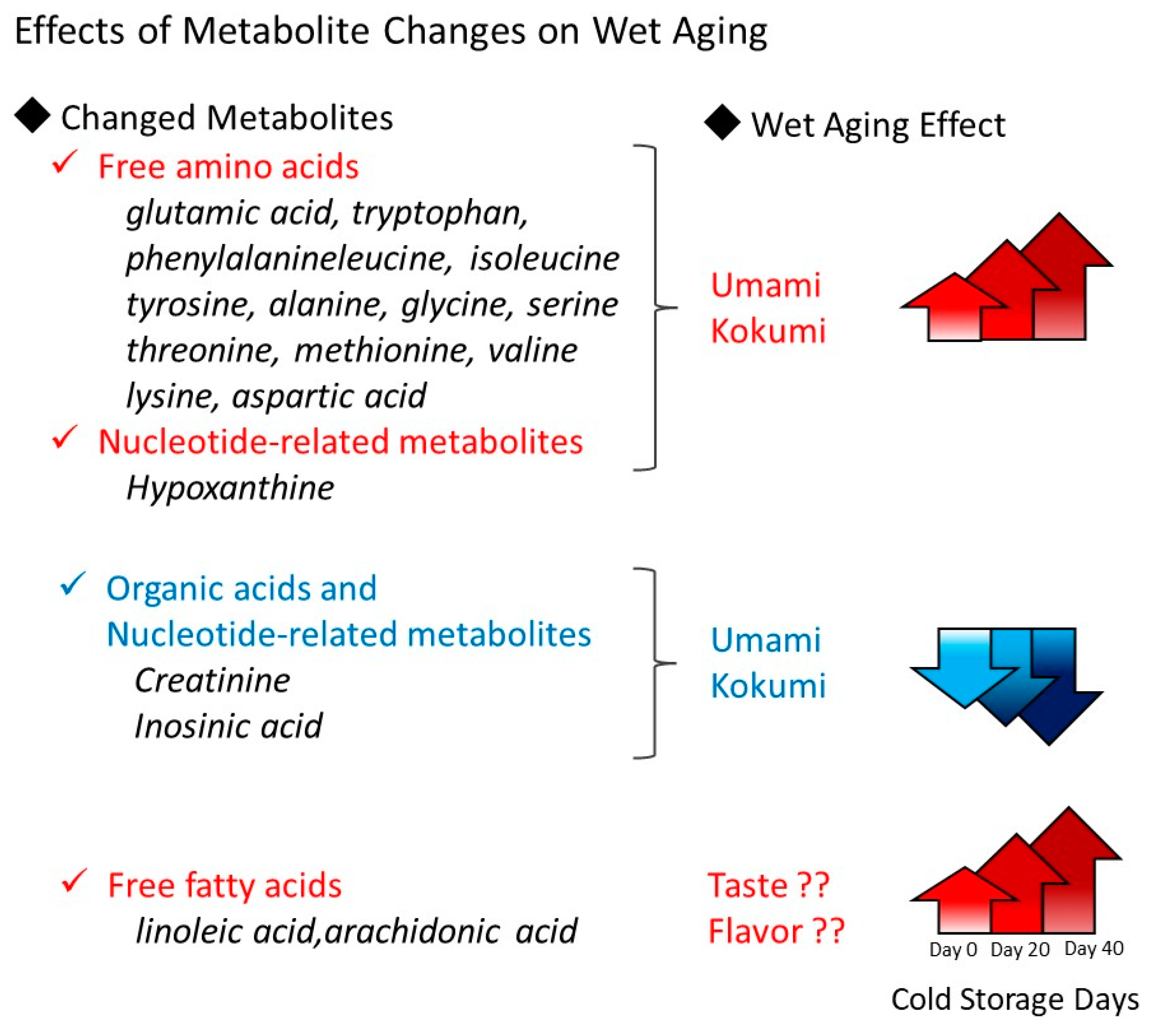
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC/MS | Gas chromatography–mass spectrometry |
| PCA | Principal component analysis |
| SD | Standard deviation |
| TG | Triacylglyceride |
| SFA | Saturated fatty acid |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| CV | Coefficient of variation |
References
- Pulina, G.; Acciaro, M.; Atzori, A.S.; Battacone, G.; Crovetto, G.M.; Mele, M.; Pirlo, G.; Rassu, S.P.G. Animal board invited review—Beef for future: Technologies for a sustainable and profitable beef industry. Animal 2021, 15, 100358. [Google Scholar] [CrossRef] [PubMed]
- Matsuishi, M. Science and technology of meat and meat products in Japan-Pursuit of their palatability under the influence of Washoku, traditional Japanese cuisine. Meat Sci. 2022, 192, 108919. [Google Scholar] [CrossRef]
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef]
- Li, G.; Yang, R.; Lu, X.; Liu, Y.; He, W.; Li, Y.; Yu, H.; Qin, L.; Cao, Y.; Zhao, Z.; et al. RNA-Seq Analysis Identifies Differentially Expressed Genes in the Longissimus dorsi of Wagyu and Chinese Red Steppe Cattle. Int. J. Mol. Sci. 2023, 24, 387. [Google Scholar] [CrossRef]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Ueda, S.; Takashima, Y.; Gotou, Y.; Sasaki, R.; Nakabayashi, R.; Suzuki, T.; Sasazaki, S.; Fukuda, I.; Kebede, B.; Kadowaki, Y.; et al. Application of Mass Spectrometry for Determining the Geographic Production Area of Wagyu Beef. Metabolites 2022, 12, 777. [Google Scholar] [CrossRef]
- Pitchford, W.S.; Deland, M.P.B.; Siebert, B.D.; Malau-Aduliand, A.E.O.; Bottema, C.D.K. Genetic variation in fatness and fatty acid composition of crossbred cattle1. J. Anim. Sci. 2002, 80, 2825–2832. [Google Scholar] [CrossRef]
- Kaewpila, C.; Sommart, K.; Mitsumori, M. Dietary fat sources affect feed intake, digestibility, rumen microbial populations, energy partition and methane emissions in different beef cattle genotypes. Animal 2018, 12, 2529–2538. [Google Scholar] [CrossRef]
- Wang, X.; Qiang, W.; Liu, X.; Yan, S.; Qi, Y.; Jia, Z.; Liu, G. The spatiotemporal patterns and network characteristics of emissions embodied in the international trade of livestock products. J. Environ. Manag. 2022, 322, 116128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, Z.; Sun, T.; Li, W.; Zhou, W.; Wang, X.; Fei, X.; Tong, H.; Yin, K. Cradle-to-grave emissions from food loss and waste represent half of total greenhouse gas emissions from food systems. Nat. Food 2023, 4, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, R.J.; Philpott, J.; Perovic, J.; Lau, J.; Davies, L.; Mudannayake, W.; Watson, R.; Tarr, G.; Thompson, J.M. The effect of packaging on consumer eating quality of beef. Meat Sci. 2018, 142, 59–64. