Struggling to Understand the NEC Spectrum—Could the Integration of Metabolomics, Clinical-Laboratory Data, and Other Emerging Technologies Help Diagnosis?
Abstract
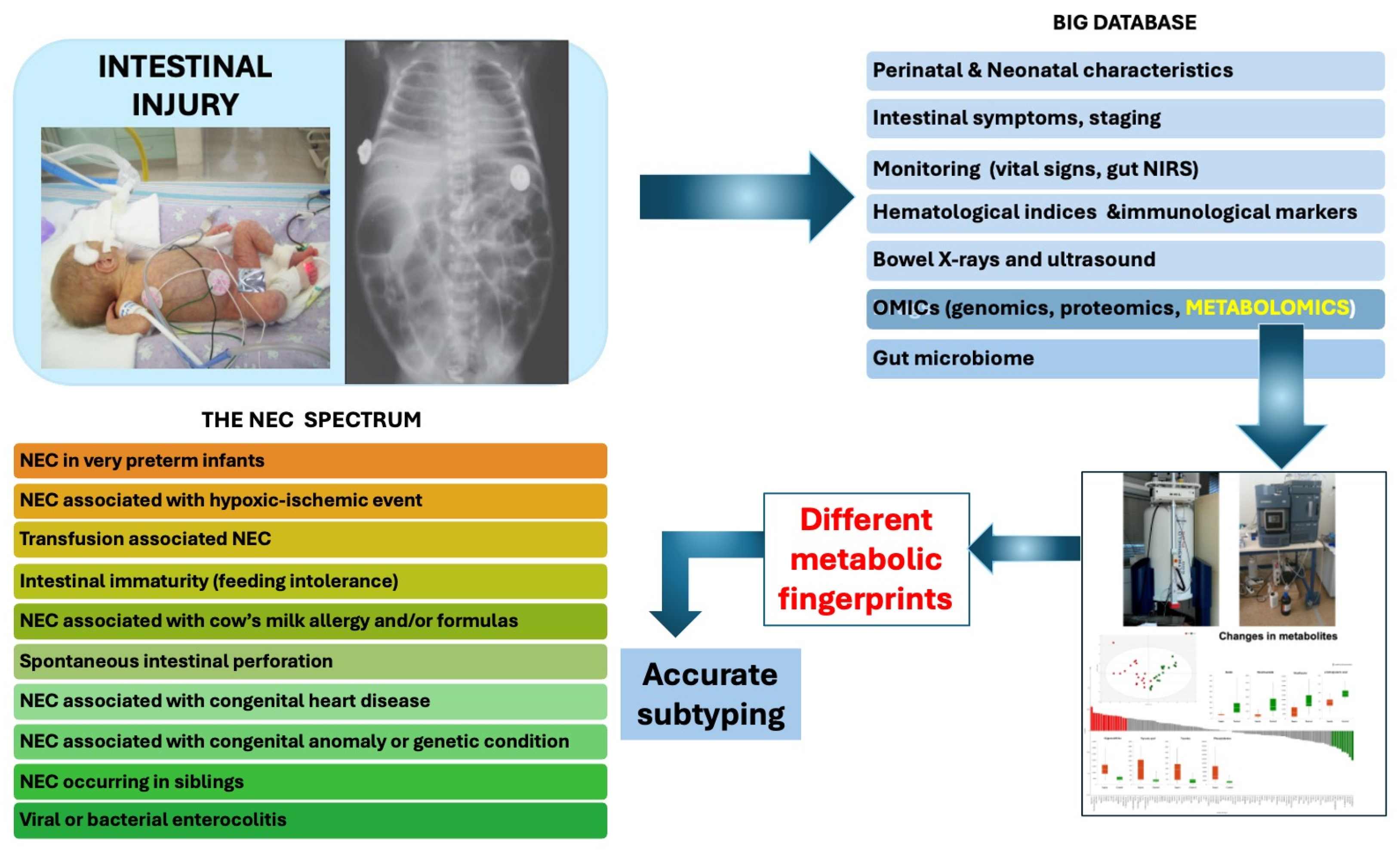
1. Introduction and Methodology
2. The Current Problem of NEC
2.1. Epidemiology
2.2. Outcome of Infants with NEC
2.3. Pathophysiology
3. Definition and Staging
4. Laboratory Investigation
4.1. Conventional and Promising Biomarkers
4.2. Ultrasound Examination
4.3. Near-Infrared Spectroscopy
5. Differential Diagnosing and Subtyping
6. Novel Technologies to Delineate NEC
6.1. Artificial Intelligence
6.2. Artificial Intelligence and NEC
7. Clinical-Laboratory Difficulties and Diagnostic Dilemmas Related to NEC
8. OMICs
9. Metabolomics
10. The Role of Metabolomics in NEC Pathogenesis and Diagnosis
10.1. Metabolomic Studies in Infants NEC
10.1.1. Urine
10.1.2. Blood/Plasma/Serum
10.1.3. Feces
10.1.4. Fecal Volatile Organic Compounds
10.2. Studies Combing Metabolomics and Gut Microbiome
11. Limitations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, M.C.; Kliegman, R.M. Necrotizing Enterocolitis: Treatment Based on Staging Criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Rich, B.S.; Dolgin, S.E. Necrotizing Enterocolitis. Pediatr. Rev. 2017, 38, 552–559. [Google Scholar] [CrossRef]
- Luig, M.; Lui, K.; NSW & ACT NICUS Group. Epidemiology of Necrotizing Enterocolitis—Part II: Risks and Susceptibility of Premature Infants during the Surfactant Era: A Regional Study. J. Paediatr. Child Health 2005, 41, 174–179. [Google Scholar] [CrossRef]
- Gordon, P.V.; Swanson, J.R.; MacQueen, B.C.; Christensen, R.D. A Critical Question for NEC Researchers: Can We Create a Consensus Definition of NEC That Facilitates Research Progress? Semin. Perinatol. 2017, 41, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, A.; Islam, N.; Thalib, L. Global Incidence of Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef]
- Heida, F.H.; Stolwijk, L.; Loos, M.-L.H.J.; van den Ende, S.J.; Onland, W.; van den Dungen, F.A.M.; Kooi, E.M.W.; Bos, A.F.; Hulscher, J.B.F.; Bakx, R. Increased Incidence of Necrotizing Enterocolitis in the Netherlands after Implementation of the New Dutch Guideline for Active Treatment in Extremely Preterm Infants: Results from Three Academic Referral Centers. J. Pediatr. Surg. 2017, 52, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Zozaya, C.; García González, I.; Avila-Alvarez, A.; Oikonomopoulou, N.; Sánchez Tamayo, T.; Salguero, E.; Saenz de Pipaón, M.; García-Muñoz Rodrigo, F.; Couce, M.L. Incidence, Treatment, and Outcome Trends of Necrotizing Enterocolitis in Preterm Infants: A Multicenter Cohort Study. Front. Pediatr. 2020, 8, 188. [Google Scholar] [CrossRef]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of Neonatal Necrotising Enterocolitis in High-Income Countries: A Systematic Review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef]
- Ostlie, D.J.; Spilde, T.L.; St Peter, S.D.; Sexton, N.; Miller, K.A.; Sharp, R.J.; Gittes, G.K.; Snyder, C.L. Necrotizing Enterocolitis in Full-Term Infants. J. Pediatr. Surg. 2003, 38, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K.; Canadian Neonatal Network. Incidence and Timing of Presentation of Necrotizing Enterocolitis in Preterm Infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Ahle, M.; Drott, P.; Andersson, R.E. Epidemiology and Trends of Necrotizing Enterocolitis in Sweden: 1987–2009. Pediatrics 2013, 132, e443–e451. [Google Scholar] [CrossRef]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis-A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Lu, M.; Huang, T.; Huang, L. Neurodevelopmental Outcomes of Preterm with Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2024, 183, 3147–3158. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.C.; Pappas, C.; Iyengar, H.; Maheshwari, A. Short Bowel Syndrome in the NICU. Clin. Perinatol. 