Abstract
Red blood cells (RBC) are the most abundant cell in the human body, with a central role in oxygen transport and its delivery to tissues. However, omics technologies recently revealed the unanticipated complexity of the RBC proteome and metabolome, paving the way for a reinterpretation of the mechanisms by which RBC metabolism regulates systems biology beyond oxygen transport. The new data and analytical tools also informed the dissection of the changes that RBCs undergo during refrigerated storage under blood bank conditions, a logistic necessity that makes >100 million units available for life-saving transfusions every year worldwide. In this narrative review, we summarize the last decade of advances in the field of RBC metabolism in vivo and in the blood bank in vitro, a narrative largely influenced by the authors’ own journeys in this field. We hope that this review will stimulate further research in this interesting and medically important area or, at least, serve as a testament to our fascination with this simple, yet complex, cell.
Keywords:
red blood cell; erythrocyte; transfusion medicine; storage lesion; hemolysis; spleen; mitochondria; iron; hematology 1. Red Blood Cell Metabolism: The Central Role of Oxygen
By recent estimates, the adult human body contains ~30 trillion cells [1,2]. Approximately 90% of these cells are derived from the hematopoietic lineage, of which the overwhelming majority are red blood cells (RBCs) [3]. The 25 trillion circulating RBCs in an adult account for ~83% of all host cells, making RBCs a type of circulating organ critical for human health [4]. The stability of the number of circulating RBCs is ensured by a delicate equilibrium between de novo erythropoiesis and erythrophagocytosis by splenic and hepatic macrophages, which recycle RBC contents, especially iron, proteins and lipids [5].
The evolution of human RBCs has maximized their capacity to transport and deliver oxygen to tissues via progressive loss of nuclei and organelles during the maturation of erythroid precursors to reticulocytes and, ultimately, mature discocytic RBCs [6]. As a result of this process, each mature RBC contains ~250–270 million copies of hemoglobin [7], with hemoglobin accounting for ~98% of the cytosolic proteome and 92% of the total proteome [8]. At full oxygen saturation, RBCs can theoretically carry up to 1 billion molecules of oxygen/cell, a function that is facilitated by the presence of all mature RBCs combined with ~2.6 g of iron (66% of the total body iron) [4]. Fenton and Haber–Weiss chemistry constantly generate the formation of hydrogen peroxide and reactive oxygen species [9,10], making the RBC’s 120 days circulatory lifespan a struggle against the oxidation [11] of proteins (especially redox-sensitive functional residues in hemoglobin such as C93 and H92 of the beta chain [12]), small molecule metabolites [13] and lipids [14]. Every day, 0.2 trillion RBCs are removed from the bloodstream and replaced by de novo erythropoiesis (2 million RBCs are produced per second [15]), accounting for ~40% of the total body mass turnover despite the small mass of each RBC (in the range of 100–300 pg) [3].
The relative simplicity of RBCs, as historically perceived, has attracted multiple efforts to leverage them as a model of simplified human cell metabolism. Indeed, RBCs exclusively rely on glycolysis (Embden–Meyerhof–Parnas pathway) to generate high-energy phosphate compounds, such as adenosine triphosphate (ATP)—whose main source in other cells is oxidative phosphorylation in mitochondria. ATP and its guanosine triphosphate equivalent GTP substantially fuel all key processes in mature RBCs, including: hemoglobin allostery [16,17] to metabolism [18], from proton pumps [19] to membrane integrity by phosphorylating structural proteins [20], from protein stabilization via fueling of transglutaminase 2 [21] to cellular mechanics [22], from cytoskeletal actin polymerization [23] to vesiculation [24], from membrane lipid symmetry by fueling phosphatidylserine flippases [25] to proteasomal activity to remove damaged proteins [26,27,28]. Ultimately, the energy-depleted erythrocyte is rapidly lost from the bloodstream [29] via intra- or, more commonly, extravascular hemolysis through splenic sequestration and erythrophagocytosis.
Two critical functional RBC pathways branch from glycolysis: the Rapoport–Luebering shunt, which generates 2,3-diphosphoglycerate (DPG), and the pentose phosphate pathway (hexose monophosphate shunt), which generates ribose phosphate and, importantly, reduced nicotinamide adenine dinucleotide phosphate (NADPH). DPG is a critical allosteric modulator of hemoglobin, promoting oxygen release from hemoglobin to counteract hypoxia (e.g., at high-altitude [30] or from hemorrhage [31]). NADPH fuels multiple antioxidant processes in RBCs [32]: (i) it is essential for the reduction in oxidized glutathione by glutathione reductases; (ii) it directly or indirectly fuels glutathione peroxidase 4 [33], catalase, peroxiredoxins [34], glutaredoxins, the thioredoxin reductase system, biliverdin reductase B [35], the ascorbate-tocopherol axis [36] and other diaphorases such as NADPH-dependent quinone oxidoreductases (NQO1) [37]. NAD(P)H-dependent methemoglobin reductases are also essential for converting (auto-oxidized) ferric hemoglobin iron back to its ferrous state. The C93 residue of hemoglobin beta also participates in antioxidant systems by buffering free glutathione [38], participating in recycling oxidized peroxiredoxin 2 [39], and contributing to nitrite reduction [40] (resulting in the pathological generation of methemoglobin in the setting of nitrite poisoning [41]). Owing to its role in redox chemistry, it has also been proposed that hemoglobin may serve as a murzyme (i.e., a redox enzyme working along the principles of the Murburn concept), thereby contributing to ATP synthesis [42].
The rate-limiting enzyme of the pentose phosphate pathway is glucose 6-phosphate dehydrogenase (G6PD). G6PD is encoded by a gene on chromosome X. Mutations of this gene are found in >500 million people around the world, with >200 G6PD mutations known in humans [43]. Individuals carrying such mutations typically present with a significant loss of enzymatic activity, ranging from <1% in the most severe forms (e.g., the Mediterranean variant-S188F) [44,45] to <10% in the common African variant (V68M); the latter is extremely common in some metropolitan areas such as New York, especially in African American communities (~13% prevalence) [46]. In China, the six most common mutations account for ~90% of G6PD-deficient alleles, with an overall national prevalence of ~2.10% [47]. Human and mouse RBCs carrying these mutations are extremely susceptible to hemolysis following oxidant insults [48,49]. As in the case of hemoglobinopathies, such as sickle cell trait [50] and beta-thalassemia [51], positive selection for these mutations in human populations is thought to be associated with the selective pressure by malaria infections in the Mediterranean and South East Asia areas, with considerable overlap between the incidence of G6PD deficiency and malaria-endemic regions [52]. Protection against mild malaria infection is also observed in heterozygous G6PD deficient females [52]. G6PD deficiency, sickle cell trait (or disease) and beta-thalassemia (minor or major) are all associated with RBC metabolic reprogramming consistent with increased susceptibility to oxidant stress-induced hemolysis (e.g., by quinone antimalarials and sulfa drugs in the setting of G6PD deficiency).
The regulation of glycolysis by oxidant stress to functional thiols in rate-limiting enzymes, including glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at cysteine residues 152 and 156 [53] and pyruvate kinase [54,55], provides a strategy to constrain metabolic fluxes through late glycolysis when oxidant stress is high while redirecting glucose oxidation to the pentose phosphate pathway to produce reducing equivalents that counteract this stress. Similarly, the most abundant RBC membrane protein, band 3 (or, equivalently, anion exchanger 1—AE1) has a very acidic N-terminus cytosolic domain that can serve as an inhibitory docking site for glycolytic enzymes at high oxygen saturation; in contrast, at low oxygen saturation deoxyhemoglobin outcompetes the glycolytic enzymes, displacing them from the membrane and boosting glycolysis to stimulate ATP and DPG production in the face of hypoxia [56,57]. Prolonged, unmitigated oxidant stress, such as during refrigerated RBC storage under blood bank conditions, promotes proteolytic or reactive oxygen species (ROS)-triggered proteolysis of the N-terminus of band 3, ultimately causing the loss of this RBC oxygen-dependent metabolic modulation pathway [58,59,60]. In addition, genetic mutations of the N-terminus region of band 3 are associated with severe hemolysis and necessitate lifelong transfusion [59].
2. RBC Metabolism beyond Glycolysis
Over the last 50 years, almost all computational efforts to simulate the RBC metabolome ex vivo were limited to the pathways described above [61,62,63]. The recent implementation of omics technologies to study RBCs has revealed an unanticipated complexity of the erythrocyte proteome, now counting ~2500–3000 unique proteins; indeed, functional metabolic tracing experiments suggest that even this list may be incomplete [8,59,64,65,66,67,68,69]. Leveraging these recent datasets, systems biology experts are redrawing connectivity maps of the human RBC metabolome (Figure 1), of relevance for basic science and translational applications [70,71]. With >77 active transporters, circulating RBCs can take up and release many metabolites from peripheral tissues, making RBCs a unique window into system health [72]. From creatinine and carnitine (as markers of renal function [73]) to conjugated bile acids (from the gut microbiome [74]), from transamination products (e.g., alanine, glutamate, aspartate) to neurotransmitters (e.g., serotonin, dopamine, acetylcholine), RBCs can directly and indirectly participate in systems metabolism throughout the body.
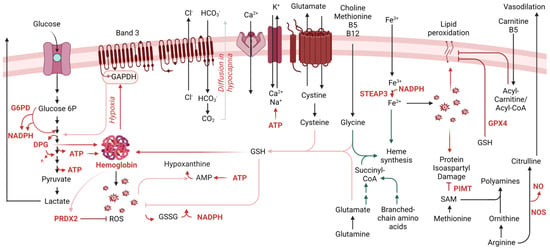
Figure 1.
Overview of the main metabolic pathways relevant to RBC physiology during aging in vivo and in vitro.
During erythropoiesis, amino acid metabolism (e.g., glycine, glutamine, branched-chain amino acids) is essential to heme synthesis as both direct and indirect substrates (e.g., succinyl-CoA from glutaminolysis and branched-chain amino acid catabolism). This process concomitantly releases ammonium, making erythropoiesis and de novo glutamine synthesis two of the leading pathways contributing to ammonium homeostasis in humans [75]. Genetic defects of rate-limiting genes in the branched-chain amino acid catabolism (e.g., in propionic [76] or methylmalonic acidemias) are associated with defects in erythropoiesis [77]. Along with cysteine, both glycine and glutamine-derived glutamate contribute to the synthesis of the glutathione tripeptide. The exchange of cystine—a cysteine disulfide—to glutamate is a critical regulator of a process previously referred to as eryptosis [78] and, more recently, recognized as ferroptosis [79] owing to its expanded relevance in other cells besides RBCs.
