Exploring the Impact of Catechins on Bone Metabolism: A Comprehensive Review of Current Research and Future Directions
Abstract
1. Introduction
2. Material and Methods
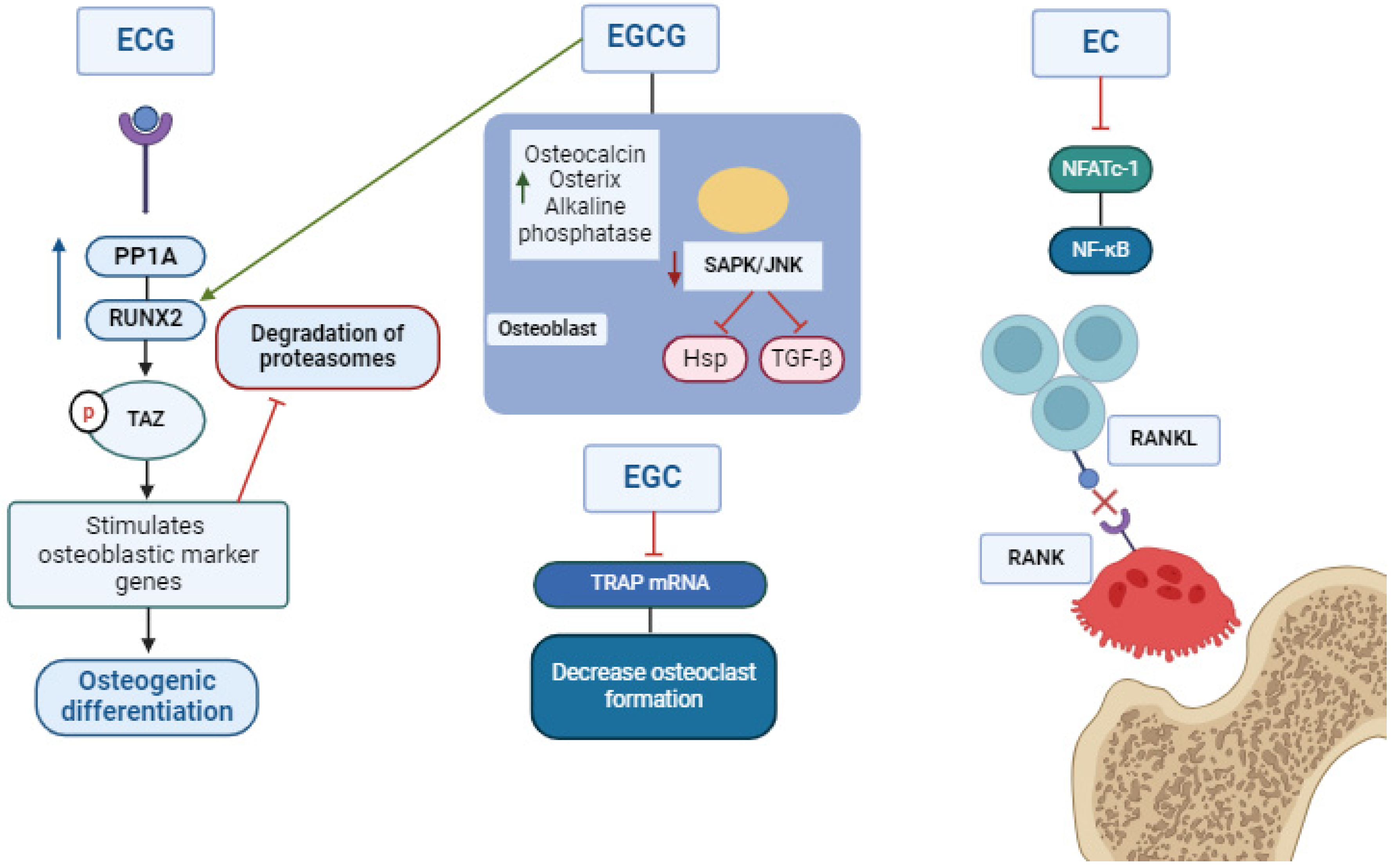
3. RANK/RANKL/OPG Signalizing
4. Catechin-Based Concentration: In Vitro Studies
| Experimental Design | Type of Polyphenol | Main Results | Author/Year |
|---|---|---|---|
| Osteoblasts were obtained from calvariae of newborn ddY mice at 6–9 weeks of age. | 25–100 µM EGCG for 24 h. | Decreased osteoclastic cells. | [34] Nakagawa et al., 2002 PMID: 11890677. |
| Osteoblast cells from the calvaria. ICR (Samtako Inc., O-San, Kyung-gi-Do, Republic of Korea). | EGCG 20, 50, and 100 µm (48 h). (Calbiochem, La Jolla, CA, USA). | Did not show action on the viability of osteoblastic cells at the concentration of 20 µm. Decreased the osteoblastic cells by 32%. | [15] Yun et al., 2004 PMID: 15324350 |
| D1 cell culture. | EGCG 1 and 10 μmol/L (48 h). | EGCG increased the activity of ALP and osteogenic genes associated with bone mineralization. | [16] Chen et al., 2005 PMID: 16170444. |
| Human osteosarcoma SAOS-2 cells. | Green tea polyphenol (GTP) 24 h. Mitsui Norin Co. (Polyphenon-E®, Tokyo, Japan). | GTP induced apoptosis of malignant cell lines. | [36] Hafeez et al., 2006 PMID: 16797629. |
| Clones similar to osteoblasts MC3T3-E1 cells. | EGCG 10 and 100 µM for 48 h. Calbiochem-Novabiochem Co. (La Jolla, CA, USA). | Activated SAPK/JNK in osteoblasts and VEGF. | [37] Tokuda et al., 2007 PMID: 17031857. |
| MC3T3-E1 cells similar to osteoblasts. | 100 µM EGCG 48 h. Calbiochem-Novabio-chem Co. (La Jolla, CA, USA). | Decreased p44/p42 MAP. In MC3T3-E1 cells, EGCG presented a reduced effect on IL-6 synthesis; however, in the primary culture of mouse osteoblasts, 30 µM of EGCG significantly reduced IL-6. | [38] Tokuda et al., 2007 PMID: 17350626. |
| MC3T3-E1 cells similar to osteoblasts. | EGCG (1 and 30 μM) for 24 h. Calbiochem-Novabiochem Corp. (La Jolla, CA, USA). | EGCG presented a partial effect on PDGF and did not show any action on osteocalcin and osteoprotegerin. | [32] Takai et al., 2008 PMID: 19148296. |
| Bone marrow cells of rats at 5 months of age. | EGCG 1–50 µM for 48 h. Sigma-Aldrich (St. Louis, MO, USA). | EGCG inactivated RANKL through the JNK/c-Jun and NF-κβ signaling pathways. | [39] Lee et al., 2010 PMID: 19828731. |
5. Catechin-Based Diet: Studies in Animals
| Experimental Design | Type of Polyphenol | Main Results | Author/Year |
|---|---|---|---|
| Male DBA/1 mice (6–7 weeks old). | EGCG (20 µg/gm por peso corporal) (Sigma (catalog no. E4143; St. Louis, MO, USA). | EGCG decreased TRAP and osteoclast gene expression. EGCG decreased NF-ATc1; however, it could not reduce NF-κβ and c-Jun. | [52] Morinobu et al., 2008 PMID: 18576345. |
| Albino female rats at the age of 95–100 days. | Black tea extract (BTE), processed by Tocklai Experimental Station, Jorhat, Assam, India. | BTE increased bone density and calcium and potassium levels in ovariectomy-induced female rats associated with bone loss. | [53] Das et al., 2004 PMID: 15228990. |
| Twenty-one female mice (20–24 weeks old). | Black tea extract (BTE) for 28 days processed and supplied by Tocklai Experimental Station, Jorhat, Assam, India. | BTE increased the estradiol levels, restoring bone density and the calcium and phosphorus levels. | [54] Das et al., 2005 PMID: 15996685. |
| Eighteen albino Wistar female rats at the age of 6 months. | Black tea extract (BTE) processed by Tocklai Experimental Station, Jorhat, Assam, India. | BTE reduced the osteoclastic factors and the cytokine levels associated with bone resorption. | [55] Das et al., 2009 PMID: 19277962. |
| Wistar rats at the age of 4–5 months, both genders. | Black tea extract (BTE) supplied by Tocklai Experimental Station, Jorhat, Assam, India. | The BTE group presented higher levels of OPG and a reduction in RANKL activity. | [56] Karmakar et al., 2011 PMID: 21772972 |
| Female rats, 14 months of age. | Green tea polyphenol (GTP). The dried leaves of Camellia sinensis were prepared daily. | GTP increased the bone mineral density of the femur. | [57] Shen et al., 2008 PMID: 18084689. |
| Twenty-four C56BL/six obese males, 4 weeks of age. | Green tea extract (GTE) 1 and 2% for 6 weeks. GTE: 5.6 mg caffeine/100 mg, 48% epigallocatechin gallate (EGCG), 31% epigallocatechin, 13% epicatechin gallate, and 8% epicatechin. | GTE reduced the body mass but reduced the cortical and medullary bone in thin and obese rats. | [58] Iwaniec et al., 2009PMID: 19710162. |