2013, 40, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Engstrand Lilja, H.; Wester, T.; Norrby, H.; Borg, H.; Persson, S.; Bjornland, K.; Brun, A.C.; Telborn, L.; Stenström, P.; et al. A Nordic Multicenter Study on Contemporary Outcomes of Pediatric Short Bowel Syndrome in 208 Patients. Clin. Nutr. Edinb. Scotl. 2023, 42, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Klerk, D.H.; van Varsseveld, O.C.; Offringa, M.; Modi, N.; Lacher, M.; Zani, A.; Pakarinen, M.P.; Koivusalo, A.; Jester, I.; Spruce, M.; et al. Core Outcome Set for Necrotizing Enterocolitis Treatment Trials. Pediatrics 2024, 153, e2023065619. [Google Scholar] [CrossRef]
- Neu, J. Necrotizing Enterocolitis: The Future. Neonatology 2020, 117, 240–244. [Google Scholar] [CrossRef]
- Pravda, J. Sepsis: Evidence-Based Pathogenesis and Treatment. World J. Crit. Care Med. 2021, 10, 66–80. [Google Scholar] [CrossRef]
- Singh, D.K.; Miller, C.M.; Orgel, K.A.; Dave, M.; Mackay, S.; Good, M. Necrotizing Enterocolitis: Bench to Bedside Approaches and Advancing Our Understanding of Disease Pathogenesis. Front. Pediatr. 2023, 10, 1107404. [Google Scholar] [CrossRef]
- Roberts, A.G.; Younge, N.; Greenberg, R.G. Neonatal Necrotizing Enterocolitis: An Update on Pathophysiology, Treatment, and Prevention. Paediatr. Drugs 2024, 26, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Rees, C.M.; Hall, N.J. Current Research on the Epidemiology, Pathogenesis, and Management of Necrotizing Enterocolitis. Neonatology 2017, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liang, H.; Li, F.; Zhang, R.; Zhu, Y.; Zhu, X.; Xu, Y. Necrotizing Enterocolitis: Current Understanding of the Prevention and Management. Pediatr. Surg. Int. 2024, 40, 32. [Google Scholar] [CrossRef] [PubMed]
- Nanthakumar, N.; Meng, D.; Goldstein, A.M.; Zhu, W.; Lu, L.; Uauy, R.; Llanos, A.; Claud, E.C.; Walker, W.A. The Mechanism of Excessive Intestinal Inflammation in Necrotizing Enterocolitis: An Immature Innate Immune Response. PLoS ONE 2011, 6, e17776. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal Dysbiosis in Preterm Infants Preceding Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Snyder, K.B.; Hunter, C.J. Bugs and the Barrier: A Review of the Gut Microbiome and Intestinal Barrier in Necrotizing Enterocolitis. Semin. Pediatr. Surg. 2023, 32, 151310. [Google Scholar] [CrossRef]
- Egan, C.E.; Sodhi, C.P.; Good, M.; Lin, J.; Jia, H.; Yamaguchi, Y.; Lu, P.; Ma, C.; Branca, M.F.; Weyandt, S.; et al. Toll-like Receptor 4-Mediated Lymphocyte Influx Induces Neonatal Necrotizing Enterocolitis. J. Clin. Investig. 2016, 126, 495–508. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Toll-Like Receptor–Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 229–238.e1. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T.; et al. Breast Milk Protects against the Development of Necrotizing Enterocolitis through Inhibition of Toll-like Receptor 4 in the Intestinal Epithelium via Activation of the Epidermal Growth Factor Receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef]
- Carlisle, E.M.; Morowitz, M.J. The Intestinal Microbiome and Necrotizing Enterocolitis. Curr. Opin. Pediatr. 2013, 25, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Ye, C.; Chen, Y.; Zhang, D.; Li, T.; Ling, X.B.; Cohen, H.J.; Shaw, G.M.; Stevenson, D.K.; Chace, D.; et al. Progressive Metabolic Dysfunction and Nutritional Variability Precedes Necrotizing Enterocolitis. Nutrients 2020, 12, 1275. [Google Scholar] [CrossRef]
- Shaw, A.G.; Sim, K.; Rose, G.; Wooldridge, D.J.; Li, M.-S.; Misra, R.V.; Gharbia, S.; Kroll, J.S. Premature Neonatal Gut Microbial Community Patterns Supporting an Epithelial TLR-Mediated Pathway for Necrotizing Enterocolitis. BMC Microbiol. 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Yeramilli, V.; Cheddadi, R.; Benjamin, H.; Martin, C. The Impact of Stress, Microbial Dysbiosis, and Inflammation on Necrotizing Enterocolitis. Microorganisms 2023, 11, 2206. [Google Scholar] [CrossRef]
