The Effect of Vitamin D Supplementation with or without Calcium on Vitamin D Epimer and Metabolites
Abstract
1. Introduction
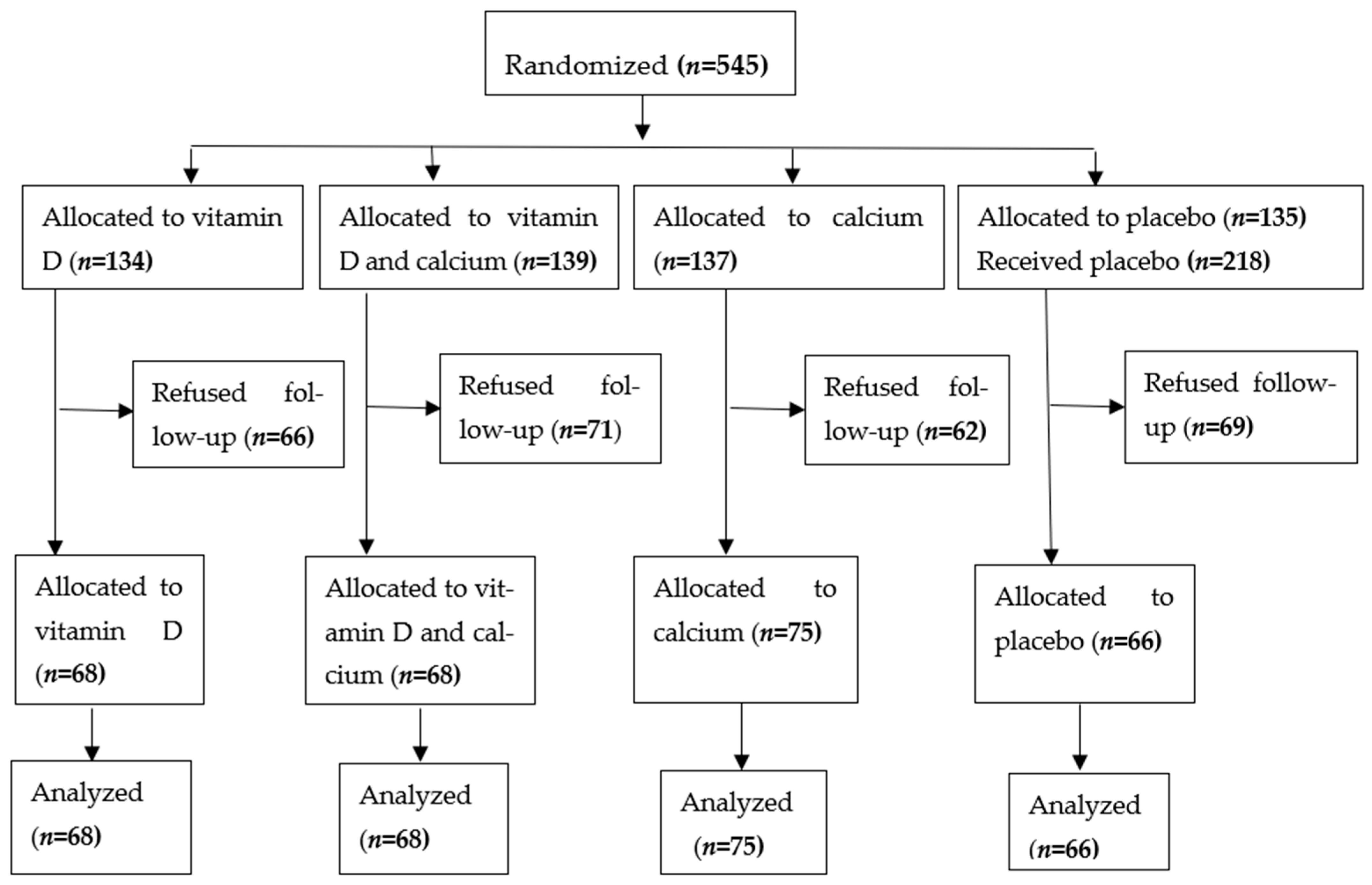
2. Methods and Study Design
2.1. Study Design
2.2. The Measurements
2.2.1. DNA Preparation and VDR SNP Genotyping Analysis [17]
2.2.2. Measurements of Vitamin D Epimers and Metabolites [17]
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
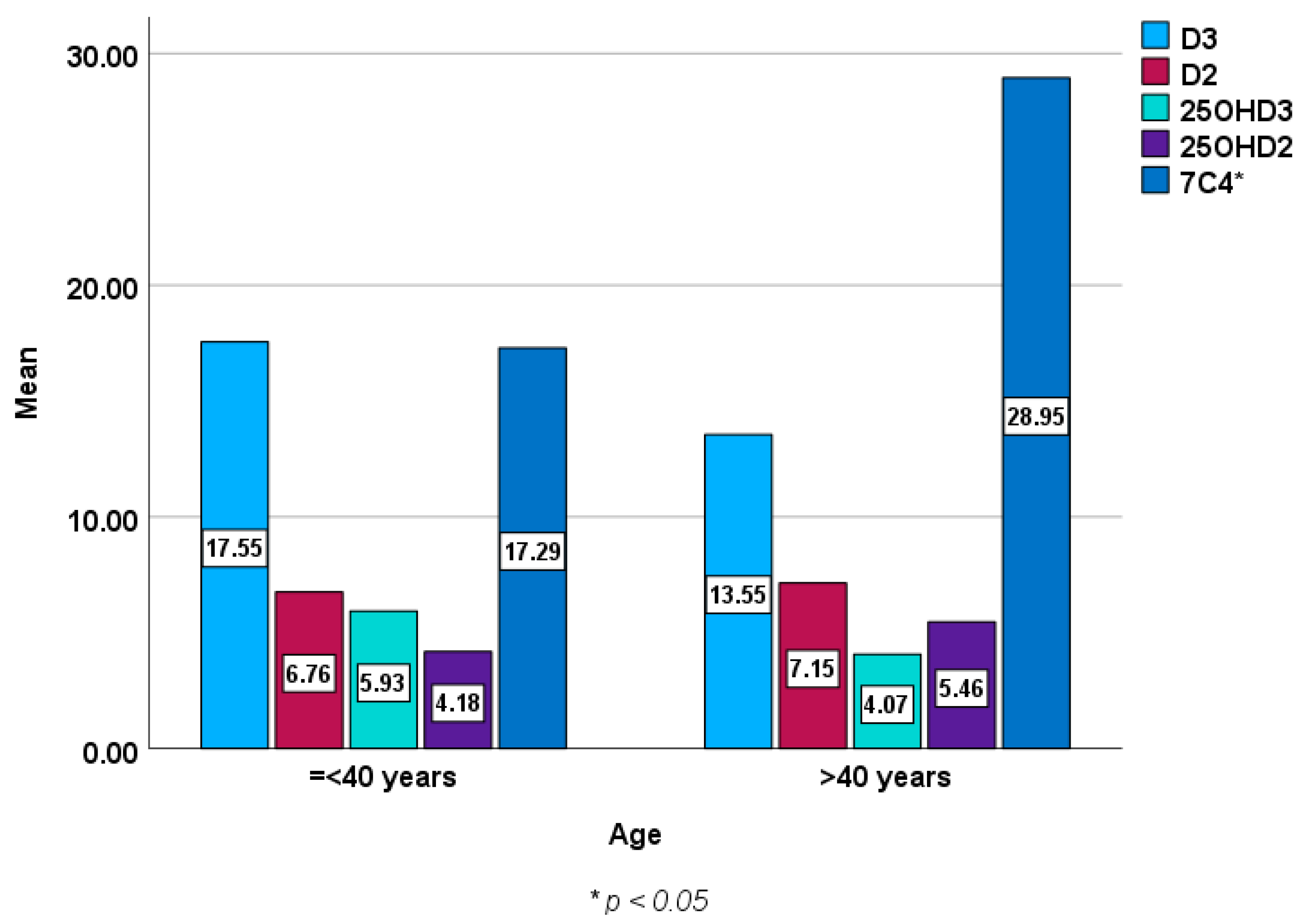
3.2. Vitamin D Epimer and Metabolite Concentrations Stratified by Age, Sex and Presence or Absence of the Genotype and Allele
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramasamy, I. Vitamin D metabolism and guidelines for vitamin D supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Alzohily, B.; AlMenhali, A.; Gariballa, S.; Munawar, N.; Yasin, J.; Shah, I. Unraveling the complex interplay between obesity and vitamin D metabolism. Sci. Rep. 2024, 14, 7583. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, S.; Gill, D.; De Silva, N.M.; Lowry, E.; Jokelainen, J.; Karhu, T.; Mutt, S.J.; Dehghan, A.; Sliz, E.; Chasman, D.I.; et al. Could vitamin D reduce obesity-associated inflammation? Observational and Mendelian randomization study. Am. J. Clin. Nutr. 2020, 111, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 2000, 894, 1–253.
