Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant
Abstract
1. Introduction
2. Materials and Methods
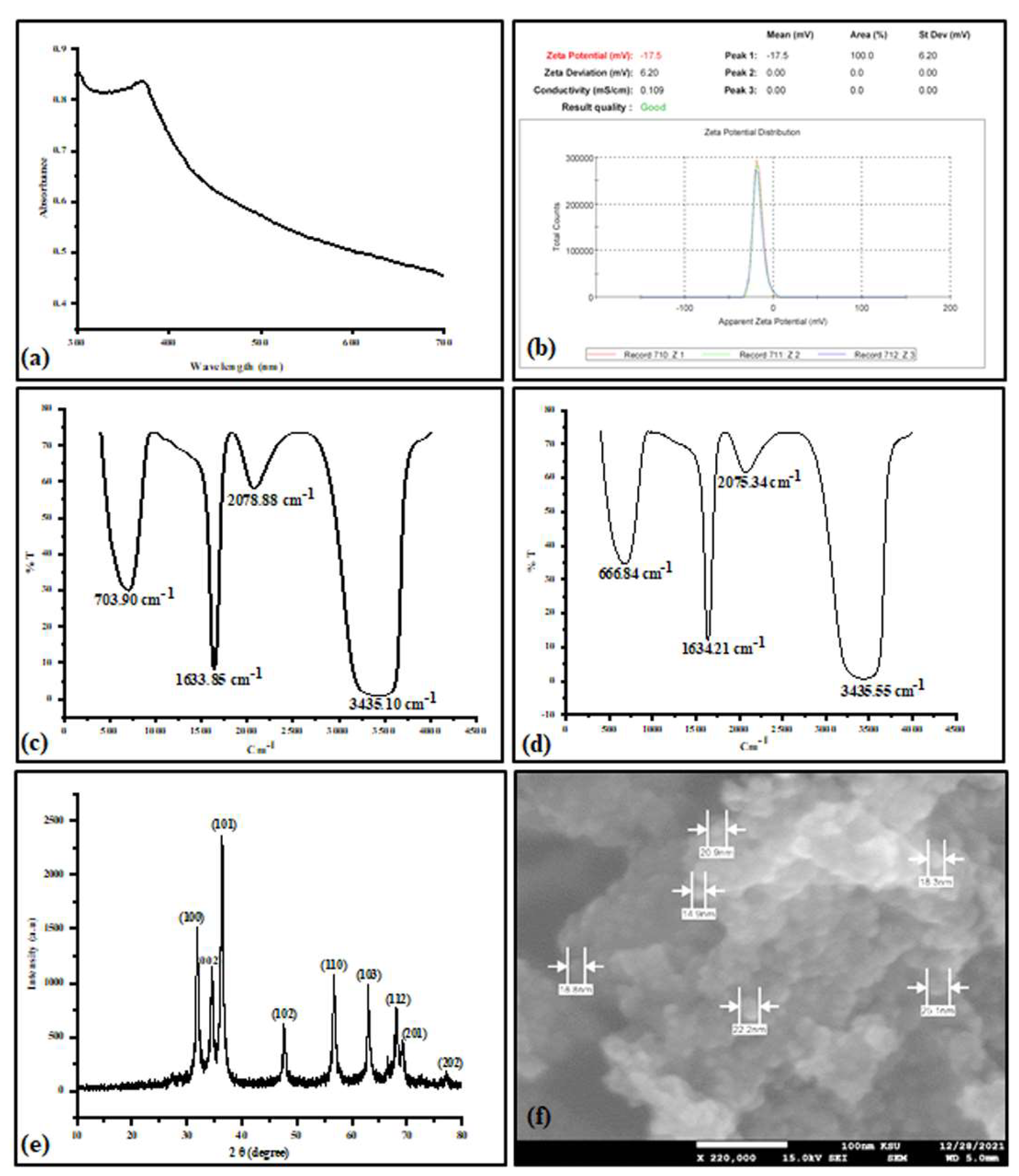
2.1. Zinc Oxide Nanoparticles Biosynthesis and Characterization
2.2. Plant Seeds Collection and Germination
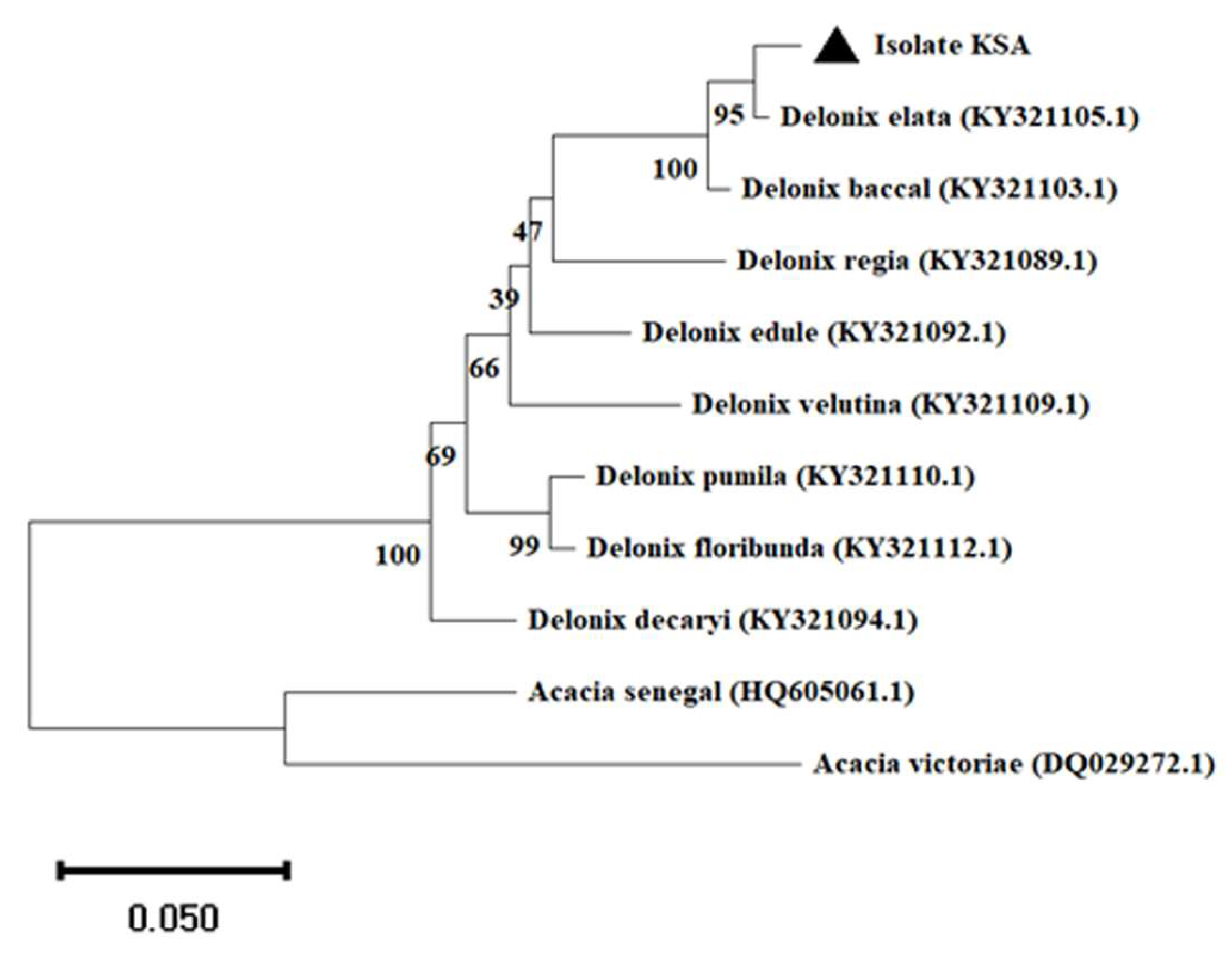
2.3. Molecular Identification
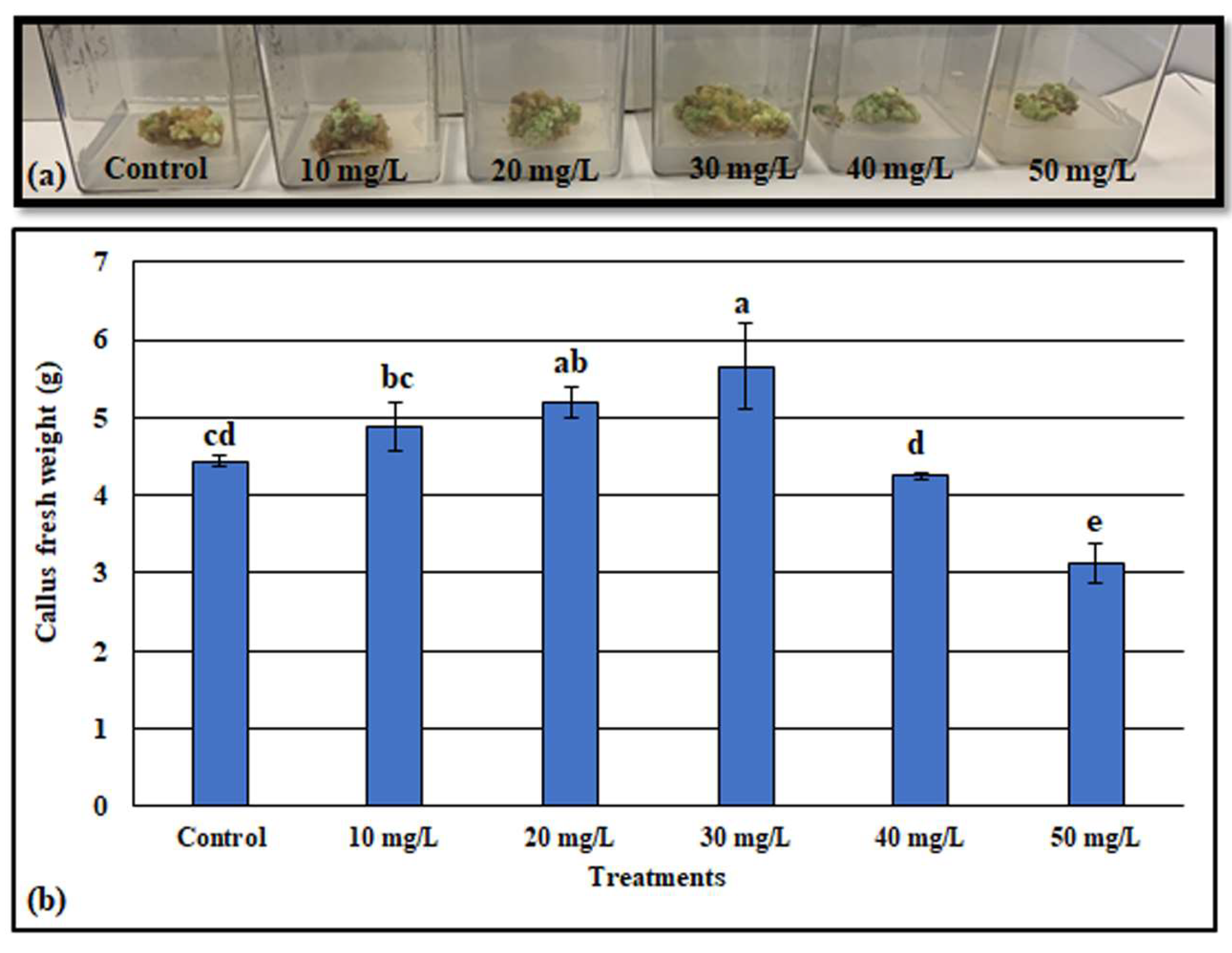
2.4. Callus Induction and ZnONP Application
2.5. Phytochemicals Extraction
2.5.1. Total Phenolic Content Estimation
2.5.2. Total Flavonoid Content Estimation
2.5.3. HPLC Quantification
2.5.4. Callus Extracts GC-MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Biosynthesis and Characterization of the ZnONPs
3.2. Delonix elata Molecular Identification
3.3. Effect of ZnONPs on Callus Growth
3.4. Phytochemical Analysis
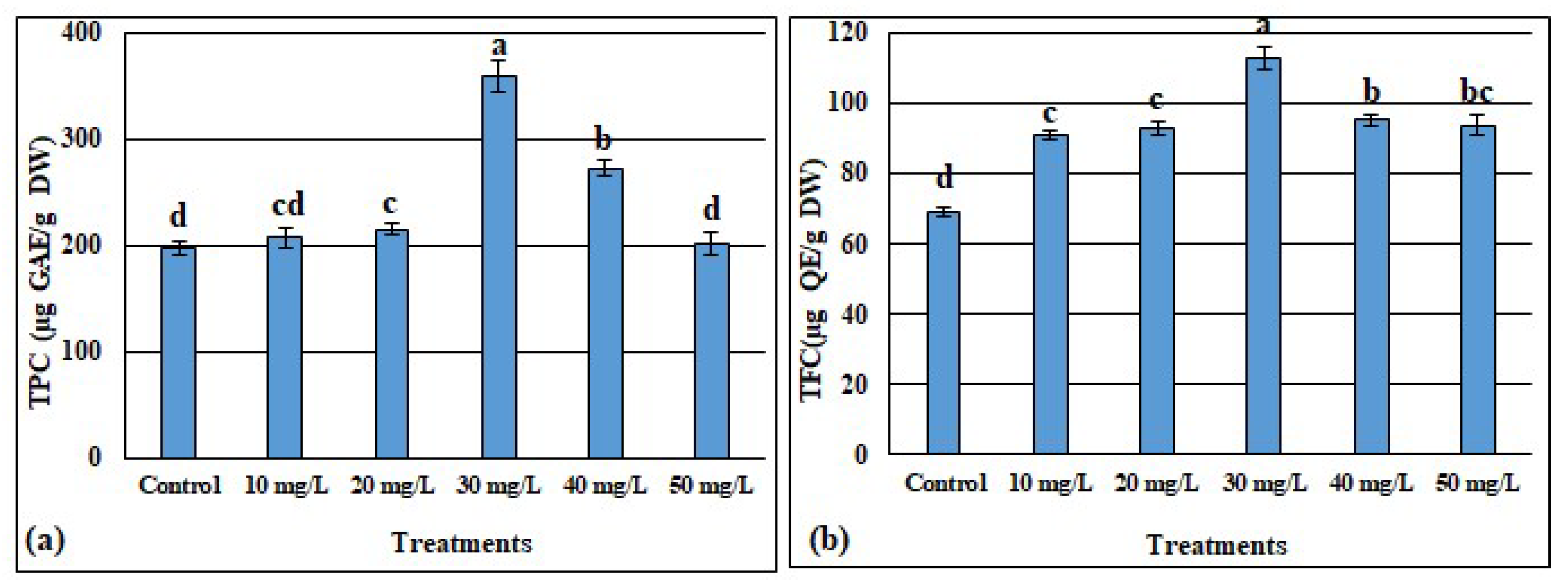
3.4.1. Effects of ZnONPs on Phenols and Flavonoids Contents
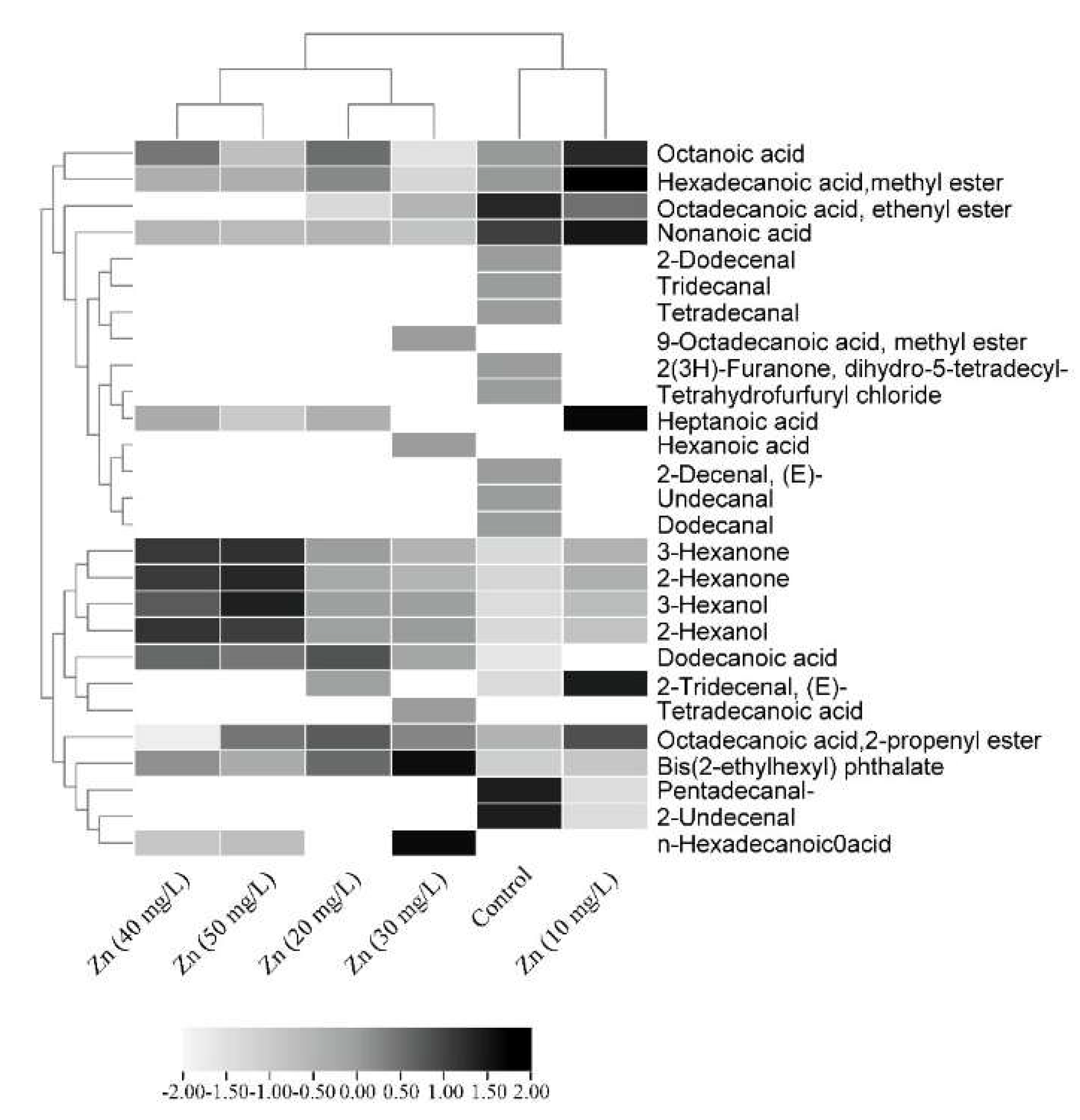
3.4.2. GC-MS for Phytochemical Compound Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, R.M.; Sharma, N.; Arya, G.; Nimesh, S. Recent progress in applied nanomaterials. In Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications; Springer Nature: Cham, Switzerland, 2019; pp. 33–64. [Google Scholar]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic nanotechnology: An approach to study scope and potential applications. Front. Chem. 2021, 9, 613343. [Google Scholar] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in sustainable green synthesis of silver nanoparticles using agri-food waste extracts and their applications in health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO nanoparticles synthesized using a novel plant extract: Application to enhance physiological and biochemical traits in maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Karam, S.T.; Abdulrahman, A.F. Green Synthesis and Characterization of ZnO Nanoparticles by Using Thyme Plant Leaf Extract. Photonics 2022, 9, 594. [Google Scholar] [CrossRef]
- Karthik, P.; Ravichandran, S.; Mukkannan, A.; Rajesh, J. Plant-mediated biosynthesis of zinc oxide nanoparticles from Delonix elata: A promising photocatalyst for crystal violet degradation. Inorg. Chem. Commun. 2022, 146, 110122. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Shobha, N.; Nanda, N.; Giresha, A.S.; Manjappa, P.; Sophiya, P.; Dharmappa, K.; Nagabhushana, B. Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng. C 2019, 97, 842–850. [Google Scholar] [CrossRef]
- Emul, E.; Asik, M.D.; Akcan, R.; Kose, K.; Uzun, L.; Saglam, S.; Korkusuz, F.; Saglam, N. Recent Advancements and New Perspectives of Nanomaterials. In Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications; Springer Nature: Cham, Switzerland, 2019; pp. 1–32. [Google Scholar]
