Bioactive Compound Diversity in a Wide Panel of Sweet Potato (Ipomoea batatas L.) Cultivars: A Resource for Nutritional Food Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Sweet Potato Root Samples
2.2. Root Flesh Color Measurement
2.3. pH and Titratable Acidity
2.4. Analysis of Proximate Composition
2.5. Determination of Starch Gelatinization Temperature
2.6. Carotenoid, Phenolic and Anthocyanin Total Contents
2.7. Identification and Quantification of Phenolic Compounds
2.8. Identification and Quantification of Carotenoids
2.9. Free Radical Scavenging Activity
2.10. Statistical Analysis
3. Results
3.1. Phenotypic Traits within the Cultivar Panel
3.2. Proximate Composition
3.3. Starch Gelatinization Temperature
3.4. Antioxidant Activity and Bioactive Compound Composition
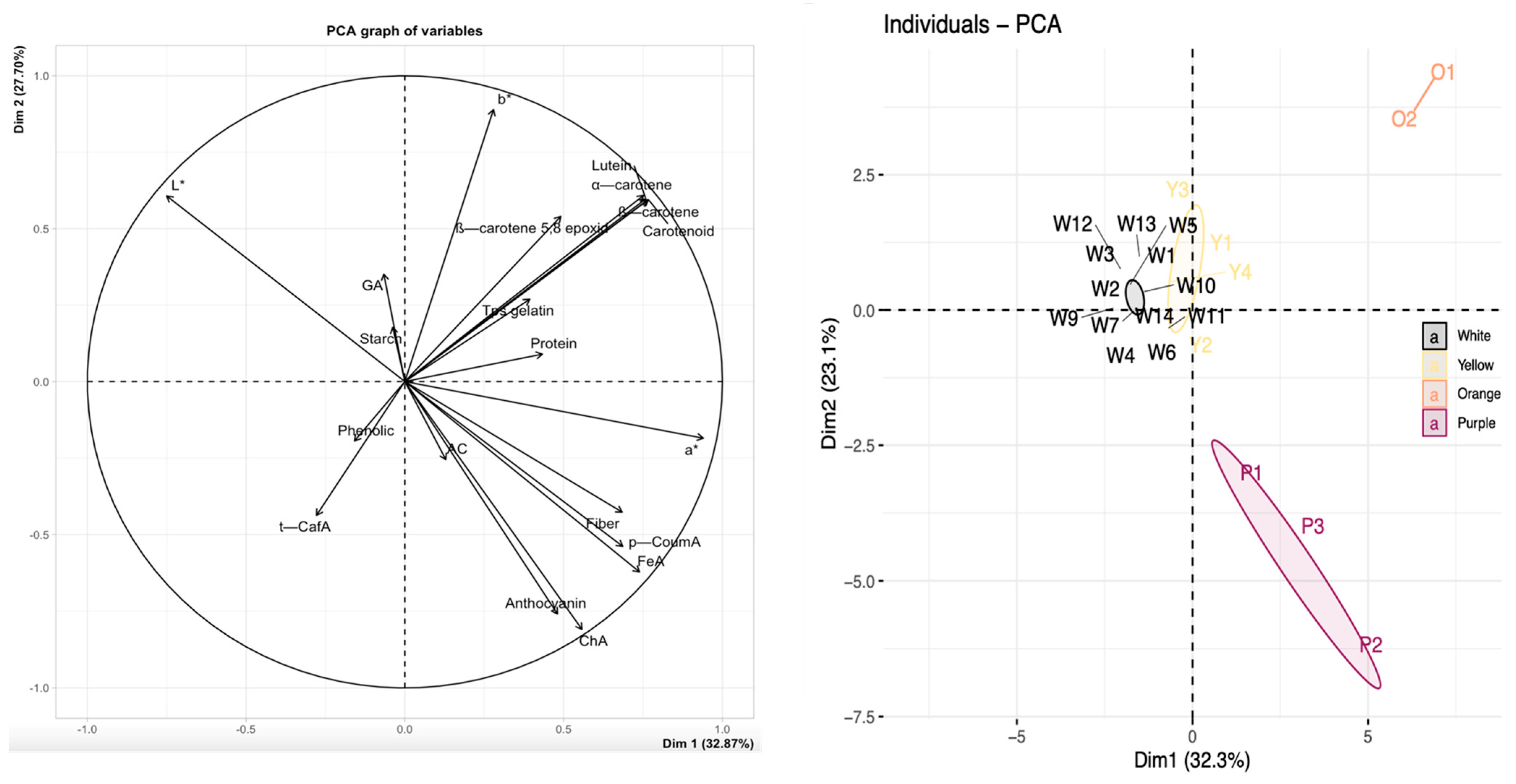
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shekhar, S.; Mishra, D.; Buragohain, A.K.; Chakraborty, S.; Chakraborty, N. Comparative analysis of phytochemicals and nutrient availability in two contrasting cultivars of sweet potato (Ipomoea batatas L.). Food Chem. 2015, 173, 957–965. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations [WWW Document]. 2022. Available online: https://www.fao.org/faostat/fr/#data/QCL (accessed on 23 January 2024).