- Moschino, L.; Verlato, G.; Duci, M.; Cavicchiolo, M.E.; Guiducci, S.; Stocchero, M.; Giordano, G.; Fascetti Leon, F.; Baraldi, E. The Metabolome and the Gut Microbiota for the Prediction of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Systematic Review. Nutrients 2022, 14, 3859. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Anne, R.P.; Meena, J.; Sundaram, V.; Dutta, S.; Kumar, P. To Feed or Not to Feed during Therapeutic Hypothermia in Asphyxiated Neonates: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2023, 182, 2759–2773. [Google Scholar] [CrossRef]
- Kelleher, S.T.; Coleman, J.; McMahon, C.J.; James, A. Outcomes and Characteristics in Term Infants with Necrotising Enterocolitis and CHD. Cardiol. Young 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.K.; Christensen, R.D.; Henry, E.; Besner, G.E.; Baer, V.L.; Wiedmeier, S.E.; Stoddard, R.A.; Miner, C.A.; Burnett, J. Necrotizing Enterocolitis in Term Neonates: Data from a Multihospital Health-Care System. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2007, 27, 437–443. [Google Scholar] [CrossRef]
- Christensen, R.D.; Lambert, D.K.; Baer, V.L.; Gordon, P.V. Necrotizing Enterocolitis in Term Infants. Clin. Perinatol. 2013, 40, 69–78. [Google Scholar] [CrossRef]
- Tian, B.; Xu, X.; Li, L.; Tian, Y.; Liu, Y.; Mu, Y.; Lu, J.; Song, K.; Lv, J.; He, Q.; et al. Epigenetic Insights Into Necrotizing Enterocolitis: Unraveling Methylation-Regulated Biomarkers. Inflammation 2024. [Google Scholar] [CrossRef]
- Frazer, L.C.; Yamaguchi, Y.; Singh, D.K.; Akopyants, N.S.; Good, M. DNA Methylation in Necrotizing Enterocolitis. Expert Rev. Mol. Med. 2024, 26, e16. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; George, L.; Sampath, V. Genetic Predisposition to Necrotizing Enterocolitis in Premature Infants: Current Knowledge, Challenges, and Future Directions. Semin. Fetal. Neonatal Med. 2018, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Obladen, M. Necrotizing Enterocolitis—150 Years of Fruitless Search for the Cause. Neonatology 2009, 96, 203–210. [Google Scholar] [CrossRef]
- Hackam, D.; Caplan, M. Necrotizing Enterocolitis: Pathophysiology from a Historical Context. Semin. Pediatr. Surg. 2018, 27, 11–18. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal Necrotizing Enterocolitis. Therapeutic Decisions Based upon Clinical Staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- D’Angelo, G.; Impellizzeri, P.; Marseglia, L.; Montalto, A.S.; Russo, T.; Salamone, I.; Falsaperla, R.; Corsello, G.; Romeo, C.; Gitto, E. Current Status of Laboratory and Imaging Diagnosis of Neonatal Necrotizing Enterocolitis. Ital. J. Pediatr. 2018, 44, 84. [Google Scholar] [CrossRef]
- Soni, R.; Katana, A.; Curry, J.I.; Humphries, P.D.; Huertas-Ceballos, A. How to Use Abdominal X-Rays in Preterm Infants Suspected of Developing Necrotising Enterocolitis. Arch. Dis. Child. Educ. Pract. Ed. 2020, 105, 50–57. [Google Scholar] [CrossRef]
- Rehan, V.K.; Seshia, M.M.; Johnston, B.; Reed, M.; Wilmot, D.; Cook, V. Observer Variability in Interpretation of Abdominal Radiographs of Infants with Suspected Necrotizing Enterocolitis. Clin. Pediatr. 1999, 38, 637–643. [Google Scholar] [CrossRef]
- Gordon, P.V.; Swanson, J.R.; Attridge, J.T.; Clark, R. Emerging Trends in Acquired Neonatal Intestinal Disease: Is It Time to Abandon Bell’s Criteria? J. Perinatol. Off. J. Calif. Perinat. Assoc. 2007, 27, 661–671. [Google Scholar] [CrossRef]
- Bell, M.J. Emerging Trends in Neonatal Intestinal Disease. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2008, 28, 383. [Google Scholar] [CrossRef]