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Meinert, L. Meat flavour in pork and beef—From animal to meal. Meat Sci. 2017, 132, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, X.; Yang, Y.; Zhang, L.; Jiao, T.; Zhao, S. Comprehensive Analysis of the Longissimus Dorsi Transcriptome and Metabolome Reveals the Regulatory Mechanism of Different Varieties of Meat Quality. J. Agric. Food Chem. 2023, 71, 1234–1245. [Google Scholar] [CrossRef]
- Ueda, S.; Sasaki, R.; Nakabayashi, R.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Iwatoh, S.; Miyaura, K.; Asikin, Y.; Kusano, M. Metabolomic profiling reveals the relationship between taste-related metabolites and roasted aroma in aged pork. LWT 2022, 155, 112928. [Google Scholar] [CrossRef]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography–mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Shimbo, K.; Kubo, S.; Harada, Y.; Oonuki, T.; Yokokura, T.; Yoshida, H.; Amao, M.; Nakamura, M.; Kageyama, N.; Yamazaki, J.; et al. Automated precolumn derivatization system for analyzing physiological amino acids by liquid chromatography/mass spectrometry. Biomed. Chromatogr. 2010, 24, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Marais, J.; Strydom, P.E.; Hoffman, L.C. Effects of increasing internal end-point temperatures on physicochemical and sensory properties of meat: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2843–2872. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Asian-Australas. J. Anim. Sci. 2019, 32, 1172–1185. [Google Scholar] [CrossRef]
- Ichimura, S.; Nakamura, Y.; Yoshida, Y.; Hattori, A. Hypoxanthine enhances the cured meat taste. Anim. Sci. J. 2017, 88, 379–385. [Google Scholar] [CrossRef]
- Shah, A.K.M.A.; Ogasawara, M.; Kurihara, H.; Takahashi, K. Effect of Drying on Creatine/Creatinine Ratios and Subsequent Taste of Herring (Clupea pallasii) Fillet. Food Sci. Technol. Res. 2013, 19, 691–696. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, Y.; Zeng, J.; Zhao, M. Six categories of amino acid derivatives with potential taste contributions: A review of studies on soy sauce. Crit. Rev. Food Sci. Nutr. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Toita, T.; Uemoto, Y. Estimates of genetic parameters for adenosine triphosphate-related compounds at different aging periods and NT5E genotypes in Japanese Black beef. Anim. Sci. J. 2022, 93, e13748. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, S.; Yang, L.; Li, Z. Flavor formation based on lipid in meat and meat products: A review. J. Food Biochem. 2022, 46, e14439. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Baek, K.H.; Ko, Y.-J.; Lee, H.J.; Yim, D.-G.; Jo, C. Characteristic Metabolic Changes of the Crust from Dry-Aged Beef Using 2D NMR Spectroscopy. Molecules 2020, 25, 3087. [Google Scholar] [CrossRef] [PubMed]
- Horbańczuk, O.K.; Wierzbicka, A. Effects of Packaging Methods on Shelf Life of Ratite Meats. J. Vet. Res. 2017, 61, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.; Witte, F.; Sieksmeyer, T.; Januschweski, E.; Terjung, N.; Hertel, C.; Heinz, V.; Juadjur, A.; Gibis, M. Metabolic and microbial analyses of the surface and inner part of wet-aged and dry-aged beef. J. Food Sci. 2023, 88, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Omori, H.; Kawabata, Y.; Yoshida, Y.; Nagamoto, Y.; Kawabata, F.; Nishimura, S.; Tabata, S. Oral expressions and functional analyses of the extracellular calcium-sensing receptor (CaSR) in chicken. Sci. Rep. 2022, 12, 17762. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, H.J.; Kim, D.; Joo, B.; Jhoo, J.W.; Jang, A. Physicochemical Properties and Volatile Organic Compounds of Dairy Beef Round Subjected to Various Cooking Methods. Food Sci. Anim. Resour. 2023, 43, 767–791. [Google Scholar] [CrossRef]
- Mitacek, R.M.; Ke, Y.; Prenni, J.E.; Jadeja, R.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Mitochondrial Degeneration, Depletion of NADH, and Oxidative Stress Decrease Color Stability of Wet-Aged Beef Longissimus Steaks. J. Food Sci. 2019, 84, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, S.; Cheng, Y.; Qian, H. The effect of aging on beef taste, aroma and texture, and the role of microorganisms: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, C.N.; Torres Filho, R.A.; Fontes, P.R.; Ramos, A.L.S.; Contreras Castillo, C.J.; Hopkins, D.L.; Ramos, E.M. Comparison of different methods for determining the extent of myofibrillar fragmentation of chilled and frozen/thawed beef across postmortem aging periods. Meat Sci. 2020, 160, 107955. [Google Scholar] [CrossRef]