When considering erythropoiesis, one-carbon metabolism is one of the first pathways that is referenced. Folates and methyl group donors such as methionine, choline and betaine—along with B6, B12 and B5 cofactors [80,81]—are essential for de novo purine nucleotide synthesis to support proliferation, as well as for generating glycine to contribute to heme synthesis described above. However, methyl-group donors also participate in RBC redox homeostasis and its dysregulation, such as in the context of folate dietary deficiency or excess [81]. This is also relevant in diseases associated with alterations of this pathway such as homocystinuria [82] or Down syndrome [83], owing to gene dosage of cystathionine beta synthase resulting in a “folate trap”-like phenotype [84]. These conditions present with macrocytic or megaloblastic anemia [82]. In addition, in keeping with the central role of oxidant stress in the economy of RBC metabolism, methionine uptake and consumption in RBCs fuel a pathway of isoaspartyl protein damage repair. As a hallmark of RBC aging in vivo [85], deamidation of asparagine residues in key structural membrane proteins and glycolytic enzymes [86,87,88,89,90,91,92] alters the protein backbone, with important structural/functional implications. Since RBCs cannot replace damaged components by de novo protein synthesis, repairing this damage is essential to their survival. The methylation of deamidated residues favors the formation of a succinimide intermediate, ultimately promoting the rescue of the protein backbone structure in 15–30% of the cases, a reaction catalyzed by the enzyme “protein isoaspartyl o-methyltransferase” (PIMT). Increased uptake of methyl donors such as methionine is observed in RBCs in response to oxidative challenges (e.g., incubation with hydrogen peroxide [93], refrigerated storage under blood bank conditions [92]). Thus, one can speculate that RBC uptake and consumption of methyl-group donors (e.g., methionine, choline) could compete with other tissues where these substrates are used to fuel epigenetic regulatory mechanisms, such as methylation of proteins (e.g., histones), RNA (N6-methyladenosine) and DNA (CpG islands), making RBCs an indirect player in systemic long-term responses to oxidant stress events.
In response to oxidant stress, ATP synthesis is reduced because of the redox sensitivity of glycolytic enzymes and the band 3-dependent mechanism, as described above. In this case, ATP breaks down into lower energy phosphate compounds, ADP and AMP, the latter being prone to deamination to IMP by RBC-specific AMP deaminase 3 [94]. Phosphoribolysis of IMP releases hypoxanthine, a substrate for xanthine oxidase to generate xanthine and urate, with concomitant production of hydrogen peroxide [94]. The presence of an active xanthine oxidase in mature RBCs was reported and challenged [95]. Exogenous urate is a potent antioxidant in mature RBCs [96]. Similarly, the breakdown of AMP into adenosine, with adenosine oxidation by adenosine deaminase was reported as contributing to hypoxanthine accumulation [97]. The recycling of hypoxanthine by X-linked hypoxanthine guanosine phosphoribosyltransferase (HPRT) preserves IMP/GMP homeostasis in RBCs. Lesch Nyhan syndrome patients exhibit genetic mutations of this enzyme and present with a range of clinical manifestations, including macrocytic anemia [98]. Purine homeostasis is relevant to RBC homeostasis for additional reasons beyond those described above. For example, ATP release by RBCs has regulatory effects on endothelial cells and on the RBCs themselves, as a type of autocrine signaling. The breakdown of extracellular ATP to ADP, AMP and adenosine by ectonucleotidases (e.g., CD38) generates agonists of P2Y receptors on endothelial cells, but adenosine can also be imported by equilibrative nucleotide transporters (ENT1) or stimulate adenosine receptors (e.g., ADORA2b). Both of these pathways contribute to RBC responses to hypoxia, the former, for example, by limiting circulating adenosine, a process that is counteracted by irreversible ENT1 degradation to facilitate acclimatization to high altitude hypoxia upon reascent [99]. In addition, ADORA2b transduces an intracellular signaling pathway that activates downstream protein kinase A and AMP-dependent kinase (AMPK), both contributing to metabolic reprogramming; for example, via phosphorylation-mediated activation of bisphosphoglycerate kinase [97,100].
Recent applications of unsupervised metabolomics and lipidomics approaches identified a sphingolipid sphingosine 1-phosphate (S1P) as a relevant metabolite regulating RBC responses to hypoxia [101]. For example, in response to high altitude-induced hypoxia [101] S1P can bind to deoxyhemoglobin following its stabilization by DPG, further promoting oxygen off-loading [102]. By further stabilizing deoxyhemoglobin, S1P also contributes to energy metabolism by promoting the release of glycolytic enzymes from band 3 into the cytosol, with subsequent activation of glycolysis at the expense of the pentose phosphate pathway. Although this adaptation is beneficial in hypoxia, it is deleterious when RBCs are challenged by oxidant stress, such as during refrigerated storage in the blood bank [103] or in sickle cell disease, where deoxy-sickle hemoglobin stabilization further promotes its crystallization [102].
Using a combination of state-of-the-art multi-omics technologies, we recently identified the presence and activity of oxidant stress-sensitive fatty acid desaturases (especially FADS2) in mature RBCs [104]. By introducing double bonds in an NADH-dependent fashion, FADS contribute to NADH homeostasis by recycling reducing equivalents back to their oxidized state, which is essential for the glycolytic step catalyzed by GAPDH. Of note, alterations of this pathway are critical for regulating abnormal hematopoiesis in aging mice and humans [105]. Dietary interventions that alter fatty acyl membrane composition predispose, or protect, lipids from peroxidation [106], a hallmark of ferroptosis that regulates RBC extravascular hemolysis; the latter is—at least in part modulated by the activity of the ferrireductase STEAP3 [107]. Pathways exist in mature RBCs to cope with lipid peroxidation; for example, via glutathionylation by GPX4—which is present and active in mature erythrocytes [33]. Genetic polymorphisms in the GPX4 coding region are common in humans and are associated with an increased propensity to hemolysis following oxidant stress [49]. Alternatively, phospholipase A2 (or PLA2-like enzymes such as peroxiredoxin 6 [108]) can release the oxidized fatty acid moiety from membrane phospholipids, thereby generating lysophospholipids. The acyl-coA/acyl-carnitine system can then fuel the transfer of new fatty acids to the lysophospholipid, restoring phospholipid composition. Carnitine availability limits the rate of this pathway, known as the Lands cycle, which is activated in response to oxidant stress (e.g., in response to exercise [109] or in patients with hemoglobinopathies, such as sickle cell trait [110] and sickle cell disease [111,112]).
Arginine metabolism in mature RBCs was found to be more complex than anticipated, despite the absence of mitochondria, where a subset of critical reactions in the urea cycle are known to occur. For example, RBCs contain high levels of arginase 1 [113], which converts arginine to ornithine, a precursor of polyamines via ornithine decarboxylase. Although this pathway is critical in erythropoiesis, owing to the role of polyamines in regulating the intracellular pH of hematopoietic precursors [114], in adult RBCs this pathway has been associated with cellular responses to iron-deficient anemia and abiotic stresses, such as exposure to radiation [115]. Arginine metabolism cross-talks with heme synthesis, glutathione homeostasis and one-carbon metabolism, in that polyamine synthesis and creatine synthesis are both affected in human RBCs by factors such as sex and age [48], and compete for rate-limiting substrates for the generation of each product. During the last decade, RBCs were found to harbor a functional nitric oxide synthase [116], which converts arginine to citrulline and concomitantly generates nitric oxide, a potent vasodilator with a crucial role in the regulation of endothelial cells and related vascular function [117,118].
As already mentioned above, carboxylic acid intermediates of the Krebs cycle, such as succinyl-CoA participate in erythropoiesis by fueling heme synthesis. In addition, small molecule dicarboxylates in this pathway (e.g., succinate, fumarate, malate) sustain erythropoiesis by the mechanism of stabilization of the Hypoxia Inducible Factor 1alpha upon exposure to hypoxia, which otherwise is degraded following hydroxylation by alpha-ketoglutarate-dependent prolyl hydroxylases [119]. The conversion of alpha-ketoglutarate to 2-hydroxyglutarate can occur under hypoxic conditions by non-canonical lactate dehydrogenase activity [120], and testosterone-induced stimulation of erythropoiesis [121]. The absence of mitochondria in healthy mature RBCs originally led the field to believe that minimal carboxylic acid metabolism would occur in this cell system. However, proteomics recently identified functional cytosolic isoforms of acetyl-CoA ligase, isocitrate dehydrogenase 1, malate dehydrogenase 1 and malic enzyme 1; thus, catabolism of pyruvate and citrate can occur in mature RBCs [122,123] via a series of reactions that can fuel the alternative generation of reducing equivalents (e.g., NADPH and NADH), especially in hypoxia [124]. The activation of these pathways may be especially beneficial as a partial compensatory mechanism in the face of genetic aberrations leading to a dysfunctional pentose phosphate pathway, such as G6PD deficiency [46,125].
Although healthy mature RBCs are indeed devoid of mitochondria, recent evidence unequivocally documented the presence of up to 6–7 mitochondria per cell in mature RBCs from sickle cell patients [126,127,128,129]. Similar observations have been reported in the context of other pathophysiological conditions, such as systemic lupus erythematosus [130] and Rett syndrome [131,132]. Whether and to what extent these organelles still function is incompletely understood, though their retention might result from a defect in mitophagy or the ubiquitin-proteasome system [126,127,132] Nonetheless, the presence of metabolically active mitochondria in mature RBCs could induce oxygen consumption and consequently oxidant stress through ROS generation [128]. The induction of reticulocytosis or heterogeneous retention of mitochondria is associated with alloimmunization in a murine model of transfusion [133], which could be relevant to blood donors with heterogeneous mitochondrial DNA content in circulating erythroid cells and recipients with inflammatory conditions such as systemic lupus erythematosus [131]. The relevance of this phenomenon in RBC aging in vivo—to the extent this may result from/contribute to processes of age-related aberrant erythropoiesis and age-related comorbidities remains to be determined. Nonetheless, one may speculate that intra- or extra-vascular hemolysis of mitochondria-containing RBCs may release prokaryotic-like RNA and DNA into the circulation or in phagocytic macrophages, thereby triggering cGAS-STING-Interferon responses [130,134], ultimately driving interferon-mediated inflammatory complications in sickle cell patients, lupus patients and in other conditions in which interferonopathies and hematological anomalies are observed (e.g., Down syndrome) [135].