- Choudhary, R.C.; Kumari, S.; Kumaraswamy, R.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Characterization methods for chitosan-based nanomaterials. In Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications; Springer Nature: Cham, Switzerland, 2019; pp. 103–116. [Google Scholar]
- Nayak, S.; Chaudhari, A.; Vaidhun, B. A review of zinc oxide nanoparticles: An evaluation of their synthesis, characterization and ameliorative properties for use in the food, pharmaceutical and cosmetic industries. J. Excip. Food Chem. 2020, 11, 79–92. [Google Scholar]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Gaafar, A.-R.Z.; Alfarraj, N.S. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Sci. Rep. 2021, 11, 19136. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.G.; Hernández, M.F.; Rangel, P.P.; Guzmán, A.P.G. ZnO nanoparticles synthesized by chemical precipitation to increase germination and bioactive compounds in sprouts of Raphanus sa-tivus L. Agro Product. 2023, 2. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C. Biogenic zinc oxide nanoparticles-enhanced biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar] [CrossRef]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khurshid, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of Biogenic Zinc Oxide Nanoparticles on Growth and Oxidative Stress Response in Flax Seedlings vs. In Vitro Cultures: A Comparative Analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef]
- Alghamdi, S.A. Antioxidative and Hypoglycemic Effects of Delonix elata and Vachellia farnesiana Leaves Extracts on Streptozotocin-Induced Diabetic Male Rats. Curr. Sci. Int. 2019, 8, 655–666. [Google Scholar]
- Yashwanth Kumar, D.; Joy Hoskeri, H. Delonix elata—A Potent Medicinal Plant: A Review. Int. J. Pharm. Pharm. Sci. 2013, 5, 1–3. [Google Scholar]
- Rao, R.V.K.; Rao, G.P.M.; Rao, B.G. Anti-inflammatory activity of the leaves and bark of Delonix elata. Anc. Sci. Life 1997, 17, 141. [Google Scholar]
- Pradeepa, K.; Venkatarangaiah, K.; Harish, B.; Venkatesh, R.; SR, S.K.; Kumar, G. Antibacterial activity of leaf extract of Delonix elata and molecular docking studies of luteolin. J. Biochem. Technol. 2014, 3, 193–197. [Google Scholar]
- Senthilkumar, M.; Sami, V. Development and validation of GC–MS methods for determination of leaf and root of Delonix elata (L.) gamble. Int. J. Adv. Res. Biol. Sci. 2014, 1, 93–107. [Google Scholar]
- Pradeepa, K.; Krishna, V.; Rajanna, S.; Gupta, R. Antioxidant and prophylactic effects of Delonix elata L., stem bark extracts, and flavonoid isolated quercetin against carbon tetrachloride-induced hepatotoxicity in rats. BioMed Res. Int. 2014, 2014, 507851. [Google Scholar]