| Seventy female F344 × BFN1/NIA rats at 14 months of age. | Green tea polyphenol (GTP) for 16 weeks. GTP, 0.1% GTP, and 0.5% GTP. | Both concentrations of GTP increased the bone mineral density. | [59] Shen et al., 2009 PMID: 19118658. |
| Female C57BL/6J rats, six weeks of age. | Catechin 30 mg/kg (Sigma-Aldrich, MO, USA) | Catechin suppressed the osteoclast expression but with no effects on RANK. | [60] Sugawara et al., 2024 PMID: 38295903. |
6. Catechin-Based Diet: Studies in Humans
| Experimental Design | Type of Polyphenol | Main Results | Author/Year |
|---|---|---|---|
| Women and men of 50 years old. | Tea | There was a 30% decrease in the risk of hip fracture in both genders. p < 0.05 | [78] Johnell et al., 1995 PMID: 8592959. |
| Women aged 65–76 years old divided into two groups: non-consumers of tea and tea consumers (1–3 glasses, 4–6 or more than 6 glasses). | Tea | The bone mineral density measurements of the greater trochanter and Ward triangle were higher in the tea consumer group; however, the femoral neck did not present a significant difference between the groups <0.05. | [79] Hegarty et al., 2000 PMID: 10731510. |
| Patients age 20–76 years, n = 1200, divided into a regular consumption group (five or more glasses of tea a day) and a control group (less than five glasses of tea a day). 10 mL of blood was collected. The BMD was analyzed. | Tea | The spine and hip BMD were higher in women (4.54% and 4.2%, respectively). In men, there were no statistical differences. Vitamin D, parathyroid hormone, and calcium presented similar values in the groups and genders. | [66] Hossein-Nezhad et al. 2007 PMID not found. |
| One hundred and seventy-one women in the postmenopausal period. Groups: - GTP 500 mg - Placebo + TC: (60 min, 3× a week) - GTP (500 mg daily) + TC (60 min of exercises 3× a week). | GTP capsules. Supplied by Zhejiang Yixin Pharmaceutical Co., Ltd., Jinhua, China (GTP IND no. 77,470 by FDA of USA). | The EGCG and ECG concentrations were higher in the GTP and GT + TC groups after 1 month of research and remained high 6 months after the intervention. GTP + TC did not present a time–dosage relationship. p = 0.05 | [80] Qian et al., 2012 PMID: 23118932 |
| Thirty patients with mild to moderate chronic periodontitis were randomly distributed into 2 groups: test (toothpaste with green tea + hygiene instruction) and control (toothpaste with fluorine and triclosan). | Green tea extract 60–90% of EGCG (Infra drug industries, Bangalore, India). | After 4 weeks of treatment, the gingival index presented significant differences: in the test group, it was 0.88, and in the control group, it was 0.54. The bleeding significantly decreased from 84.38% to 25.5% in the test group and from 78.12% to 31.25% in the control group. The test group’s depth was higher than 4 mm, with a depth of 5.38 mm and a decrease of 3.89 mm. In the control group, it was 5.34 mm, which decreased to 0.51 mm. | [81] Hrishi et al., 2016 PMID: 25690541. |
7. Catechin in Periodontal Tissue
8. Application of Catechins on Membranes, Hydrogel, and Dental Materials
9. Discussion
10. Conclusions
11. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Häussler, B.; Gothe, H.; Göl, D.; Glaeske, G.; Pientka, L.; Felsenberg, D. Epidemiology, Treatment and Costs of Osteoporosis in Germany—the BoneEVA Study. Osteoporos. Int. 2007, 18, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Arabi, A. Vitamins and Bone Health: Beyond Calcium and Vitamin D. Nutr. Rev. 2011, 69, 584–598. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The Cell Biology of Bone Metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and Pro-Oxidant Activity of Rutin and Quercetin Derivatives. J. Pharm. Pharmacol. 2010, 55, 131–142. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins Enhance Skeletal Muscle Performance. Crit. Rev. Food. Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. Biomed. Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Byun, H.; Kim, S.; Joo, J.; Park, H.H.; Shin, H. Evaluation of the Anti-Oxidative and ROS Scavenging Properties of Biomaterials Coated with Epigallocatechin Gallate for Tissue Engineering. Acta Biomater. 2021, 124, 166–178. [Google Scholar] [CrossRef]
- Liao, S.; Tang, Y.; Chu, C.; Lu, W.; Baligen, B.; Man, Y.; Qu, Y. Application of Green Tea Extracts Epigallocatechin-3-Gallate in Dental Materials: Recent Progress and Perspectives. J. Biomed. Mater. Res. A 2020, 108, 2395–2408. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M. Effects of Tea Consumption on Nutrition and Health. J. Nutr. 2000, 130, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H. Green Tea Polyphenol Sensing. Proc. Jpn. Acad. Ser. B 2011, 87, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Chyu, M.C.; Wang, J.S. Tea and Bone Health: Steps Forward in Translational Nutrition1-5. Am. J. Clin. Nutr. 2013, 98, 1694S–1699S. [Google Scholar] [CrossRef] [PubMed]
