Abstract
Cholesterol has many critical functions in cells. It is a key component of membranes and cell-signalling processes, and it functions as a chemical precursor in several biochemical pathways, such as Vitamin D and steroid synthesis. Cholesterol has also been implicated in the development and progression of various cancers, in which it is thought to promote cell proliferation, migration, and invasion. Chronic lymphocytic leukemia (CLL) is an example of a lipid-avid cancer that relies on lipid metabolism, rather than glycolysis, to fuel cell proliferation. However, data regarding the role of cholesterol in CLL are conflicting. Studies have shown that dyslipidaemia is more common among CLL patients than age-matched healthy controls, and that CLL patients who take cholesterol-lowering drugs, such as statins, appear to have improved survival rates. Therefore, defining the roles of cholesterol in CLL may highlight the importance of monitoring and managing hyperlipidaemia as part of the routine management of patients with CLL. In this review, we discuss the roles of cholesterol in the context of CLL by examining the literature concerning the trafficking, uptake, endogenous synthesis, and intracellular handling of this lipid. Data from clinical trials investigating various classes of cholesterol and lipid-lowering drugs in CLL are also discussed.
1. Introduction—The Role of Cholesterol in Cancer
For almost a century, it has been acknowledged that nutrients play an important role in cancer [1]. There is growing evidence that cholesterol, a type of lipid, plays a significant role in carcinogenesis. Numerous studies, both in vitro and clinical, have found that hypercholesterolemia can affect the development of cancer [2]. For example, a 10mg/dL increase in cholesterol levels was associated with a 9% increase in prostate cancer recurrence [3]. In a study of almost 300,000 Danish patients, lowering cholesterol levels through the administration of drugs such as statins was found to cause a slight reduction in cancer-related mortality of patients with 13 different types of cancer [4].
There is evidence that serum cholesterol levels start to decline up to 6 years before the detection of colon cancer and chronic myeloid leukemia [5,6], suggesting that cholesterol levels may be an early predictor of cancer development for some tumours. Conversely, epidemiological studies have shown that increased serum cholesterol is positively correlated with a higher incidence of some cancers, such as those of the colon, prostate, and testes [2,7]. While data from these studies suggest that cholesterol levels may be critical in some tumours, a review by Ding et al. [8] suggests that the link between cancer progression and cholesterol is much more complicated than a simple two-factor association, and that other factors, such as the cholesterol requirement for the tissue of cancer origin and dietary habits, may be more critical. For example, in patients with bladder cancer, more aggressive disease was observed among patients taking statins [9], and large studies carried out in the 1980s determined that low cholesterol levels were associated with colon and lung cancer [6,10]. These studies, among many others, highlight the need for further investigation into whether targeting cholesterol metabolism may represent a novel therapeutic avenue for cancer treatment.
2. Cholesterol Synthesis and Exogenous Sources
In humans, cholesterol can be generated endogenously or derived from exogenous dietary sources. Endogenous cholesterol generation is regulated by the enzyme 3-hydroxy-3-methylglutaryl co-enzyme reductase (HMG-CoA reductase) and is predominately synthesized in the liver, where it is packaged into low-density lipoproteins (LDL). Cholesterol can also be sourced from the diet in a process that involves the packaging of the cholesterol into chylomicrons in the intestine, which are then transported into the circulation via capillaries. Cells expressing lipoprotein lipase (LPL) can degrade the chylomicrons, releasing free fatty acids, which can then be absorbed into cells. Chylomicrons, or their remnants, are then transported to the liver where they are either degraded or re-processed into VLDL particles.
Cholesterol homeostasis is tightly regulated by a number of processes, which are summarized in Figure 1. When dietary cholesterol levels are low, more is synthesized via the endogenous pathway. Cells that rely on endogenously synthesized cholesterol are often incapable of accumulating excess cholesterol due to homeostatic regulation at various points in the cholesterol synthesis pathway. In cancer, cholesterol homeostasis is often disordered, which may be an early event that occurs within 2 weeks of exposure to carcinogens [11]. However, it is unclear whether cholesterol increases the risk of cancer development, or whether increased cholesterol levels are a consequence of malignancy. Here, we focus on the role of cholesterol in the B-cell malignancy, chronic lymphocytic leukemia (CLL). We have recently shown significant differences in lipid metabolism in CLL cells compared to healthy B-cells [12], suggesting that cholesterol may play a crucial role in this disease.
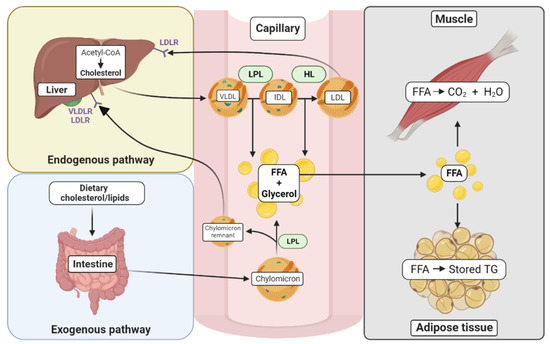
Figure 1.
Cholesterol synthesis and trafficking in various tissues. The exogenous pathway begins with the uptake of dietary cholesterol where lipids are incorporated into chylomicrons in the intestine. Once in the circulation, the chylomicrons are metabolized by tissues that contain lipoprotein lipase (LPL), and their contents, free fatty acids (FFA) and glycerol, are metabolized by tissues such as muscle and adipose tissue. In muscle, FFAs are used for energy. In the adipose tissue, they can be stored in the form of triglycerides (TG). The resulting chylomicron remnants in the circulation are absorbed in the liver by apolipoprotein-specific receptors (VLDLR and LDLR). The endogenous pathway begins in the liver with the formation of cholesterol from acetyl-CoA, catalyzed by the enzyme HMG-CoA reductase. The VLDL particles, containing triglycerides and cholesterol esters, can be hydrolyzed by LPL to form IDL with the release of FFAs. IDLs can further be hydrolyzed to LDLs by the action of hepatic lipase (HL), which also results in the release of FFAs. LDL can be internalized by other tissues, such as the liver by the LDL receptor (LDLR).
3. Chronic Lymphocytic Leukemia (CLL)
CLL is the most common leukemia in the Western world. While the median age of diagnosis is 72 years, CLL is often diagnosed in younger individuals, with almost 15% of patients being 55 years or younger [13]. The clinical course of CLL is heterogenous, with many patients not requiring treatment at diagnosis. Various genetic markers, such as the presence of genomic aberrations and the mutational status of the immunoglobulin heavy chain gene (IGHV), are predictive of prognosis in CLL. Approximately 30% of patients will never require treatment [14], while the outcome for the remaining 70% will depend on various factors, such as comorbidities, the ability to deliver optimal treatment, and cytogenomic profiles. Historically, combined chemoimmunotherapy regimens were the preferred first-line treatments for symptomatic CLL patients (and remains so in some parts of the world due to drug accessibility). Increasingly, small molecular inhibitors, such as the BTK-inhibitor ibrutinib and the BCL-2 antagonist venetoclax, are being used as first-line treatment and at relapse. Despite high-response rates following treatment with these agents [14], most patients will ultimately relapse. Therefore, CLL is still widely considered incurable.
