Anti-Inflammatory Potential of Pteropodine in Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
2.2. Description of the Murine Model
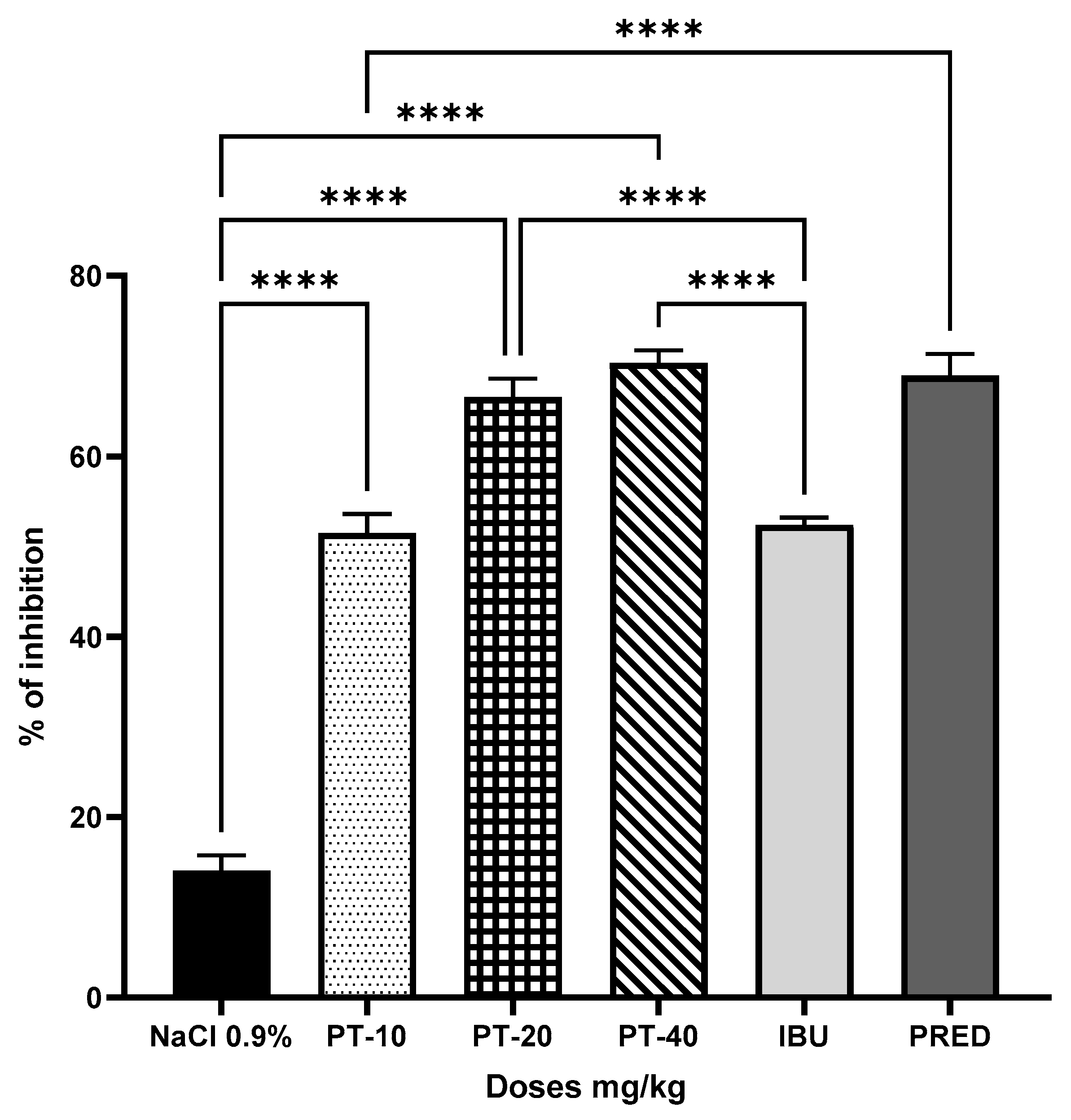
2.3. Induction of Rat Paw Edema
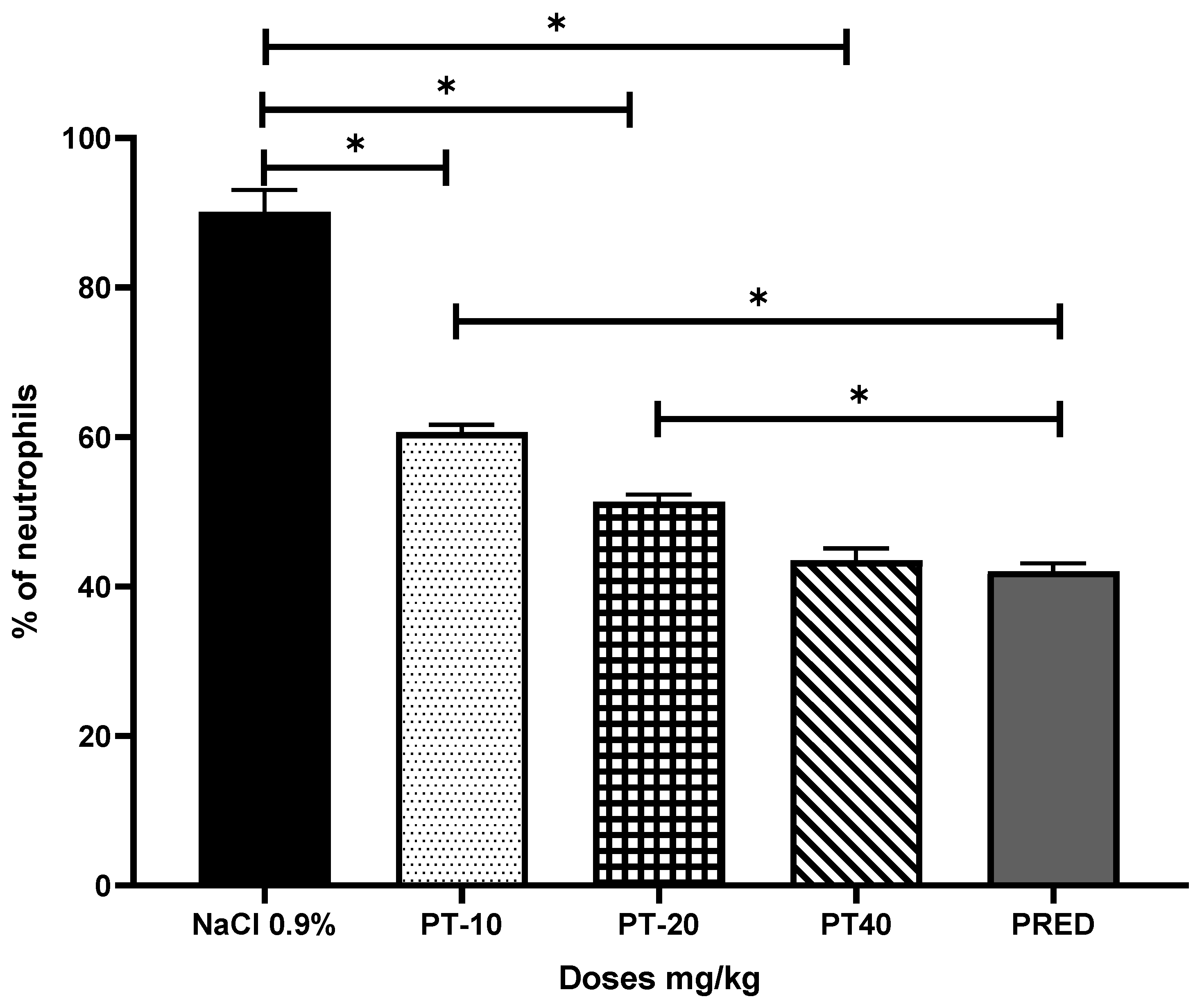
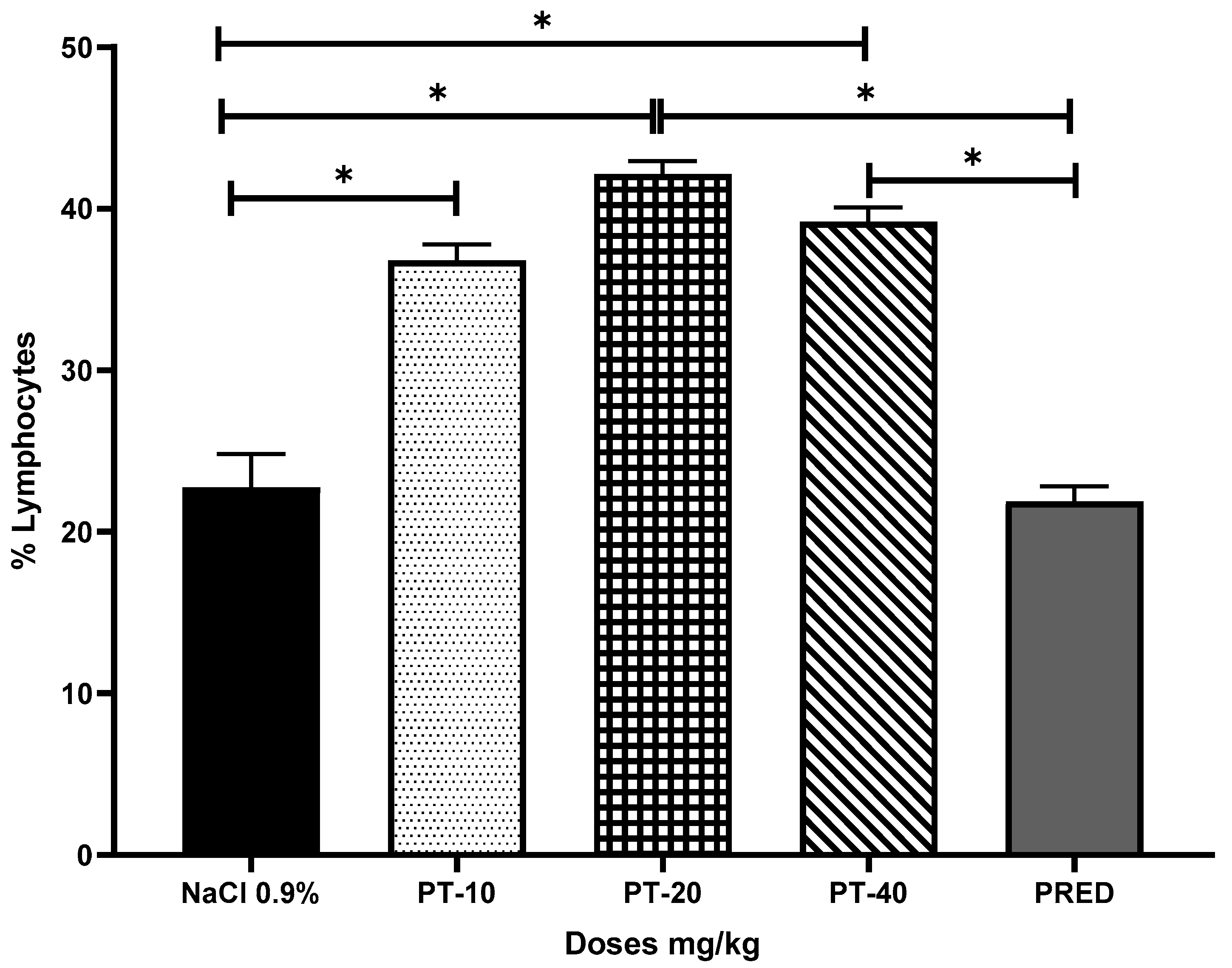
2.4. Pleurisy Assay
2.5. Mouse Ear Edema Model
2.6. Myeloperoxidase Inhibition
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Oh, H.C.; Park, J.W.; Kim, I.S.; Kim, J.Y.; Kim, K.C.; Chae, D.S.; Jo, W.L.; Song, J.H. Diagnosis and Treatment of Inflammatory Joint Disease. Hip Pelvis 2017, 29, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, D. Inflammatory arthritides of the spine: Surgical versus nonsurgical treatment. Clin. Orthop. Relat. Res. 2006, 443, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Garbedian, S.; Wai, E.K. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin. Orthop. Relat. Res. 2008, 67, 623–637. [Google Scholar] [CrossRef]
- Murray, C.; Marshall, M.; Rathod, T.; Bowen, C.J.; Menz, H.B.; Roddy, E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS ONE 2018, 13, e0193662. [Google Scholar] [CrossRef]
- Von Koskull, S.; Truckenbrodt, H.; Holle, R.; Hormann, A. Incidence and prevalence of juvenile arthritis in an urban population of southern Germany: A prospective study. Ann. Rheum. Dis. 2001, 60, 940–945. [Google Scholar] [CrossRef]
- Kaipiainen-Seppanen, O.; Kautiainen, H. Declining trend in the incidence of rheumatoid factor-positive rheumatoid arthritis in Finland 1980–2000. J. Rheumatol. 2006, 33, 2132–2138. [Google Scholar] [PubMed]
- Hukuda, S.; Minami, M.; Saito, T.; Mitsui, H.; Matsui, N.; Komatsubara, Y.; Makino, H.; Shibata, T.; Shingu, M.; Sakou, T.; et al. Spondyloarthropathies in Japan: Nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J. Rheumatol. 2001, 28, 554–559. [Google Scholar]