- German, I.J.S.; Pomini, K.T.; Andreo, J.C.; Shindo, J.V.T.C.; Castro, M.V.M.D.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Fornari Laurindo, L.; Bueno, P.C.D.S.; et al. New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients 2024, 16, 326. [Google Scholar] [CrossRef]
- Yun, J.H.; Pang, E.K.; Kim, C.S.; Yoo, Y.J.; Cho, K.S.; Chai, J.K.; Kim, C.K.; Choi, S.H. Inhibitory Effects of Green Tea Polyphenol (–)-Epigallocatechin Gallate on the Expression of Matrix Metalloproteinase-9 and on the Formation of Osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green Tea Catechin Enhances Osteogenesis in a Bone Marrow Mesenchymal Stem Cell Line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef]
- Hayashi, K.; Takai, S.; Matsushima-Nishiwaki, R.; Hanai, Y.; Kato, K.; Tokuda, H.; Kozawa, O. (–)-Epigallocatechin Gallate Reduces Transforming Growth Factor β-Stimulated HSP27 Induction through the Suppression of Stress-Activated Protein Kinase/c-Jun N-Terminal Kinase in Osteoblasts. Life Sci. 2008, 82, 1012–1017. [Google Scholar] [CrossRef]
- Hsiao, H.B.; Wu, J.B.; Lin, W.C. (–)-Epicatechin 3-O-β-d-Allopyranoside Prevent Ovariectomy-Induced Bone Loss in Mice by Suppressing RANKL-Induced NF-ΚB and NFATc-1 Signaling Pathways. BMC Complement. Altern. Med. 2017, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Lau, K.M.; Choy, W.Y.; Leung, P.C. Effects of Tea Catechins, Epigallocatechin, Gallocatechin, and Gallocatechin Gallate, on Bone Metabolism. J. Agric. Food Chem. 2009, 57, 7293–7297. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Bendall, A.J.; Abate-Shen, C. Roles for Msx and Dlx Homeoproteins in Vertebrate Development. Gene 2000, 247, 17–31. [Google Scholar] [CrossRef]
- Xuan, B.; Yang, P.; Wu, S.; Li, L.; Zhang, J.; Zhang, W. Expression of Dlx-5 and Msx-1 in Craniofacial Skeletons and Ilia of Rats Treated with Zoledronate. J. Oral Maxillofac. Surg. 2017, 75, 994.e1–994.e9. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, M.; Behringer, R.R.; De Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell. 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Shalan, N.A.A.M.; Mustapha, N.M.; Mohamed, S. Noni Leaf and Black Tea Enhance Bone Regeneration in Estrogen-Deficient Rats. Nutrition 2017, 33, 42–51. [Google Scholar] [CrossRef]
- Chu, C.; Liu, L.; Wang, Y.; Yang, R.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Evaluation of Epigallocatechin-3-Gallate (EGCG)-Modified Scaffold Determines Macrophage Recruitment. Mater. Sci. Eng. C 2019, 100, 505–513. [Google Scholar] [CrossRef]
- Chu, C.; Wang, Y.; Wang, Y.; Yang, R.; Liu, L.; Rung, S.; Xiang, L.; Wu, Y.; Du, S.; Man, Y.; et al. Evaluation of Epigallocatechin-3-Gallate (EGCG) Modified Collagen in Guided Bone Regeneration (GBR) Surgery and Modulation of Macrophage Phenotype. Mater. Sci. Eng. C 2019, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Matsushima-Nishiwaki, R.; Adachi, S.; Natsume, H.; Minamitani, C.; Mizutani, J.; Otsuka, T.; Tokuda, H.; Kozawa, O. (–)-Epigallocatechin Gallate Reduces Platelet-Derived Growth Factor-BB-Stimulated Interleukin-6 Synthesis in Osteoblasts: Suppression of SAPK/JNK. Mediators Inflamm. 2008, 2008, 291808. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Kainuma, S.; Fujita, K.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Matsushima-Nishiwaki, R.; Harada, A.; et al. (–)-Epigallocatechin Gallate Synergistically Potentiates Prostaglandin E2-Stimulated Osteoprotegerin Synthesis in Osteoblasts. Prostaglandins Other Lipid Mediat. 2017, 128–129, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Wachi, M.; Kato, M.; Kasai, S.; Takahashi, F.; Woo, J.T.; Nagai, K.; Lee, I.S. Fenton Reaction Is Primarily Involved in a Mechanism of (–)-Epigallocatechin-3-Gallate to Induce Osteoclastic Cell Death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Ahmed, S.; Wang, N.; Gupta, S.; Zhang, A.; Haqqi, T.M. Green Tea Polyphenols-Induced Apoptosis in Human Osteosarcoma SAOS-2 Cells Involves a Caspase-Dependent Mechanism with Downregulation of Nuclear Factor-ΚB. Toxicol. Appl. Pharmacol. 2006, 216, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Takai, S.; Matsushima-Nishiwaki, R.; Akamatsu, S.; Hanai, Y.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (–)-Epigallocatechin Gallate Enhances Prostaglandin F2α- Induced VEGF Synthesis via Upregulating SAPK/JNK Activation in Osteoblasts. J. Cell. Biochem. 2007, 100, 1146–1153. [Google Scholar] [CrossRef]