- Lueschow, S.R.; Boly, T.J.; Jasper, E.; Patel, R.M.; McElroy, S.J. A Critical Evaluation of Current Definitions of Necrotizing Enterocolitis. Pediatr. Res. 2022, 91, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Leistner, R.; Piening, B.; Gastmeier, P.; Geffers, C.; Schwab, F. Nosocomial Infections in Very Low Birthweight Infants in Germany: Current Data from the National Surveillance System NEO-KISS. Klin. Padiatr. 2013, 225, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging Biomarkers for Prediction and Early Diagnosis of Necrotizing Enterocolitis in the Era of Metabolomics and Proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef] [PubMed]
- Zani, A.; Eaton, S.; Puri, P.; Rintala, R.; Lukac, M.; Bagolan, P.; Kuebler, J.F.; Hoellwarth, M.E.; Wijnen, R.; Tovar, J.; et al. International Survey on the Management of Necrotizing Enterocolitis. Eur. J. Pediatr. Surg. Off. J. Austrian Assoc. Pediatr. Surg. Al Z. Kinderchir. 2015, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Knell, J.; Han, S.M.; Jaksic, T.; Modi, B.P. Current Status of Necrotizing Enterocolitis. Curr. Probl. Surg. 2019, 56, 11–38. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Konstantopoulos, P.; Karampetsou, N.; Koutaki, D.; Gkioka, E.; Perrea, D.N.; Papantoniou, N. Calprotectin Levels in Necrotizing Enterocolitis: A Systematic Review of the Literature. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2016, 65, 847–852. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Jiang, X. Diagnostic Value of Intestinal Fatty-Acid-Binding Protein in Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Indian J. Pediatr. 2016, 83, 1410–1419. [Google Scholar] [CrossRef]
- Coufal, S.; Kokesova, A.; Tlaskalova-Hogenova, H.; Frybova, B.; Snajdauf, J.; Rygl, M.; Kverka, M. Urinary I-FABP, L-FABP, TFF-3, and SAA Can Diagnose and Predict the Disease Course in Necrotizing Enterocolitis at the Early Stage of Disease. J. Immunol. Res. 2020, 2020, 3074313. [Google Scholar] [CrossRef]
- Esposito, F.; Mamone, R.; Di Serafino, M.; Mercogliano, C.; Vitale, V.; Vallone, G.; Oresta, P. Diagnostic Imaging Features of Necrotizing Enterocolitis: A Narrative Review. Quant. Imaging Med. Surg. 2017, 7, 336–344. [Google Scholar] [CrossRef]
- Dördelmann, M.; Rau, G.A.; Bartels, D.; Linke, M.; Derichs, N.; Behrens, C.; Bohnhorst, B. Evaluation of Portal Venous Gas Detected by Ultrasound Examination for Diagnosis of Necrotising Enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F183–F187. [Google Scholar] [CrossRef]
- Muchantef, K.; Epelman, M.; Darge, K.; Kirpalani, H.; Laje, P.; Anupindi, S.A. Sonographic and Radiographic Imaging Features of the Neonate with Necrotizing Enterocolitis: Correlating Findings with Outcomes. Pediatr. Radiol. 2013, 43, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.C.; Reddy, N.; Robinson, A.L.; Chan, S.S. Bowel Ultrasound for Predicting Surgical Management of Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Pediatr. Radiol. 2018, 48, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Senarathna, J.; Kovler, M.; Prasad, A.; Bhargava, A.; Thakor, N.V.; Sodhi, C.P.; Hackam, D.J.; Pathak, A.P. In Vivo Phenotyping of the Microvasculature in Necrotizing Enterocolitis with Multicontrast Optical Imaging. Microcirculation 2022, 29, e12768. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Cody, J.; Maldonado, Y.; Ramakrishna, H. Near-Infrared Spectroscopy (NIRS) for Cerebral and Tissue Oximetry: Analysis of Evolving Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Pellicer, A.; Hyttel-Sørensen, S.; Ergenekon, E.; Szczapa, T.; Hagmann, C.; Naulaers, G.; Mintzer, J.; Fumagalli, M.; Dimitriou, G.; et al. Cerebral Oximetry Monitoring in Extremely Preterm Infants. N. Engl. J. Med. 2023, 388, 1501–1511. [Google Scholar] [CrossRef]