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.; Pols, H.A.; Van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef]
- Rezende, V.B.; Barbosa, F., Jr.; Montenegro, M.F.; Sandrim, V.C.; Gerlach, R.F.; Tanus-Santos, J.E. An interethnic comparison of the distribution of vitamin D receptor genotypes and haplotypes. Clin. Chim. Acta 2007, 384, 155–159. [Google Scholar] [CrossRef]
- Qin, W.H.; Wang, H.X.; Qiu, J.L.; Huang, X.B.; Huang, Y.; Wu, N.R.; Liang, H.S. A meta-analysis of association of vitamin D receptor BsmI gene polymorphism with the risk of type 1 diabetes mellitus. J. Recept. Signal Transduct. Res. 2014, 34, 372–377. [Google Scholar] [CrossRef]
- Holvik, K.; Meyer, H.E.; Søgaard, A.J.; Selmer, R.; Haug, E.; Falch, J.A. Biochemical markers of bone turnover and their relation to forearm bone mineral density in persons of Pakistani and Norwegian background living in Oslo Norway. Eur. J. Endocrinol. 2006, 155, 693–699. [Google Scholar] [CrossRef][Green Version]
- Lowe, N.M.; Mitra, S.R.; Foster, P.C.; Bhojani, I.; McCann, J. Vitamin D status and markers of bone turnover in Caucasian and Southy Asian postmenopausal women living in the UK. Br. J. Nutr. 2010, 103, 1706–1710. [Google Scholar] [CrossRef]
- Farrar, M.D.; Kift, R.; Felton, S.J.; Berry, J.L.; Durkin, M.T.; Allan, D.; Vail, A.; Webb, A.R.; Rhodes, L.E. Recommended summer sunlight exposure amounts fail to produce sufficie vitamin D status in UK adults of South Asian origin. Am. J. Clin. Nutr. 2011, 94, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Saadi, H.; Dawodu, A.; Afandi, B.; Zayed, R.; Benedict, S.; Ngelkerke, N. Efficacy of daily and monthly high-dose calciferol in Vitamin D deficient nulliparous and lactating women. Am. J. Clin. Nutr. 2007, 85, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, M.; Kärkkäinen, M.; Outila, T.; Vanninen, T.; Ray, C.; Lamberg-Allardt, C. Vitamin D receptor gene BsmI-polymorphism in Finnish premenopausal and postmenopausal women: Its association with bone mineral density, markers of bone turnover, and intestinal calcium absorption, with adjustment for lifestyle factors. J. Bone Miner. Metab. 2002, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Al-Dabbagh, B.; Gariballa, S.; Al-Menhali, A.; Muhammad, N.; Yasin, J.; Ashraf, S.S. Application of a new vitamin D blood test on the Emirati population. J. Steroid Biochem. Mol. Biol. 2018, 180, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Yasin, J.; Alessa, A. A randomized, double-blind, placebo-controlled trial of vitamin D supplementation with or without calcium in community-dwelling vitamin D deficient subjects. BMC Musculoskelet. Disord. 2022, 23, 415. [Google Scholar] [CrossRef]
- Gariballa, S.; Al-Bluwi, G.S.M.; Yasin, J. Frequency of Vitamin D Receptor Gene Polymorphisms in a Population with a very High Prevalence of Vitamin D Deficiency, Obesity, Diabetes and Hypertension. Biomedicines 2023, 11, 1202. [Google Scholar] [CrossRef]