- Peláez-Ballestas, I.; Sanin, L.H.; Moreno-Montoya, J.; Alvarez-Nemegyei, J.; Burgos-Vargas, R.; Garza-Elizondo, M.; Rodríguez-Amado, J.; Goycochea-Robles, M.V.; Madariaga, M.; Zamudio, J.; et al. Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J. Rheumatol. Suppl. 2011, 86, 3–8. [Google Scholar] [CrossRef]
- Tobon, G.J.; Youinou, P.; Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 2010, 35, 10–14. [Google Scholar] [CrossRef]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014, 506, 376–381. [Google Scholar] [CrossRef]
- Raychaudhuri, S. Recent advances in the genetics of rheumatoid arthritis. Curr. Opin. Rheumatol. 2010, 22, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Bennett, J.S.; Daugherty, A.; Furberg, C.; Roberts, H.; Taubert, K.A. Use of nonsteroidal Antiinflammatory drugs an update for clinicians—A scientific statement from the American Heart Association. Circulation 2007, 115, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Boers, M.; Verhoeven, A.C.; Markusse, H.M.; van de Laar, M.A.; Westhovens, R.; van Denderen, J.C.; van Zeben, D.; Dijkmans, B.A.; Peeters, A.J.; Jacobs, P.; et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997, 350, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Burmester, G.R.; Straub, R.H.; Seibel, M.J.; Zhou, H. Exogenous and endogenous glucocorticoids in rheumatic diseases. Arthritis Rheum. 2011, 63, 1–9. [Google Scholar] [CrossRef]
- Ethgen, O.; Esteves, F.D.; Bruyere, O.; Reginster, J.Y. What do we know about the safety of corticosteroids in rheumatoid arthritis? Curr. Med. Res. Opin. 2013, 29, 1147–1160. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Ahmed Mohamed, A.A.; Emery, P. Glucocorticoids and rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2016, 42, 33–46. [Google Scholar] [CrossRef]