3. RBC Metabolism and Blood Storage for Clinical Transfusion Purposes
Understanding RBC metabolism holds critical translational implications in modern medicine. Transfusion of packed RBCs is a life-saving intervention for 4–5 million Americans every year. With over 110 million units of packed RBCs collected and transfused annually worldwide, RBC transfusion is the most common hospital iatrogenic intervention after vaccination [136]. Storage in the blood bank for up to 42 days in most countries is a logistic necessity to make RBC units available for transfusion to acutely or chronically ill recipients, such as those with trauma [137] or beta-thalassemia/sickle cell disease [138], respectively. Unfortunately, during refrigerated storage under blood bank conditions, RBCs undergo a series of biochemical, metabolic and morphological changes, collectively referred to as the “storage lesion” [139]. Application of omics technologies to the investigation of the metabolic storage lesion has documented a plethora of changes [140], with a temporal sequence of events [141] first started by slower kinetics of metabolic enzymes at 4 °C [142]. As temperature-sensitive ion pumps fail under refrigerated storage conditions, increased ATP demands to counteract these effects through the active transport of potassium and calcium ions against gradients [143] results in additional metabolic strains for RBC glycolysis. While glucose consumption and the generation of lactate are still observed in stored erythrocytes, the rate at which these fluxes occur is insufficient to meet the demand for ATP and DPG of refrigerator-stored RBCs [144]. Slow glycolytic rates are aggravated by multiple mechanisms beyond storage temperature. First, in the closed system of a blood bag, accumulation of lactic acid for up to 42 days is accompanied by the progressive acidification of the intracellular and extracellular pH, ultimately leading to slower kinetics for pH-sensitive enzymes, such as phosphofructokinase, bisphosphoglycerate mutase and G6PD, rate-limiting enzymes of glycolysis, the Rapoport–Luebering shunt and the pentose phosphate pathway, respectively [145]. Strategies have been envisaged to counteract this phenomenon, such as the development of alkaline additives with low/no chloride and high bicarbonate content [146,147]. As ATP and DPG are consumed, the latter >95% depleted by storage weeks 2–3 [148], and RBC oxygen saturation increases up to 95% by storage day 21, with concomitant accumulation of ROS [141]. ROS attack on functional residues of hemoglobin and glycolytic enzymes such as GAPDH further negatively affects glycolytic fluxes, resulting in the transient activation of the PPP to generate NADPH and counteract storage-induced oxidant stress [53]. These antioxidant systems are insufficient to cope with oxidant stress, ultimately resulting in the irreversible oxidation of functional enzymes and structural proteins, such as band 3, ankyrin and spectrin [141,149], with consequent alteration of the membrane band 3 interactome [59]. Mechanisms of isoaspartyl protein damage methylation—such as the ones described above as a function of PIMT activity in aging RBCs in vivo—are activated to cope with oxidant stress to structural proteins and glycolytic enzymes in stored RBCs [92]. However, calcium-activated caspase and oxidative stress both contribute to the fragmentation of the N-terminus of band 3 [150], ultimately depriving RBCs of the capacity to inhibit GAPDH (and other glycolytic enzymes) by mechanism of inhibitory binding to this region [59,60]. Interestingly, a similar failure of this mechanism is observed upon exposure to chronic oxidant stress in vivo in sickle cell disease [151]. Since fragmentation to band 3 is irreversible, and no new band 3 protein can be synthesized, stored transfused RBCs cannot respond to oxidant stress in vivo by activating the pentose phosphate pathway at the same rate that fresh RBCs would do—as observed in storage-biotinylation-recovery studies in humans [152]. Interestingly, transient activation of the pentose phosphate pathway at the second week of storage is a measurable transient metabolic state in the unsupervised elaboration of omics data of stored RBCs [70]. The activation of glycolysis at the expense of the pentose phosphate pathway by exogenous supplementation (or inter-donor heterogeneity in the levels at donation) of S1P is associated with an increased susceptibility of stored RBCs to extravascular hemolysis [103]. Genetic ablation of the S1P synthesizing enzyme Sphk1 in mice improves storability and post-transfusion recovery (PTR) [103]. Polymorphisms in the S1P transporter Mtfsd2b are relatively common in the blood donor population and are associated with increased susceptibility to osmotic fragility [49].
In RBCs obtained from donors with non-hemolytic G6PD deficiency, whose RBCs have naturally aberrant flux through the pentose phosphate pathway at baseline, storage results in higher basal levels of glycolysis and ATP, despite increased oxidant stress; this unusual combination yields RBCs that have better-preserved morphology by the end of the storage period [44,45] yet suffer from an exacerbated redox storage lesion, ultimately resulting in increased susceptibility to storage, osmotic and oxidant stress-induced hemolysis [48,49], as well as poorer PTR, i.e., the percentage of stored RBCs that still circulates at 24 h upon transfusion. As the ATP synthesis rate does not meet demand, lower energy phosphate compounds such as AMP accumulate and are deaminated by AMPD3 into IMP and hypoxanthine, a phenomenon exponentially activated after storage week 3 [94]. The timeline is not casual, in that by storage day 21 we observe a peak in consumption of DPG, with concomitant significant accumulation of intracellular calcium—both factors promoting AMPD3 activity [94]. The accumulation of hypoxanthine, a biomarker of the RBC metabolic storage lesion and a predictor of PTR in stored human and murine RBCs, is observed not just in human RBCs, but also in other primates (macaques [153], baboons [154]) and mammals (e.g., mouse [155], rats [156], guinea pigs [157], cows, dogs, donkey and horses [158]), though at different rates as a function of species genotypes, suggesting a genetic regulation of this pathway—which is relevant for veterinary transfusion considerations [159]. Since HPRT (see above) is an X-linked gene, sex dimorphisms in this pathway are observed in the stored RBCs [48]. One additional confounder related to sex pertains to the relative age of circulating RBCs at the time of donation, generally younger in pre-menopausal females [160], further confirming a sex dimorphism in RBC storability [161]. Indeed, small-scale studies have demonstrated RBC storability differences in the hemolytic propensity and membrane binding of stress protein markers between pre- and post-menopausal women [162,163]. Similarly, and still part of the purine oxidation pathway, inter-donor heterogeneity in uric acid levels is observed and contributes to the overall variability in antioxidant capacity across blood units [96].
Ultimately, unmitigated oxidant stress contributes to increased fatty acid desaturation, oxidation and migration to the membrane of oxidized proteins (e.g., peroxiredoxin 2 [34]), vesiculation of oxidized proteins [12] and lipids [164], a process that promotes the loss of the RBC discocytic phenotype and the acquisition of an echinocytic, spheroechinocytic and spherocytic morphology [141,143,165,166]. Losing cell volume increases the surface-to-volume ratio, making the small microcytic erythrocyte (<43 µm2) less deformable and more susceptible to sequestration in the splenic slits, ultimately priming erythrophagocytosis. It should be noted that the extracellular vesicles that accumulate in stored RBC supernatant could act as biological response modifiers, potentially affecting post-transfusion responses, especially in light of recent studies that demonstrate the presence of several RNA transcripts in RBC-derived vesicles [167].
The implementation of high-throughput metabolomics technologies is now informing the data-driven development of novel storage additives [168]. In the meantime, the summary above generally holds true for RBCs stored in almost all currently licensed storage additives, from saline adenine glucose mannitol (SAGM), to additive solution (AS) 1, 3, 5, and phosphate adenine glucose gluconate saline mannitol (PAGGSM), with slightly different kinetics [145,169,170,171,172]. The data are so consistent that biomarkers of the metabolic storage lesion have been calculated [70] and used to predict stored RBC metabolic states throughout the time course even from just a single data point [173]. Heterogeneity in blood storage solutions is indeed a critical parameter modulating the storage lesion, one that explains almost the same percentage of the total metabolic variance across stored units as storage duration itself [174].
Nonetheless, the extent to which these storage-induced changes correlate with functional and clinically relevant outcomes is only partially understood. For example, it is now evident that DPG and ATP depletion perfectly predict alterations in RBC oxygen kinetics, with faster oxygen binding and slower oxygen off-loading kinetics [18]. Of note, RBC storage under hypoxic and hypocapnic (reduced carbon dioxide) conditions promotes intracellular alkalinization by boosting the exchange of chloride for bicarbonate (strong vs. weak acid) via carbonic anhydrase/band 3 activity—which is dependent on residues 559–630 and 681 as per Uniprot entry P02730—phenocopying the benefits of alkaline additives [175]. In so doing, hypoxic storage boosts RBC DPG levels [176], preserving oxygen kinetics of the stored RBC [177]. At the same time, hypoxic storage deprives the system of oxygen, a substrate for the chemistry driving the formation of ROS, ultimately mitigating PTR of murine [178] and human RBCs [179]. It is tempting to hypothesize that improved ATP preservation under hypoxic conditions [177,179] could additionally benefit stored RBCs through enhanced proteostatic regulation, including efficient chaperoning and proteasome activity, a feature that is crucial for a cell without the ability to produce new protein molecules.
4. All Blood Units Are Created Equal, but Some Blood Units Are More Equal than Others
A meta-analysis of multiple PTR studies in healthy volunteers has clearly shown that the quality of donated blood is heterogeneous across donors [180] (Figure 2). In recent years, a series of studies have shed light on inter-donor heterogeneity that manifests itself in variable hemolytic propensity and post-transfusion hemoglobin increment as a function of donor biology, including donor sex, age, ethnicity and body mass index/obesity as well as genetic mutations in enzymes or Hb [48,51,181,182,183]. Further studies in monozygotic (identical) and dizygotic (non-genetically identical) twins have shown that hemolysis and metabolite levels, especially of antioxidants such as glutathione, are heritable in the blood donor population [184,185,186]. Leveraging this concept, studies in murine models of blood storage and PTR have clearly shown cross-strain heterogeneity in extravascular hemolysis [155,187].

Figure 2.
Summary of the main factors impacting RBC storage metabolism and post-transfusion performances.
Elegant breeding strategies were executed to cross good and poor storing mouse strains, ultimately leading to the identification of the ferrireductase STEAP3 as a critical mediator of lipid peroxidation and extravascular hemolysis of stored, transfused murine RBCs [107]. The rationale driving the aforementioned studies is now being expanded to large-scale studies in humans, with investigations such as the Recipient Epidemiology and Donor Evaluation Study (REDS) [188,189]. As part of this study, ~14,000 volunteers were enrolled to donate blood at four different blood centers across the United States. Units were stored for up to 42 days and, at the end of the storage period, they were tested for spontaneous hemolysis or hemolysis induced by oxidative or osmotic insults [190]. Donors with extreme hemolytic propensity (5th and 95th percentile) were invited to donate a second unit of blood, which was tested again for the same parameters, showing significant reproducibility of intra-donor hemolytic propensity across multiple donations [190]. These donors were genotyped for 879,000 Single Nucleotide Polymorphisms (SNPs) [191], which identified genetic underpinnings of hemolytic propensity, including G6PD and GPX4 status, among others [49]. The linkage of genetic polymorphisms, hemolytic propensity, and hemoglobin increments in recipients of units from these donors via a vein-to-vein linkage database [181,192] and metabolite levels (metabolite quantitative trait loci—mQTL) [193] is currently underway, and early results are already significantly expanding our understanding of the impact of donor genetics on RBC metabolism during aging in vitro in the blood bank on in vivo RBC recovery and function following transfusion.