- Gariballa, S.; Shah, I.; Yasin, J.; Alessa, A. Vitamin D [25(OH)D] metabolites and epimers in obese subject: Interaction and correlations with adverse metabolic health risk factors. J. Steroid Biochem. Mol. Biol. 2022, 215, 106023. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693.38. [Google Scholar] [CrossRef]
- Maki, K.C.; Rubin, M.R.; Wong, L.G.; McManus, J.F.; Jensen, C.D.; Lawless, A. Effects of vitamin D supplementation on 25-hydroxyvitamin D, high-density lipoprotein cholesterol, and other cardiovascular disease risk markers in subjects with elevated waist circumference. Int. J. Food Sci. Nutr. 2011, 62, 318–332. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, P.J.; Pham, T.M.; Ekwaru, J.P. Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients 2015, 7, 10189–10208. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020, 43, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Gandini, S.; Mullie, P. A Systematic Review: Influence of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentration. J. Clin. Endocrinol. Metab. 2012, 97, 2606–2613. [Google Scholar] [CrossRef]
- Al-Zohily Al Menhali, B.; Gariballa, S.; Haq, A.; Shah, I. Epimers of vitamin D: A review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef]
- Chailurkit, L.; Aekplakorn, W.; Ongphiphadhanakul, B. Serum C3 epimer of 25-hydroxyvitamin D and its determinants in adults: A national health examination survey in Thais. Osteoporos. Int. 2015, 26, 2339–2344. [Google Scholar] [CrossRef]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef]
- Ghaly, S.; Bliuc, D.; Center, J.R.; Clarke, M.W.; Jones, A.P.; Trend, S.; Kermode, A.G.; Neale, R.E.; Hart, P.H. Vitamin D C3-epimer levels are proportionally higher with oral vitamin D supplementation compared to ultraviolet irradiation of skin in mice but not humans. J. Steroid Biochem. Mol. Biol. 2019, 186, 110–116. [Google Scholar] [CrossRef]
- Bouillon, R.; Bikle, D. Vitamin D metabolism revised: Fall of dogmas. J. Bone Miner. Res. 2019, 34, 1985–1992. [Google Scholar] [CrossRef]


| Vitamin D3 (n = 68) | Vitamin D3 + Calcium (n = 68) | Calcium (n = 75) | Placebo (n = 66) | p Value | ||
|---|---|---|---|---|---|---|
| Age (years) | 42 (11) | 41 (13) | 41 (13) | 41 (13) | 0.89 | |
| Gender, n (%) | Female | 55 (78) | 55 (73) | 48 (73) | 48 (70) | 0.28 |
| Smoking, n (%) | 0.94 | |||||
| Yes | 8 (11) | 10 (13) | 7 (11) | 10 (15) | ||
| Occasionally | 5 (7) | 3 (4) | 2 (3) | 2 (3) | ||
| No | 55 (79) | 63 (82) | 55 (83) | 55 (80) | ||
| Diabetes mellitus, n (%) | 7 (10) | 10 (13) | 12 (18) | 17 (25) | 0.02 | |
| Hypertension, n (%) | 8 (11) | 11 (15) | 9 (14) | 14 (20) | 0.14 | |
| BMI | 29.3 (5) | 28.7 (5.5) | 29 (6) | 28.6 (5) | 0.99 | |
| Physical activity, n (%) | 0.65 | |||||
| Very active | 18 (25) | 17 (23) | 12 (18) | 8 (12) | ||
| Moderate | 41 (59) | 47 (62) | 37 (57) | 49 (72) | ||
| Not active | 11 (16) | 17 (22) | 16 (25) | 11 (16) | ||
| Systolic blood pressure, (mm/Hg) | 118 (8) | 120 (9) | 116 (10) | 122 (14) | 0.35 | |
| Diastolic blood pressure, (mm/Hg) | 75 (6) | 79 (5) | 76 (6) | 77 (6) | 0.28 | |
| Cholesterol, (mmol/L) | 7 (10) | 8 (11) | 4 (6) | 14 (20) | 0.08 | |