- Tokuda, H.; Takai, S.; Hanai, Y.; Matsushima-Nishiwaki, R.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (–)-Epigallocatechin Gallate Suppresses Endothelin-1-Induced Interleukin-6 Synthesis in Osteoblasts: Inhibition of P44/P42 MAP Kinase Activation. FEBS Lett. 2007, 581, 1311–1316. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, H.; Shim, H.E.; Kim, H.N.; Ha, H.; Lee, Z.H. Epigallocatechin-3-Gallate Inhibits Osteoclastogenesis by down-Regulating c-Fos Expression and Suppressing the Nuclear Factor-ΚB Signal. Mol. Pharmacol. 2010, 77, 17–25. [Google Scholar] [CrossRef]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea Polyphenols Inhibit Rat Osteoclast Formation and Differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef]
- Vali, B.; Rao, L.G.; El-Sohemy, A. Epigallocatechin-3-Gallate Increases the Formation of Mineralized Bone Nodules by Human Osteoblast-like Cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Kang, L.; Wang, C.Z.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (−)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green Tea Polyphenols Benefits Body Composition and Improves Bone Quality in Long-Term High-Fat Diet-Induced Obese Rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.-C.; Cao, J.J. Green Tea Supplementation Benefits Body Composition and Improves Bone Properties in Obese Female Rats Fed with High-Fat Diet and Caloric Restricted Diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lin, R.W.; Fu, Y.C.; Lin, Y.S.; Chang, J.K.; Chen, H.T.; Chen, C.H.; Lin, S.Y.; Wang, G.J.; et al. (–)-Epigallocatechin-3-Gallate Improves Bone Microarchitecture in Ovariectomized Rats. Menopause 2013, 20, 687–694. [Google Scholar] [CrossRef]
- Lin, S.Y.; Kan, J.Y.; Lu, C.C.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Ho, C.J.; Lee, T.C.; Chuang, S.C.; Lin, Y.S.; et al. Green Tea Catechin (–)-Epigallocatechin-3-Gallate (EGCG) Facilitates Fracture Healing. Biomolecules 2020, 10, 620. [Google Scholar] [CrossRef]
- Xi, J.; Li, Q.; Luo, X.; Li, J.; Guo, L.; Xue, H.; Wu, G. Epigallocatechin-3-gallate Protects against Secondary Osteoporosis in a Mouse Model via the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2018, 18, 4555–4562. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Tenner, T.E.; Chyu, M.C.; Yeh, J.K. Supplementation with Green Tea Polyphenols Improves Bone Microstructure and Quality in Aged, Orchidectomized Rats. Calcif. Tissue. Int. 2011, 88, 455–463. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Green Tea Polyphenols Mitigate Bone Loss of Female Rats in a Chronic Inflammation-Induced Bone Loss Model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Synergistic Effects of Green Tea Polyphenols and Alphacalcidol on Chronic Inflammation-Induced Bone Loss in Female Rats. Osteoporos. Int. 2010, 21, 1841–1852. [Google Scholar] [CrossRef]
- Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. Int. J. Mol. Sci. 2019, 20, 6042. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (–)-Epigallocatechin-3-Gallate Suppresses Osteoclast Differentiation and Ameliorates Experimental Arthritis in Mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Mukherjee, M.; Mitra, C. Evidence for a Prospective Anti-Osteoporosis Effect of Black Tea (Camellia Sinensis) Extract in a Bilaterally Ovariectomized Rat Model. Asia Pac. J. Clin. Nutr. 2004, 13, 210–216. [Google Scholar]
- Das, A.S.; Das, D.; Mukherjee, M.; Mukherjee, S.; Mitra, C. Phytoestrogenic Effects of Black Tea Extract (Camellia Sinensis) in an Oophorectomized Rat (Rattus Norvegicus) Model of Osteoporosis. Life Sci. 2005, 77, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Mukherjee, M.; Das, D.; Mitra, C. Protective Action of Aqueous Black Tea (Camellia Sinensis) Extract (BTE) against Ovariectomy-Induced Oxidative Stress of Mononuclear Cells and Its Associated Progression of Bone Loss. Phytother. Res. 2009, 23, 1287–1294. [Google Scholar] [CrossRef]
- Karmakar, S.; Majumdar, S.; Maiti, A.; Choudhury, M.; Ghosh, A.; Das, A.S.; Mitra, C. Protective Role of Black Tea Extract against Nonalcoholic Steatohepatitis-Induced Skeletal Dysfunction. J. Osteoporos. 2011, 2011, 426863. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective Effect of Green Tea Polyphenols on Bone Loss in Middle-Aged Female Rats. Osteoporos. Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T.; Koo, S.I.; Kaur, R.; Ho, E.; Wong, C.P.; Bruno, R.S. Consumption of Green Tea Extract Results in Osteopenia in Growing Male Mice. J. Nutr. 2009, 139, 1914–1919. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Stoecker, B.J.; Chyu, M.C.; Wang, J.S. Green Tea Polyphenols Mitigate Deterioration of Bone Microarchitecture in Middle-Aged Female Rats. Bone 2009, 44, 684–690. [Google Scholar] [CrossRef]