- Iese, V.; Holland, E.; Wairiu, M.; Havea, R.; Patolo, S.; Nishi, M.; Hoponoa, T.; Bourke, R.M.; Dean, A.; Waqainabete, L. Facing food security risks: The rise and rise of the sweet potato in the Pacific Islands. Glob. Food Secur. 2018, 18, 48–56. [Google Scholar] [CrossRef]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huaccho, L.; Huaccho, R.; Zhang, D. Description and analysis of a geo-referenced database of the global distribution of sweetpotato area. Acta Hortic. 2002, 583, 41–49. [Google Scholar] [CrossRef]
- Mu, T.-H.; Zhang, M. Sweet potato starch. In Sweet Potato; Elsevier: Cambridge, MA, USA, 2019; pp. 27–68. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of Glycemic Index and Glycemic Load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.-D.; Yencho, G.C.; Lila, M.A. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef]
- Amagloh, F.C.; Yada, F.; Tumuhimbise, G.A.; Amagloh, F.K.; Kaaya, A.N. The potential of sweetpotato as a functional food in Sub-Saharan Africa and its implications for health: A review. Molecules 2021, 26, 2971. [Google Scholar] [CrossRef]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, vitamin C, and effect of thermal processing on carotenoids composition in nine varieties orange-fleshed sweet potato (Ipomoea batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- CRB Vatel. CRB Vatel/Prestations—Plateforme technologique du Pôle de Protection des Plantes (3P) [WWW Document]. 2024. Available online: https://3p.cirad.fr/prestations/crb-vatel (accessed on 5 April 2024).