Beyond donor genetics, metabolites were identified that do not change with storage, but are rather associated with donor exposures—the so-called exposome, factors that are now being associated with hemolytic propensity [194]. From these studies, it is emerging that donor habits, such as smoking or other nicotine exposures, consumption of alcohol, coffee and caffeinated, taurine-rich beverages, all impact RBC energy and redox metabolism in a way that affects storage biology and, potentially, post-transfusion performance, especially when combined with invasive processing such as RBC unit irradiation [195,196,197,198]. While phthalate plasticizers were historically added to polyvinylchloride bags to decrease the rigidity of the blood bag, these compounds leach from the unit and intercalate into the RBC membranes, resulting in an erythrocyte with altered deformability, decreased hemolytic propensity [199,200], and altered increased risk for toxicity (e.g., infertility, cardiodepression), especially in certain categories of recipients such as pediatric patients [201]. Controversial reports on the detection of metabolites of professional exposures such as Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in high-fidelity recurring donors such as firefighters have been described [202], though the potential toxicity profiles of these compounds in transfusion recipients remain to be assessed. Similarly, a long list of over-the-counter or prescription drugs that are not grounds for blood donor deferral have been detected, at least in traces, in blood units, including acetaminophen/paracetamol, ibuprofen, statins, xartans, proton pump inhibitors, antidepressants, just to mention few [194]. Inter-donor heterogeneity in diets results in differential lipid composition of RBC membranes, which ultimately affects membrane fluidity and fragility [203,204,205]. Other antioxidant molecules of dietary origin such as carnitine, vitamin C, vitamin E, resveratrol, quercetin and ergothioneine have all been detected at variable levels in donated blood and could theoretically affect RBC storability [194,206,207,208,209,210,211,212]. While exercise is known to significantly impact RBC metabolism and deformability [109], little is known as to whether RBCs from athletes store and recover better than RBCs from sedentary donors. Similar considerations can be made for blood donors living at high altitudes vs. sea level [30]. RBCs from subjects previously infected with corona- or flavi-viruses such as SARS-CoV-2, present metabolic alterations consistent with anomalous activation of the pentose phosphate pathway and band 3 oxidation/fragmentation [213,214]. In the case of Zika virus-infected donors, clearance of viremia and seroconversion were still accompanied by months-long (up to >100 days) alterations of RBC metabolism, suggesting potential long-lasting effects of infection on circulating RBC biology, perhaps relevant to the blood donors and recipient populations, e.g., in the context of sepsis [215]. Altogether, all the factors listed above contribute to the metabolic heterogeneity of the stored RBC beyond the chronological age of the unit (i.e., the days elapsed since donation), suggesting a more relevant role for the metabolic age of the unit as a critical predictor of transfusion outcomes in the future [216].
Although all these changes are well documented, it is unclear whether and to what extent they are reversed in vivo following transfusion, and whether they ultimately affect RBC performance in vivo. Autologous blood transfusion studies in healthy volunteers have shown that stored RBCs significantly impact healthy recipient plasma metabolism [113], an observation that may inform strategies for autologous blood doping detection in sports [217]. Studies on autologous volunteers and recovery of biotinylated RBCs suggest that part of the storage lesion is reversible in vivo, such as the rescue of ATP and DPG levels, a process that may require up to 24–72 h [152,218] and thus be sufficiently slow that stored RBC transfusions may not correct oxygen kinetics within the golden hour of hypoxic patients undergoing massive bleeding, to mention a key category of recipients [137]. Studies on the metabolic impact of transfusion in massively transfused trauma patients are currently underway [137], while clear evidence of an association with cardiorenal dysfunction and systemic hypoxia has been reported in sickle cell patients [111,138]. Interestingly, studies of ex vivo preservation of murine RBCs have shown that erythrocytes from mice with good or poor storage quality cross-regulate, suggestive of as yet under-investigated mechanisms of metabolic cross-regulation, ultimately impacting post-transfusion performance [219]. It remains to be determined whether these “good apple, bad apple” mechanisms occur in vivo, for example in sickle cell patients undergoing exchange therapy, or even when the donors’ RBCs are exposed to the recipients’ erythrocytes.
As our understanding of RBC metabolism in vivo and in vitro refines, novel pathways are discovered and mechanisms elucidated, the simple cell of the early days of biochemistry keeps surprising us with novel pathways and novel roles in systems metabolism beyond oxygen transport. Multi-omics studies on RBC propensity to hemolysis upon storage and transfusion are finding new mechanisms even for well-investigated proteins such as band 3; for example, while the extracellular domain of this protein has long been recognized as the Diego blood group in transfusion medicine [220], it has only recently emerged that genetic polymorphisms in the region coding for this protein impact RBC hemolytic propensity and post-transfusion performances [49]. It is easy to anticipate a near future where RBCs will no longer be considered an inert background participant in human biology, but rather a targetable vulnerability of human health, longevity and disease, beyond the current focus in transfusion medicine.
Author Contributions
A.D. wrote the first draft of this review, which was then expanded, finalized and approved by all co-authors. All authors have read and agreed to the published version of the manuscript.
Funding
A.D. was supported by funds by the National Heart, Lung, and Blood Institute (R01HL146442, R01HL149714, R01HL148151, R01HL161004). The authors acknowledge support from the REDS-IV-P CTLS project, sponsored by the National Heart, Lung, and Blood Institute contract 75N2019D00033, and from the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) RBC Omics project, which was supported by NHLBI contracts HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and -00009I. AA received support from the European Haematology Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare that A.D. is a founder of Omix Technologies Inc. (Aurora, CO, USA) and Altis Biosciences LLC (Aurora, CO, USA). A.D. is also a consultant for Hemanext Inc. (Lexington, MA, USA) and Macopharma Inc. (Tourcoing, France). S.L.S. is a consultant for Hemanext Inc. and ALCOR Scientific LLC (Smithfield, RI, USA). All the other authors have no relevant conflict to disclose.
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F.; et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Xia, Y.; Zimring, J.C.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteom. 2018, 15, 855–864. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Meinderts, S.M.; van den Berg, T.K.; van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Righetti, P.G.; Zolla, L. The red blood cell proteome and interactome: An update. J. Proteome Res. 2010, 9, 144–163. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef]
- Kanias, T.; Acker, J.P. Biopreservation of red blood cells—The struggle with hemoglobin oxidation. Febs J. 2010, 277, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wither, M.; Dzieciatkowska, M.; Nemkov, T.; Strop, P.; D’Alessandro, A.; Hansen, K.C. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion 2016, 56, 421–426. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Blasi, B.; D’Amici, G.M.; Marrocco, C.; Zolla, L. Red blood cell subpopulations in freshly drawn blood: Application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013, 11, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dreischer, P.; Duszenko, M.; Stein, J.; Wieder, T. Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells. Cells 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, Function and Allostery. Subcell. Biochem. 2020, 94, 345–382. [Google Scholar] [CrossRef]
- Yuan, Y.; Tam, M.F.; Simplaceanu, V.; Ho, C. New look at hemoglobin allostery. Chem. Rev. 2015, 115, 1702–1724. [Google Scholar] [CrossRef]
- Donovan, K.; Meli, A.; Cendali, F.; Park, K.C.; Cardigan, R.; Stanworth, S.; McKechnie, S.; D’Alessandro, A.; Smethurst, P.A.; Swietach, P. Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter. Haematologica 2022, 107, 298–302. [Google Scholar] [CrossRef]
- Gatto, C.; Milanick, M. Red blood cell Na pump: Insights from species differences. Blood Cells Mol. Dis. 2009, 42, 192–200. [Google Scholar] [CrossRef]
- Longo, V.; Marrocco, C.; Zolla, L.; Rinalducci, S. Label-free quantitation of phosphopeptide changes in erythrocyte membranes: Towards molecular mechanisms underlying deformability alterations in stored red blood cells. Haematologica 2014, 99, e122–e125. [Google Scholar] [CrossRef]
- Xu, P.; Chen, C.; Zhang, Y.; Dzieciatkowska, M.; Brown, B.C.; Zhang, W.; Xie, T.; Abdulmalik, O.; Song, A.; Tong, C.; et al. Erythrocyte transglutaminase-2 combats hypoxia and chronic kidney disease by promoting oxygen delivery and carnitine homeostasis. Cell Metab. 2022, 34, 299–316.e6. [Google Scholar] [CrossRef]
- Betz, T.; Lenz, M.; Joanny, J.-F.; Sykes, C. ATP-dependent mechanics of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15320–15325. [Google Scholar] [CrossRef]
- Gokhin, D.S.; Fowler, V.M. Feisty filaments: Actin dynamics in the red blood cell membrane skeleton. Curr. Opin. Hematol. 2016, 23, 206–214. [Google Scholar] [CrossRef]
- Wagner, G.M.; Chiu, D.T.; Yee, M.C.; Lubin, B.H. Red cell vesiculation—A common membrane physiologic event. J. Lab. Clin. Med. 1986, 108, 315–324. [Google Scholar]
