Low Fasting Concentrations of Glucagon in Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency
Abstract
1. Introduction
2. Materials and Methods
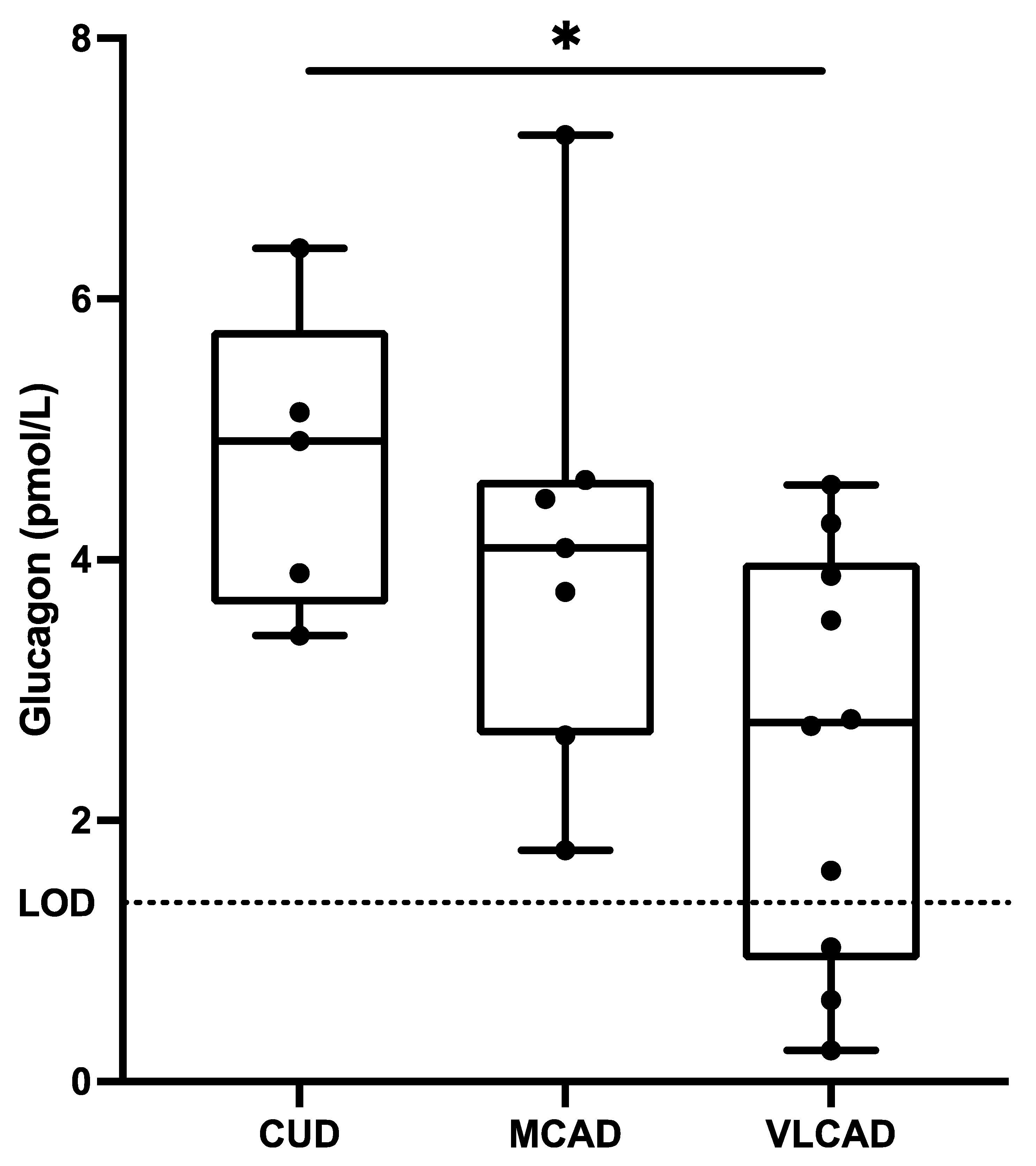
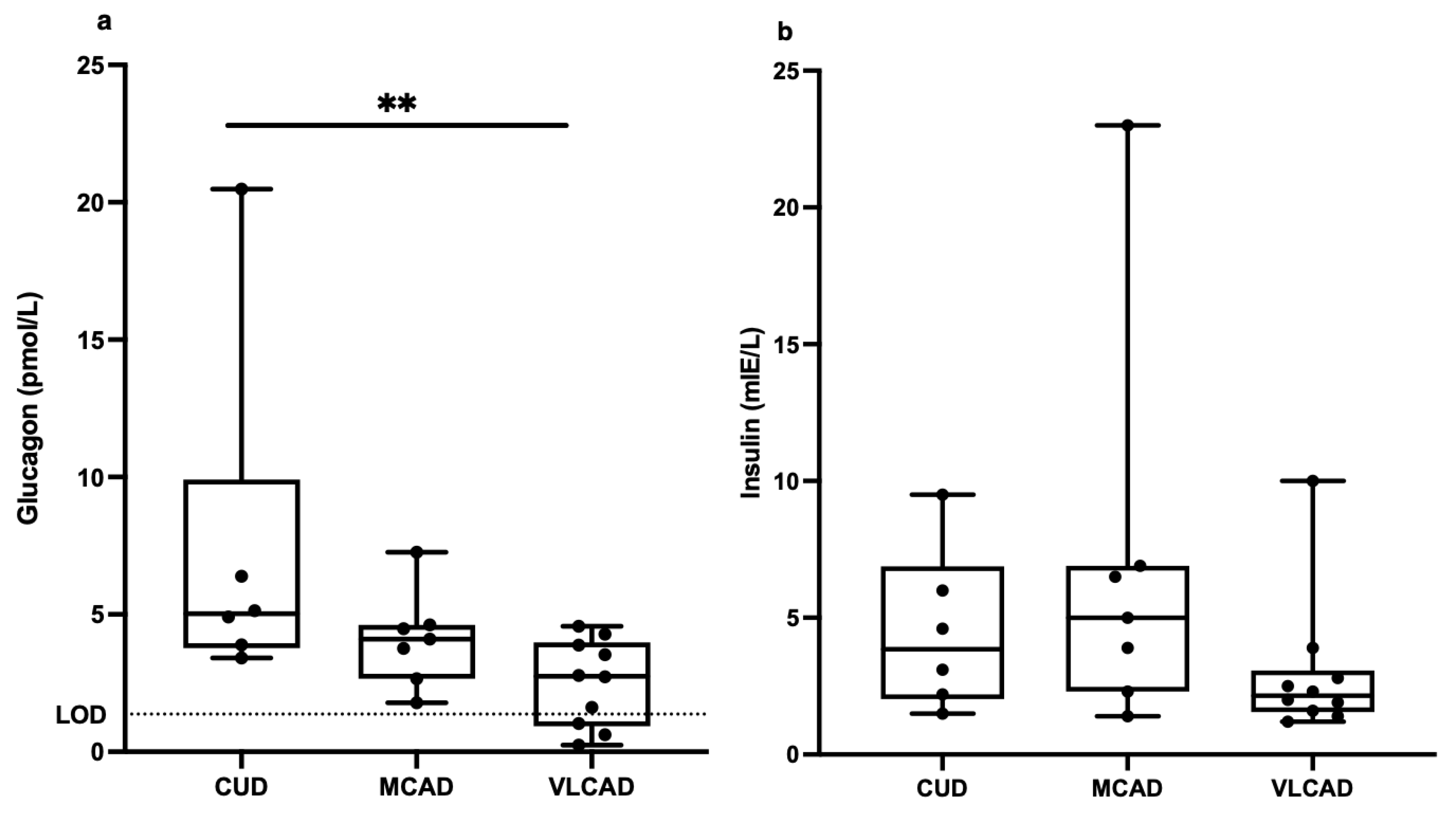
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilcken, B.; Wiley, V.; Hammond, J.; Carpenter, K. Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry. N. Engl. J. Med. 2003, 348, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.J. Inborn Errors of Metabolism: Advances in Diagnosis and Therapy. JAMA Pediatr. 2015, 169, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Olsson, D.; Barbaro, M.; Haglind, C.; Halldin, M.; Lajic, S.; Tucci, S.; Zetterström, R.H.; Nordenström, A. Very long-chain acyl-CoA dehydrogenase deficiency in a Swedish cohort: Clinical symptoms, newborn screening, enzyme activity, and genetics. JIMD Rep. 2022, 63, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hesse, J.; Braun, C.; Behringer, S.; Matysiak, U.; Spiekerkoetter, U.; Tucci, S. The diagnostic challenge in very-long chain acyl-CoA dehydrogenase deficiency (VLCADD). J. Inherit. Metab. Dis. 2018, 41, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Tajika, M.; Shimura, M.; Carey, K.T.; Stroud, D.A.; Murayama, K.; Ohtake, A.; McKenzie, M. Loss of the Mitochondrial Fatty Acid β-Oxidation Protein Medium-Chain Acyl-Coenzyme A Dehydrogenase Disrupts Oxidative Phosphorylation Protein Complex Stability and Function. Sci. Rep. 2018, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.; Mohsen, A.-W.; Karunanidhi, A.; McCracken, E.; Yeasted, R.; Vockley, J. Molecular and cellular pathology of very-long-chain acyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 2013, 109, 21–27. [Google Scholar] [CrossRef]
- Gillingham, M.B.; Heitner, S.B.; Martin, J.; Rose, S.; Goldstein, A.; El-Gharbawy, A.H.; Deward, S.; Lasarev, M.R.; Pollaro, J.; DeLany, J.; et al. Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: A double blinded, randomized controlled trial. J. Inherit. Metab. Dis. 2017, 40, 831–843. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef]
- Holst, J.J.; Albrechtsen, N.J.W.; Pedersen, J.; Knop, F.K. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver–α-Cell Axis. Diabetes 2017, 66, 235–240. [Google Scholar] [CrossRef]