- Huamàn, Z.; AVRDC; IBPGR; CIP (Eds.) Descriptors for Sweet Potato; International Board for Plant Genetic Ressources: Rome, Italy, 1991. [Google Scholar]
- AOAC. Solids (Total) and Moisture in Flour, Method 925.10; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giraldo Toro, A.; Gibert, O.; Ricci, J.; Dufour, D.; Mestres, C.; Bohuon, P. Digestibility prediction of cooked plantain flour as a function of water content and temperature. Carbohydr. Polym. 2015, 118, 257–265. [Google Scholar] [CrossRef]
- AOAC. Total, Soluble, and Insoluble Dietary Fibre in Foods; AOAC: Rockville, MD, USA, 1996. [Google Scholar]
- Barba, F.; Esteve, M.J.; Tedeschi, P.; Brandolini, V.; Frigola, A. A comparative study of the analysis of antioxidant activities of liquid foods employing spectrophotometric, fluorometric, and chemiluminescent methods. Food Anal. Methods 2013, 6, 317–327. [Google Scholar] [CrossRef]
- Schierle, J.; Pietsch, B.; Ceresa, A.; Fizet, C.; Waysek, E.H. Method for the determination of b-carotene in supplements and raw materials by Reversed-Phase Liquid Chromatography: Single laboratory validation. J. AOAC Int. 2004, 87, 1070–1082. [Google Scholar] [CrossRef]
- Barral, B.; Chillet, M.; Léchaudel, M.; Lugan, R.; Schorr-Galindo, S. Coumaroyl-isocitric and caffeoyl-isocitric acids as markers of pineapple fruitlet core rot disease. Fruits 2019, 74, 11–17. [Google Scholar] [CrossRef]