- Oliviero, M.; Langellotti, A.L.; Russo, G.L.; Baselice, M.; Donadio, A.; Ritieni, A.; Graziani, G.; Masi, P. Use of different organic carbon sources in Cynara cardunculus cells: Effects on biomass productivity and secondary metabolites. Plants 2022, 11, 701. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Nadeem, M.; Tarroum, M.; Shaikhaldein, H.O. Biogenic silver nanoparticles improve bioactive compounds in medicinal plant Juniperus procera in vitro. Front. Plant Sci. 2022, 13, 962112. [Google Scholar] [CrossRef]
- Hegazi, G. In vitro studies on Delonix elata L., an endangered medicinal plant. World Appl. Sci. J. 2011, 14, 679–686. [Google Scholar]
- Shafique, S.; Jabeen, N.; Ahmad, K.S.; Irum, S.; Anwaar, S.; Ahmad, N.; Alam, S.; Ilyas, M.; Khan, T.F.; Hussain, S.Z. Green fabricated zinc oxide nanoformulated media enhanced callus induction and regeneration dynamics of Panicum virgatum L. PLoS ONE 2020, 15, e0230464. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurainy, F.; Tarroum, M.; Khan, S.; Nadeem, M.; Gaafar, A.-R.Z.; Alansi, S.; Alfarraj, N.S. Genome estimation and phytochemical compound identification in the leaves and callus of Abrus precatorius: A locally endangered plant from the flora of Saudi Arabia. Plants 2022, 11, 567. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Schulz, S.; Biwer, P.; Harig, T.; Koteska, D.; Schlawis, C. Chemical ecology of bacterial volatiles. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Bioactivity of short-chain aliphatic ketones against adults of the granary weevil, Sitophilus granarius (L.). Pest Manag. Sci. 2012, 68, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O.; Vreeland, N.S. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J. Bacteriol. 1980, 144, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, W.; Fu, Y. (±)-2-Hexanol from Pterocarpus indicus leaves as attractant for female Aleurodicus dispersus (Hemiptera: Aleyrodidae). Afr. Entomol. 2014, 22, 267–272. [Google Scholar] [CrossRef]
- Kamata, Y.; Shiraga, H.; Tai, A.; Kawamoto, Y.; Gohda, E. Induction of neurite outgrowth in PC12 cells by the medium-chain fatty acid octanoic acid. Neuroscience 2007, 146, 1073–1081. [Google Scholar] [CrossRef]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Shoko, T.; Manhivi, V.E.; Mtlhako, M.; Sivakumar, D. Changes in functional compounds, volatiles, and antioxidant properties of culinary herb coriander leaves (Coriandrum sativum) stored under red and blue LED light for different storage times. Front. Nutr. 2022, 9, 856484. [Google Scholar] [CrossRef]
- Sahin, N.; Kula, I.; Erdogan, Y. Investigation of antimicrobial activities of nonanoic acid derivatives. Fresenius Environ. Bull. 2006, 15, 141–143. [Google Scholar]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 2017, 40, 2042–2067. [Google Scholar] [CrossRef] [PubMed]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Zárate-Reyes, J.A.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatile composition and biological activities of the leaf essential oil from Zanthoxylum limoncello grown in Oaxaca, México. Chem. Biodivers. 2019, 16, e1800498. [Google Scholar] [CrossRef] [PubMed]