- Arashiki, N.; Takakuwa, Y.; Mohandas, N.; Hale, J.; Yoshida, K.; Ogura, H.; Utsugisawa, T.; Ohga, S.; Miyano, S.; Ogawa, S.; et al. ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica 2016, 101, 559–565. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Arvaniti, V.Z.; Dzieciatkowska, M.; Stamoulis, K.; Lekka, M.E.; Papassideri, I.S.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Red Blood Cell Proteasome in Beta-Thalassemia Trait: Topology of Activity and Networking in Blood Bank Conditions. Membranes 2021, 11, 716. [Google Scholar] [CrossRef]
- Song, A.; Wen, A.Q.; Wen, Y.E.; Dzieciatkowska, M.; Kellems, R.E.; Juneja, H.S.; D’Alessandro, A.; Xia, Y. p97 dysfunction underlies a loss of quality control of damaged membrane proteins and promotes oxidative stress and sickling in sickle cell disease. FASEB J. 2022, 36, e22246. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Dzieciatkowska, M.; Anastasiadi, A.T.; Karadimas, D.G.; Vergaki, A.; Siourounis, P.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; D’Alessandro, A.; et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. 2022, 20, 27–39. [Google Scholar] [CrossRef]
- van Wijk, R.; van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.A.; Subudhi, A.W.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef]
- Reisz, J.A.; Slaughter, A.L.; Culp-Hill, R.; Moore, E.E.; Silliman, C.C.; Fragoso, M.; Peltz, E.D.; Hansen, K.C.; Banerjee, A.; D’Alessandro, A. Red blood cells in hemorrhagic shock: A critical role for glutaminolysis in fueling alanine transamination in rats. Blood Adv. 2017, 1, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Hansen, K.C.; Eisenmesser, E.Z.; Zimring, J.C. Protect, repair, destroy or sacrifice: A role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. 2019, 17, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.M.; Stefely, J.A.; Veling, M.T.; van ‘t Erve, T.J.; Wagner, B.A.; Raife, T.J.; Buettner, G.R. Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 2021, 46, 102073. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; D’Amici, G.M.; Blasi, B.; Vaglio, S.; Grazzini, G.; Zolla, L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011, 51, 1439–1449. [Google Scholar] [CrossRef]
- Paukovich, N.; Xue, M.; Elder, J.R.; Redzic, J.S.; Blue, A.; Pike, H.; Miller, B.G.; Pitts, T.M.; Pollock, D.D.; Hansen, K.; et al. Biliverdin Reductase B Dynamics Are Coupled to Coenzyme Binding. J. Mol. Biol. 2018, 430, 3234–3250. [Google Scholar] [CrossRef]
- Corti, A.; Casini, A.F.; Pompella, A. Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Arch. Biochem. Biophys. 2010, 500, 107–115. [Google Scholar] [CrossRef]
- Pey, A.L.; Megarity, C.F.; Timson, D.J. NAD(P)H quinone oxidoreductase (NQO1): An enzyme which needs just enough mobility, in just the right places. Biosci. Rep. 2019, 39, BSR20180459. [Google Scholar] [CrossRef]
- Fenk, S.; Melnikova, E.V.; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y.V.; Kaestner, L.; Minetti, G.; Mairbäurl, H.; et al. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef]
- Harper, V.M.; Oh, J.Y.; Stapley, R.; Marques, M.B.; Wilson, L.; Barnes, S.; Sun, C.W.; Townes, T.; Patel, R.P. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid. Redox Signal. 2015, 22, 294–307. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef]
- Katabami, K.; Hayakawa, M.; Gando, S. Severe Methemoglobinemia due to Sodium Nitrite Poisoning. Case Rep. Emerg. Med. 2016, 2016, 9013816. [Google Scholar] [CrossRef]
- Parashar, A.; Jacob, V.D.; Gideon, D.A.; Manoj, K.M. Hemoglobin catalyzes ATP-synthesis in human erythrocytes: A murburn model. J. Biomol. Struct. Dyn. 2022, 40, 8783–8795. [Google Scholar] [CrossRef]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med. 2016, 96, 152–165. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Data on how several physiological parameters of stored red blood cells are similar in glucose 6-phosphate dehydrogenase deficient and sufficient donors. Data Brief. 2016, 8, 618–627. [Google Scholar] [CrossRef]
- Francis, R.O.; D’Alessandro, A.; Eisenberger, A.; Soffing, M.; Yeh, R.; Coronel, E.; Sheikh, A.; Rapido, F.; La Carpia, F.; Reisz, J.A.; et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Investig. 2020, 130, 2270–2285. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Chen, X.; Wang, Q.; Ling, L.; Xu, Y. Glucose-6-phosphate dehydrogenase deficiency in the Han Chinese population: Molecular characterization and genotype-phenotype association throughout an activity distribution. Sci. Rep. 2020, 10, 17106. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Fu, X.; Kanias, T.; Reisz, J.A.; Culp-Hill, R.; Guo, Y.; Gladwin, M.T.; Page, G.; Kleinman, S.; Lanteri, M.; et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2021, 106, 1290–1302. [Google Scholar] [CrossRef]
- Page, G.P.; Kanias, T.; Guo, Y.J.; Lanteri, M.C.; Zhang, X.; Mast, A.E.; Cable, R.G.; Spencer, B.R.; Kiss, J.E.; Fang, F.; et al. Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J. Clin. Investig. 2021, 131, e146077. [Google Scholar] [CrossRef]
- Archer, N.M.; Petersen, N.; Clark, M.A.; Buckee, C.O.; Childs, L.M.; Duraisingh, M.T. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Stefanoni, D.; Cendali, F.; Bertolone, L.; Gamboni, F.; Dzieciatkowska, M.; Rousakis, P.; Vergaki, A.; Soulakis, V.; et al. β-thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2022, 107, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, E.C.; Ahmed, A.M.; Titouna, A.; Elmaraezy, A.; Trang, N.T.; Phuoc Long, N.; Hoang Anh, N.; Diem Nghi, T.; The Hung, B.; Van Hieu, M.; et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 45963. [Google Scholar] [CrossRef]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, C.; Manuel, A.M.; Sharafi, M.; Aboushousha, R.; Qian, X.; Erickson, C.; MacPherson, M.; Chan, G.; Adcock, I.M.; ZounematKermani, N.; et al. Glutathione-S-transferase P promotes glycolysis in asthma in association with oxidation of pyruvate kinase M2. Redox Biol. 2021, 47, 102160. [Google Scholar] [CrossRef] [PubMed]
- Aboushousha, R.; Elko, E.; Chia, S.B.; Manuel, A.M.; van de Wetering, C.; van der Velden, J.; MacPherson, M.; Erickson, C.; Reisz, J.A.; D’Alessandro, A.; et al. Glutathionylation chemistry promotes interleukin-1 beta-mediated glycolytic reprogramming and pro-inflammatory signaling in lung epithelial cells. Faseb J. 2021, 35, e21525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.A.; Campanella, M.E.; Markley, J.L.; Low, P.S. Role of band 3 in regulating metabolic flux of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18515–18520. [Google Scholar] [CrossRef]
- Chu, H.; Low, P.S. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem. J. 2006, 400, 143–151. [Google Scholar] [CrossRef]
- Messana, I.; Ferroni, L.; Misiti, F.; Girelli, G.; Pupella, S.; Castagnola, M.; Zappacosta, B.; Giardina, B. Blood bank conditions and RBCs: The progressive loss of metabolic modulation. Transfusion 2000, 40, 353–360. [Google Scholar] [CrossRef]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef]
- Rogers, S.C.; Ge, X.; Brummet, M.; Lin, X.; Timm, D.D.; d’Avignon, A.; Garbow, J.R.; Kao, J.; Prakash, J.; Issaian, A.; et al. Quantifying dynamic range in red blood cell energetics: Evidence of progressive energy failure during storage. Transfusion 2021, 61, 1586–1599. [Google Scholar] [CrossRef]
- Wiback, S.J.; Palsson, B.O. Extreme pathway analysis of human red blood cell metabolism. Biophys. J. 2002, 83, 808–818. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Pajerowski, J.D.; Jamshidi, N.; Palsson, B.O.; Edwards, J.S. Description and Analysis of Metabolic Connectivity and Dynamics in the Human Red Blood Cell. Biophys. J. 2002, 83, 646–662. [Google Scholar] [CrossRef]
- Nakayama, Y.; Kinoshita, A.; Tomita, M. Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor. Biol. Med. Model. 2005, 2, 18. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Dzieciatkowska, M.; Nemkov, T.; Hansen, K.C. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017, 15, 182–187. [Google Scholar] [CrossRef]
- Gautier, E.F.; Leduc, M.; Cochet, S.; Bailly, K.; Lacombe, C.; Mohandas, N.; Guillonneau, F.; El Nemer, W.; Mayeux, P. Absolute proteome quantification of highly purified populations of circulating reticulocytes and mature erythrocytes. Blood Adv. 2018, 2, 2646–2657. [Google Scholar] [CrossRef]
- Pasini, E.M.; Kirkegaard, M.; Mortensen, P.; Lutz, H.U.; Thomas, A.W.; Mann, M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 2006, 108, 791–801. [Google Scholar] [CrossRef]
- Sae-Lee, W.; McCafferty, C.L.; Verbeke, E.J.; Havugimana, P.C.; Papoulas, O.; McWhite, C.D.; Houser, J.R.; Vanuytsel, K.; Murphy, G.J.; Drew, K.; et al. The protein organization of a red blood cell. Cell Rep. 2022, 40, 111103. [Google Scholar] [CrossRef]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell. Proteom. 2016, 15, 1938–1946. [Google Scholar] [CrossRef]
- Goodman, S.R.; Daescu, O.; Kakhniashvili, D.G.; Zivanic, M. The proteomics and interactomics of human erythrocytes. Exp. Biol. Med. 2013, 238, 509–518. [Google Scholar] [CrossRef]
- Paglia, G.; D’Alessandro, A.; Rolfsson, O.; Sigurjonsson, O.E.; Bordbar, A.; Palsson, S.; Nemkov, T.; Hansen, K.C.; Gudmundsson, S.; Palsson, B.O. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016, 128, e43–e50. [Google Scholar] [CrossRef]
- Bordbar, A.; McCloskey, D.; Zielinski, D.C.; Sonnenschein, N.; Jamshidi, N.; Palsson, B.O. Personalized Whole-Cell Kinetic Models of Metabolism for Discovery in Genomics and Pharmacodynamics. Cell Syst. 2015, 1, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, A.; Jamshidi, N.; Palsson, B.O. iAB-RBC-283: A proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst. Biol. 2011, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, R.; Nemkov, T.; D’Alessandro, A.; Grau, M.; Dietz, T.; Bohnert, B.N.; Essigke, D.; Wörn, M.; Schaefer, L.; Xiao, M.; et al. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability and metabolism. Kidney Int. 2021, 100, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- La Carpia, F.; Wojczyk, B.S.; Annavajhala, M.K.; Rebbaa, A.; Culp-Hill, R.; D’Alessandro, A.; Freedberg, D.E.; Uhlemann, A.C.; Hod, E.A. Transfusional iron overload and intravenous iron infusions modify the mouse gut microbiota similarly to dietary iron. NPJ Biofilms Microbiomes 2019, 5, 26. [Google Scholar] [CrossRef]
- Hamza, I.; Dailey, H.A. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 2012, 1823, 1617–1632. [Google Scholar] [CrossRef]