- Glyn, J. The discovery and early use of cortisone. J. R. Soc. Med. 1998, 91, 513–517. [Google Scholar] [CrossRef]
- Gemmell, D.K.; Cottney, J.; Lewis, A.J. Comparative effects of drugs on four paw oedema models in the rat. Agents Actions 1979, 9, 107–116. [Google Scholar] [CrossRef]
- Kma, L. Plant extracts and plant-derived compounds: Promising players in countermeasure strategy against radiological exposure: A review. Asian Pac. J. Cancer Prev. 2014, 15, 2405–2425. [Google Scholar]
- Bors, M.; Michałowicz, J.; Pilarski, R.; Sicińska, P.; Gulewicz, K.; Bukowska, B. Studies of biological properties of Uncaria tomentosa extracts on human blood mononuclear cells. J. Ethnopharmacol. 2012, 142, 669–678. [Google Scholar]
- Allen-Hall, L.; Cano, P.; Arnason, J.; Rojas, R.; Lock, O.; Lafrenie, R. Treatment of THP-1 cells with Uncaria tomentosa extracts differentially regulates the expression if IL-1 and TNF. J. Ethnopharmacol. 2007, 109, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.; Sartori, A.; Valente, L.; Golim, M.; Siani, A.; Viero, R. Uncaria tomentosa Aqueous-ethanol Extract Triggers an Immunomodulation toward a Th2 Cytokine Profile. Phytother. Res. 2011, 25, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Dinis, T.; Batista, M. Antioxidant properties of proanthocyanidins of bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [PubMed]
- Pilarski, R.; Filip, B.; Wietrzyk, J.; Kura´s, M.; Gulewicz, K. Anticancer activity of the Uncaria tomentosa (Willd.) DC. preparations with different oxindole alkaloid composition. Phytomedicine 2010, 17, 1133–1139. [Google Scholar]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Molina-Jasso, D.; Gallaga, J.P.; Silva-Miranda, A.; Velazco, O.; Hernández, N.; Chamorro, G. Genotoxic and cytotoxic studies of beta-sitosterol and pteropodine in mouse. J. Biomed. Biotechnol. 2005, 3, 242–247. [Google Scholar] [CrossRef]