- Martini, S.; Corvaglia, L. Splanchnic NIRS Monitoring in Neonatal Care: Rationale, Current Applications and Future Perspectives. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Schat, T.E.; van Zoonen, A.G.J.F.; van der Laan, M.E.; Mebius, M.J.; Bos, A.F.; Hulzebos, C.V.; Boezen, H.M.; Hulscher, J.B.F.; Kooi, E.M.W. Early Cerebral and Intestinal Oxygenation in the Risk Assessment of Necrotizing Enterocolitis in Preterm Infants. Early Hum. Dev. 2019, 131, 75–80. [Google Scholar] [CrossRef]
- Schat, T.E.; Schurink, M.; van der Laan, M.E.; Hulscher, J.B.F.; Hulzebos, C.V.; Bos, A.F.; Kooi, E.M.W. Near-Infrared Spectroscopy to Predict the Course of Necrotizing Enterocolitis. PLoS ONE 2016, 11, e0154710. [Google Scholar] [CrossRef]
- Howarth, C.; Banerjee, J.; Leung, T.; Aladangady, N. Could Near Infrared Spectroscopy (NIRS) Be the New Weapon in Our Fight against Necrotising Enterocolitis? Front. Pediatr. 2022, 10, 1024566. [Google Scholar] [CrossRef]
- Pumberger, W.; Mayr, M.; Kohlhauser, C.; Weninger, M. Spontaneous Localized Intestinal Perforation in Very-Low-Birth-Weight Infants: A Distinct Clinical Entity Different from Necrotizing Enterocolitis. J. Am. Coll. Surg. 2002, 195, 796–803. [Google Scholar] [CrossRef]
- Garg, P.M.; Garg, P.P.; Shenberger, J.S. Is Necrotizing Enterocolitis and Spontaneous Intestinal Perforation Part of Same Disease Spectrum—New Insights? Curr. Pediatr. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Keane, O.A.; Dantes, G.; Dutreuil, V.L.; Do, L.; Rumbika, S.; Sylvestre, P.B.; Bhatia, A.M. Comparison of Preoperative and Intraoperative Surgeon Diagnosis and Pathologic Findings in Spontaneous Intestinal Perforation vs Necrotizing Enterocolitis. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2024, 44, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; NHLBI ARDS Network. Subphenotypes in Acute Respiratory Distress Syndrome: Latent Class Analysis of Data from Two Randomised Controlled Trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.S.; et al. Genomic Landscape of the Individual Host Response and Outcomes in Sepsis: A Prospective Cohort Study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- O’Connell, A.E. Applying the Bronchopulmonary Dysplasia Framework to Necrotizing Enterocolitis. Front. Pediatr. 2024, 12, 1388392. [Google Scholar] [CrossRef]
- Lure, A.C.; Du, X.; Black, E.W.; Irons, R.; Lemas, D.J.; Taylor, J.A.; Lavilla, O.; de la Cruz, D.; Neu, J. Using Machine Learning Analysis to Assist in Differentiating between Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Novel Predictive Analytic Tool. J. Pediatr. Surg. 2021, 56, 1703–1710. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Tsai, D.H.-T.; Wu, I.C.-Y.; Boselli, E.; Hughes, C.; Padmanabhan, D.; Hsia, Y. Machine Learning Applications on Neonatal Sepsis Treatment: A Scoping Review. BMC Infect. Dis. 2023, 23, 441. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, L.; Wang, J.; Chen, Y.; Wu, L. Bioinformatics Analysis of Potential Key Genes and Pathways in Neonatal Necrotizing Enterocolitis. BMC Pediatr. 2022, 22, 658. [Google Scholar] [CrossRef]
- Tang, B.-H.; Li, Q.-Y.; Liu, H.-X.; Zheng, Y.; Wu, Y.-E.; van den Anker, J.; Hao, G.-X.; Zhao, W. Machine Learning: A Potential Therapeutic Tool to Facilitate Neonatal Therapeutic Decision Making. Paediatr. Drugs 2024, 26, 355–363. [Google Scholar] [CrossRef]
- McElroy, S.J.; Lueschow, S.R. State of the Art Review on Machine Learning and Artificial Intelligence in the Study of Neonatal Necrotizing Enterocolitis. Front. Pediatr. 2023, 11, 1182597. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Aghaeepour, N.; Neu, J. Multiomics, Artificial Intelligence, and Precision Medicine in Perinatology. Pediatr. Res. 2023, 93, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial Intelligence in Healthcare: Past, Present and Future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Maddali, M.V.; Churpek, M.; Pham, T.; Rezoagli, E.; Zhuo, H.; Zhao, W.; He, J.; Delucchi, K.L.; Wang, C.; Wickersham, N.; et al. Validation and Utility of ARDS Subphenotypes Identified by Machine-Learning Models Using Clinical Data: An Observational, Multicohort, Retrospective Analysis. Lancet Respir. Med. 2022, 10, 367–377. [Google Scholar] [CrossRef]