- Chanprapai, P.; Kubo, I.; Chavasiri, W. Anti-rice pathogenic microbial activity of Persicaria sp. extracts. Sci. Technol. Asia 2018, 23, 32–41. [Google Scholar]
- Naqvi, S.F.; Khan, I.H.; Javaid, A. Hexane soluble bioactive components of Chenopodium murale stem. Pak. J. Weed Sci. Res. 2020, 26, 425. [Google Scholar] [CrossRef]
- Soosairaj, S.; Dons, T. Bio-active compounds analysis and characterization in ethanolic plant extracts of Justicia tranquebariensis L. (Acanthaceae)-using GC-MS. Int. J. Chem. Tech. Res. 2016, 9, 260–265. [Google Scholar]
- Renugadevi, K.; Nachiyar, V.C.; Zaveri, M. Bioactivity of dodecanoic acid extracted from Geitlerinema sp. TRV57. Indian J. Pharm. Educ. Res. 2021, 55, 224–231. [Google Scholar] [CrossRef]
- Ricciardelli, A.; Casillo, A.; Papa, R.; Monti, D.M.; Imbimbo, P.; Vrenna, G.; Artini, M.; Selan, L.; Corsaro, M.M.; Tutino, M.L.; et al. Pentadecanal inspired molecules as new anti-biofilm agents against Staphylococcus epidermidis. Biofouling 2018, 34, 1110–1120. [Google Scholar] [CrossRef]
- El-Din, S.M.M.; El-Ahwany, A.M. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J. Taibah Univ. Sci. 2016, 10, 471–484. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Hanafy, S.M.; El-Shafea, Y.M.A.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021, 19, 80. [Google Scholar] [CrossRef]
- Beschi, D.A.; Appavoo, M.R.; Wilsy, J.I. GC-MS analysis, collected from kavalkinaru area, tirunelveli district, Tamil nadu, India. Eur. J. Mol. Clin. Med. 2021, 8, 2021. [Google Scholar]
- Koudehi, M.F.; Ardalan, A.A.; Zibaseresht, R. Chemical constituents of an Iranian grown Capsicum annuum and their cytotoxic activities evaluation. Org. Med. Chem. Int. J. 2020, 9, 148–154. [Google Scholar]
- Javed, M.R.; Salman, M.; Tariq, A.; Tawab, A.; Zahoor, M.K.; Naheed, S.; Shahid, M.; Ijaz, A.; Ali, H. The Antibacterial and Larvicidal Potential of Bis-(2-Ethylhexyl) Phthalate from Lactiplantibacillus plantarum. Molecules 2022, 27, 7220. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramirez, S.-G.; Lopez-Saiz, C.-M.; Rosas-Burgos, E.-C.; Cinco-Moroyoqui, F.-J.; Velazquez, C.; Hernandez, J.; Burgos-Hernandez, A. Antimutagenic bis (2-ethylhexyl) phthalate isolated from octopus (Paraoctopus vulgaris). Food Sci. Technol. 2020, 41, 314–320. [Google Scholar] [CrossRef]