4. Serum Cholesterol Levels and Prognosis in CLL
Studies suggest that rates of dyslipidemia are higher among CLL patients than age-matched healthy controls [15]. One study showed that CLL patients suffer from hypercholesterolemia, observing elevated LDL levels in 75% of CLL patients [16]. Another study reported lower serum levels in CLL patients compared to healthy individuals, with CLL patients averaging 151.2 +/− 11.2 mg/dL compared to 195.3 +/− 8.5 mg/dL in healthy, aged-matched controls [17]. CLL patients with hypocholesterolemia have a shorter overall and treatment-free survival [18], possibly due to increased catabolism of LDL and impaired hepatic lipoprotein synthesis [17], or increased expression of the LDL receptor (LDLR) on CLL cells [19], leading to increased cellular uptake.
Hypercholesteremia may be due to an increase in the hepatic secretion of cholesterol into the blood stream; this may potentially fuel the rapid proliferation of leukemic cells in patients with a poor prognosis [20]. This notion is supported by a study that focused on patients with high-risk disease, defined by unmutated IGHV status (UM–IGHV). Using a metabolomic approach, significantly higher levels of serum cholesterol and LDL fatty acid side chains were observed in patients with high-risk disease [20]. This study also determined that UM–IGHV patients had higher levels of VLDL and lower levels of HDL cholesterol compared to patients with mutated IGHV genes (M–IGHV), while LDL levels were similar between the two groups of patients [20]. Hyperlipidemia may lead to inflammation, which helps drive CLL cell proliferation [21]. It is not surprising that statins, commonly used to treat hypercholesteremia, delayed the need for treatment by nearly 3 years [16].
In another study, no association between serum levels of LDL, HDL, total cholesterol and triglycerides, and clinical outcomes was observed [22]. However, this study included samples from a relatively small number of patients (n = 26), which raises the possibility that discrepancies between the studies may, at least in part, be due to the heterogeneity among CLL patients, and that much larger cohort studies are warranted. While there are conflicting reports concerning the role of cholesterol in CLL, there is clear evidence that cholesterol uptake and metabolism is disordered in CLL cells compared to healthy B-cells and other blood cancers, including acute lymphoblastic (ALL), acute myeloid (AML), and chronic myeloid leukemia (CML) [23].
5. The Role of Intracellular Cholesterol in CLL
Under normal physiological conditions, free cholesterol in the plasma membrane is not static and continuously shuttled between the cell interior and surface [24]. Generally, cytoplasmic cholesterol exists in the form of cholesterol esters, whereas free cholesterol is located almost entirely within the plasma membrane. The esterification of intracellular cholesterol is essential, as free cholesterol in the cytoplasm is toxic [25,26]. Intracellular free cholesterol is esterified by acyl-coenzyme-1 (ACAT-1) and released by the action of neutral cholesterol ester hydrolase 1 (nCEH-1). In normal lymphocytes, approximately 90% of intracellular cholesterol is free, and 8% is in the esterified form. However, in leukemic cells (CLL and ALL), approximately 40% is esterified, and ACAT-1 expression is increased two-fold [27]. Leukemic cells appear to have a unique ability to trap and store cholesterol in the form of lipid droplets, which may restrict the shuttling of cholesterol to cell membranes. This presents a potentially novel therapeutic avenue in CLL; by inhibiting cholesterol esterification and its incorporation into lipid droplets, increased free cholesterol levels in the cytoplasm would lead to lipotoxic cell death. This is supported by a study in which the inhibition of cholesterol esterification by progesterone or SaH 58-035 led to a reduction in cholesterol esterification and leukemic cell proliferation [27].
Intracellular Cholesterol Levels in CLL
Almost 50 years ago, Hildebrand et al. [28] determined that CLL cells contain higher concentrations of lipids than non-leukemic cells or malignant cells from patients with CML or acute leukemia. The study determined that a significant proportion of the neutral lipid content of CLL cells is cholesterol, and that the ratio of free-to-total cholesterol is elevated in CLL cells compared to healthy B-cells. CLL cells were also determined to contain high levels of phospholipids. However, another study, published around the same time as the Hildebrand study, contradicted these findings by demonstrating that the cholesterol content of CLL cells was significantly lower than that of healthy B-cells [29]. Lower intracellular levels of cholesterol have also been observed in CLL cells than in leukemic cells from patients with hairy cell leukemia [30]. A recent study also concluded that there was no difference in total intracellular cholesterol or phospholipid levels in CLL cells compared to healthy B-cells, but that the leukemic cells have lower levels of free cholesterol and higher levels of cholesterol esters than their healthy counterpart [27]. Reasons for the discrepancies between these studies are unclear but could be due to the proliferative state of the CLL cells examined. CLL cells migrate from tissue microenvironments in lymph nodes and bone marrow to the peripheral blood, with concomitant changes in their metabolic demands; quiescent CLL cells in the peripheral circulation likely have lower metabolic demands, which may be reflected in their cholesterol content.
6. Cholesterol Uptake in CLL
6.1. HDL Uptake
Many malignant cells overexpress SR-B1, a high affinity receptor located in lipid rafts that functions as an HDL receptor and mediates cellular uptake of HDL-derived cholesterol esters [31,32]. When HDL particles bind to SR-B1, the diffusion of free cholesterol occurs from the HDL particle into the plasma membrane, resulting in a reduction in the size of the HDL particle. Decreased levels of HDL have been observed in the peripheral blood of CLL patients compared to aged matched healthy controls [33], which may be due to the overexpression of SR-B1 on CLL cells [34], leading to an increase in the sequestration of HDL into tumour cells (Figure 2).
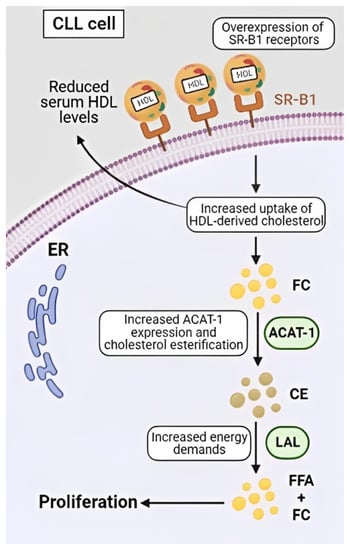
Figure 2.
The high-affinity HDL receptor, SR-B1, is overexpressed on CLL cells, which leads to an increased uptake of HDL-derived cholesterol esters and the diffusion of free cholesterol (FC) into the plasma membrane and cytoplasm. As cytoplasmic FC is toxic, it undergoes esterification by acyl-coenzyme-1 (ACAT-1) to protect the cells from ER stress and apoptosis. Cholesterol esters (CE) represent a safe way to store intracellular cholesterol and act as a repository, which can be exploited by the cancers cells to fuel rapid proliferation by lysosomal acid lipase (LAL) degradation of CE to free fatty acids (FFA) and FC.