- Roy, M.K.; Cendali, F.I.; Ooyama, G.; Gamboni, F.; Morton, H.; D’Alessandro, A. Red Blood Cell Metabolism in Patients with Propionic Acidemia. Separations 2021, 8, 142. [Google Scholar] [CrossRef]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and propionic acidemias: Clinical management update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Nemkov, T.; Qadri, S.M.; Sheffield, W.P.; D’Alessandro, A. Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus. 2020, 18, 130–142. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Mian, S.A.; Philippe, C.; Maniati, E.; Protopapa, P.; Bergot, T.; Piganeau, M.; Nemkov, T.; Di Bella, D.; Morales, V.; Finch, A.J.; et al. Vitamin B5 and succinyl-CoA improve ineffective erythropoiesis in SF3B1-mutated myelodysplasia. Sci. Transl. Med. 2023, 15, eabn5135. [Google Scholar] [CrossRef]
- Henry, C.J.; Nemkov, T.; Casás-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.C.; Serkova, N.J.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Sharma, R.; Sharma, M. Homocystinuria: A rare condition presenting as stroke and megaloblastic anemia. J. Pediatr. Neurosci. 2010, 5, 129–131. [Google Scholar] [CrossRef]
- Culp-Hill, R.; Zheng, C.; Reisz, J.A.; Smith, K.; Rachubinski, A.; Nemkov, T.; Butcher, E.; Granrath, R.; Hansen, K.C.; Espinosa, J.M.; et al. Red blood cell metabolism in Down syndrome: Hints on metabolic derangements in aging. Blood Adv. 2017, 1, 2776–2780. [Google Scholar] [CrossRef]
- Pogribna, M.; Melnyk, S.; Pogribny, I.; Chango, A.; Yi, P.; James, S.J. Homocysteine metabolism in children with Down syndrome: In Vitro modulation. Am. J. Hum. Genet. 2001, 69, 88–95. [Google Scholar] [CrossRef]
- Inaba, M.; Gupta, K.C.; Kuwabara, M.; Takahashi, T.; Benz, E.J., Jr.; Maede, Y. Deamidation of human erythrocyte protein 4.1: Possible role in aging. Blood 1992, 79, 3355–3361. [Google Scholar] [CrossRef]
- Janson, C.A.; Clarke, S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J. Biol. Chem. 1980, 255, 11640–11643. [Google Scholar] [CrossRef]
- Kim, E.; Lowenson, J.D.; MacLaren, D.C.; Clarke, S.; Young, S.G. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 6132–6137. [Google Scholar] [CrossRef]
- Lowenson, J.D.; Kim, E.; Young, S.G.; Clarke, S. Limited accumulation of damaged proteins in l-isoaspartyl (D-aspartyl) O-methyltransferase-deficient mice. J. Biol. Chem. 2001, 276, 20695–20702. [Google Scholar] [CrossRef]
- McFadden, P.N.; Clarke, S. Methylation at D-aspartyl residues in erythrocytes: Possible step in the repair of aged membrane proteins. Proc. Natl. Acad. Sci. USA 1982, 79, 2460–2464. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Clarke, S. Analysis of erythrocyte protein methyl esters by two-dimensional gel electrophoresis under acidic separating conditions. Anal. Biochem. 1985, 148, 79–86. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Clarke, S. Methylation of erythrocyte membrane proteins at extracellular and intracellular D-aspartyl sites in vitro. Saturation of intracellular sites in vivo. J. Biol. Chem. 1983, 258, 8485–8492. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Nemkov, T.; Dzieciatkowska, M.; Culp-Hill, R.; Stefanoni, D.; Hill, R.C.; Yoshida, T.; Dunham, A.; Kanias, T.; Dumont, L.J.; et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018, 58, 2978–2991. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Hay, A.; Dzieciatkowska, M.; Brown, B.C.; Morrison, E.J.; Hansen, K.C.; Zimring, J.C. Protein-L-isoaspartate O-methyltransferase is required for in vivo control of oxidative damage in red blood cells. Haematologica 2021, 106, 2726–2739. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Rosencrance, C.B.; De Vallance, E.; Giromini, A.; Williams, X.M.; Oh, J.Y.; Schmidt, H.; Straub, A.C.; Chantler, P.D.; Patel, R.P.; et al. Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO. Free Radic. Biol. Med. 2021, 174, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Papageorgiou, E.G.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion 2015, 55, 2659–2671. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wu, H.; D’Alessandro, A.; Yegutkin, G.G.; Song, A.; Sun, K.; Li, J.; Cheng, N.Y.; Huang, A.; et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation 2016, 134, 405–421. [Google Scholar] [CrossRef]
- Cakmakli, H.F.; Torres, R.J.; Menendez, A.; Yalcin-Cakmakli, G.; Porter, C.C.; Puig, J.G.; Jinnah, H.A. Macrocytic anemia in Lesch-Nyhan disease and its variants. Genet. Med. 2019, 21, 353–360. [Google Scholar] [CrossRef]
- Song, A.; Zhang, Y.; Han, L.; Yegutkin, G.G.; Liu, H.; Sun, K.; D’Alessandro, A.; Li, J.; Karmouty-Quintana, H.; Iriyama, T.; et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat. Commun. 2017, 8, 14108. [Google Scholar] [CrossRef]
- Qiang, Q.; Manalo, J.M.; Sun, H.; Zhang, Y.; Song, A.; Wen, A.Q.; Wen, Y.E.; Chen, C.; Liu, H.; Cui, Y.; et al. Erythrocyte adenosine A2B receptor prevents cognitive and auditory dysfunction by promoting hypoxic and metabolic reprogramming. PLoS Biol. 2021, 19, e3001239. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef]
- Sun, K.; D’Alessandro, A.; Ahmed, M.H.; Zhang, Y.; Song, A.; Ko, T.P.; Nemkov, T.; Reisz, J.A.; Wu, H.; Adebiyi, M.; et al. Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci. Rep. 2017, 7, 15281. [Google Scholar] [CrossRef]
- Hay, A.; Nemkov, T.; Gamboni, F.; Dzieciatkowska, M.; Key, A.; Galbraith, M.; Bartsch, K.; Sun, K.; Xia, Y.; Stone, M.; et al. Sphingosine 1-phosphate has a negative effect on RBC storage quality. Blood Adv. 2023, 7, 1379–1393. [Google Scholar] [CrossRef]
- Thomas, T.; Cendali, F.; Fu, X.; Gamboni, F.; Morrison, E.J.; Beirne, J.; Nemkov, T.; Antonelou, M.H.; Kriebardis, A.; Welsby, I.; et al. Fatty acid desaturase activity in mature red blood cells and implications for blood storage quality. Transfusion 2021, 61, 1867–1883. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Q.; Kao, Y.R.; Diaz, A.; Tasset, I.; Kaushik, S.; Thiruthuvanathan, V.; Zintiridou, A.; Nieves, E.; Dzieciatkowska, M.; et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature 2021, 591, 117–123. [Google Scholar] [CrossRef]
- Kim, C.Y.; Johnson, H.; Peltier, S.; Spitalnik, S.L.; Hod, E.A.; Francis, R.O.; Hudson, K.E.; Stone, E.F.; Gordy, D.E.; Fu, X.; et al. Deuterated Linoleic Acid Attenuates the RBC Storage Lesion in a Mouse Model of Poor RBC Storage. Front. Physiol. 2022, 13, 868578. [Google Scholar] [CrossRef]
- Howie, H.L.; Hay, A.M.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.D.; Ranson, M.S.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef]
- Fisher, A.B. The phospholipase A(2) activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef]
- Nemkov, T.; Skinner, S.C.; Nader, E.; Stefanoni, D.; Robert, M.; Cendali, F.; Stauffer, E.; Cibiel, A.; Boisson, C.; Connes, P.; et al. Acute Cycling Exercise Induces Changes in Red Blood Cell Deformability and Membrane Lipid Remodeling. Int. J. Mol. Sci. 2021, 22, 896. [Google Scholar] [CrossRef]
- Nemkov, T.; Skinner, S.; Diaw, M.; Diop, S.; Samb, A.; Connes, P.; D’Alessandro, A. Plasma Levels of Acyl-Carnitines and Carboxylic Acids Correlate With Cardiovascular and Kidney Function in Subjects With Sickle Cell Trait. Front. Physiol. 2022, 13, 916197. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nouraie, S.M.; Zhang, Y.; Cendali, F.; Gamboni, F.; Reisz, J.A.; Zhang, X.; Bartsch, K.W.; Galbraith, M.D.; Gordeuk, V.R.; et al. In Vivo evaluation of the effect of sickle cell hemoglobin S, C and therapeutic transfusion on erythrocyte metabolism and cardiorenal dysfunction. Am. J. Hematol. 2023, 98, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bogdanov, M.; Zhang, Y.; Sun, K.; Zhao, S.; Song, A.; Luo, R.; Parchim, N.F.; Liu, H.; Huang, A.; et al. Hypoxia-mediated impaired erythrocyte Lands’ Cycle is pathogenic for sickle cell disease. Sci. Rep. 2016, 6, 29637. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Reisz, J.A.; Zhang, Y.; Gehrke, S.; Alexander, K.; Kanias, T.; Triulzi, D.J.; Donadee, C.; Barge, S.; Badlam, J.; et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019, 3, 884–896. [Google Scholar] [CrossRef]
- Kumar, S.; Vassallo, J.D.; Nattamai, K.J.; Hassan, A.; Karns, R.; Vollmer, A.; Soller, K.; Sakk, V.; Sacma, M.; Nemkov, T.; et al. pH regulates hematopoietic stem cell potential via polyamines. EMBO Rep. 2023, 24, e55373. [Google Scholar] [CrossRef] [PubMed]
- Kalani Roy, M.; La Carpia, F.; Cendali, F.; Fernando, S.; Moriconi, C.; Wojczyk, B.S.; Wang, L.; Nemkov, T.; Hod, E.A.; D’Alessandro, A. Irradiation Causes Alterations of Polyamine, Purine, and Sulfur Metabolism in Red Blood Cells and Multiple Organs. J. Proteome Res. 2022, 21, 519–534. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric oxide, vasodilation and the red blood cell. Biorheology 2014, 51, 121–134. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Wang, B.; Liu, H.; Shah, H.; Carmona-Fontaine, C.; Rustenburg, A.S.; Salah, S.; Gunner, M.R.; Chodera, J.D.; Cross, J.R.; et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 2017, 13, 494–500. [Google Scholar] [CrossRef]
- Roy, M.K.; Wilkerson, R.B.; Alexander, K.; Nokoff, N.J.; Cree-Green, M.; D’Alessandro, A. Longitudinal metabolic study of red blood cells from patients undergoing gender-affirming testosterone therapy. Blood Adv. 2022. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Bordbar, A.; Yurkovich, J.T.; Paglia, G.; Rolfsson, O.; Sigurjónsson, Ó.E.; Palsson, B.O. Elucidating dynamic metabolic physiology through network integration of quantitative time-course metabolomics. Sci. Rep. 2017, 7, 46249. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Yoshida, T.; Dunham, A.; Wen, E.Y.; Wen, A.Q.; Roach, R.C.; Hansen, K.C.; Xia, Y.; et al. Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage. Front. Med. 2017, 4, 175. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Howie, H.L.; Hay, A.M.; Dziewulska, K.H.; Brown, B.C.; Wither, M.J.; Karafin, M.; Stone, E.F.; Spitalnik, S.L.; Hod, E.A.; et al. Hematologic and systemic metabolic alterations due to Mediterranean class II G6PD deficiency in mice. JCI Insight 2021, 6, e147056. [Google Scholar] [CrossRef]
- Moriconi, C.; Dzieciatkowska, M.; Roy, M.; D’Alessandro, A.; Roingeard, P.; Lee, J.Y.; Gibb, D.R.; Tredicine, M.; McGill, M.A.; Qiu, A.; et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br. J. Haematol. 2022, 198, 574–586. [Google Scholar] [CrossRef]
- Jagadeeswaran, R.; Vazquez, B.A.; Thiruppathi, M.; Ganesh, B.B.; Ibanez, V.; Cui, S.; Engel, J.D.; Diamond, A.M.; Molokie, R.E.; DeSimone, J.; et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp. Hematol. 2017, 50, 46–52. [Google Scholar] [CrossRef]