- Drucker, D.J. The Ascending GLP-1 Road From Clinical Safety to Reduction of Cardiovascular Complications. Diabetes 2018, 67, 1710–1719. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Gui, B.; Fu, R.; Ma, F.; Yu, J.; Qu, P.; Dong, L.; Chen, C. Acute stimulation of glucagon secretion by linoleic acid results from GPR40 activation and [Ca2+]i increase in pancreatic islet α-cells. J. Endocrinol. 2011, 210, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Flodgren, E.; Olde, B.; Meidute-Abaraviciene, S.; Winzell, M.S.; Ahrén, B.; Salehi, A. GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem. Biophys. Res. Commun. 2007, 354, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.; Sargsyan, E.; Manell, H.; Smith, D.M.; Göpel, S.O.; Bergsten, P. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Sci. Rep. 2017, 7, 4657. [Google Scholar] [CrossRef] [PubMed]
- Bataille, D. Pro-protein convertases in intermediary metabolism: Islet hormones, brain/gut hormones and integrated physiology. J. Mol. Med. 2007, 85, 673–684. [Google Scholar] [CrossRef]
- Manell, H.; Kristinsson, H.; Kullberg, J.; Ubhayasekera, S.J.K.; Mörwald, K.; Staaf, J.; Cadamuro, J.; Zsoldos, F.; Göpel, S.; Sargsyan, E.; et al. Hyperglucagonemia in youth is associated with high plasma free fatty acids, visceral adiposity, and impaired glucose tolerance. Pediatr. Diabetes 2019, 20, 880–891. [Google Scholar] [CrossRef]
- Briant, L.J.; Dodd, M.S.; Chibalina, M.V.; Rorsman, N.J.; Johnson, P.R.; Carmeliet, P.; Rorsman, P.; Knudsen, J.G. CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets. Cell Rep. 2018, 23, 3300–3311. [Google Scholar] [CrossRef]
- Stenlid, R.; Manell, H.; Halldin, M.; Kullberg, J.; Ahlström, H.; Manukyan, L.; Weghuber, D.; Paulmichl, K.; Zsoldos, F.; Bergsten, P.; et al. High DPP-4 Concentrations in Adolescents Are Associated with Low Intact GLP-1. J. Clin. Endocrinol. Metab. 2018, 103, 2958–2966. [Google Scholar] [CrossRef]
- Manell, H.; Staaf, J.; Manukyan, L.; Kristinsson, H.; Cen, J.; Stenlid, R.; Ciba, I.; Forslund, A.; Bergsten, P. Altered Plasma Levels of Glucagon, GLP-1 and Glicentin During OGTT in Adolescents With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1181–1189. [Google Scholar] [CrossRef]
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine Inborn Errors of Metabolism. Molecules 2019, 24, 3251. [Google Scholar] [CrossRef]
- Stenlid, R.; Olsson, D.; Cen, J.; Manell, H.; Haglind, C.; Chowdhury, A.I.; Bergsten, P.; Nordenström, A.; Halldin, M. Altered mitochondrial metabolism in peripheral blood cells from patients with inborn errors of β-oxidation. Clin. Transl. Sci. 2021, 15, 182–194. [Google Scholar] [CrossRef]