- Anjana, G.; Nisha, K.L.; Sankar, A. Improving Sepsis Classification Performance with Artificial Intelligence Algorithms: A Comprehensive Overview of Healthcare Applications. J. Crit. Care 2024, 83, 154815. [Google Scholar] [CrossRef]
- Grapov, D.; Fahrmann, J.; Wanichthanarak, K.; Khoomrung, S. Rise of Deep Learning for Genomic, Proteomic, and Metabolomic Data Integration in Precision Medicine. Omics J. Integr. Biol. 2018, 22, 630–636. [Google Scholar] [CrossRef]
- Martin, C.R. Definitions of Necrotizing Enterocolitis: What Are We Defining and Is Machine Learning the Answer? Pediatr. Res. 2022, 91, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using Machine Learning Approaches for Multi-Omics Data Analysis: A Review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef] [PubMed]
- Sitek, A.; Seliga-Siwecka, J.; Płotka, S.; Grzeszczyk, M.K.; Seliga, S.; Włodarczyk, K.; Bokiniec, R. Artificial Intelligence in the Diagnosis of Necrotising Enterocolitis in Newborns. Pediatr. Res. 2023, 93, 376–381. [Google Scholar] [CrossRef]
- Meeus, M.; Beirnaert, C.; Mahieu, L.; Laukens, K.; Meysman, P.; Mulder, A.; Van Laere, D. Clinical Decision Support for Improved Neonatal Care: The Development of a Machine Learning Model for the Prediction of Late-Onset Sepsis and Necrotizing Enterocolitis. J. Pediatr. 2024, 266, 113869. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, Y.J.; Son, J.; Jung, D.; Kim, D.; Ryu, S.R.; Na, J.Y.; Hwang, J.K.; Kim, T.H.; Park, H.-K. Machine Learning-Based Analysis for Prediction of Surgical Necrotizing Enterocolitis in Very Low Birth Weight Infants Using Perinatal Factors: A Nationwide Cohort Study. Eur. J. Pediatr. 2024, 183, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Gipson, D.R.; Chang, A.L.; Lure, A.C.; Mehta, S.A.; Gowen, T.; Shumans, E.; Stevenson, D.; de la Cruz, D.; Aghaeepour, N.; Neu, J. Reassessing Acquired Neonatal Intestinal Diseases Using Unsupervised Machine Learning. Pediatr. Res. 2024, 96, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.L.; Kalish, L.A.; Duggan, C.; Johnston, P.; Brandt, M.L.; Dunn, J.C.Y.; Ehrenkranz, R.A.; Jaksic, T.; Nobuhara, K.; Simpson, B.J.; et al. Clinical Parameters Do Not Adequately Predict Outcome in Necrotizing Enterocolitis: A Multi-Institutional Study. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2008, 28, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Niemarkt, H.J.; de Meij, T.G.J.; van de Velde, M.E.; van der Schee, M.P.; van Goudoever, J.B.; Kramer, B.W.; Andriessen, P.; de Boer, N.K.H. Necrotizing Enterocolitis: A Clinical Review on Diagnostic Biomarkers and the Role of the Intestinal Microbiota. Inflamm. Bowel Dis. 2015, 21, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.Y.; Poon, T.C.W.; Lam, H.S.; Cheung, H.M.; Ma, T.P.Y.; Chan, K.Y.Y.; Wong, R.P.O.; Leung, K.T.; Lam, M.M.T.; Li, K.; et al. Gut-Associated Biomarkers L-FABP, I-FABP, and TFF3 and LIT Score for Diagnosis of Surgical Necrotizing Enterocolitis in Preterm Infants. Ann. Surg. 2013, 258, 1111–1118. [Google Scholar] [CrossRef]
- Ng, P.C.; Ma, T.P.Y.; Lam, H.S. The Use of Laboratory Biomarkers for Surveillance, Diagnosis and Prediction of Clinical Outcomes in Neonatal Sepsis and Necrotising Enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F448–F452. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Stronati, L.; Cucchiara, S.; De Curtis, M. Serum Markers of Necrotizing Enterocolitis: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e120–e132. [Google Scholar] [CrossRef]