- Esperti, S.; Nader, E.; Stier, A.; Boisson, C.; Carin, R.; Marano, M.; Robert, M.; Martin, M.; Horand, F.; Cibiel, A.; et al. Increased retention of functional mitochondria in mature sickle red blood cells is associated with increased sickling tendency, hemolysis and oxidative stress. Haematologica 2023. [Google Scholar] [CrossRef]
- Tumburu, L.; Ghosh-Choudhary, S.; Seifuddin, F.T.; Barbu, E.A.; Yang, S.; Ahmad, M.M.; Wilkins, L.H.W.; Tunc, I.; Sivakumar, I.; Nichols, J.S.; et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood 2021, 137, 3116–3126. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Caielli, S.; Cardenas, J.; de Jesus, A.A.; Baisch, J.; Walters, L.; Blanck, J.P.; Balasubramanian, P.; Stagnar, C.; Ohouo, M.; Hong, S.; et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 2021, 184, 4464–4479.e4419. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Tundo, G.R.; Campagnolo, L.; Valacchi, G.; Orlandi, A.; Curatolo, P.; Borsellino, G.; D’Esposito, M.; Ciaccio, C.; Cesare, S.D.; et al. Retention of Mitochondria in Mature Human Red Blood Cells as the Result of Autophagy Impairment in Rett Syndrome. Sci. Rep. 2017, 7, 12297. [Google Scholar] [CrossRef]
- Thomas, T.A.; Qiu, A.; Kim, C.Y.; Gordy, D.E.; Miller, A.; Tredicine, M.; Dzieciatkowska, M.; Zotti, F.D.; Hod, E.A.; D’Alessandro, A.; et al. Reticulocytes in donor RBC units enhance RBC alloimmunization. bioRxiv 2023. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Culp-Hill, R.; Ludwig, M.P.; Smith, K.P.; Waugh, K.A.; Minter, R.; Tuttle, K.D.; Lewis, H.C.; Rachubinski, A.L.; Granrath, R.E.; et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nat. Commun. 2019, 10, 4766. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015, 55, 205–219. [Google Scholar] [CrossRef]
- LaCroix, I.S.; Cohen, M.; Moore, E.E.; Dzieciatkowska, M.; Nemkov, T.; Schaid, T.R., Jr.; Debot, M.; Jones, K.; Silliman, C.C.; Hansen, K.C.; et al. Omics Markers of Red Blood Cell Transfusion in Trauma. Int. J. Mol. Sci. 2022, 23, 13815. [Google Scholar] [CrossRef]
- Culp-Hill, R.; Srinivasan, A.J.; Gehrke, S.; Kamyszek, R.; Ansari, A.; Shah, N.; Welsby, I.; D’Alessandro, A. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion 2018, 58, 2797–2806. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Hess, J.R.; D’Alessandro, A. Red blood cell metabolism and preservation. In Rossi’s Principles of Transfusion Medicine; Wiley: Hoboken, NJ, USA, 2022; pp. 143–157. [Google Scholar]
- D’Alessandro, A.; D’Amici, G.M.; Vaglio, S.; Zolla, L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 2012, 97, 107–115. [Google Scholar] [CrossRef]
- Yurkovich, J.T.; Zielinski, D.C.; Yang, L.; Paglia, G.; Rolfsson, O.; Sigurjónsson, Ó.E.; Broddrick, J.T.; Bordbar, A.; Wichuk, K.; Brynjólfsson, S.; et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J. Biol. Chem. 2017, 292, 19556–19564. [Google Scholar] [CrossRef]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef]
- D’Alessandro, A. Red Blood Cell Omics and Machine Learning in Transfusion Medicine: Singularity Is Near. Transfus. Med. Hemotherapy 2023, 1–10. [Google Scholar] [CrossRef]
- Gevi, F.; D’Alessandro, A.; Rinalducci, S.; Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J. Proteom. 2012, 76, 168–180. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Korsten, H.; van Bruggen, R.; de Korte, D. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion 2018, 58, 1992–2002. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Hansen, K.C.; Szczepiorkowski, Z.M.; Dumont, L.J. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: A metabolomics approach. Transfusion 2015, 55, 2955–2966. [Google Scholar] [CrossRef]
- Yoshida, T.; Blair, A.; D’Alessandro, A.; Nemkov, T.; Dioguardi, M.; Silliman, C.C.; Dunham, A. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017, 15, 172–181. [Google Scholar] [CrossRef]
- Kriebardis, A.G.; Antonelou, M.H.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J. Cell. Mol. Med. 2007, 11, 148–155. [Google Scholar] [CrossRef]
- Rinalducci, S.; Ferru, E.; Blasi, B.; Turrini, F.; Zolla, L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012, 10 (Suppl. S2), s55–s62. [Google Scholar] [CrossRef]
- Rogers, S.C.; Ross, J.G.; d’Avignon, A.; Gibbons, L.B.; Gazit, V.; Hassan, M.N.; McLaughlin, D.; Griffin, S.; Neumayr, T.; Debaun, M.; et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood 2013, 121, 1651–1662. [Google Scholar] [CrossRef]
- de Bruin, S.; Peters, A.L.; Wijnberge, M.; van Baarle, F.; AbdelRahman, A.H.A.; Vermeulen, C.; Beuger, B.M.; Reisz, J.A.; D’Alessandro, A.; Vlaar, A.P.J.; et al. Storage of red blood cells in alkaline PAGGGM improves metabolism but has no effect on recovery after transfusion. Blood Adv. 2022, 6, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Shin, H.K.H.; Baek, J.H.; Champagne, D.P.; Nemkov, T.; Thomas, T.; Francis, R.O.; Zimring, J.C.; Yoshida, T.; Reisz, J.A.; et al. Red blood cell metabolism in Rhesus macaques and humans: Comparative biology of blood storage. Haematologica 2020, 105, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Bertolone, L.; Shin, H.K.; Stefanoni, D.; Baek, J.H.; Gao, Y.; Morrison, E.J.; Nemkov, T.; Thomas, T.; Francis, R.O.; Hod, E.A.; et al. ZOOMICS: Comparative Metabolomics of Red Blood Cells from Old World Monkeys and Humans. Front. Physiol. 2020, 11, 593841. [Google Scholar] [CrossRef] [PubMed]
- Zimring, J.C.; Smith, N.; Stowell, S.R.; Johnsen, J.M.; Bell, L.N.; Francis, R.O.; Hod, E.A.; Hendrickson, J.E.; Roback, J.D.; Spitalnik, S.L. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion 2014, 54, 137–148. [Google Scholar] [CrossRef]
- Williams, A.T.; Jani, V.P.; Nemkov, T.; Lucas, A.; Yoshida, T.; Dunham, A.; D’Alessandro, A.; Cabrales, P. Transfusion of Anaerobically or Conventionally Stored Blood After Hemorrhagic Shock. Shock 2020, 53, 352–362. [Google Scholar] [CrossRef]
- Bertolone, L.; Shin, H.K.H.; Baek, J.H.; Gao, Y.; Spitalnik, S.L.; Buehler, P.W.; D’Alessandro, A. ZOOMICS: Comparative Metabolomics of Red Blood Cells From Guinea Pigs, Humans, and Non-human Primates During Refrigerated Storage for Up to 42 Days. Front. Physiol. 2022, 13, 845347. [Google Scholar] [CrossRef]
- Miglio, A.; Maslanka, M.; Di Tommaso, M.; Rocconi, F.; Nemkov, T.; Buehler, P.W.; Antognoni, M.T.; Spitalnik, S.L.; D’Alessandro, A. ZOOMICS: Comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days. Blood Transfus. 2022. [Google Scholar] [CrossRef]
- Miglio, A.; Cremonini, V.; Leonardi, L.; Manuali, E.; Coliolo, P.; Barbato, O.; Dall’Aglioa, C.; Antognoni, M.T. Omics Technologies in Veterinary Medicine: Literature Review and Perspectives in Transfusion Medicine. Transfus. Med. Hemotherapy 2023, 5, 198–207. [Google Scholar] [CrossRef]
- Mykhailova, O.; Olafson, C.; Turner, T.R.; D’Alessandro, A.; Acker, J.P. Donor-dependent aging of young and old red blood cell subpopulations: Metabolic and functional heterogeneity. Transfusion 2020, 60, 2633–2646. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Dzieciatkowska, M.; Arvaniti, V.-Z.; Papageorgiou, E.G.; Papassideri, I.S.; Stamoulis, K.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Innate Variability in Physiological and Omics Aspects of the Beta Thalassemia Trait-Specific Donor Variation Effects. Front. Physiol. 2022, 13, 999. [Google Scholar] [CrossRef]
- Raval, J.S.; Waters, J.H.; Seltsam, A.; Scharberg, E.A.; Richter, E.; Kameneva, M.V.; Yazer, M.H. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011, 100, 418–421. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Drossos, P.V.; Karadimas, D.G.; Valsami, S.; Stamoulis, K.E.; Papassideri, I.S.; Politou, M.; Antonelou, M.H.; Kriebardis, A.G. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. 2021, 19, 224–236. [Google Scholar] [CrossRef]
- Fu, X.; Felcyn, J.R.; Odem-Davis, K.; Zimring, J.C. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: A targeted metabolomics study. Transfusion 2016, 56, 2560–2570. [Google Scholar] [CrossRef]
- D’Alessandro, A. In vivo clearance of stored red blood cells. Blood 2021, 137, 2275–2276. [Google Scholar] [CrossRef]
- Roussel, C.; Morel, A.; Dussiot, M.; Marin, M.; Colard, M.; Fricot-Monsinjon, A.; Martinez, A.; Chambrion, C.; Henry, B.; Casimir, M.; et al. Rapid clearance of storage-induced microerythrocytes alters transfusion recovery. Blood 2021, 137, 2285–2298. [Google Scholar] [CrossRef]
- Kerkelä, E.; Lahtela, J.; Larjo, A.; Impola, U.; Mäenpää, L.; Mattila, P. Exploring Transcriptomic Landscapes in Red Blood Cells, in Their Extracellular Vesicles and on a Single-Cell Level. Int. J. Mol. Sci. 2022, 23, 12897. [Google Scholar] [CrossRef]
- Nemkov, T.; Yoshida, T.; Nikulina, M.; D’Alessandro, A. High-Throughput Metabolomics Platform for the Rapid Data-Driven Development of Novel Additive Solutions for Blood Storage. Front. Physiol. 2022, 13, 833242. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Kelher, M.; West, F.B.; Schwindt, R.K.; Banerjee, A.; Moore, E.E.; Silliman, C.C.; Hansen, K.C. Routine storage of red blood cell (RBC) units in additive solution-3: A comprehensive investigation of the RBC metabolome. Transfusion 2015, 55, 1155–1168. [Google Scholar] [CrossRef]
- Rolfsson, Ó.; Sigurjonsson, Ó.E.; Magnusdottir, M.; Johannsson, F.; Paglia, G.; Guðmundsson, S.; Bordbar, A.; Palsson, S.; Brynjólfsson, S.; Guðmundsson, S.; et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017, 112, 326–335. [Google Scholar] [CrossRef]
- Bordbar, A.; Johansson, P.I.; Paglia, G.; Harrison, S.J.; Wichuk, K.; Magnusdottir, M.; Valgeirsdottir, S.; Gybel-Brask, M.; Ostrowski, S.R.; Palsson, S.; et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion 2016, 56, 852–862. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Hansen, K.C.; Silliman, C.C.; Moore, E.E.; Kelher, M.; Banerjee, A. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015, 108, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yurkovich, J.T.; Yang, L.; Palsson, B.O. Biomarkers are used to predict quantitative metabolite concentration profiles in human red blood cells. PLoS Comput. Biol. 2017, 13, e1005424. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Thomas, T.; Akpan, I.J.; Reisz, J.A.; Cendali, F.I.; Gamboni, F.; Nemkov, T.; Thangaraju, K.; Katneni, U.; Tanaka, K.; et al. Biological and Clinical Factors Contributing to the Metabolic Heterogeneity of Hospitalized Patients with and without COVID-19. Cells 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Dumont, L.J.; D’Alessandro, A.; Szczepiorkowski, Z.M.; Yoshida, T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion 2016, 56, 392–403. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Gevi, F.; Zolla, L. Red blood cell metabolism under prolonged anaerobic storage. Mol. Biosyst. 2013, 9, 1196–1209. [Google Scholar] [CrossRef]