In B-cell lymphomas, the administration of synthetic HDL particles, which mimic endogenous HDL particles but are devoid of any free cholesterol or cholesterol esters, have been proposed as a therapeutic option [35]. These HDL nanoparticles were found to potently induce apoptosis of the leukemic cells in animal models without any evidence of systemic side effects or toxicity [35]. A similar study in CLL [34] determined that expression of SR-B1 on CLL cells, but not healthy B-cells, correlated with the cytotoxic effects of the nanoparticles towards the leukemic cells, but not their healthy counterpart. Although HDL nanoparticles are yet to be tested in human trials, the preclinical data suggests they may represent a novel therapeutic approach for CLL and other lipid-avid cancers.
6.2. LDL Uptake
The internalization of exogenous cholesterol is predominately mediated by LDL receptors (LDLR) and endocytosis [36]. When LDL particles come in contact with cancer cells, they bind to specific LDLRs. The LDL particle and its associated receptor are then internalized by clathrin-mediated endocytosis. The LDL and LDLR are then transported to lysosomes and hydrolyzed to release cholesterol and fatty acids, which are then incorporated into various cellular structures, including membranes [37]. LDLR expression is regulated by sterols via sterol regulatory elements (SRE), which bind to transcription factors known as SRE-binding proteins (SREBPs). In response to elevated levels of intracellular cholesterol, the expression of LDLRs and SREBP2 is downregulated [38]. In healthy cells, SREBP2 is a potent regulator of the LDLR promoter, but this does not appear to be true in cancer cells [39] (Figure 3).
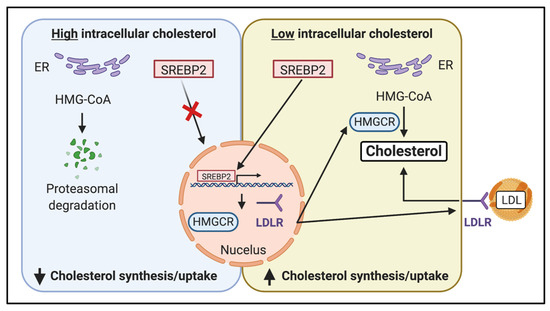
Figure 3.
The sterol regulatory element-binding protein transcription factor 2 (SREBP2) pathway regulates endogenous cholesterol synthesis. Cholesterol levels in the endoplasmic reticulum (ER) act as a sensor for intracellular cholesterol homeostasis. When high-intracellular cholesterol levels are detected, SREBP2 remains in the ER, resulting in a decreased expression of HMG–CoA reductase (HMGCR). HMG–CoA also undergoes proteasomal degradation, further reducing its activity. When cholesterol levels in the ER decrease, SREBP2 translocates to the nucleus and activates the transcription of genes, including HMGCR, which regulates the expression of HMG–CoA reductase and the LDL receptor (LDLR), resulting in an increase in cholesterol uptake into the cell.
In CLL, LDLRs may play a critical role in regulating CLL proliferation. Sankanagoudar et al. [24] observed hypocholesteremia and hyper-expression of LDLR in CLL cells, which was associated with increased levels of cholesterol in both the nucleus and cytoplasm. The study demonstrated that the uptake of fluorescently conjugated cholesterol (Dil–LDL) was significantly higher in CLL lymphocytes than in healthy B-cells [19]. However, the study also determined that the rate of LDL degradation is lower in CLL cells than in B-cells from healthy individuals, and that LDLR activity was no different in CLL cells than in healthy B-cells [40]. Similar results were observed by Juliussion and Vitols, in which the authors used radio-labelled LDL (125I-LDL) to demonstrate that the rate of LDL degradation is significantly lower in CLL cells compared to healthy B-cells [40]. LDLR activity has also been shown to be lower in CLL cells than in leukemic cells from patients with acute myeloid leukemia (AML) [41]. The low LDLR activity in CLL cells observed in these studies may be due to the quiescent nature of CLL cells in the peripheral circulation, which are morphologically similar to small resting B-cells [42,43]. A similar observation was noted in quiescent fibroblasts. When these cells were stimulated with growth factors, there was an increased expression of the LDLR at the cell surface [44] However, no studies to date have compared LDLR activity in CLL cells to that of small resting lymphocytes, probably due to the rarity of the latter in healthy individuals. As the authors suggest, the variability in LDL activity observed may also be due to heterogeneity between the CLL patients studied [40]. Although further studies are required to determine whether LDL uptake correlates with clinical parameters in CLL, it is conceivable that LDL utilization may promote leukemic cell proliferation and may, therefore, be associated with a more aggressive disease course.
6.3. Chylomicron Metabolism
Chylomicrons are lipoproteins that transport lipids in the blood. Chylomicrons are degraded through lipolysis by the enzyme lipoprotein lipase (LPL) at the cell surface. LPL hydrolyses phospholipids in VLDL particles, resulting in an increase in serum triglycerides, a release of free fatty acids, and the conversion of VLDL particles to LDL particles. Remnants of chylomicrons are then either degraded or re-processed into VLDL particles by the liver. LPL is highly expressed on the surface of CLL cells [45,46] but not healthy lymphocytes, suggesting the leukemic cells are capable of metabolizing chylomicrons. Furthermore, high LPL expression on CLL cells is associated with a poor prognosis [47] and significantly worse survival [48]. Data from our laboratory have shown that CLL cells include high amounts of free fatty acids [49], which supports the hypothesis that free fatty acids may provide a crucial energy source for these cells.
Chylomicron degradation is very difficult to assess in vivo. Sakashita et al. [50] created a double-labelled chylomicron-resembling emulsion, which was injected into 10 CLL patients and 11 healthy controls. The kinetics of triglycerides (TAGS) and cholesterol ester levels were then determined in plasma samples collected over a 30-min time course. No significant difference in the fractional clearance rate of TAGS and cholesterol esters was observed between CLL cells and controls, suggesting that the rates of chylomicron lipolysis and remnant removal are not altered in CLL. However, ~90% of the patients in this study had indolent disease suggesting that the energy requirements and proliferation rates of the leukemic cells may be lower than in patients with rapidly progressing disease.
7. LDLs as Signaling Molecules
Numerous pathways mediate the growth and proliferation of CLL cells, but three appear to be critical: the extracellular signal-regulated kinase, mitogen-activated protein kinase (ERK1/2-MAPK), and Ak-strain transforming (AKT) and signal transducer and activator of transcription-3 (STAT3) pathways, which are all activated by LDLs in CLL cells [23]. STAT3 phosphorylation drives LPL expression by binding to transcriptional activators [51]. The breakdown of VLDL and chylomicrons by LPL, and the resulting free fatty acids, activate STAT3 and, in turn, increase the expression of LPL [52]. However, they do so without cross-linking of the B-cell receptor (BCR) [23]. LDL uptake has been observed in healthy PBMCs, although no change in plasma cholesterol levels or activation of STAT3 was observed [23], reinforcing the notion that CLL cells handle cholesterol differently than normal B-cells. Interestingly, the downregulation of several genes involved in intracellular cholesterol transport has been observed in CLL cells compared to healthy B-cells. These include sortilin-related receptor-1 (SORL1), which inhibits lipolysis of intracellular lipid droplets. CLL cells also appear to handle cholesterol differently than tumour cells from other blood cancers, including the Burkitts lymphoma cell line Daudi, which showed no increase in STAT3 phosphorylation in response to the addition of exogenous LDLs. STAT3 phosphorylation in CLL cells in response to LDLs can be suppressed by anti-IL-10 antibodies and by the inhibition of Janus kinase (JAK) [53], suggesting that there is some overlap in the function of these pathways. In their study, McCaw et al. [23] investigated which constituents of LDL molecules were responsible for activation of STAT3 and determined that long chain fatty acids and free cholesterol were critical, and that short- and medium-chain fatty acids, which are not transported in LDLs, did not activate STAT3 [23]. This is supported by our recent study, in which we have shown that CLL cells preferentially include long chain fatty acids rather than medium- and short-chain fatty acids [49].