- Huang, F.; Ding, Z.; Chen, J.; Guo, B.; Wang, L.; Liu, C.; Zhang, C. Contribution of mitochondria to postmortem muscle tenderization: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.K.; Luckemeyer, T.J.; Kerth, C.R.; Adhikari, K. Descriptive beef flavor and texture attributes relationships with consumer acceptance of US light beef eaters. Meat Sci. 2023, 204, 109252. [Google Scholar] [CrossRef]
- Muroya, S.; Nomura, R.; Nagai, H.; Ojima, K.; Matsukawa, K. Metabolomic profiling of postmortem aged muscle in Japanese Brown beef cattle revealed an interbreed difference from Japanese Black beef. Anim. Biosci. 2023, 36, 506–520. [Google Scholar] [CrossRef]
- Foster, D.B.; Liu, T.; Rucker, J.; O’Meally, R.N.; Devine, L.R.; Cole, R.N.; O’Rourke, B. The cardiac acetyl-lysine proteome. PLoS ONE 2013, 8, e67513. [Google Scholar] [CrossRef]
- Ueda, S.; Hosoda, M.; Kasamatsu, K.; Horiuchi, M.; Nakabayashi, R.; Kang, B.; Shinohara, M.; Nakanishi, H.; Ohto-Nakanishi, T.; Yamanoue, M.; et al. Production of Hydroxy Fatty Acids, Precursors of γ-Hexalactone, Contributes to the Characteristic Sweet Aroma of Beef. Metabolites 2022, 12, 332. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhang, X.; Liu, S.; Yu, J.; Cui, H.; Xia, S.; Ho, C.-T. Changes of lipid oxidation, volatile and taste-active compounds during pan-heating of pork belly. Food Res. Int. 2023, 172, 113106. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Tatiyaborworntham, N.; Oz, F.; Richards, M.P.; Wu, H. Paradoxical effects of lipolysis on the lipid oxidation in meat and meat products. Food Chem. X 2022, 14, 100317. [Google Scholar] [CrossRef] [PubMed]
- Shanmugamprema, D.; Muthuswamy, K.; Subramanian, G.; Ponnusamy, V.; Krishnan, V.; Subramaniam, S. Fat taste signal transduction and its possible negative modulator components. Prog. Lipid Res. 2020, 79, 101035. [Google Scholar] [CrossRef]

| Item | Capabilities |
|---|---|
| Physical Properties | Measured Value |
| Oxygen permeability | 24 mL/m2·day·MPa |
| Humidity permeability | 2 g/m2/day |
| Outer layer | Ethylene vinyl acetate copolymer and polyamide copolymer |
| Core layer | Vinylidene chloride copolymer |
| Fatty Acid | % of All Fatty Acid ± SD | |||
|---|---|---|---|---|
| Name | Abbreviation | Symbol | Loin | Round |
| Myristic acid | C14:0 | M | 2.7 ± 0.32 | 2.4 ± 0.30 |
| Myristoleic acid | C14:1 | Mo | 0.9 ± 0.16 | 1.1± 0.22 |
| Pentadecanoic acid | C15:0 | Pe | 0.3 ± 0.09 | 0.4 ± 0.10 |
| Palmitic acid | C16:0 | P | 26.5 ± 1.04 | 25.8 ± 1.59 |
| Palmitoleic acid | C16:1 | Po | 3.2 ± 0.61 | 4.3 ± 0.60 |
| Margaric acid | C17:0 | Ma | 0.8 ± 0.21 | 0.7 ± 0.13 |
| Stearic acid | C18:0 | S | 11.9 ± 0.83 | 8.9 ± 0.50 |