- Sugawara, D.; Sakai, N.; Sato, Y.; Azetsu, Y.; Karakawa, A.; Chatani, M.; Mizuno, M.; Maruoka, Y.; Myers, M.; Fukuhara, K.; et al. Planar Catechin Increases Bone Mass by Regulating Differentiation of Osteoclasts in Mice. J. Oral Biosci. 2024, 66, 196–204. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, N.; Wang, Q.; Feng, J.; Sun, D.; Zhang, Q.; Huang, J.; Wen, Q.; Hu, R.; Wang, L.; et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J. Bone Miner. Res. 2019, 34, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-Analysis of How Well Measures of Bone Mineral Density Predict Occurrence of Osteoporotic Fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pettinger, M.B.; Ritenbaugh, C.; LaCroix, A.Z.; Robbins, J.; Caans, B.J.; Barad, D.H.; Hakim, I.A. Habitual Tea Consumption and Risk of Osteoporosis: A Prospective Study in the Women’s Health Initiative Observational Cohort. Am. J. Epidemiol. 2003, 158, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, Y.C.; Yao, W.J.; Lu, F.H.; Wu, J.S.; Chang, C.J. Epidemiological Evidence of Increased Bone Mineral Density in Habitual Tea Drinkers. Arch. Intern. Med. 2002, 162, 1001–1006. [Google Scholar] [CrossRef]
- Du, F.; Qiukui, H.; Birong, D.; Changquan, H.; Hongmei, W.; Yanling, Z.; Wen, Z.; Li, L. Association of Osteoporotic Fracture with Smoking, Alcohol Consumption, Tea Consumption and Exercise among Chinese Nonagenarians/Centenarians. J. Nutr. Health Aging 2011, 15, 327–331. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Maghbooli, Z.; Shafaei, A.R.; Javadi, E.; Larijani, B. Relationship between Tea Drinking and Bone Mineral Density in Iranian Population. J. Public Health 2007, 36, 57–62. [Google Scholar]
- Kyriazopoulos, P.; Trovas, G.; Charopoulos, J.; Antonogiannakist, E.; Galanos, A.; Lyritis, G. Lifestyle Factors and Forearm Bone Density in Young Greek Men. Clin. Endocrinol. 2006, 65, 234–238. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liu, C.Y.; Wang, C.J. Factors Associated with Low Bone Density among Women with Major Depressive Disorder. Int. J. Psychiatry Med. 2012, 44, 77–90. [Google Scholar] [CrossRef]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Nakamura, K. Diet and Lifestyle Associated with Increased Bone Mineral Density: Cross-Sectional Study of Japanese Elderly Women at an Osteoporosis Outpatient Clinic. J. Orthop. Sci. 2007, 12, 317–320. [Google Scholar] [CrossRef]
- Hoover, P.A.; Webber, C.E.; Beaumont, L.F.; Blake, J.M. Postmenopausal Bone Mineral Density: Relationship to Calcium Intake, Calcium Absorption, Residual Estrogen, Body Composition, and Physical Activity. Can. J. Physiol. Pharmacol. 1996, 74, 911–917. [Google Scholar] [CrossRef]
- Vestergaard, P.; Hermann, A.P.; Gram, J.; Jensen, L.B.; Eiken, P.; Abrahamsen, B.; Brot, C.; Kolthoff, N.; Sørensen, O.H.; Beck Nielsen, H.; et al. Evaluation of Methods for Prediction of Bone Mineral Density by Clinical and Biochemical Variables in Perimenopausal Women. Maturitas 2001, 40, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.H.; Aydin, S.; Gemalmaz, A.; Aktürk, Z.; Yaman, H.; Bozdemir, N.; Kurdak, H.; Sitmapinar, K.; Sencar, I.D.; Başak, O.; et al. Habitual Tea Drinking and Bone Mineral Density in Postmenopausal Turkish Women: Investigation of Prevalence of Postmenopausal Osteoporosis in Turkey (IPPOT Study). Int. J. Vitam. Nutr. Res. 2013, 77, 389–397. [Google Scholar] [CrossRef]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; MacDonald, H.M. Associations between Dietary Flavonoid Intakes and Bone Health in a Scottish Population. J. Bone Min. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.M.; Mithal, A.; Malhotra, N.; Brown, E.M. BioMed Central Pilot Case-Control Investigation of Risk Factors for Hip Fractures in the Urban Indian Population. BMC Musculoskelet. Disord. 2010, 11, 49. [Google Scholar] [CrossRef]
- Kreiger, N.; Gross, A.; Hunter, G. Dietary Factors and Fracture in Postmenopausal Women: A Case-Control Study. Int. J. Epidemiol. 1992, 21, 953–958. [Google Scholar] [CrossRef]
- Zeng, F.-F.; Wu, B.-H.; Fan, F.; Xie, H.-L.; Xue, W.-Q.; Zhu, H.-L.; Chen, Y.-M. Dietary Patterns and the Risk of Hip Fractures in Elderly Chinese: A Matched Case-Control Study. J. Clin. Endocrinol. Metab. 2009, 98, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Negri, E.; La Vecchia, C. La Coffee Intake and Risk of Hip Fracture in Women in Northern Italy. Prev. Med. 1995, 24, 396–400. [Google Scholar] [CrossRef]