8. Endogenous Sterol Synthesis Pathways
CLL cells respond to extracellular LDL by reducing cholesterol synthesis [54]. However, it has been suggested that CLL cells may be more dependent on cholesterol synthesized by the endogenous pathway than on the LDLR-mediated uptake [55]. Sterol synthesis is tightly regulated by the enzyme HMG–CoA reductase and cholesterol levels in the endoplasmic reticulum (ER) act as a sensor for intracellular cholesterol homeostasis. When levels of cholesterol at the ER decrease, sterol regulatory element-binding protein transcription factor 2 (SREBF2) translocates to the nucleus and activates transcription of genes, including HMG–CoA reductase (HMGCR), which induce cholesterol synthesis and increase LDLR expression, resulting in increased uptake of cholesterol into the cell. When intracellular levels of cholesterol rise, the expression of HMG–CoA reductase is decreased through proteasomal degradation and a reduction in gene transcription (Figure 3).
HMG–CoA reductase activity is significantly elevated (up to 20-fold) in a wide variety of hematological malignancies, including in CLL cells, (35.1 ± 5 pmol/min/mg) compared to healthy B-cells (10.3 ± 0.8 pmol/min/mg) [55]. HMG–CoA reductase activity has been demonstrated to be regulated by the fluidity of the microsomal membrane, which are small vesicle-like artifacts formed from the ER. Increased membrane fluidity has been described as a feature of CLL lymphocytes [56,57], possibly due to low membrane cholesterol levels and hypolipidemia observed in several studies [58,59,60]. It is possible that the increase in membrane fluidity in CLL cells drives the increase in HMG–CoA reductase activity in these cells [61]. Several reports suggest that the basal phosphorylation state of the enzyme may be altered in cancer cells [62,63] leading to an increase in activity of the enzyme. Since HMG–CoA reductase expression is higher in CLL cells than in their healthy counterpart [55], it is likely that CLL cells may be more sensitive to HMG–CoA reductase inhibitors, such as statins, which will be discussed in more detail later in this review. High levels of endogenous sterol synthesis are likely to be crucial, particularly during periods of rapid cell proliferation, as high amounts of cholesterol are essential for the generation of new cellular structures, including plasma membranes. Newly synthesized cholesterol produced via the endogenous pathway can be incorporated into the plasma membrane in less than 15 min after synthesis [64] facilitating this rapid cell proliferation.
9. The Use of Cholesterol Lowering Drugs in CLL
Numerous cell types obtain cholesterol from LDLs, which circulate in the blood. Statins, a widely used class of cholesterol-lowering drugs, decrease LDL levels by inhibiting HMG–CoA reductase [65]. This, in turn, increases LDL clearance from the circulation by increasing expression of the LDL receptor on the cell surface. While the benefits of statins in cardiovascular disease are well-documented, they have also been associated with a reduction in cancer-associated deaths [66,67]. Multiple cancer-related studies have been published in which the effects of statins, and a range of other lipid-lowering medications, have been examined both in vitro (summarized in Table 1) and in clinical studies (summarized in Table 2).

Table 1.
Outcomes from in-vitro and animal studies on the effects of targeting cholesterol-associated pathways in CLL.

Table 2.
Clinical studies assessing the use of cholesterol lowering drugs on various clinical outcomes in CLL patients.
9.1. Statins
Statins are a broad class of drugs that include hydrophilic (rosuvastatin and pravastatin) and lipophilic statins (atorvastatin, simvastatin, lovastatin, fluvastatin, cerivastatin, and pitavastatin). Hydrophilic statins are liver-specific, whereas lipophilic statins are widely distributed in different tissues. Their mechanism of action and systemic effects are summarized in Figure 4.
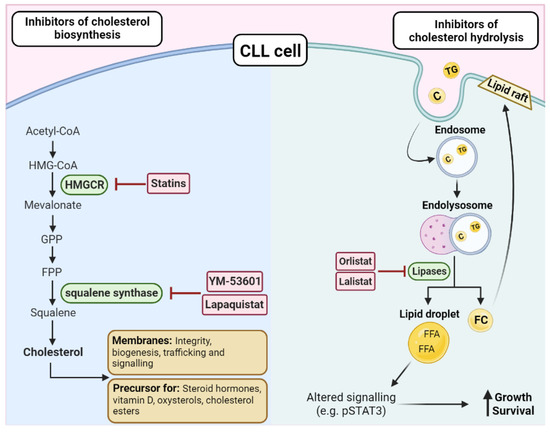
Figure 4.
Targeting cholesterol biosynthesis or hydrolysis in CLL. There are two key enzymes in the biosynthesis of cholesterol, HMG–CoA reductase (HMGCR), and squalene synthase, which can be targeted by a range of inhibitors such as statins, YM-53601, and lapaquistat. As cholesterol is essential for membranes and as a precursor for cholesterol esters and other factors, disrupting the biosynthesis pathway is an attractive treatment option in CLL. On the other hand, triglycerides (TG) and cholesterol (C) can directly enter the cell via endosomes and be hydrolyzed by lipases. The resulting free fatty acids (FFAs) and free cholesterol (FC) can have downstream effects that promote the growth and survival of the CLL cells, such as altering the expression of transcriptional regulators, such as STAT3 or by increasing the lipid content of the membrane, leading to altered cellular functions, such as increased activation of oncogenic pathways and trafficking. Orlistat and lalistat are two inhibitors that have been investigated in CLL due to their ability to block lipases and prevent the hydrolysis of cholesterol and triacylglycerols.
As HMG–CoA reductase levels are elevated in CLL cells [55], it is likely the leukemic cells are particularly sensitive to inhibitors of the enzyme, including statins. In general, CLL patients who are prescribed statins tend to be older and male [16]. Compared to patients who were not taking statins, CLL patients given these drugs were determined to have significantly lower LDL levels, lower amounts of intracellular cholesterol, fewer circulating CLL cells, and longer lymphocyte doubling times [23].