| Glucose, (mmol/L) | 6.5 (2) | 6.2 (2.5) | 6.2 (2.3) | 6.3 (2.5) | 0.88 | |
| HbA1c | 5.9 (1) | 5.8 (0.9) | 5.8 (1) | 5.8 (0.9) | 0.97 | |
| Urea, (mmol/L) | 4.4 (1.7) | 3.9 (1.2) | 3.9 (1.2) | 4.2 (1.6) | 0.07 | |
| Creatinine, (mmol/L) | 52 (12) | 52 (12) | 51 (12) | 53 (15) | 0.69 | |
| Vitamin D, (ng/mL) | 23 (9) | 19 (11) | 25 (11) | 25 (11) | 0.58 |
| AA | GG | AG | A | G | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Vitamin D3 (ng/mL) | 12.49 (5.61) | 12.52 (6.36) | 11.85 (5.29) | 12.92 (6.67) | 13.15 (7.18) | 12.07 (5.38) | 12.93 (6.71) | 11.83 (5.24) | 12.53 (6.37) | 12.46 (5.56) |
| Vitamin D2 (ng/mL) | 5.72 (1.15) | 5.87 (1.17) | 5.84 (1.18) | 5.84 (1.16) | 5.90 (1.17) | 5.80 (1.16) | 5.84 (1.17) | 5.84 (1.17) | 5.87 (1.17) | 5.72 (1.14) |
| 25OHD3 (ng/mL) | 3.80 (0.82) | 3.98 (1.43) | 3.83 (0.89) | 4.02 (1.52) | 4.13 (1.77) | 3.82 (0.86) | 4.02 (1.53) | 3.83 (0.88) | 3.98 (1.43) | 3.80 (0.81) |
| 3-epi-25OHD3 (ng/mL) | 1.29 (0.21) | 1.31 (0.25) | 1.29 (0.25) | 1.31 (0.23) | 1.32 (0.25) | 1.29 (0.24) | 1.31 (0.24) | 1.29 (0.25) | 1.31 (0.25) | 1.29 (0.21) |
| 7αC4 * (ng/mL) | 11.99 (11.74) | (11.93) 11.43 | 12.13 (11.86) | 11.83 (11.27) | 11.80 (11.11) | 12.04 (11.76) | 11.89 (11.32) | 12.02 (11.77) | 11.95 (11.44) | 11.89 (11.67) |
| AA | GG | AG | A | G | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Vitamin D3 (ng/mL) | 12.06 (6.09) | 12.72 (6.26) | 11.61 (4.54) | 12.73 (6.52) | 13.17 (6.79) | 11.88 (5.52) | 12.74 (6.54) | 11.59 (4.50) | 12.74 (6.27) | 12.04 (6.06) |
| Vitamin D2 (ng/mL) | 5.81 (1.21) | 5.85 (1.15) | 5.69 (1.20) | 5.88 (1.16) | 5.92 (1.13) | 5.77 (1.19) | 5.88 (1.16) | 5.70 (1.19) | 5.85 (1.15) | 1.20 (5.81) |
| 25OHD3 (ng/mL) | 3.84 (1.08) | 3.99 (1.42) | 3.88 (1.03) | 3.96 (1.39) | 4.03 (1.55) | 3.86 (1.05) | 3.96 (1.39) | 3.89 (1.02) | 3.99 (1.42) | 3.84 (1.07) |
| 3-epi-25OHD3 (ng/mL) | 1.33 (0.27) | 1.29 (0.23) | 1.29 (0.22) | 1.30 (0.25) | 1.29 (0.23) | 1.31 (0.25) | 1.31 (0.25) | 1.29 (0.22) | 1.29 (0.23) | 1.33 (0.27) |
| 7αC4 (ng/mL) | 10.68 (10.24) | 12.51 (11.97) | 12.12 (12.89) | 11.90 (11.14) | 12.71 (11.67) | 11.19 (11.27) | 11.92 (11.16) | 12.00 (12.80) | 12.54 (11.99) | 10.62 (10.19) |
| AA | CC | AC | A | C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Vitamin D3 (ng/mL) | 13.16 (6.76) | 12.01 (5.70) | 11.28 (3.43) | 12.77 * (6.60) | 12.34 (6.45) | 12.63 (6.06) | 12.78 (6.61) | 11.26 (3.40) | 12.02 (5.72) | 13.14 (6.74) |
| Vitamin D2 (ng/mL) | 5.77 (1.03) | 5.90 (1.27) | 5.64 (1.05) | 5.88 (1.19) | 6.01 (1.34) | 5.73 (1.03) | 5.88 (1.19) | 5.64 (1.04) | 5.90 (1.27) | 5.77 (1.02) |
| 25OHD3 (ng/mL) | 4.00 (1.51) | 3.91 (1.15) | 3.74 (0.74) | 3.987 (1.41) | 3.98 (1.29) | 3.93 (1.35) | 3.99 (1.41) | 3.74 (0.73) | 3.90 (1.16) | 4.00 (1.50) |
| 3-epi-25OHD3 (ng/mL) | 1.33 (0.23) | 1.28 (0.25) | 1.32 (0.26) | 1.30 (0.24) | 1.27 (0.25) | 1.32 (0.23) | 1.30 (0.24) | 1.32 (0.25) | 1.28 (0.25) | 1.32 (0.22) |
| 7αC4 (ng/mL) | 12.18 (11.34) | 11.75 (11.60) | 13.18 (13.29) | 11.69 (11.09) | 11.19 (10.86) | 12.41 11.85 | 11.72 (11.11) | 13.18 (13.03) | 11.79 (11.63) | 12.13 (11.31) |