- Johnell, O.; Gullberg, B. Risk factors for hip fracture in European women: The MEDOS Study. Mediterranean Osteoporosis Study. J. Bone Min. Res 1995, 10, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, V.M.; May, H.M.; Khaw, K.T. Tea Drinking and Bone Mineral Density in Older Women. Am. J. Clin. Nutr. 2000, 71, 1003–1007. [Google Scholar] [CrossRef]
- Qian, G.; Xue, K.; Tang, L.; Wang, F.; Song, X.; Chyu, M.C.; Pence, B.C.; Shen, C.L.; Wang, J.S. Mitigation of Oxidative Damage by Green Tea Polyphenols and Tai Chi Exercise in Postmenopausal Women with Osteopenia. PLoS ONE 2012, 7, e48090. [Google Scholar] [CrossRef]
- Hrishi, T.S.; Kundapur, P.P.; Naha, A.; Thomas, B.S.; Kamath, S.; Bhat, G.S. Effect of Adjunctive Use of Green Tea Dentifrice in Periodontitis Patients—A Randomized Controlled Pilot Study. Int. J. Dent. Hyg. 2016, 14, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Dudonné, S.; Desjardins, Y.; Grenier, D. Wild Blueberry (Vaccinium Angustifolium Ait.) Polyphenols Target Fusobacterium Nucleatum and the Host Inflammatory Response: Potential Innovative Molecules for Treating Periodontal Diseases. J. Agric. Food. Chem. 2015, 63, 6999–7008. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A Systematic Review of the Effect of Surgical Debridement vs. Non-Surgical Debridement for the Treatment of Chronic Periodontitis. J. Clin. Periodontol. 2002, 29, 92–102. [Google Scholar] [CrossRef]
- Cho, A.R.; Kim, J.H.; Lee, D.E.; Lee, J.S.; Jung, U.W.; Bak, E.J.; Yoo, Y.J.; Chung, W.G.; Choi, S.H. The Effect of Orally Administered Epigallocatechin-3-Gallate on Ligature-Induced Periodontitis in Rats. J. Periodontal Res. 2013, 48, 781–789. [Google Scholar] [CrossRef]
- Nakamura, H.; Ukai, T.; Yoshimura, A.; Kozuka, Y.; Yoshioka, H.; Yoshinaga, Y.; Abe, Y.; Hara, Y. Green Tea Catechin Inhibits Lipopolysaccharide-Induced Bone Resorption in Vivo. J. Periodontal Res. 2010, 45, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Aizawa, M.; Kim, M.; Yamamoto, T. Inhibitory Effects of Green Tea Polyphenols on Growth and Cellular Adherence of an Oral Bacterium, Porphyromonas Gingivalis. Biosci. Biotechnol. Biochem. 1996, 60, 745–749. [Google Scholar] [CrossRef]
- Javadkhani, A.; Shokouhi, B.; Mosayebzadeh, A.; Safa, S.; Fahimi, M.; Sharifi, S.; Maleki Dizaj, S.; Salatin, S. Nano-Catechin Gel as a Sustained Release Antimicrobial Agent against Clinically Isolated Porphyromonas gingivalis for Promising Treatment of Periodontal Diseases. Biomedicines 2023, 11, 1932. [Google Scholar] [CrossRef]
- Hong, J.Y.; Yon, J.; Lee, J.S.; Lee, I.K.; Yang, C.; Kim, M.S.; Choi, S.H.; Jung, U.W. Effects of Epigallocatechin-3-Gallate on the Healing of Extraction Sockets with a Periapical Lesion: A Pilot Study in Dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, Z.; Liu, H.; Xuan, Y.; Wang, X.; Luan, Q. Green Tea Epigallocatechin-3-Gallate Alleviates Porphyromonas Gingivalis-Induced Periodontitis in Mice. Int. Immunopharmacol. 2015, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, Y.; Ukai, T.; Nakatsu, S.; Kuramoto, A.; Nagano, F.; Yoshinaga, M.; Montenegro, J.L.; Shiraishi, C.; Hara, Y. Green Tea Extract Inhibits the Onset of Periodontal Destruction in Rat Experimental Periodontitis. J. Periodontal Res. 2014, 49, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Chava, V.K.; Vedula, B.D. Thermo-Reversible Green Tea Catechin Gel for Local Application in Chronic Periodontitis: A 4-Week Clinical Trial. J. Periodontol. 2013, 84, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Roodgaryan, R.; Jenabian, N.; Akbar Moghadamnia, A.; Pouramir, M.; Khadir, F. Clinical and Biochemical Effects of Dark Chocolate in Moderate Chronic Periodontitis. Casp. J. Dent. Res. 2015, 4, 43–49. [Google Scholar] [CrossRef]
- Gennaro, G.; Claudino, M.; Cestari, T.M.; Ceolin, D.; Germino, P.; Garlet, G.P.; De Assis, G.F.; Gronthos, S. Green Tea Modulates Cytokine Expression in the Periodontium and Attenuates Alveolar Bone Resorption in Type 1 Diabetic Rats. PLoS ONE 2015, 10, e0134784. [Google Scholar] [CrossRef]
- Yao, D.; Guo, J.; Qin, T.; Chen, H.; Jin, S. Effect of Alleviating Fibrosis with EGCG-Modified Bone Graft in Murine Model Depended on Less Accumulation of Inflammatory Macrophage. Biomed. Res. Int. 2023, 2023, 9466110. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of Epigallocatechin-3-Gallate (EGCG) Cross-Linked Collagen Membranes and Concerns on Osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 386–394. [Google Scholar] [CrossRef]