There is strong in-vitro evidence that statins also have direct tumoricidal effects against CLL cells, inducing apoptosis-mediated cytotoxicity [68,69]. Although the mechanisms of action of statins against CLL cells still have not been fully elucidated, statins have been shown to inhibit signaling via the NF-κB transcription factor in myeloid cells [81]. NF-kB signaling also plays a critical role in promoting CLL cell viability [82], and an in-vitro study determined that the statin, simvastatin, induces apoptosis of CLL cells by lowering the BCL-2/Bax ratio and through activation of caspase 9 [69]. Importantly, these pro-apoptotic effects were induced specifically in the leukemic cells and not in normal lymphocytes. There is also evidence from a study involving the short-term ex-vivo culture of primary CLL cells that simvastatin may act in synergy with the purine analogues, fludarabine, and cladribine [71]. Furthermore, simvastatin was found to be equally effective at inducing apoptosis in cells from high-risk CLL patients and patients with a more favorable prognosis [71].
To date, there have been seven clinical studies (summarized in Table 2) that have investigated the effects of statins on the pathogenesis of CLL [15,16,22,77,78,79,80]. Of these studies, five concluded that statins have favorable effects on CLL patient outcomes [15,16,78,79,80] by either reducing disease progression [16], reducing the risk of CLL development [80] or by increasing overall survival [15,78,79]. The outcome data in these studies were derived from a large number of patients (> 4000), with varying ages and included treatment naïve patients and patients with relapsed/refractory disease. The other two studies [22,77] observed no impact of statins on clinical outcomes. One of these studies, in which statins were administered to newly diagnosed Rai Stage 0 patients [77], determined that statins had no effect on time to initial therapy. The study also included patients that were already taking statins at the time of their CLL diagnosis but concluded that statins had no impact on the time to first treatment in this group of patients. Similarly, Friedman et al. observed no effect of statins on treatment free survival but did determine that patients who were taking statins at the time of CLL diagnosis were less likely to require immediate therapy. However, as the authors pointed out, the retrospective analysis and short follow-up time may be confounding factors that limit the power of the study to identify differences between the patient groups.
9.2. Other Cholesterol Lowering Drugs in CLL
The lipase inhibitors, lalistat and orlistat, also function through effects on cholesterol homeostasis. Lalistat is a small molecular inhibitor that inhibits lysosomal lipases (Figure 4). Following cellular uptake, LDL particles are degraded in lysosomes, releasing cholesterol and free fatty acids. In CLL cells, cholesterol esters are further degraded by lysosomal lipases, which generate free fatty acids and increase the activity of the pro-survival STAT3-mediated signaling pathway. Lalistat inhibits this process by preventing degradation of cholesterol esters, which leads to a reduction in the phosphorylation and activity of STAT3 [23]. Orlistat, marketed under the name xenical, is a lipase inhibitor that prevents adsorption of dietary fats. Orlistat inhibits lipoprotein lipase (LPL) and, therefore, prevents the release of free fatty acids from LDL particles. In the only published study to date, orlistat was observed to induce apoptosis of primary CLL cells in vitro [70].
Statins have been demonstrated to reduce surface expression and induce conformational changes of the CD20 molecule on B-cells and decrease the cytotoxic effects of the anti-CD20 monoclonal antibody, rituximab, towards CLL cells [83]. The possibility that statins may reduce the clinical efficacy of CD20-targeted therapies [84] is an important consideration as chemoimmunotherapeutic regimens that include anti-CD20 antibodies are still commonly used to treat CLL. An alternative approach may be to use agents that target other critical regulators of cholesterol biosynthesis, particularly for patients who are concurrently receiving anti-CD20 therapies. A study of two inhibitors of squalene synthase (YM-53601 and TAK-475) and an inhibitor of 2-3,oxidosqualene cyclase (BIBB-515) determined that these compounds reduce intracellular cholesterol levels by around 20% and increase CD20 surface expression in the MEC2 cell line [73]. Consistent with the increase in CD20 expression, both inhibitors were found to significantly increase the sensitivity of the cell line and primary CLL cells to rituximab and, also the purine analogue, fludarabine. These data suggest that certain drugs that target regulators of cholesterol biosynthesis may have both direct cytotoxic effects and increase the sensitivity of CLL cells to current therapies, including regimens containing anti-CD20 therapies.
10. Conclusions
Cholesterol is well recognized as an essential nutrient for cells throughout the body. It is an important precursor in the production of hormones, bile acids, and Vitamin D, and it is a critical structural component of cell membranes. The malignant transformation of healthy B-cells to CLL cells is associated with the reorganization of membrane components, which is dependent on cholesterol. CLL cells appear capable of overriding normal feedback mechanisms that regulate cholesterol levels, resulting in elevated intracellular levels of the lipid, a greater intracellular mass, modified membrane composition, and alterations in signaling pathways. However, there are conflicting reports in the literature concerning the role of dietary cholesterol in the pathobiology of CLL.
Metabolic syndrome is a well-known risk factor for the development and prognosis of cancer, and one important aspect of metabolic syndrome is dyslipidemia. Although further research is required to determine the causes of cholesterol dysregulation or dyslipidemia in cancer, targeting pathways involved in cholesterol metabolism has been proposed as a unique therapeutic approach in many cancers, including glioblastoma [85] and cancers of the breast [86] and prostate [87]. There is also evidence that cholesterol-lowering drugs, such as statins, may reduce the risk of tumorigenesis. Several studies have examined the potential impact of statins on the outcome of patients with CLL, but short follow-up times and retrospective analyses limit interpretation of the data, particularly concerning serum triglyceride and cholesterol levels, which must be assessed under fasting conditions. However, the findings of these studies raise some potentially important points, including the possibility that concurrent administration of statins may significantly impact the response of patients to regimens containing anti-CD20 antibodies [84].
While the tumoricidal effects of drugs that inhibit both endogenous cholesterol synthesis and inhibitors of HMG–CoA reductase highlight the critical role of cholesterol in CLL cell survival and proliferation, there are still many unanswered questions. For example, does cholesterol play a role in drug resistance in CLL or in the transformation of the disease to a more aggressive B-cell malignancy, known as Richter’s transformation? In other cancers, cholesterol is known to play a role in drug-resistance [reviewed in [88]]. Therefore, it is conceivable that agents that target cholesterol metabolism may restrict the development of drug resistance and transformation in CLL.
Microenvironments within lymph nodes and bone marrow promote survival and drive the proliferation of CLL cells. Cholesterol may play an important role in supporting the high energy demands associated with the rapid CLL cell proliferation that occurs in these microenvironments. However, no studies to date have examined whether lipids and lipoproteins represent a crucial energy source for CLL cell proliferation and play a role in mediating the interaction of CLL cells with accessory cells within the tumour microenvironment.
Further studies concerning the potentially significant role of dietary cholesterol in CLL are also required, particularly as dietary intervention studies are relatively easy to conduct, with little risk to the patients. As many CLL patients are often initially managed with “watch-and-wait” approaches, it remains to be explored whether dietary changes may delay the need for treatment, particularly in patients with low- or medium-risk disease. Addressing these critical questions and gaining a better understanding of the role of cholesterol in the pathogenesis of CLL may identify novel treatment or management strategies.