- Saad, A.; Man, C.D.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal Pattern to Insulin Secretion and Insulin Action in Healthy Individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Færch, K.; Vistisen, D.; Pacini, G.; Torekov, S.S.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; et al. Insulin Resistance Is Accompanied by Increased Fasting Glucagon and Delayed Glucagon Suppression in Individuals with Normal and Impaired Glucose Regulation. Diabetes 2016, 65, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Stinson, S.E.; Jonsson, A.E.; de Retana Alzola, I.F.; Lund, M.A.; Frithioff-Bøjsøe, C.; Aas Holm, L.; Fonvig, C.E.; Pedersen, O.; Ängquist, L.; Sørensen, T.I.; et al. Hyperglucagonemia in Pediatric Adiposity Associates with Cardiometabolic Risk Factors but Not Hyperglycemia. J. Clin. Endocrinol. Metab. 2022, 107, 1569–1576. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Færch, K.; Jensen, T.M.; Witte, D.R.; Pedersen, J.; Mahendran, Y.; Jonsson, A.E.; Galsgaard, K.D.; Winther-Sørensen, M.; Torekov, S.S.; et al. Evidence of a liver–alpha cell axis in humans: Hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 2018, 61, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Herrema, H.; Brinke, H.T.; Denis, S.; Ruiter, J.P.; van Dijk, T.H.; Argmann, C.A.; Ottenhoff, R.; Müller, M.; Groen, A.K.; et al. Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum. Mol. Genet. 2013, 22, 5249–5261. [Google Scholar] [CrossRef] [PubMed]
- Gelling, R.W.; Du, X.Q.; Dichmann, D.S.; Rømer, J.; Huang, H.; Cui, L.; Obici, S.; Tang, B.; Holst, J.J.; Fledelius, C.; et al. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1438–1443. [Google Scholar] [CrossRef]
- Chlouverakis, C.; Harris, P. Composition of the Free Fatty Acid Fraction in the Plasma of Human Arterial Blood. Nature 1960, 188, 1111–1112. [Google Scholar] [CrossRef]
- Ubhayasekera, S.J.K.A.; Staaf, J.; Forslund, A.; Bergsten, P.; Bergquist, J. Free fatty acid determination in plasma by GC-MS after conversion to Weinreb amides. Anal. Bioanal. Chem. 2013, 405, 1929–1935. [Google Scholar] [CrossRef]
- Vergari, E.; Knudsen, J.G.; Ramracheya, R.; Salehi, A.; Zhang, Q.; Adam, J.; Asterholm, I.W.; Benrick, A.; Briant, L.J.B.; Chibalina, M.V.; et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat. Commun. 2019, 10, 139. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Sun, E.W.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms Controlling Glucose-Induced GLP-1 Secretion in Human Small Intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef]