- Verma, N.K.; Kar, A.K.; Singh, A.; Jagdale, P.; Satija, N.K.; Ghosh, D.; Patnaik, S. Control Release of Adenosine Potentiate Osteogenic Differentiation within a Bone Integrative EGCG- g-NOCC/Collagen Composite Scaffold toward Guided Bone Regeneration in a Critical-Sized Calvarial Defect. Biomacromolecules 2021, 22, 3069–3083. [Google Scholar] [CrossRef]
- Hara, E.; Honda, Y.; Suzuki, O.; Tanaka, T.; Matsumoto, N. Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones. Int. J. Mol. Sci. 2018, 19, 3232. [Google Scholar] [CrossRef]
- Kook, Y.J.; Tian, J.; Jeon, Y.S.; Choi, M.J.; Song, E.; Park, C.H.; Reis, R.L.; Khang, G.; Eun, J.; Shin Jeon, Y. Nature-Derived Epigallocatechin Gallate/Duck’s Feet Collagen/Hydroxyapatite Composite Sponges for Enhanced Bone Tissue Regeneration. J. Biomater. Sci. 2018, 29, 984–996. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Evaluation of Nanohydroxyapaptite (Nano-HA) Coated Epigallocatechin-3-Gallate (EGCG) Cross-Linked Collagen Membranes. Mater. Sci. Eng. C 2017, 78, 258–264. [Google Scholar] [CrossRef]
- Honda, Y.; Huang, A.; Tanaka, T.; Han, X.; Gao, B.; Liu, H.; Wang, X.; Zhao, J.; Hashimoto, Y.; Yamamoto, K.; et al. Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics. Int. J. Mol. Sci. 2020, 21, 4213. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Du, X.; Huang, C.; Fu, D.; Ouyang, X.; Wang, Y. Antibacterial and Physical Properties of EGCG-Containing Glass Ionomer Cements. J. Dent. 2013, 41, 927–934. [Google Scholar] [CrossRef]
- Lee, S.; Chang, Y.Y.; Lee, J.; Perikamana, S.K.M.; Kim, E.M.; Jung, Y.H.; Yun, J.H.; Shin, H. Surface Engineering of Titanium Alloy Using Metal-Polyphenol Network Coating with Magnesium Ions for Improved Osseointegration. Biomater. Sci. 2020, 8, 3404–3417. [Google Scholar] [CrossRef]
- Shin, Y.S.; Seo, J.Y.; Oh, S.H.; Kim, J.H.; Kim, S.T.; Park, Y.B.; Moon, H.S. The Effects of ErhBMP-2-/EGCG-Coated BCP Bone Substitute on Dehiscence around Dental Implants in Dogs. Oral Dis. 2014, 20, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, X.; Yin, M.; Xu, T.; Guo, F. Long Non-Coding RNA in Osteogenesis: A New World to Be Explored. Bone Jt. Res. 2019, 8, 73–80. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A Receptor for Green Tea Polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, S.M.; Lee, J.; Ahmad, T.; Lee, M.S.; Yang, H.S.; Shin, H. Oxidative Epigallocatechin Gallate Coating on Polymeric Substrates for Bone Tissue Regeneration. Macromol. Biosci. 2019, 19, e1800392. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable Anti-Inflammatory Hyaluronic Acid Hydrogel for Osteoarthritic Cartilage Repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Miyaura, C.; Inada, M. Epigallocatechin Gallate (EGCG) Suppresses Lipopolysaccharide-Induced Inflammatory Bone Resorption, and Protects against Alveolar Bone Loss in Mice. FEBS Open Bio. 2015, 5, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.W.; Chen, C.H.; Wang, Y.H.; Ho, M.L.; Hung, S.H.; Chen, I.S.; Wang, G.J. (-)-Epigallocatechin Gallate Inhibition of Osteoclastic Differentiation via NF-KappaB. Biochem. Biophys. Res. Commun. 2009, 379, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Karsenty, G. Two Distinct Osteoblast-Specific Cis-Acting Elements Control Expression of a Mouse Osteocalcin Gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.-H.; Inada, M.; et al. Targeted Disruption of Results in a Complete Lack of Bone Formation Owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Huh, J.-E.; Kwon, N.-H.; Baek, Y.-H.; Lee, J.-D.; Choi, D.-Y.; Jingushi, S.; Kim, K.; Park, D.-S. Formononetin Promotes Early Fracture Healing through Stimulating Angiogenesis by Up-Regulating VEGFR-2/Flk-1 in a Rat Fracture Model. Int. Immunopharmacol. 2009, 9, 1357–1365. [Google Scholar] [CrossRef]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef]
- Shen, C.L.; Chyu, M.C.; Yeh, J.K.; Zhang, Y.; Pence, B.C.; Felton, C.K.; Brismée, J.M.; Arjmandi, B.H.; Doctolero, S.; Wang, J.S. Effect of Green Tea and Tai Chi on Bone Health in Postmenopausal Osteopenic Women: A 6-Month Randomized Placebo-Controlled Trial. Osteoporos. Int. 2012, 23, 1541–1552. [Google Scholar] [CrossRef]