Author Contributions
A.M.W. constructed the tables and figures for the article; A.M.W., O.G.B. and L.A.T. contributed equally to the writing of the paper; A.K.H. and B.J.K. edited and reviewed the final submission and L.A.T. oversaw all stages of preparation and submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Cancer Council of South Australia Beat Cancer Project on behalf of its donors and the South Australian Department of Health.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Radišauskas, R.; Kuzmickienė, I.; Milinavičienė, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas Lith.) 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef]
- Marini, A.; Carulli, G.; Azzara, A.; Grassi, B.; Ambrogi, F. Serum cholesterol and triglycerides in hematological malignancies. Acta Haematol. 1989, 81, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.R.; Sorlie, P.D.; Feinleib, M.; McNamara, P.M.; Kannel, W.B.; Dawber, T.R. Cancer incidence by levels of cholesterol. JAMA 1981, 245, 247–252. [Google Scholar] [CrossRef]
- Murai, T. Cholesterol lowering: Role in cancer prevention and treatment. Biol. Chem. 2015, 396, 1–11. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Hoffmann, P.; Roumeguère, T.; Schulman, C.; van Velthoven, R. Use of statins and outcome of BCG treatment for bladder cancer. N. Engl. J. Med. 2006, 355, 2705–2707. [Google Scholar] [CrossRef]
- Chang, A.K.; Barrett-Connor, E.; Edelstein, S. Low plasma cholesterol predicts an increased risk of lung cancer in elderly women. Prev. Med. 1995, 24, 557–562. [Google Scholar] [CrossRef]
- Horton, B.J.; Sabine, J.R. Metabolic controls in precancerous liver: Defective control of cholesterol synthesis in rats fed N-2-fluorenylacetamide. Eur. J. Cancer 1971, 7, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Thurgood, L.A.; Dwyer, E.S.; Lower, K.M.; Chataway, T.K.; Kuss, B.J. Altered expression of metabolic pathways in CLL detected by unlabelled quantitative mass spectrometry analysis. Br. J. Haematol. 2019, 185, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, L.; Ferreri, A.J.M.; Ghia, P. Chronic lymphocytic leukaemia. Crit. Rev. Oncol./Hematol. 2016, 104, 169–182. [Google Scholar] [CrossRef]
- Burger, J.A. Treatment of Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2020, 383, 460–473. [Google Scholar] [CrossRef]
- Mozessohn, L.; Earle, C.; Spaner, D.; Cheng, S.Y.; Kumar, M.; Buckstein, R. The Association of Dyslipidemia with Chronic Lymphocytic Leukemia: A Population-Based Study. JNCI J. Natl. Cancer Inst. 2016, 109, djw226. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Buckstein, R.; Spaner, D.E. A link between hypercholesterolemia and chronic lymphocytic leukemia. Leuk. Lymphoma 2016, 57, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Cucuianu, A.; Malide, D.; Petrov, L.; Patiu, M.; Vlaicu, S.; Cucuianu, M. Serum cholesterol and apoprotein B levels and serum cholinesterase activity in selected hematologic malignancies. Rom. J. Intern. Med. = Rev. Roum. De Med. Interne 1992, 30, 261–268. [Google Scholar]
- Gao, R.; Du, K.; Liang, J.; Xia, Y.; Wu, J.; Li, Y.; Pan, B.; Wang, L.; Li, J.; Xu, W. Low Serum Cholesterol Level Is a Significant Prognostic Factor That Improves CLL-IPI in Chronic Lymphocytic Leukaemia. Int. J. Mol. Sci. 2023, 24, 7396. [Google Scholar] [CrossRef]
- Sankanagoudar, S.; Singh, G.; Mahapatra, M.; Kumar, L.; Chandra, N. Cholesterol Homeostasis in Isolated Lymphocytes: A Differential Correlation Between Male Control and Chronic Lymphocytic Leukemia Subjects. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 23–30. [Google Scholar] [CrossRef]
- MacIntyre, D.A.; Jiménez, B.; Lewintre, E.J.; Martín, C.R.; Schäfer, H.; Ballesteros, C.G.; Mayans, J.R.; Spraul, M.; García-Conde, J.; Pineda-Lucena, A. Serum metabolome analysis by 1H-NMR reveals differences between chronic lymphocytic leukaemia molecular subgroups. Leukemia 2010, 24, 788–797. [Google Scholar] [CrossRef]
- Tomic, J.; Lichty, B.; Spaner, D.E. Aberrant interferon-signaling is associated with aggressive chronic lymphocytic leukemia. Blood 2011, 117, 2668–2680. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.R.; Magura, L.A.; Warren, H.A.; Harrison, J.D.; Diehl, L.F.; Weinberg, J.B. Statin use and need for therapy in chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 2295–2298. [Google Scholar] [CrossRef] [PubMed]
- McCaw, L.; Shi, Y.; Wang, G.; Li, Y.J.; Spaner, D.E. Low Density Lipoproteins Amplify Cytokine-signaling in Chronic Lymphocytic Leukemia Cells. EBioMedicine 2017, 15, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, D.Y.; Savushkin, E.V.; Dergunov, A.D. Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis. J. Lipids 2018, 2018, 3965054. [Google Scholar] [CrossRef]
- Tabas, I. Free cholesterol-induced cytotoxicity a possible contributing factor to macrophage foam cell necrosis in advanced atherosclerotic lesions. Trends Cardiovasc. Med. 1997, 7, 256–263. [Google Scholar] [CrossRef]
- Warner, G.J.; Stoudt, G.; Bamberger, M.; Johnson, W.J.; Rothblat, G.H. Cell toxicity induced by inhibition of acyl coenzyme A: Cholesterol acyltransferase and accumulation of unesterified cholesterol. J. Biol. Chem. 1995, 270, 5772–5778. [Google Scholar] [CrossRef]
- Mulas, M.F.; Abete, C.; Pulisci, D.; Pani, A.; Massidda, B.; Dessì, S.; Mandas, A. Cholesterol esters as growth regulators of lymphocytic leukaemia cells. Cell Prolif. 2011, 44, 360–371. [Google Scholar] [CrossRef]
- Hildebrand, J.; Stryckmans, P.; Stoffyn, P. Neutral glycolipids in leukemic and nonleukemic leukocytes. J. Lipid Res. 1971, 12, 361–366. [Google Scholar] [CrossRef]
- Gottfried, E.L. Lipids of human leukocytes: Relation to celltype. J. Lipid Res. 1967, 8, 321–327. [Google Scholar] [CrossRef]
- Golomb, H.M.; Saffold, C.W.; Nathans, A.H.; Dawson, G. Phospholipid and cholesterol differences amongst leukemic cell types with special reference to hairy cell leukemia: A preliminary report. Clin. Chim. Acta 1981, 116, 311–318. [Google Scholar] [CrossRef]