- Rabcuka, J.; Blonski, S.; Meli, A.; Sowemimo-Coker, S.; Zaremba, D.; Stephenson, D.; Dzieciatkowska, M.; Nerguizian, D.; Cardigan, R.; Korczyk, P.M.; et al. Metabolic reprogramming under hypoxic storage preserves faster oxygen unloading from stored red blood cells. Blood Adv. 2022, 6, 5415–5428. [Google Scholar] [CrossRef]
- Hay, A.; Dziewulska, K.; Gamboni, F.; Nerguizian, D.; Dzieciatkowska, M.; Zimring, J.C.; D’Alessandro, A. Hypoxic storage of murine red blood cells improves energy metabolism and post-transfusion recoveries. Blood Transfus. 2022, 21. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Yoshida, T.; Nestheide, S.; Nemkov, T.; Stocker, S.; Stefanoni, D.; Mohmoud, F.; Rugg, N.; Dunham, A.; Cancelas, J.A. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion 2020, 60, 786–798. [Google Scholar] [CrossRef]
- Dumont, L.J.; AuBuchon, J.P. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 2008, 48, 1053–1060. [Google Scholar] [CrossRef]
- Roubinian, N.H.; Reese, S.E.; Qiao, H.; Plimier, C.; Fang, F.; Page, G.P.; Cable, R.G.; Custer, B.; Gladwin, M.T.; Goel, R.; et al. Donor genetic and nongenetic factors affecting red blood cell transfusion effectiveness. JCI Insight 2022, 7, e152598. [Google Scholar] [CrossRef]
- Hazegh, K.; Fang, F.; Bravo, M.D.; Tran, J.Q.; Muench, M.O.; Jackman, R.P.; Roubinian, N.; Bertolone, L.; D’Alessandro, A.; Dumont, L.; et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 2021, 61, 435–448. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Paronis, E.C.; Arvaniti, V.Z.; Velentzas, A.D.; Apostolidou, A.C.; Balafas, E.G.; Dzieciatkowska, M.; Kostomitsopoulos, N.G.; Stamoulis, K.; Papassideri, I.S.; et al. The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile. Int. J. Mol. Sci. 2021, 22, 12281. [Google Scholar] [CrossRef]
- van ‘t Erve, T.J.; Doskey, C.M.; Wagner, B.A.; Hess, J.R.; Darbro, B.W.; Ryckman, K.K.; Murray, J.C.; Raife, T.J.; Buettner, G.R. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic. Biol. Med. 2014, 76, 107–113. [Google Scholar] [CrossRef]
- Van ‘t Erve, T.J.; Wagner, B.A.; Martin, S.M.; Knudson, C.M.; Blendowski, R.; Keaton, M.; Holt, T.; Hess, J.R.; Buettner, G.R.; Ryckman, K.K.; et al. The heritability of hemolysis in stored human red blood cells. Transfusion 2015, 55, 1178–1185. [Google Scholar] [CrossRef]
- van ‘t Erve, T.J.; Wagner, B.A.; Martin, S.M.; Knudson, C.M.; Blendowski, R.; Keaton, M.; Holt, T.; Hess, J.R.; Buettner, G.R.; Ryckman, K.K.; et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion 2014, 54, 2055–2063. [Google Scholar] [CrossRef]
- Hay, A.M.; Howie, H.L.; Gorham, J.D.; D’Alessandro, A.; Spitalnik, S.L.; Hudson, K.E.; Zimring, J.C. Mouse background genetics in biomedical research: The devil’s in the details. Transfusion 2021, 61, 3017–3025. [Google Scholar] [CrossRef]
- Endres-Dighe, S.M.; Guo, Y.; Kanias, T.; Lanteri, M.; Stone, M.; Spencer, B.; Cable, R.G.; Kiss, J.E.; Kleinman, S.; Gladwin, M.T.; et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion 2019, 59, 46–56. [Google Scholar] [CrossRef]
- Josephson, C.D.; Glynn, S.; Mathew, S.; Birch, R.; Bakkour, S.; Baumann Kreuziger, L.; Busch, M.P.; Chapman, K.; Dinardo, C.; Hendrickson, J.; et al. The Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P): A research program striving to improve blood donor safety and optimize transfusion outcomes across the lifespan. Transfusion 2022, 62, 982–999. [Google Scholar] [CrossRef]
- Lanteri, M.C.; Kanias, T.; Keating, S.; Stone, M.; Guo, Y.; Page, G.P.; Brambilla, D.J.; Endres-Dighe, S.M.; Mast, A.E.; Bialkowski, W.; et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: Results of recall phase of the REDS-III RBC-Omics study. Transfusion 2019, 59, 79–88. [Google Scholar] [CrossRef]
- Guo, Y.; Busch, M.P.; Seielstad, M.; Endres-Dighe, S.; Westhoff, C.M.; Keating, B.; Hoppe, C.; Bordbar, A.; Custer, B.; Butterworth, A.S.; et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion 2019, 59, 101–111. [Google Scholar] [CrossRef]
- Roubinian, N.H.; Plimier, C.; Woo, J.P.; Lee, C.; Bruhn, R.; Liu, V.X.; Escobar, G.J.; Kleinman, S.H.; Triulzi, D.J.; Murphy, E.L.; et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood 2019, 134, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Busch, M.P.; Dziewulska, K.; Francis, R.O.; Hod, E.A.; Zimring, J.C.; D’Alessandro, A.; Page, G.P. Genome-wide metabolite quantitative trait loci analysis (mQTL) in red blood cells from volunteer blood donors. J. Biol. Chem. 2022, 298, 102706. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Stefanoni, D.; Bordbar, A.; Issaian, A.; Palsson, B.O.; Dumont, L.J.; Hay, A.M.; Song, A.; Xia, Y.; Redzic, J.S.; et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight 2021, 6, e146175. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Fu, X.; Reisz, J.A.; Kanias, T.; Nemkov, T.; Page, G.P.; Dumont, L.; Roubinian, N.; Stone, M.; Kleinman, S.; et al. Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion 2020, 60, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.A.; Plimier, C.; Lee, C.; Kanias, T.; Cushing, M.M.; Sachais, B.S.; Kleinman, S.; Busch, M.P.; Roubinian, N.H. Additive effects of blood donor smoking and gamma irradiation on outcome measures of red blood cell transfusion. Transfusion 2020, 60, 1175–1182. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Fu, X.; Reisz, J.A.; Stone, M.; Kleinman, S.; Zimring, J.C.; Busch, M. Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion 2020, 60, 1183–1196. [Google Scholar] [CrossRef]
- Bertolone, L.; Roy, M.K.; Hay, A.M.; Morrison, E.J.; Stefanoni, D.; Fu, X.; Kanias, T.; Kleinman, S.; Dumont, L.J.; Stone, M.; et al. Impact of taurine on red blood cell metabolism and implications for blood storage. Transfusion 2020, 60, 1212–1226. [Google Scholar] [CrossRef]
- Hill, H.R.; Oliver, C.K.; Lippert, L.E.; Greenwalt, T.J.; Hess, J.R. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sang. 2001, 81, 161–166. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Hansen, K.C. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016, 14, 140–144. [Google Scholar] [CrossRef]
- Swift, L.M.; Roberts, A.; Pressman, J.; Guerrelli, D.; Allen, S.; Haq, K.T.; Reisz, J.A.; D’Alessandro, A.; Posnack, N.G. Toxins in plastic: Evidence for the cardiodepressive effects of di-2-ethylhexylphthalate (DEHP). bioRxiv 2023. [Google Scholar] [CrossRef]
- Gasiorowski, R.; Forbes, M.K.; Silver, G.; Krastev, Y.; Hamdorf, B.; Lewis, B.; Tisbury, M.; Cole-Sinclair, M.; Lanphear, B.P.; Klein, R.A.; et al. Effect of Plasma and Blood Donations on Levels of Perfluoroalkyl and Polyfluoroalkyl Substances in Firefighters in Australia: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e226257. [Google Scholar] [CrossRef]
- Himbert, S.; D’Alessandro, A.; Qadri, S.M.; Majcher, M.J.; Hoare, T.; Sheffield, W.P.; Nagao, M.; Nagle, J.F.; Rheinstädter, M.C. The bending rigidity of the red blood cell cytoplasmic membrane. PLoS ONE 2022, 17, e0269619. [Google Scholar] [CrossRef]
- Himbert, S.; Qadri, S.M.; Sheffield, W.P.; Schubert, P.; D’Alessandro, A.; Rheinstädter, M.C. Blood bank storage of red blood cells increases RBC cytoplasmic membrane order and bending rigidity. PLoS ONE 2021, 16, e0259267. [Google Scholar] [CrossRef]
- Bicalho, B.; Holovati, J.L.; Acker, J.P. Phospholipidomics reveals differences in glycerophosphoserine profiles of hypothermically stored red blood cells and microvesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 317–326. [Google Scholar] [CrossRef]
- Huyut, Z.; Şekeroğlu, M.R.; Balahoroğlu, R.; Huyut, M.T. Characteristics of resveratrol and serotonin on antioxidant capacity and susceptibility to oxidation of red blood cells in stored human blood in a time-dependent manner. J. Int. Med. Res. 2018, 46, 272–283. [Google Scholar] [CrossRef]
- Antosik, A.; Czubak, K.; Cichon, N.; Nowak, P.; Zbikowska, H. Vitamin E Analogue Protects Red Blood Cells against Storage-Induced Oxidative Damage. Transfus. Med. Hemother. 2018, 45, 347–354. [Google Scholar] [CrossRef]
- Pallotta, V.; Gevi, F.; D’Alessandro, A.; Zolla, L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: A metabolomics overview. Blood Transfus. 2014, 12, 376–387. [Google Scholar] [CrossRef]
- Stowell, S.R.; Smith, N.H.; Zimring, J.C.; Fu, X.; Palmer, A.F.; Fontes, J.; Banerjee, U.; Yazer, M.H. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion 2013, 53, 2248–2257. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Arduini, A. L-carnitine and its possible role in red cell and platelet storage. Transfus. Med. Rev. 2004, 18, 58–65. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Açaì (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence for structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Catala, A.; Stone, M.; Busch, M.P.; D’Alessandro, A. Reprogramming of red blood cell metabolism in Zika virus–infected donors. Transfusion 2022, 62, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Zimring, J.C.; Busch, M. Chronological storage age and metabolic age of stored red blood cells: Are they the same? Transfusion 2019, 59, 1620–1623. [Google Scholar] [CrossRef]
- Marrocco, C.; Pallotta, V.; D’Alessandro, A.; Alves, G.; Zolla, L. Red blood cell populations and membrane levels of peroxiredoxin 2 as candidate biomarkers to reveal blood doping. Blood Transfus. 2012, 10 (Suppl. S2), s71–s77. [Google Scholar] [CrossRef]
- Scott, A.V.; Nagababu, E.; Johnson, D.J.; Kebaish, K.M.; Lipsitz, J.A.; Dwyer, I.M.; Zuckerberg, G.S.; Barodka, V.M.; Berkowitz, D.E.; Frank, S.M. 2,3-Diphosphoglycerate Concentrations in Autologous Salvaged Versus Stored Red Blood Cells and in Surgical Patients After Transfusion. Anesth. Analg. 2016, 122, 616–623. [Google Scholar] [CrossRef]
- Hay, A.; Howie, H.L.; Waterman, H.R.; de Wolski, K.; Zimring, J.C. Murine red blood cells from genetically distinct donors cross-regulate when stored together. Transfusion 2017, 57, 2657–2664. [Google Scholar] [CrossRef]
- Figueroa, D. The Diego blood group system: A review. Immunohematology 2013, 29, 73–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).