- Nash, L.A.; Ward, W.E. Tea and Bone Health: Findings from Human Studies, Potential Mechanisms, and Identification of Knowledge Gaps. Crit. Rev. Food. Sci. Nutr. 2017, 57, 1603–1617. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R.; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens Attenuate Oxidative Stress and the Differentiation and Apoptosis of Osteoblasts by DNA-Binding-Independent Actions of the ERalpha. J. Bone Min. Res. 2010, 25, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of Green Tea Polyphenols in the Inhibition of Collagenolytic Activity by Collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Makimura, M.; Hirasawa, M.; Kobayashi, K.; Indo, J.; Sakanaka, S.; Taguchi, T.; Otake, S. Inhibitory effect of tea catechins on collagenase activity. J. Periodontol. 1993, 64, 630-6. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Takai, S.; Hanai, Y.; Matsushima-Nishiwaki, R.; Yamauchi, J.; Harada, A.; Hosoi, T.; Ohta, T.; Kozawa, O. (–)-Epigallocatechin Gallate Inhibits Basic Fibroblast Growth Factor-Stimulated Interleukin-6 Synthesis in Osteoblasts. Horm. Metab. Res. 2008, 40, 674–678. [Google Scholar] [CrossRef]
- Han, Y.; Pei, D.; Li, W.; Luo, B.; Jiang, Q. Epigallocatechin gallate attenuates tumor necrosis factor (TNF)-α-induced inhibition of osteoblastic differentiation by up-regulating lncRNA TUG1 in osteoporosis. Bioengineered. 2022, 13, 8950–8961. [Google Scholar] [CrossRef]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (Poly)Phenols Inhibit Periodontal Pathogen Growth and Biofilm Formation. Food Func. 2015, 6, 649–1022. [Google Scholar] [CrossRef]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef]
- Turcotte, A.F.; O’Connor, S.; Morin, S.N.; Gibbs, J.C.; Willie, B.M.; Jean, S.; Gagnon, C. Association between Obesity and Risk of Fracture, Bone Mineral Density and Bone Quality in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0252487. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK Signaling Cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Marie, P.J.; Debiais, F.; Haÿ, E. Regulation of Human Cranial Osteoblast Phenotype by FGF-2, FGFR-2 and BMP-2 Signaling. Histol. Histopathol. 2002, 17, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Rawadi, G. Wnt Signaling and Potential Applications in Bone Diseases. Curr. Drug Targets 2008, 9, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Meleth, S.; Katiyar, S.K. Growth Inhibitory and Antimetastatic Effect of Green Tea Polyphenols on Metastasis-Specific Mouse Mammary Carcinoma 4T1 Cells In Vitro and In Vivo Systems. Clin. Cancer Res. 2005, 11, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary Polyphenols and Periodontitis—A Mini-Review of Literature. Molecules 2018, 23, 1786. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Green tea: A novel functional food for the oral health of older adults. Geriatr. Gerontol. Int. 2014, 14, 238-50. [Google Scholar] [CrossRef]
- Higuchi, M.; Abiko, Y.; Washio, J.; Takahashi, N. Antimicrobial Effects of Epigallocatechin-3-Gallate, a Catechin Abundant in Green Tea, on Periodontal Disease-Associated Bacteria. Arch. Oral. Biol. 2024, 167, 106063. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Nakamoto, T.; Kawazoe, N.; Chen, G. Gelatin Scaffolds with Controlled Pore Structure and Mechanical Property for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2016, 22, 189–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
German, I.J.S.; Barbalho, S.M.; Andreo, J.C.; Zutin, T.L.M.; Laurindo, L.F.; Rodrigues, V.D.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Pomini, K.T.; et al. Exploring the Impact of Catechins on Bone Metabolism: A Comprehensive Review of Current Research and Future Directions. Metabolites 2024, 14, 560. https://doi.org/10.3390/metabo14100560
German IJS, Barbalho SM, Andreo JC, Zutin TLM, Laurindo LF, Rodrigues VD, Araújo AC, Guiguer EL, Direito R, Pomini KT, et al. Exploring the Impact of Catechins on Bone Metabolism: A Comprehensive Review of Current Research and Future Directions. Metabolites. 2024; 14(10):560. https://doi.org/10.3390/metabo14100560
Chicago/Turabian StyleGerman, Iris Jasmin Santos, Sandra Maria Barbalho, Jesus Carlos Andreo, Tereza Lais Menegucci Zutin, Lucas Fornari Laurindo, Victória Dogani Rodrigues, Adriano Cressoni Araújo, Elen Landgraf Guiguer, Rosa Direito, Karina Torres Pomini, and et al. 2024. "Exploring the Impact of Catechins on Bone Metabolism: A Comprehensive Review of Current Research and Future Directions" Metabolites 14, no. 10: 560. https://doi.org/10.3390/metabo14100560
APA StyleGerman, I. J. S., Barbalho, S. M., Andreo, J. C., Zutin, T. L. M., Laurindo, L. F., Rodrigues, V. D., Araújo, A. C., Guiguer, E. L., Direito, R., Pomini, K. T., & Shinohara, A. L. (2024). Exploring the Impact of Catechins on Bone Metabolism: A Comprehensive Review of Current Research and Future Directions. Metabolites, 14(10), 560. https://doi.org/10.3390/metabo14100560