- Ohneda, A.; Ohneda, K.; Nagsaki, T.; Sasaki, K. Insulinotropic action of human glicentin in dogs. Metabolism 1995, 44, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Enteroglucagon. Annu. Rev. Physiol. 1997, 59, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Lundquist, I. Effects of Glicentine on Insulin Secretion. Horm. Metab. Res. 1980, 12, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Mantzoros, C.S. The Role of Glicentin and Oxyntomodulin in Human Metabolism: New Evidence and New Directions. J. Clin. Endocrinol. Metab. 2020, 105, e3003–e3005. [Google Scholar] [CrossRef]
- Kompare, M.; Rizzo, W.B. Mitochondrial Fatty-Acid Oxidation Disorders. Semin. Pediatr. Neurol. 2008, 15, 140–149. [Google Scholar] [CrossRef]
- Vockley, J.; Marsden, D.; McCracken, E.; DeWard, S.; Barone, A.; Hsu, K.; Kakkis, E. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment—A retrospective chart review. Mol. Genet. Metab. 2015, 116, 53–60. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; Ijlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef]
- Vockley, J.; Charrow, J.; Ganesh, J.; Eswara, M.; Diaz, G.; McCracken, E.; Conway, R.; Enns, G.; Starr, J.; Wang, R.; et al. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol. Genet. Metab. 2016, 119, 223–231. [Google Scholar] [CrossRef]
- Shirley, M. Triheptanoin: First Approval. Drugs 2020, 80, 1595–1600. [Google Scholar] [CrossRef]
- Galsgaard, K.D.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Albrechtsen, N.J.W. Glucagon Receptor Signaling and Lipid Metabolism. Front. Physiol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Zhang, D.; Guerra, M.T.; Brill, A.L.; Goedeke, L.; Nasiri, A.R.; Rabin-Court, A.; Wang, Y.; Peng, L.; Dufour, S.; et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 2020, 579, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.P.; Benson, J.W.; Walter, R.M.; Ensinck, J.W. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J. Clin. Investig. 1976, 58, 565–570. [Google Scholar] [CrossRef]
- Haglind, C.B.; Nordenström, A.; Ask, S.; Von Dobeln, U.; Gustafsson, J.; Stenlid, M.H. Increased and early lipolysis in children with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency during fast. J. Inherit. Metab. Dis. 2014, 38, 315–322. [Google Scholar] [CrossRef] [PubMed]


| CUD (n = 6) | MCAD (n = 7) | VLCAD (n = 10) | p Value | |
|---|---|---|---|---|