- Handford, C.E.; Dean, M.; Henchion, M.; Spence, M.; Elliott, C.T.; Campbell, K. Implications of nanotechnology for the agri-food industry: Opportunities, benefits and risks. Trends Food Sci. Technol. 2014, 40, 226–241. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology-Big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; O’Brien, P. Castor oil: A suitable green source of capping agent for nanoparticle syntheses and facile surface functionalization. R. Soc. Open Sci. 2018, 5, 180824. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Maqbool, Q.; Bibi, T.; Nazar, M.; Hussain, S.Z.; Hussain, T.; Jan, T.; Ahmad, I.; Maaza, M.; Anwaar, S. Optimised synthesis of ZnO-nano-fertiliser through green chemistry: Boosted growth dynamics of economically important L. esculentum. IET Nanobiotechnology 2018, 12, 405–411. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, C.; Ren, Y.; Lu, Y.; Song, Y.; Ji, Y.; Han, C.; He, J. Green synthesis of zinc oxide nanoparticles using Cinnamomum camphora (L.) Presl leaf extracts and its antifungal activity. J. Environ. Chem. Eng. 2021, 9, 106659. [Google Scholar] [CrossRef]
- Alenezi, N.A.; Al-Qurainy, F.; Tarroum, M.; Nadeem, M.; Khan, S.; Salih, A.M.; Shaikhaldein, H.O.; Alfarraj, N.S.; Gaafar, A.-R.Z.; Al-Hashimi, A. Zinc Oxide Nanoparticles (ZnO NPs), Biosynthesis, Characterization and Evaluation of Their Impact to Improve Shoot Growth and to Reduce Salt Toxicity on Salvia officinalis In Vitro Cultivated. Processes 2022, 10, 1273. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Chennimalai, M.; Do, J.Y.; Kang, M.; Senthil, T. A facile green approach of ZnO NRs synthesized via Ricinus communis L. leaf extract for Biological activities. Mater. Sci. Eng. C 2019, 103, 109844. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Al-Mur, B.A. Green Zinc Oxide (ZnO) Nanoparticle Synthesis Using Mangrove Leaf Extract from Avicenna marina: Properties and Application for the Removal of Toxic Metal Ions (Cd2+ and Pb2+). Water 2023, 15, 455. [Google Scholar] [CrossRef]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of aquilegia pubiflora: Their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxidative Med. Cell. Longev. 2021, 2021, 4786227. [Google Scholar] [CrossRef] [PubMed]
- Lingegowda, D.C.; Kumar, J.K.; Prasad, A.D.; Zarei, M.; Gopal, S. FTIR spectroscopic studies on cleome gynandra-Comparative analysis of functional group before and after extraction. Rom. J. Biophys. 2012, 22, 137–143. [Google Scholar]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Kumari, M.; Kandwal, A.; Singh, A.; Bisht, N.S. Biofabrication of zinc oxide nanoparticles from two different zinc sources and their antimicrobial activity. BioNanoScience 2021, 11, 793–809. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef] [PubMed]
- Chabattula, S.; Gupta, P.; Tripathi, S.; Gahtori, R.; Padhi, P.; Mahapatra, S.; Biswal, B.; Singh, S.; Dua, K.; Ruokolainen, J. Anticancer therapeutic efficacy of biogenic Am-ZnO nanoparticles on 2D and 3D tumor models. Mater. Today Chem. 2021, 22, 100618. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Alfarraj, N.S.; Tarroum, M.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Salih, A.M.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alansi, S.; Perveen, K. Biosynthesis of Silver Nanoparticles and Exploring Their Potential of Reducing the Contamination of the In Vitro Culture Media and Inducing the Callus Growth of Rumex nervosus Explants. Molecules 2023, 28, 3666. [Google Scholar] [CrossRef] [PubMed]