- Fessard, A.; Bourdon, E.; Payet, B.; Remize, F. Identification, stress tolerance, and antioxidant activity of lactic acid bacteria isolated from tropically grown fruits and leaves. Can. J. Microbiol. 2016, 62, 550–561. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Rosalie, R.; Joas, J.; Deytieux-Belleau, C.; Vulcain, E.; Payet, B.; Dufossé, L.; Léchaudel, M. Réponses antioxydantes et enzymatiques au stress oxydatif induit par la réduction de l’apport en eau avant la récolte et la maturation de la mangue (Mangifera indica L, cv. ’Cogshall’) en relation avec la teneur en caroténoïdes. J. Plant Physiol. 2015, 184, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schols, H.A.; Voragen, A. Physicochemical properties of starches obtained from three varieties of Chinese sweet potatoes. J. Food Sci. 2003, 68, 431–437. [Google Scholar] [CrossRef]
- Dako, E.; Retta, N.; Desse, G. Comparison of three sweet potato (Ipomoea batatas (L.) lam) varieties on nutritional and anti-nutritional factors. Agric. Vet. D Agric. Vet. 2016, 16, 1–11. [Google Scholar]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. Int. J. Food Sci. Technol. 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Mello, A.F.S.; da Silva, G.O.; da Silva Minguita, A.P.; dos Santos, F.N.; Samborski, T.; Ferreira, J.C.; de Carvalho, J.L.V.; Nuti, M.R.; Siquieroli, A.C.S.; Severo, J. Quality parameters in orange flesh sweetpotato grown in different Brazilian states. J. Food Compos. Anal. 2022, 107, 104406. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet potato is not simply an abundant food crop: A comprehensive review of its phytochemical constituents, biological activities, and the effects of processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.J.; Rodriquez-Amaya, D. Pro-vitamin A carotenoid conversion factors: Retinol equivalents—Fact or fiction? Food Chem. 2000, 69, 125–127. [Google Scholar] [CrossRef]
- Tomlins, K.; Owori, C.; Bechoff, A.; Menya, G.; Westby, A. Relationship among the carotenoid content, dry matter content and sensory attributes of sweet potato. Food Chem. 2012, 131, 14–21. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, S.A.; Ranaweera, K.K.D.S.; Gunaratne, A.; Bamunuarachchi, A. Comparative analysis of nutritional quality of five different cultivars of sweet potatoes (Ipomea batatas (L) Lam) in Sri Lanka. Food Sci. Nutr. 2013, 1, 284–291. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Ray, R.C.; Sivakumar, P.S. Traditional and novel fermented foods and beverages from tropical root and tuber crops: Review. Int. J. Food Sci. Technol. 2009, 44, 1073–1087. [Google Scholar] [CrossRef]
- Panda, S.H.; Parmanick, M.; Ray, R.C. Lactic acid fermentation of sweet potato (Ipomoea batatas L.) into pickles. J. Food Process. Preserv. 2007, 31, 83–101. [Google Scholar] [CrossRef]
- Sanni, L.O.; Babajide, J.M.; Ojerinde, M.W. Effect of chemical pretreatments on the physico-chemical and sensory attributes of sweet potato-gari. Int. J. Agric. Sci. Sci. Environ. Technol. (Ser. B) ASSET Int. J. (Ser. B) 2010, 6, 41–49. [Google Scholar]