| AA | GG | AG | A | G | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Vitamin D3 (ng/mL) | 11.21 (3.89) | 12.68 (6.43) | 12.38 (6.85) | 12.62 (5.70) | 12.99 (6.04) | 12.12 (6.33) | 12.13 (6.35) | 12.97 (6.02) | 12.64 (6.41) | 11.59 (4.42) |
| Vitamin D2 (ng/mL) | 5.57 (1.11) | 5.88 (1.17) | 5.75 (1.10) | 5.91 (1.21) | 5.99 (1.23) | 5.72 (1.10) | 5.71 (1.10) | 5.99 (1.23) | 5.88 (1.17) | 5.59 (1.08) |
| 25OHD3 (ng/mL) | 3.95 (1.53) | 3.95 (1.30) | 3.84 (0.96) | 4.03 (1.54) | 4.05 (1.55) | 3.86 (1.10) | 3.86 (1.10) | 4.05 (1.55) | 3.95 (1.30) | 3.95 (1.48) |
| 3-epi-25OHD3 (ng/mL) | 1.24 (0.20) | 1.31 (0.25) | 1.33 (0.25) | 1.28 (0.23) | 1.30 (0.24) | 1.31 (0.24) | 1.31 (0.24) | 1.30 (0.24) | 1.31 (0.25) | 1.24 (0.19) |
| 7αC4 (ng/mL) | 12.80 (9.44) | 11.83 (11.72) | 12.37 (12.96) | 11.62 (10.26) | 11.36 (10.50) | 12.42 (12.23) | 12.47 (12.26) | 11.32 (10.47) | 11.87 (11.76) | 12.46 (9.26) |
| Clinical Variable | Vitamin D3 (n = 68) | RG2 Vitamin D3 + Calcium (n = 68) | RG3 Calcium (n = 75) | RG4 Placebo (n = 66) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow Up | Baseline | Follow Up | Baseline | Follow Up | Baseline | Follow Up | |
| Vitamin D3 | 12.37 (5.82) | 37.41 (21.21) | 11.81 (5.84) | 37.08 (24.02) | 13.43 (6.67) | 41.467 (29.14) | 12.35(6.41) | 46.15 (36.22) |
| Vitamin D2 | 5.71 (1.10) | 6.97 (1.71) | 5.66 (1.05) | 6.78 (1.36) | 6.05 (1.12) | 7.17 (1.57) | 5.93 (1.36) | 7.77 (2.71) |
| 25 OHD3 | 3.94 (1.18) | 7.34 (3.44) | 3.84 (1.28) | 7.83 (4.36) | 4.20 (1.78) | 6.82 (3.60) | 3.78 (0.79) | 7.62 (3.89) |
| 25OHD2 | 4.18 (0.85) | 6.62 (2.90) | 3.58 (0.46) | 3.14 (0.24) | 4.26 (0.84) | 8.89 (6.28) | 6.19 (4.04) | 6.12 (3.12) |
| 1α,25(OH)2D3 | 5.63 (2.25) | 6.19 (3.12) | 5.96 (3.12) | 5.81 (2.79) | 4.85 (2.61) | 6.18 (2.90) | 5.06 (2.32) | 6.79 (3.79) |
| 3-epi-25OHD3 | 1.28 (0.20) | 2.21 (0.92) | 1.24 (0.23) | 1.97 (1.06) | 1.42 (0.24) | 1.73 (0.93) | 1.35 (0.21) | 2.19 (1.29) |
| 7αC4 | 12.17 (9.49) | 11.90 (8.27) | 10.97 (10.92) | 14.63 (13.36) | 11.50 (11.52) | 11.52 (8.57) | 13.21 (13.75) | 14.71 (10.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariballa, S.; Al-Bluwi, G.S.M.; Yasin, J. The Effect of Vitamin D Supplementation with or without Calcium on Vitamin D Epimer and Metabolites. Metabolites 2024, 14, 524. https://doi.org/10.3390/metabo14100524
Gariballa S, Al-Bluwi GSM, Yasin J. The Effect of Vitamin D Supplementation with or without Calcium on Vitamin D Epimer and Metabolites. Metabolites. 2024; 14(10):524. https://doi.org/10.3390/metabo14100524
Chicago/Turabian StyleGariballa, Salah, Ghada S. M. Al-Bluwi, and Javed Yasin. 2024. "The Effect of Vitamin D Supplementation with or without Calcium on Vitamin D Epimer and Metabolites" Metabolites 14, no. 10: 524. https://doi.org/10.3390/metabo14100524
APA StyleGariballa, S., Al-Bluwi, G. S. M., & Yasin, J. (2024). The Effect of Vitamin D Supplementation with or without Calcium on Vitamin D Epimer and Metabolites. Metabolites, 14(10), 524. https://doi.org/10.3390/metabo14100524