| Oleic acid | C18:1 | O | 46.7 ± 2.13 | 49.1 ± 1.60 |
| Linoleic acid | C18:2 | L | 2.9 ± 0.47 | 3.0 ± 0.53 |
| Linolenic acid | C18:3 | Al | 0.2 ± 0.03 | 0.2 ± 0.04 |
| Dihomo-γ-linolenic acid | C20:3 | D | 0.1 ± 0.02 | 0.1 ± 0.02 |
| Arachidonic acid | C20:4 | Ar | 0.1 ± 0.02 | 0.2 ± 0.04 |
| Triacylglycerides | % of All TGs ± SD | ||
|---|---|---|---|
| Name | Symbol | Longissimus Thoracis | Adductor Muscle |
| TG (C16:0/C18:1/C18:1) | POO a | 29.8 ± 0.3 | 31.8 ± 1.9 |
| TG (C18:0/C18:1/C18:1) | SOO | 9.1 ± 1.9 | 6.5 ± 0.5 |
| TG (C16:0/C18:1/C16:0) | POP b | 8.5 ± 0.9 | 8.0 ± 0.9 |
| TG (C18:1/C18:1/C18:1) | OOO | 7.9 ± 1.9 | 9.2 ± 0.9 |
| TG (C16:0/C18:1/C18:0) | POS | 7.7 ± 0.5 | 5.4 ± 0.4 |
| TG (C16:0/C16:1/C18:1) | PPoO c | 7.2 ± 1.9 | 9.0 ± 1.3 |
| TG (C16:0/C18:2/C18:1) | PLO | 5.0 ± 1.9 | 4.3 ± 1.5 |
| TG (C14:0/C18:1/C16:0) | MOP d | 4.7 ± 0.6 | 4.2 ± 0.3 |
| TG (C18:1/C18:1/C18:2) | OOL | 3.9 ± 0.6 | 6.0 ± 0.4 |
| TG (C18:0/C18:1/C18:0) | SOS | 2.7 ± 1.0 | 0.9 ± 0.6 |
| TG (C16:0/C16:0/C16:0) | PPP | 2.7 ± 0.2 | 2.1 ± 0.3 |
| TG (C16:0/C16:0/C18:0) | PPS | 1.4 ± 0.2 | 1.0 ± 0.2 |
| TG (C16:0/C18:1/C17:0) | POMa | 0.8 ± 0.7 | 0.7 ± 0.1 |
| TG (C16:0/C18:0/C18:0) | PSS | 0.5 ± 0.4 | 1.2 ± 0.7 |
| Other e | - | 8.1 ± 2.2 | 9.9 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Yoshida, Y.; Kebede, B.; Kitamura, C.; Sasaki, R.; Shinohara, M.; Fukuda, I.; Shirai, Y. New Implications of Metabolites and Free Fatty Acids in Quality Control of Crossbred Wagyu Beef during Wet Aging Cold Storage. Metabolites 2024, 14, 95. https://doi.org/10.3390/metabo14020095
Ueda S, Yoshida Y, Kebede B, Kitamura C, Sasaki R, Shinohara M, Fukuda I, Shirai Y. New Implications of Metabolites and Free Fatty Acids in Quality Control of Crossbred Wagyu Beef during Wet Aging Cold Storage. Metabolites. 2024; 14(2):95. https://doi.org/10.3390/metabo14020095
Chicago/Turabian StyleUeda, Shuji, Yuka Yoshida, Biniam Kebede, Chiaki Kitamura, Ryo Sasaki, Masakazu Shinohara, Itsuko Fukuda, and Yasuhito Shirai. 2024. "New Implications of Metabolites and Free Fatty Acids in Quality Control of Crossbred Wagyu Beef during Wet Aging Cold Storage" Metabolites 14, no. 2: 95. https://doi.org/10.3390/metabo14020095
APA StyleUeda, S., Yoshida, Y., Kebede, B., Kitamura, C., Sasaki, R., Shinohara, M., Fukuda, I., & Shirai, Y. (2024). New Implications of Metabolites and Free Fatty Acids in Quality Control of Crossbred Wagyu Beef during Wet Aging Cold Storage. Metabolites, 14(2), 95. https://doi.org/10.3390/metabo14020095