- Li, Y.; Zhao, L.; Shi, L.; Lin, L.; Cao, Q.; Wei, C. Sizes, components, crystalline structure, and thermal properties of starches from sweet potato varieties originating from different countries. Molecules 2022, 27, 1905. [Google Scholar] [CrossRef]
- Kim, J.; Ren, C.; Shin, M. Physicochemical properties of starch isolated from eight different varieties of Korean sweet potatoes. Starch 2013, 65, 923–930. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Abegunde, O.K.; Mu, T.-H.; Chen, J.-W.; Deng, F.-M. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
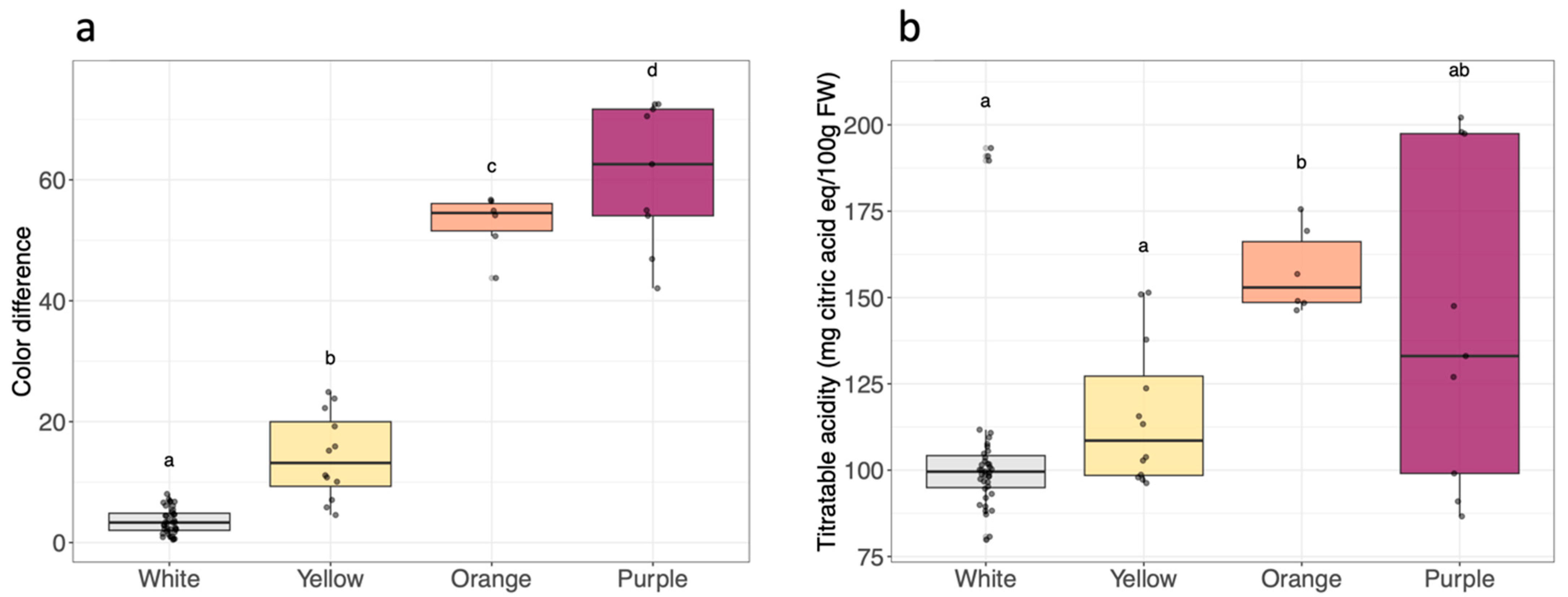
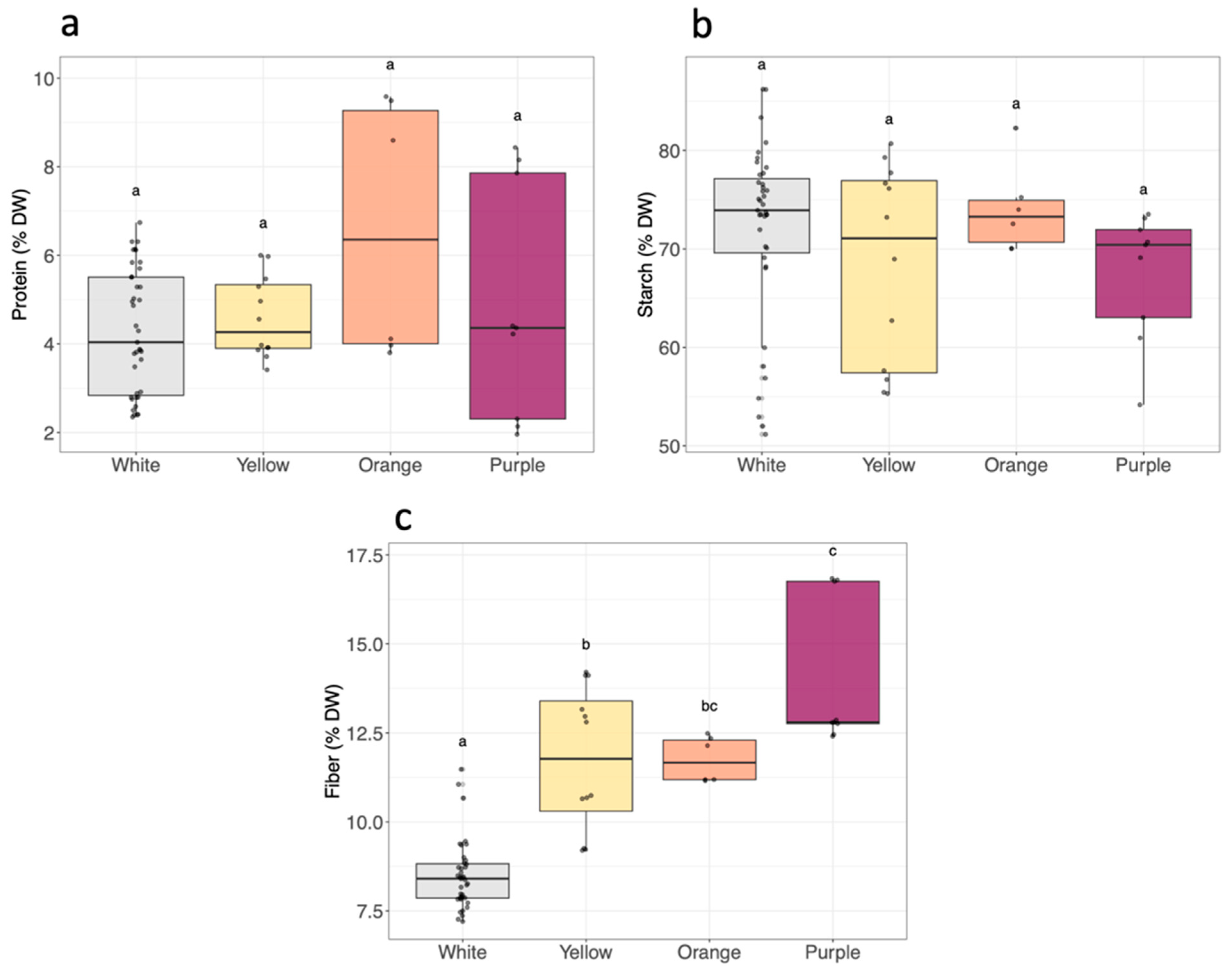
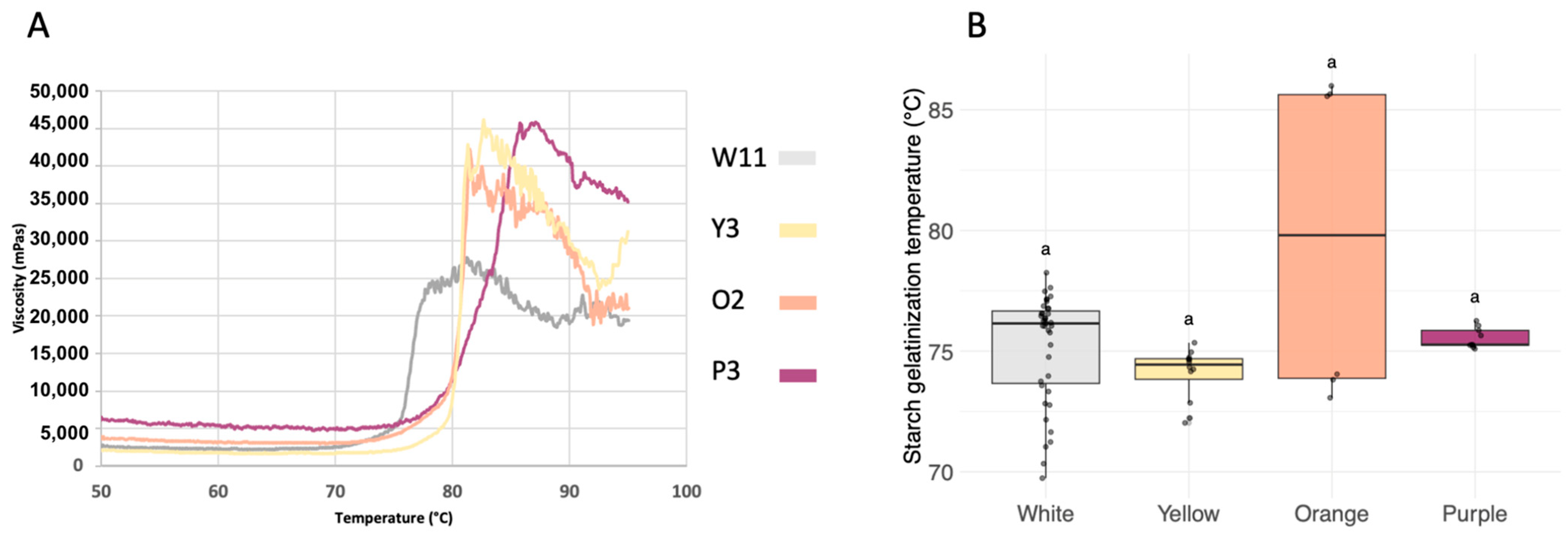
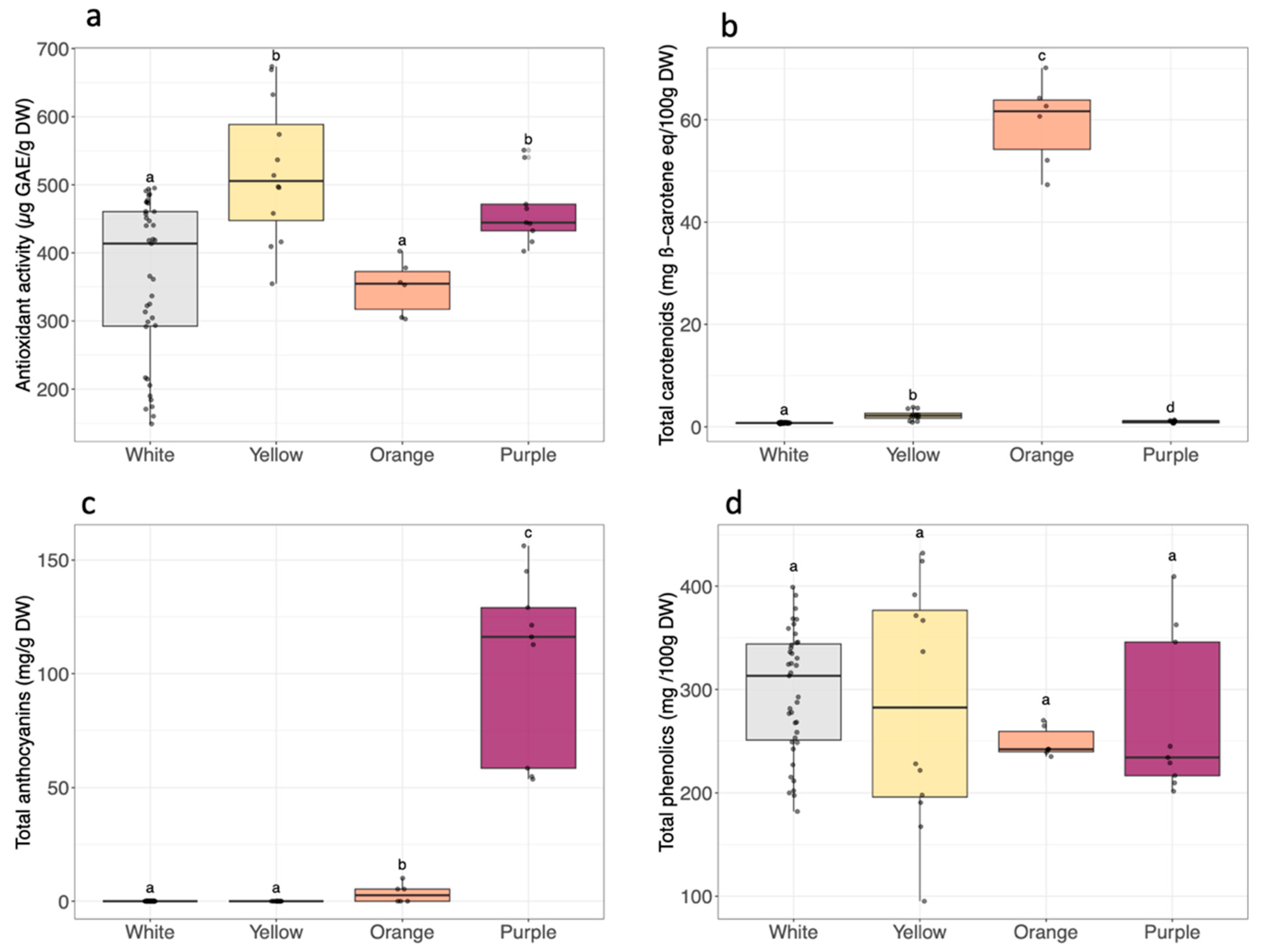
| Sweet Potato Flesh Color | All | White | Yellow | Orange | Purple |
|---|---|---|---|---|---|
| L* | 78.4 (22%) | 86.9 A (2%) | 85.2 A (3%) | 67.3 B (2%) | 39.5 C (26%) |
| a* | 4.8 (285%) | −2.6 A (41%) | −2.3 A (36%) | 32.5 B (8%) | 27.9 C (21%) |
| b* | 20.2 (72%) | 16.8 B (18%) | 32.2 C (22%) | 51.5 D (11%) | −2.3 A (231%) |
| β-carotene (mg/100 g DW) | 4.3 (316%) | ND | 0.3 ± 0.2 B | 47.2 ± 0.7 A | ND |
| α-carotene (mg/100 g DW) | - | ND | 0.4 ± 0.5 B | 4.8 ± 1.2 A | ND |
| β-car. 5,8 epoxide (mg/100 g DW) | - | ND | 0.08 ± 0.07 B | 0.1 ± 0.1 A | ND |
| lutein (mg/100 g DW) | - | ND | 0.03 ± 0.02 B | 1.3 ± 0.4 A | ND |
| chlorogenic acid (µg/g DW) | 122.6 (207%) | 24.6 ± 33.6 A | 7.7 ± 13.9 A | 81.1 ± 89.3 AB | 728.3 ± 175.1 C |
| ferulic acid (µg/g DW) | 106.6 (74%) | 60.1 ± 18.1 A | 106 ± 26.5 B | 148.2 ± 21.1 C | 281.1 ± 34.9 D |