- Paniagua-Perez, R.; Madrigal-Bujaidar, E.; Molina-Jasso, D.; Reyes-Cadena, S.; Alvarez-Gonzalez, I.; Sanchez-Chapul, L.; Perez-Gallaga, J. Antigenotoxic, antioxidant and lymphocyte induction effects produced by pteropodine. Basic Clin. Pharmacol. 2009, 104, 222–227. [Google Scholar] [CrossRef]
- Pflum, L.R.; Graeme, M.L. The arthus reaction in rats, a possible test for anti-inflammatory and antirheumatic drugs. Agents Actions 1979, 9, 184–189. [Google Scholar] [CrossRef]
- Cong, H.H.; Khaziakhmetova, V.N.; Zigashina, L.E. Rat paw oedema modeling and NSAIDs: Timing of effects. Int. J. Risk Saf. Med. 2015, 27 (Suppl. S1), S76–S77. [Google Scholar]
- Paniagua-Pérez, R.; Flores-Mondragón, G.; Reyes-Legorreta, C.; Herrera-López, B.; Cervantes-Hernández, I.; Madrigal-Santillán, O.; Morales-González, J.A.; Álvarez-González, I.; Madrigal-Bujaidar, E. Evaluation of the anti-inflammatory capacity of beta-sitosterol in rodent assays. Afr. J. Tradit Complement Altern. Med. 2017, 14, 123–130. [Google Scholar] [CrossRef]
- Young, J.M.; Spires, D.A.; Bedord, C.J.; Wagner, B.; Ballaron, S.J.; De Young, L.M. The mouse ear inflammatory response to topical arachidonic acid. J. Investig. Dermatol. 1984, 82, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.L.; Rojas, P.; Marcelo, A.; Plaza, A.; Bauer, R.; Reininger, E.; Klaas, C.A.; Merfort, I. Antiinflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae). J. Ethnopharmacol. 2002, 81, 271–276. [Google Scholar] [PubMed]
- Akesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int. Immunopharmacol. 2003, 3, 1889–1900. [Google Scholar]