- Tymoszuk, A.; Kulus, D. Silver nanoparticles induce genetic, biochemical, and phenotype variation in chrysanthemum. Plant Cell Tissue Organ Cult. PCTOC 2020, 143, 331–344. [Google Scholar] [CrossRef]
- Alharby, H.F.; Metwali, E.M.; Fuller, M.P.; Aldhebiani, A.Y. Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch. Biol. Sci. 2017, 68, 723–735. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of biogenic ZnO nanoparticles on growth, physiological, biochemical traits and antioxidants on olive tree in vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Saeed, F.; Younas, M.; Fazal, H.; Mushtaq, S.; Rahman, F.U.; Shah, M.; Anjum, S.; Ahmad, N.; Ali, M.; Hano, C.; et al. Green and chemically synthesized zinc oxide nanoparticles: Effects on in-vitro seedlings and callus cultures of Silybum marianum and evaluation of their antimicrobial and anticancer potential. Artif. Cells Nanomed. Biotechnol. 2021, 49, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri-Tirani, M.; Dayani, S. In vitro effect of zinc oxide nanoparticles on Nicotiana tabacum callus compared to ZnO micro particles and zinc sulfate (ZnSO4). Plant Cell Tissue Organ Cult. PCTOC 2020, 140, 279–289. [Google Scholar] [CrossRef]
- Park, J.-S.; Seong, Z.-K.; Kim, M.-S.; Ha, J.-H.; Moon, K.-B.; Lee, H.-J.; Lee, H.-K.; Jeon, J.-H.; Park, S.U.; Kim, H.-S. Production of flavonoids in callus cultures of Sophora flavescens Aiton. Plants 2020, 9, 688. [Google Scholar] [CrossRef]
- Shehzad, M.a.; Khan, M.A.; Ali, A.; Mohammad, S.; Noureldeen, A.; Darwish, H.; Ali, A.; Ahmad, A.; Khan, T.; Khan, R.S. Interactive effects of zinc oxide nano particles and different light regimes on growth and silymarin biosynthesis in callus cultures of Silybum marianum L. Artif. Cells Nanomed. Biotechnol. 2021, 49, 523–535. [Google Scholar] [CrossRef]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Wang, H.; Reeves, S.; Brooks, N., Jr.; Saganti, P.; Weerasooriya, A.; Peace, E. Effect of zinc oxide nanoparticles synthesized from Carya illinoinensis leaf extract on growth and antioxidant properties of mustard (Brassica juncea). Front. Plant Sci. 2023, 14, 1108186. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, T.; Khan, M.A.; Nadhman, A.; Aasim, M.; Khan, N.Z.; Ali, W.; Nazir, N.; Zahoor, M. Iron-doped zinc oxide nanoparticles-triggered elicitation of important phenolic compounds in cell cultures of Fagonia indica. Plant Cell Tissue Organ Cult. PCTOC 2021, 147, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]