- Schörghofer, D.; Kinslechner, K.; Preitschopf, A.; Schütz, B.; Röhrl, C.; Hengstschläger, M.; Stangl, H.; Mikula, M. The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reprod. Biol. Endocrinol. RBE 2015, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Yavasoglu, I.; Sargin, G.; Yilmaz, F.; Altındag, S.; Akgun, G.; Tombak, A.; Toka, B.; Dal, S.; Ozbas, H.; Cetin, G.; et al. Cholesterol Levels in Patients with Chronic Lymphocytic Leukemia. J. Natl. Med. Assoc. 2017, 109, 23–27. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.M.; Scielzo, C.; Angeloni, N.L.; Deiss-Yehiely, E.; Scarfo, L.; Ranghetti, P.; Ma, S.; Kaplan, J.; Barbaglio, F.; Gordon, L.I.; et al. Synthetic high-density lipoproteins as targeted monotherapy for chronic lymphocytic leukemia. Oncotarget 2017, 8, 11219–11227. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Damiano, M.G.; Zhang, H.; Tripathy, S.; Luthi, A.J.; Rink, J.S.; Ugolkov, A.V.; Singh, A.T.; Dave, S.S.; Gordon, L.I.; et al. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc. Natl. Acad. Sci. USA. 2013, 110, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977, 46, 897–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.L.; Ruan, X.Z.; Liu, B.C. Dysregulation of the Low-Density Lipoprotein Receptor Pathway Is Involved in Lipid Disorder-Mediated Organ Injury. Int. J. Biol. Sci. 2016, 12, 569–579. [Google Scholar] [CrossRef]
- Chen, Y.; Hughes-Fulford, M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer 2001, 91, 41–45. [Google Scholar] [CrossRef]
- Juliusson, G.; Vitols, S. Impaired low-density lipoprotein receptor activity in chronic B-lymphocytic leukaemia cells. Eur. J. Haematol. 1988, 40, 18–24. [Google Scholar] [CrossRef]
- Vitols, S.; Gahrton, G.; Ost, A.; Peterson, C. Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 1984, 63, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Damle, R.N.; Calissano, C.; Chiorazzi, N. Chronic lymphocytic leukaemia: A disease of activated monoclonal B cells. Best Pract. Res. Clin. Haematol. 2010, 23, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Calissano, C.; Damle, R.N.; Marsilio, S.; Yan, X.-J.; Yancopoulos, S.; Hayes, G.; Emson, C.; Murphy, E.J.; Hellerstein, M.K.; Sison, C.; et al. Intraclonal Complexity in Chronic Lymphocytic Leukemia: Fractions Enriched in Recently Born/Divided and Older/Quiescent Cells. Mol. Med. 2011, 17, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T.; Basheeruddin, K.; Ping, L.; Frazer, S.; Getz, G.S. Mechanism of the growth-related activation of the low density lipoprotein receptor pathway. J. Biol. Chem. 1989, 264(3), 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Seija, N.; Uriepero, A.; Souto-Padron, T.; Oliver, C.; Irigoin, V.; Guillermo, C.; Navarrete, M.A.; Inés Landoni, A.; Dighiero, G.; et al. LPL protein in Chronic Lymphocytic Leukaemia have different origins in Mutated and Unmutated patients. Advances for a new prognostic marker in CLL. Br. J. Haematol. 2018, 182, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Rombout, A.; Verhasselt, B.; Philippé, J. Lipoprotein lipase in chronic lymphocytic leukemia: Function and prognostic implications. Eur. J. Haematol. 2016, 97, 409–415. [Google Scholar] [CrossRef]
- Heintel, D.; Kienle, D.; Shehata, M.; Kröber, A.; Kroemer, E.; Schwarzinger, I.; Mitteregger, D.; Le, T.; Gleiss, A.; Mannhalter, C.; et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1216–1223. [Google Scholar] [CrossRef]
- Weinberg, J.B.; Volkheimer, A.D.; Mihovilovic, M.; Jiang, N.; Chen, Y.; Bond, K.; Moore, J.O.; Gockerman, J.P.; Diehl, L.F.; de Castro, C.M.; et al. Apolipoprotein E genotype as a determinant of survival in chronic lymphocytic leukemia. Leukemia 2008, 22, 2184–2192. [Google Scholar] [CrossRef]
- Thurgood, L.A.; Best, O.G.; Rowland, A.; Lower, K.M.; Brooks, D.A.; Kuss, B.J. Lipid uptake in chronic lymphocytic leukemia. Exp. Hematol. 2022, 106, 58–67. [Google Scholar] [CrossRef]
- Sakashita, A.M.; Bydlowski, S.P.; Chamone, D.A.; Maranhão, R.C. Plasma kinetics of an artificial emulsion resembling chylomicrons in patients with chronic lymphocytic leukemia. Ann. Hematol. 2000, 79, 687–690. [Google Scholar] [CrossRef]
- Rozovski, U.; Grgurevic, S.; Bueso-Ramos, C.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Jain, P.; Wierda, W.; Burger, J.; et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol. Cancer Res. MCR 2015, 13, 944–953. [Google Scholar] [CrossRef]
- Spaner, D.E.; Lee, E.; Shi, Y.; Wen, F.; Li, Y.; Tung, S.; McCaw, L.; Wong, K.; Gary-Gouy, H.; Dalloul, A.; et al. PPAR-alpha is a therapeutic target for chronic lymphocytic leukemia. Leukemia 2013, 27, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Severin, F.; Frezzato, F.; Visentin, A.; Martini, V.; Trimarco, V.; Carraro, S.; Tibaldi, E.; Brunati, A.M.; Piazza, F.; Semenzato, G.; et al. In Chronic Lymphocytic Leukemia the JAK2/STAT3 Pathway Is Constitutively Activated and Its Inhibition Leads to CLL Cell Death Unaffected by the Protective Bone Marrow Microenvironment. Cancers 2019, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.A.; Melnykovych, G.; Fiskin, A.M. Compactin (ML-236B) reduces the content of filipin-cholesterol complexes in the plasma membrane of chronic lymphocytic leukemia cells. Exp. Cell Res. 1984, 153, 91–98. [Google Scholar] [CrossRef]
- Harwood, H.J., Jr.; Alvarez, I.M.; Noyes, W.D.; Stacpoole, P.W. In vivo regulation of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase: Increased enzyme protein concentration and catalytic efficiency in human leukemia and lymphoma. J. Lipid Res. 1991, 32, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.; Shinitzky, M. Increase of cholesterol level in the surface membrane of lymphoma cells and its inhibitory effect on ascites tumor development. Proc. Natl. Acad. Sci. USA 1974, 71, 2128–2130. [Google Scholar] [CrossRef] [PubMed]
- Shinitzky, M.; Inbar, M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J. Mol. Biol. 1974, 85, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Deliconstantinos, G.; Daefler, S.; Krueger, G.R. Cholesterol modulation of membrane fluidity and ecto-nucleotide triphosphatase activity in human normal and CLL lymphocytes. Anticancer Res. 1987, 7, 347–352. [Google Scholar]
- Daefler, S.; Krueger, G.R.; Mödder, B.; Deliconstantinos, G. Cell membrane fluidity in chronic lymphocytic leukemia (CLL) lymphocytes and its relation to membrane receptor expression. J. Exp. Pathol. 1987, 3, 147–154. [Google Scholar]
- Inbar, M.; Shinitzky, M. Cholesterol as a bioregulator in the development and inhibition of leukemia. Proc. Natl. Acad. Sci. USA 1974, 71, 4229–4231. [Google Scholar] [CrossRef]