- Eberlin, S.; dos Santos, L.M.; Queiroz, M.L. Uncaria tomentosa extract increases the number of myeloid progenitor cells in the bone marrow of mice infected with Listeria monocytogenes. Int. Immunopharmacol. 2005, 5, 1235–1246. [Google Scholar]
- Passos, G.F.; Medeiros, R.; Marcon, R.; Nascimento, A.F.; Calixto, J.B.; Pianowski, L.F. The role of PKC/ERK1/2 signaling in the anti-inflammatory effect of tetracyclic triterpene euphol on TPA-induced skin inflammation in mice. Eur. J. Pharmacol. 2013, 698, 413–420. [Google Scholar] [CrossRef]
- Sandoval-Chacón, M.; Thompson, J.H.; Zhang, X.J.; Liu, X.; Mannick, E.E.; Sadowska-Krowicka, H.; Charbonnet, R.M.; Clark, D.A.; Miller, M.J. Antiinflammatory actions of cat’s claw: The role of NF-ΚB. Aliment. Pharmacol. Ther. 1998, 12, 1279–1289. [Google Scholar]
- Wirth, C.; Wagner, H. Pharmacologically active procyanidines from the bark of Uncaria tomentosa. Phytomedicine 1997, 4, 265–266. [Google Scholar]
- Aquino, R.; de Feo, V.; de Simone, F.; Pizza, C.; Cirino, G. Plant metabolites. New compounds and anti-inflammatory activity of Uncaria tomentosa. J. Nat. Prod. 1991, 54, 453–459. [Google Scholar]
- Sandoval, M.; Ronzio, R.A.; Muanza, D.N.; Clark, D.A.; Miller, M.J. Peroxynitrite-induced apoptosis in epithelial (T84) and macrophage (RAW 264.7) cell lines: Effect of legume-derived polyphenols (phytolens). Nitric Oxide 1997, 1, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Squadrito, F.; Bitto, A.; Polito, F.; Burnett, B.; Di Stefano, V.; Minutoli, L. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br. J. Pharmacol. 2009, 157, 1410–1418. [Google Scholar] [CrossRef] [PubMed]

| Group | Dose (mg/ear) | Edema (mg) | Inhibition % |
|---|---|---|---|
| Control TPA | 0.0025 | 23.7 ± 0.3 | 0 |
| Indomethacin | 0.5 | 6.5 ± 0.12 | 72.6 * |