| NO | RT (min) | % | Compounds |
|---|---|---|---|
| 1 | 5.267 | 5.25 | Silanediol, dimethyl- |
| 2 | 6.989 | 1.34 | 2-Furanmethanol |
| 3 | 8.156 | 1.57 | 1,2-Cyclopentanedione |
| 4 | 8.34 | 2.37 | Undecane |
| 5 | 8.391 | 2.46 | Dodecanoic acid, 1,1-dimethylpropyl ester |
| 6 | 9.118 | 2.36 | Cyclopentasiloxane, decamethyl- |
| 7 | 9.192 | 1.08 | 2(5H)-Furanone |
| 8 | 9.936 | 2.70 | Benzeneacetaldehyde |
| 9 | 11.601 | 2.96 | 2-Propen-1-ol |
| 10 | 11.984 | 0.93 | p-Cresol |
| 11 | 12.442 | 2.69 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 12 | 14.971 | 5.69 | 2-Methoxy-4-vinylphenol |
| 13 | 15.601 | 1.96 | 5-Hydroxymethylfurfural |
| 14 | 19.446 | 1.27 | p-Hydroxyphenylacetone |
| 15 | 28.738 | 65.36 | Ricinine |
| Compounds | Control | Zn (10 mg/L) | Zn (20 mg/L) | Zn (30 mg/L) | Zn (40 mg/L) | Zn (50 mg/L) | Biological Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | % | RT | % | RT | % | RT | % | RT | % | RT | % | ||
| Tetrahydrofurfuryl chloride | 3.38 | 2.10 | - | - | - | - | - | - | - | - | - | - | Not reported |
| 3-Hexanone | 3.522 | 2.22 | 3.493 | 5.65 | 3.447 | 7.46 | 3.294 | 5.58 | 3.417 | 11.84 | 3.393 | 12.20 | Antifungal activity [35] |
| 2-Hexanone | 3.653 | 3.99 | 3.622 | 7.59 | 3.571 | 8.22 | 3.395 | 7.11 | 3.535 | 13.64 | 3.506 | 14.48 | Insecticide [36] |
| 3-Hexanol | 3.789 | 1.73 | 3.743 | 3.62 | 3.681 | 5.28 | 3.494 | 5.30 | 3.640 | 7.26 | 3.610 | 9.00 | Antibacterial [37] |
| 2-Hexanol | 3.929 | 2.82 | 3.880 | 4.50 | 3.811 | 6.79 | 3.601 | 7.04 | 3.765 | 10.54 | 3.730 | 10.18 | Biological control of pests [38] |
| Heptanoic acid | - | - | 9.468 | 13.37 | 9.383 | 9.00 | - | - | 9.312 | 9.17 | 9.261 | 7.75 | Marker of neuronal differentiation [39] |
| Hexanoic acid | - | - | - | - | - | - | 7.415 | 0.83 | - | - | - | - | Antifungal activity [40] |
| Octanoic acid | 11.054 | 6.09 | 10.988 | 7.90 | 10.908 | 6.80 | 10.679 | 3.86 | 10.848 | 6.65 | 10.805 | 4.95 | Antimicrobial activity [41] |
| 2-Decenal, (E)- | 12.107 | 6.05 | - | - | - | - | - | - | - | - | - | - | Nematicidal activity [42] |
| 2-Tridecenal, (E)- | - | - | 12.073 | 4.16 | 13.540 | 1.93 | - | - | - | - | - | - | Antioxidant activity [43] |
| Nonanoic acid | 12.469 | 4.15 | 12.406 | 4.65 | 12.339 | 2.47 | 12.149 | 2.12 | 12.288 | 2.44 | 12.248 | 2.32 | Antimicrobial activities [44] |
| Undecanal | 12.713 | 3.69 | - | - | - | - | - | - | - | - | - | - | Antifungal activity [45] |
| 2-Undecenal | 13.607 | 6.10 | 13.571 | 4.94 | - | - | - | - | - | - | - | - | Mosquito-repellent activity [46] |
| Dodecanal | 14.170 | 4.28 | - | - | - | - | - | - | - | - | - | - | Antimicrobial activity [47] |
| 2-Dodecenal | 15.010 | 0.95 | - | - | - | - | - | - | - | - | - | - | Nematicidal activity [42] |
| Tridecanal | 15.530 | 2.95 | - | - | - | - | - | - | - | - | - | - | Antimicrobial [48] |