| p-coumaric acid (µg/g DW) | 118.7 (84%) | ND | 89.2 ± 162.5 AB | 805.2 ± 246.2 B | 1606.4 ± 1425.3 B |
| trans-caffeic acid (µg/g DW) | 308.5 (247%) | 147.1 ± 92.9 A | 74.8 ± 87.2 AB | ND | 133.3 ± 146.1 AB |
| gallic acid (µg/g DW) | 74.4 (119%) | 56.2 ± 20.7 A | 185 ± 178.2 B | 83.7 ±17 B | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabot, M.; Garcia, C.; Seguin, M.; Ricci, J.; Brabet, C.; Remize, F. Bioactive Compound Diversity in a Wide Panel of Sweet Potato (Ipomoea batatas L.) Cultivars: A Resource for Nutritional Food Development. Metabolites 2024, 14, 523. https://doi.org/10.3390/metabo14100523
Nabot M, Garcia C, Seguin M, Ricci J, Brabet C, Remize F. Bioactive Compound Diversity in a Wide Panel of Sweet Potato (Ipomoea batatas L.) Cultivars: A Resource for Nutritional Food Development. Metabolites. 2024; 14(10):523. https://doi.org/10.3390/metabo14100523
Chicago/Turabian StyleNabot, Marion, Cyrielle Garcia, Marc Seguin, Julien Ricci, Catherine Brabet, and Fabienne Remize. 2024. "Bioactive Compound Diversity in a Wide Panel of Sweet Potato (Ipomoea batatas L.) Cultivars: A Resource for Nutritional Food Development" Metabolites 14, no. 10: 523. https://doi.org/10.3390/metabo14100523
APA StyleNabot, M., Garcia, C., Seguin, M., Ricci, J., Brabet, C., & Remize, F. (2024). Bioactive Compound Diversity in a Wide Panel of Sweet Potato (Ipomoea batatas L.) Cultivars: A Resource for Nutritional Food Development. Metabolites, 14(10), 523. https://doi.org/10.3390/metabo14100523