| PT-D1 | 0.010 | 5.7 ± 0.69 | 75.3 * |
| PT-D2 | 0.020 | 4.4 ± 1.5 | 74.2 * |
| PT-D3 | 0.040 | 3.1 ± 0.93 | 81.4 * |
| Group | Dose (mg/ear) | Inhibition % |
|---|---|---|
| Control TPA | 0.0025 | 0 |
| IND | 0.50 | 94.66 * |
| PT-D1 | 0.5 | 47.59 * |
| PT-D2 | 1.0 | 72.24 * |
| PT-D3 | 1.5 | 97.19 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paniagua-Pérez, R.; Sánchez-Chapul, L.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Cruz-Hernández, L.; Martínez-Canseco, C.; Reyes-Legorreta, C.; Ruiz-Rosano, L.; Hernández-Flores, C.; et al. Anti-Inflammatory Potential of Pteropodine in Rodents. Metabolites 2023, 13, 907. https://doi.org/10.3390/metabo13080907
Paniagua-Pérez R, Sánchez-Chapul L, Madrigal-Bujaidar E, Álvarez-González I, Madrigal-Santillán E, Cruz-Hernández L, Martínez-Canseco C, Reyes-Legorreta C, Ruiz-Rosano L, Hernández-Flores C, et al. Anti-Inflammatory Potential of Pteropodine in Rodents. Metabolites. 2023; 13(8):907. https://doi.org/10.3390/metabo13080907
Chicago/Turabian StylePaniagua-Pérez, Rogelio, Laura Sánchez-Chapul, Eduardo Madrigal-Bujaidar, Isela Álvarez-González, Eduardo Madrigal-Santillán, Lidia Cruz-Hernández, Carlos Martínez-Canseco, Celia Reyes-Legorreta, Lidia Ruiz-Rosano, Cecilia Hernández-Flores, and et al. 2023. "Anti-Inflammatory Potential of Pteropodine in Rodents" Metabolites 13, no. 8: 907. https://doi.org/10.3390/metabo13080907
APA StylePaniagua-Pérez, R., Sánchez-Chapul, L., Madrigal-Bujaidar, E., Álvarez-González, I., Madrigal-Santillán, E., Cruz-Hernández, L., Martínez-Canseco, C., Reyes-Legorreta, C., Ruiz-Rosano, L., Hernández-Flores, C., Valdez-Mijares, R., & Quintana-Armenta, A. (2023). Anti-Inflammatory Potential of Pteropodine in Rodents. Metabolites, 13(8), 907. https://doi.org/10.3390/metabo13080907