| Tetradecanal | 16.814 | 3.47 | - | - | - | - | - | - | - | - | - | - | Antibacterial activity [49] |
| Dodecanoic acid | - | - | - | - | 16.264 | 2.32 | 16.119 | 1.38 | 16.217 | 2.09 | 16.194 | 1.94 | Antibacterial, antioxidant, and anti-apoptotic [50] |
| Pentadecanal- | 18.031 20.283 | 4.27 1.06 | 17.995 | 2.14 | - | - | - | - | - | - | - | - | Antibacterial activity [51] |
| Tetradecanoic acid | - | - | - | - | - | - | 18.478 | 1.21 | - | - | - | - | Antibacterial activity [52] |
| Hexadecanoic acid, methyl ester | 20.351 | 5.66 | 20.314 | 8.77 | 20.288 | 5.99 | 20.216 | 3.26 | 20.264 | 4.89 | 20.254 | 4.88 | Antibacterial activities [53] |
| n-Hexadecanoic acid | - | - | - | - | - | - | 20.637 | 2.91 | 20.724 | 1.96 | 20.703 | 2.03 | Antioxidant and antibacterial activities [54] |
| Octadecanoic acid, 2-propenyl ester | 21.868 | 10.04 | 21.831 | 13.31 | 21.806 23.681 | 8.64 4.34 | 21.736 | 11.84 | 21.782 | 6.88 | 21.773 23.650 | 8.27 4.01 | Antibacterial activity [55] |
| 9-Octadecanoic acid, methyl ester | - | - | - | - | - | - | 22.003 | 2.81 | - | - | - | - | Antioxidant and anticancer [56] |
| 2(3H)-Furanone, dihydro-5-tetradecyl- | 22.325 | 9.16 | - | - | - | - | - | - | - | - | - | - | Not reported |
| Octadecanoic acid, ethenyl ester | 23.331 | 9.83 | 23.293 | 8.13 | 23.681 | 4.34 | 23.611 | 6.06 | - | - | - | - | Antimicrobial [57] |
| Bis(2-ethylhexyl) phthalate | 26.194 | 9.40 | 26.153 | 11.27 | 26.126 | 27.33 | 26.041 | 38.68 | 26.098 | 22.66 | 26.087 | 17.98 | Antibacterial and antimutagenic activities [58,59] |
| Total compounds | 20 | 14 | 13 | 15 | 12 | 12 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarroum, M.; Alfarraj, N.S.; Al-Qurainy, F.; Al-Hashimi, A.; Khan, S.; Nadeem, M.; Salih, A.M.; Shaikhaldein, H.O. Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant. Metabolites 2023, 13, 905. https://doi.org/10.3390/metabo13080905
Tarroum M, Alfarraj NS, Al-Qurainy F, Al-Hashimi A, Khan S, Nadeem M, Salih AM, Shaikhaldein HO. Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant. Metabolites. 2023; 13(8):905. https://doi.org/10.3390/metabo13080905
Chicago/Turabian StyleTarroum, Mohamed, Norah S. Alfarraj, Fahad Al-Qurainy, Abdulrahman Al-Hashimi, Salim Khan, Mohammad Nadeem, Abdalrhaman M. Salih, and Hassan O. Shaikhaldein. 2023. "Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant" Metabolites 13, no. 8: 905. https://doi.org/10.3390/metabo13080905
APA StyleTarroum, M., Alfarraj, N. S., Al-Qurainy, F., Al-Hashimi, A., Khan, S., Nadeem, M., Salih, A. M., & Shaikhaldein, H. O. (2023). Improving the Production of Secondary Metabolites via the Application of Biogenic Zinc Oxide Nanoparticles in the Calli of Delonix elata: A Potential Medicinal Plant. Metabolites, 13(8), 905. https://doi.org/10.3390/metabo13080905