- Chatziioannou, A.C.; Wolters, J.C.; Sarafidis, K.; Thomaidou, A.; Agakidis, C.; Govorukhina, N.; Kuivenhoven, J.A.; Bischoff, R.; Theodoridis, G. Targeted LC-MS/MS for the Evaluation of Proteomics Biomarkers in the Blood of Neonates with Necrotizing Enterocolitis and Late-Onset Sepsis. Anal. Bioanal. Chem. 2018, 410, 7163–7175. [Google Scholar] [CrossRef]
- Thomaidou, A.; Deda, O.; Begou, O.; Lioupi, A.; Kontou, A.; Gika, H.; Agakidou, E.; Theodoridis, G.; Sarafidis, K. A Prospective, Case-Control Study of Serum Metabolomics in Neonates with Late-Onset Sepsis and Necrotizing Enterocolitis. J. Clin. Med. 2022, 11, 5270. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Loddo, C.; Fanni, C.; Fanos, V. Metabolomics in Pharmacology—A Delve into the Novel Field of Pharmacometabolomics. Expert Rev. Clin. Pharmacol. 2020, 13, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Qian, S.; Yu, D. Multilevel Omics for the Discovery of Biomarkers in Pediatric Sepsis. Pediatr. Investig. 2023, 7, 277–289. [Google Scholar] [CrossRef]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics Enables Precision Medicine: “A White Paper, Community Perspective”. Metabolomics Off. J. Metabolomic Soc. 2016, 12, 149. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Morris, G.A.J.; Fornace, A.J.; Howie, S.R.C. Metabolomic Analysis in Severe Childhood Pneumonia in the Gambia, West Africa: Findings from a Pilot Study. PLoS ONE 2010, 5, e12655. [Google Scholar] [CrossRef]
- Demicheva, E.; Dordiuk, V.; Polanco Espino, F.; Ushenin, K.; Aboushanab, S.; Shevyrin, V.; Buhler, A.; Mukhlynina, E.; Solovyova, O.; Danilova, I.; et al. Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review. Metabolites 2024, 14, 54. [Google Scholar] [CrossRef]
- Banoei, M.M.; Donnelly, S.J.; Mickiewicz, B.; Weljie, A.; Vogel, H.J.; Winston, B.W. Metabolomics in Critical Care Medicine: A New Approach to Biomarker Discovery. Clin. Investig. Med. Med. Clin. Exp. 2014, 37, E363–E376. [Google Scholar] [CrossRef]
- Bosco, A.; Piu, C.; Picciau, M.E.; Pintus, R.; Fanos, V.; Dessì, A. Metabolomics in NEC: An Updated Review. Metabolites 2023, 14, 14. [Google Scholar] [CrossRef]
- Thomaidou, A.; Chatziioannou, A.C.; Deda, O.; Benaki, D.; Gika, H.; Mikros, E.; Agakidis, C.; Raikos, N.; Theodoridis, G.; Sarafidis, K. A Pilot Case-Control Study of Urine Metabolomics in Preterm Neonates with Necrotizing Enterocolitis. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2019, 1117, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.-C.; De Magistris, A.; Mussap, M.; Corbu, S.; Dessì, A.; Noto, A.; Fanos, V.; Cesare Marincola, F. Urine NMR Metabolomics Profile of Preterm Infants With Necrotizing Enterocolitis Over the First Two Months of Life: A Pilot Longitudinal Case-Control Study. Front. Mol. Biosci. 2021, 8, 680159. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, A.; Begley, P.; Stevens, A.; Whatmore, A.; Victor, S. The Metabolomics of Necrotising Enterocolitis in Preterm Babies: An Exploratory Study. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2016, 29, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, K.G.; Kastenberg, Z.J.; Moss, R.L.; Enns, G.M.; Cowan, T.M.; Shaw, G.M.; Stevenson, D.K.; Sinclair, T.J.; Scharfe, C.; Ryckman, K.K.; et al. Acylcarnitine Profiles Reflect Metabolic Vulnerability for Necrotizing Enterocolitis in Newborns Born Premature. J. Pediatr. 2017, 181, 80–85.e1. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Nelson, A.; Treumann, A.; Skeath, T.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E. Metabolomic and Proteomic Analysis of Serum from Preterm Infants with Necrotising Entercolitis and Late-Onset Sepsis. Pediatr. Res. 2016, 79, 425–431. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.L.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E.; et al. Temporal Bacterial and Metabolic Development of the Preterm Gut Reveals Specific Signatures in Health and Disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, B.; Jiang, X.; Sidhu, R.; Ory, D.S.; Warner, B.B.; Tarr, P.I. Gut Sphingolipid Composition as a Prelude to Necrotizing Enterocolitis. Sci. Rep. 2018, 8, 10984. [Google Scholar] [CrossRef]