- Davis, P.J.; Poznansky, M.J. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase by changes in microsomal cholesterol content or phospholipid composition. Proc. Natl. Acad. Sci. USA 1987, 84, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Wiley, M.H.; Moser, A.H.; Siperstein, M.D. Altered activation state of hydroxymethylglutaryl-coenzyme A reductase in liver tumors. Arch. Biochem. Biophys. 1983, 226, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.G.; Wilce, P.A. Reversible phosphorylation of 3-hydroxy-3-methylglutaryl CoA reductase in Morris hepatomas. Biochem. Biophys. Res. Commun. 1983, 114, 473–478. [Google Scholar] [CrossRef]
- Kaplan, M.R.; Simoni, R.D. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J. Cell Biol. 1985, 101, 446–453. [Google Scholar] [CrossRef]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. 1992. Atheroscler. Suppl. 2004, 5, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-L.; Qin, X. Does adherence to lipid-lowering medications improve cancer survival? A nationwide study of breast and colorectal cancer, and melanoma. Br. J. Clin. Pharmacol. 2021, 87, 1847–1858. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A. Current perspectives on statins as potential anti-cancer therapeutics: Clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res. 2019, 8, 692–699. [Google Scholar] [CrossRef]
- Vitols, S.; Angelin, B.; Juliusson, G. Simvastatin impairs mitogen-induced proliferation of malignant B-lymphocytes from humans—In Vitro and In Vivo studies. Lipids 1997, 32, 255–262. [Google Scholar] [CrossRef]
- Chapman-Shimshoni, D.; Yuklea, M.; Radnay, J.; Shapiro, H.; Lishner, M. Simvastatin induces apoptosis of B-CLL cells by activation of mitochondrial caspase 9. Exp. Hematol. 2003, 31, 779–783. [Google Scholar] [CrossRef]
- Pallasch, C.P.; Schwamb, J.; Königs, S.; Schulz, A.; Debey, S.; Kofler, D.; Schultze, J.L.; Hallek, M.; Ultsch, A.; Wendtner, C.M. Targeting lipid metabolism by the lipoprotein lipase inhibitor orlistat results in apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 2008, 22, 585–592. [Google Scholar] [CrossRef]
- Podhorecka, M.; Halicka, D.; Klimek, P.; Kowal, M.; Chocholska, S.; Dmoszynska, A. Simvastatin and purine analogs have a synergic effect on apoptosis of chronic lymphocytic leukemia cells. Ann. Hematol. 2010, 89, 1115–1124. [Google Scholar] [CrossRef]
- Żołnierczyk, J.D.; Borowiak, A.; Hikisz, P.; Cebula-Obrzut, B.; Błoński, J.Z.; Smolewski, P.; Robak, T.; Kiliańska, Z.M. Promising anti-leukemic activity of atorvastatin. Oncol. Rep. 2013, 29, 2065–2071. [Google Scholar] [CrossRef]
- Benakanakere, I.; Johnson, T.; Sleightholm, R.; Villeda, V.; Arya, M.; Bobba, R.; Freter, C.; Huang, C. Targeting cholesterol synthesis increases chemoimmuno-sensitivity in chronic lymphocytic leukemia cells. Exp. Hematol. Oncol. 2014, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.M.; Riganti, C.; Vitale, C.; Griggio, V.; Campia, I.; Robino, M.; Foglietta, M.; Castella, B.; Sciancalepore, P.; Buondonno, I.; et al. Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in IGHV unmutated CLL cells. Oncotarget 2015, 6, 29833–29846. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Roberts, A.; Juarez, D.; Vo, T.T.; Bhatt, S.; Herzog, L.O.; Mallya, S.; Bellin, R.J.; Agarwal, S.K.; Salem, A.H.; et al. Statins enhance efficacy of venetoclax in blood cancers. Sci Transl. Med. 2018, 10, eaaq1240. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, N.; Tripathi, R.; Giró, A.; Rosich, L.; López-Guerra, M.; López-Oreja, I.; Playa-Albinyana, H.; Arenas, F.; Mas, J.M.; Pérez-Galán, P.; et al. Systems biology drug screening identifies statins as enhancers of current therapies in chronic lymphocytic leukemia. Sci. Rep. 2020, 10, 22153. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Rabe, K.G.; Kay, N.E.; Zent, C.S.; Call, T.G.; Slager, S.L.; Bowen, D.A.; Schwager, S.M.; Nowakowski, G.S. Statin and non-steroidal anti-inflammatory drug use in relation to clinical outcome among patients with Rai stage 0 chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 1233–1240. [Google Scholar] [CrossRef]
- Khan, A.M.; Dave, V.; Srinivas, S.; Chang, V.T.-S.; McPherson, M.; Kasimis, B. Statins and survival in a cohort of veterans with chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2014, 32, 1610. [Google Scholar] [CrossRef]
- Chae, Y.K.; Trinh, L.; Jain, P.; Wang, X.; Rozovski, U.; Wierda, W.G.; Keating, M.J.; Estrov, Z. Statin and aspirin use is associated with improved outcome of FCR therapy in relapsed/refractory chronic lymphocytic leukemia. Blood 2014, 123, 1424–1426. [Google Scholar] [CrossRef]
- Righolt, C.H.; Zhang, G.; Ye, X.; Banerji, V.; Johnston, J.B.; Gibson, S.; Mahmud, S.M. Statin Use and Chronic Lymphocytic Leukemia Incidence: A Nested Case-Control Study in Manitoba, Canada. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1495–1501. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Cuní, S.; Pérez-Aciego, P.; Pérez-Chacón, G.; Vargas, J.A.; Sánchez, A.; Martín-Saavedra, F.M.; Ballester, S.; García-Marco, J.; Jordá, J.; Durántez, A. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia 2004, 18, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Cragg, M.S. The potential effect of statins on rituximab immunotherapy. PLoS Med. 2008, 5, e77. [Google Scholar] [CrossRef]
- Winiarska, M.; Bil, J.; Wilczek, E.; Wilczynski, G.M.; Lekka, M.; Engelberts, P.J.; Mackus, W.J.; Gorska, E.; Bojarski, L.; Stoklosa, T.; et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008, 5, e64. [Google Scholar] [CrossRef]
- Pirmoradi, L.; Seyfizadeh, N.; Ghavami, S.; Zeki, A.A.; Shojaei, S. Targeting cholesterol metabolism in glioblastoma: A new therapeutic approach in cancer therapy. J. Investig. Med. 2019, 67, 715. [Google Scholar] [CrossRef]
- Segala, G.; de Medina, P.; Iuliano, L.; Zerbinati, C.; Paillasse, M.R.; Noguer, E.; Dalenc, F.; Payré, B.; Jordan, V.C.; Record, M.; et al. 5,6-Epoxy-cholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem. Pharmacol. 2013, 86, 175–189. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Jia, Z.; Qiao, C.; Wang, M.; Ding, X. Cholesterol metabolism in drug-resistant cancer (Review). Int. J. Oncol. 2020, 57, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).