- Deianova, N.; El Manouni El Hassani, S.; Struijs, E.A.; Jansen, E.E.W.; Bakkali, A.; van de Wiel, M.A.; de Boode, W.P.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; et al. Fecal Amine Metabolite Analysis before Onset of Severe Necrotizing Enterocolitis in Preterm Infants: A Prospective Case-Control Study. Sci. Rep. 2022, 12, 12310. [Google Scholar] [CrossRef]
- Probert, C.; Greenwood, R.; Mayor, A.; Hughes, D.; Aggio, R.; Jackson, R.E.; Simcox, L.; Barrow, H.; García-Finana, M.; Ewer, A.K. Faecal Volatile Organic Compounds in Preterm Babies at Risk of Necrotising Enterocolitis: The DOVE Study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 474–479. [Google Scholar] [CrossRef]
- Garner, C.E.; Ewer, A.K.; Elasouad, K.; Power, F.; Greenwood, R.; Ratcliffe, N.M.; de Costello, B.L.; Probert, C.S. Analysis of Faecal Volatile Organic Compounds in Preterm Infants Who Develop Necrotising Enterocolitis: A Pilot Study. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 559–565. [Google Scholar] [CrossRef]
- De Meij, T.G.J.; van der Schee, M.P.C.; Berkhout, D.J.C.; van de Velde, M.E.; Jansen, A.E.; Kramer, B.W.; van Weissenbruch, M.M.; van Kaam, A.H.; Andriessen, P.; van Goudoever, J.B.; et al. Early Detection of Necrotizing Enterocolitis by Fecal Volatile Organic Compounds Analysis. J. Pediatr. 2015, 167, 562–567.e1. [Google Scholar] [CrossRef]
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early Microbial and Metabolomic Signatures Predict Later Onset of Necrotizing Enterocolitis in Preterm Infants. Microbiome 2013, 1, 13. [Google Scholar] [CrossRef]
- Brehin, C.; Dubois, D.; Dicky, O.; Breinig, S.; Oswald, E.; Serino, M. Evolution of Gut Microbiome and Metabolome in Suspected Necrotizing Enterocolitis: A Case-Control Study. J. Clin. Med. 2020, 9, 2278. [Google Scholar] [CrossRef]
- Du, T.-T.; Liu, X.-C.; He, Y.; Gao, X.; Liu, Z.-Z.; Wang, Z.-L.; Li, L.-Q. Changes of Gut Microbiota and Tricarboxylic Acid Metabolites May Be Helpful in Early Diagnosis of Necrotizing Enterocolitis: A Pilot Study. Front. Microbiol. 2023, 14, 1119981. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Xiong, J.; Liao, X.-S.; Yin, T.; Liu, X.-C.; Bao, L.; Li, L.-Q. Alterations of the Gut Microbiota and Short Chain Fatty Acids in Necrotizing Enterocolitis and Food Protein-Induced Allergic Protocolitis Infants: A Prospective Cohort Study. Front. Cell. Infect. Microbiol. 2022, 12, 1030588. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 2018, 3, e00104-18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafidis, K.; Agakidou, E.; Kontou, A.; Agakidis, C.; Neu, J. Struggling to Understand the NEC Spectrum—Could the Integration of Metabolomics, Clinical-Laboratory Data, and Other Emerging Technologies Help Diagnosis? Metabolites 2024, 14, 521. https://doi.org/10.3390/metabo14100521
Sarafidis K, Agakidou E, Kontou A, Agakidis C, Neu J. Struggling to Understand the NEC Spectrum—Could the Integration of Metabolomics, Clinical-Laboratory Data, and Other Emerging Technologies Help Diagnosis? Metabolites. 2024; 14(10):521. https://doi.org/10.3390/metabo14100521
Chicago/Turabian StyleSarafidis, Kosmas, Eleni Agakidou, Angeliki Kontou, Charalampos Agakidis, and Josef Neu. 2024. "Struggling to Understand the NEC Spectrum—Could the Integration of Metabolomics, Clinical-Laboratory Data, and Other Emerging Technologies Help Diagnosis?" Metabolites 14, no. 10: 521. https://doi.org/10.3390/metabo14100521
APA StyleSarafidis, K., Agakidou, E., Kontou, A., Agakidis, C., & Neu, J. (2024). Struggling to Understand the NEC Spectrum—Could the Integration of Metabolomics, Clinical-Laboratory Data, and Other Emerging Technologies Help Diagnosis? Metabolites, 14(10), 521. https://doi.org/10.3390/metabo14100521