| Age, y | 6.8 ± 3.2 | 7.6 ± 3.5 | 5.25 ± 2.58 | 0.29 |
| Weight, kg | 27.0 ± 14.0 | 36.1 ± 31.6 | 22.5 ± 8.0 | 0.41 |
| Height, cm | 117.0 ± 24.2 | 126.7 ± 20.6 | 113.3 ± 14.3 | 0.39 |
| BMI | 17.8 ± 4.0 | 21.1 ± 8.6 | 17.1 ± 2.6 | 0.37 |
| BMI SDS | 0.70 ± 1.21 | 1.27 ± 1.6 | 0.64 ± 1.22 | 0.64 |
| Sex (female/male) | 2/4 | 3/4 | 3/7 | 0.87 |
| CUD (n = 5) | MCAD (n = 6) | VLCAD (n = 9) | p Value | |
|---|---|---|---|---|
| BCAA | 398 ± 93 | 323 ± 78 | 362 ± 66 | 0.68 |
| Non-BCAA | 2399 ± 297 | 2113 ± 330 | 2056 ± 245 | 0.99 |
| Glucogenic AA | 1875 ± 173 | 1698 ± 294 | 1667 ± 201 | 0.27 |
| α-Aminobutyric acid | 22 ± 9 | 25 ± 11 | 19 ± 6 | 0.99 |
| Alanine | 322 ± 66 | 289 ± 103 | 251 ± 55 | 0.70 |
| Arginine | 79 ± 28 | 63 ± 17 | 62 ± 21 | 0.99 |
| Asparagine | 46 ± 3 | 44 ± 8 | 44 ± 9 | 0.99 |
| Aspartic acid | 15 ± 3 | 16 ± 4 | 12 ± 1 | 0.99 |
| Citrulline | 34 ± 4 | 36 ± 5 | 40 ± 9 | 0.99 |
| Cysteine | <4 | 4 ± 3 | 5 ± 4 | 0.95 |
| Glutamic acid | 118 ± 39 | 76 ± 12 | 67 ± 12 | 0.98 |
| Glutamine | 473 ± 60 | 475 ± 50 | 436 ± 66 | 0.67 |
| Glycine | 211 ± 33 | 177 ± 36 | 216 ± 29 | 0.66 |
| Histidine | 73 ± 5 | 62 ± 6 | 64 ± 8 | 0.99 |
| Isoleucine | 66 ± 24 | 45 ± 11 | 53 ± 16 | 0.98 |
| Leucine | 123 ± 29 | 93 ± 21 | 106 ± 22 | 0.95 |
| Lysine | 157 ± 54 | 127 ± 27 | 131 ± 36 | 0.99 |
| Methionine | 23 ± 6 | 20 ± 5 | 22 ± 6 | 0.99 |
| Ornithine | 50 ± 20 | 54 ± 20 | 43 ± 10 | 0.97 |
| Phenylalanine | 83 ± 65 | 58 ± 10 | 48 ± 6 | 0.97 |
| Proline | 192 ± 33 | 194 ± 93 | 180 ± 51 | 0.95 |
| Serine | 113 ± 28 | 98 ± 13 | 110 ± 31 | 0.96 |
| Taurine | 149 ± 69 | 110 ± 27 | 88 ± 32 | 0.89 |
| Threonine | 107 ± 22 | 91 ± 24 | 118 ± 45 | 0.83 |
| Tryptophan | 44 ± 9 | 30 ± 8 | 38 ± 11 | 0.98 |
| Tyrosine | 86 ± 26 | 71 ± 16 | 64 ± 13 | 0.99 |
| Valine | 209 ± 41 | 186 ± 47 | 203 ± 35 | 0.92 |
| Alloisoleucine | <4 | <4 | 5 ± 2 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenlid, R.; Manell, H.; Seth, R.; Cerenius, S.Y.; Chowdhury, A.; Roa Cortés, C.; Nyqvist, I.; Lundqvist, T.; Halldin, M.; Bergsten, P. Low Fasting Concentrations of Glucagon in Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Metabolites 2023, 13, 780. https://doi.org/10.3390/metabo13070780
Stenlid R, Manell H, Seth R, Cerenius SY, Chowdhury A, Roa Cortés C, Nyqvist I, Lundqvist T, Halldin M, Bergsten P. Low Fasting Concentrations of Glucagon in Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Metabolites. 2023; 13(7):780. https://doi.org/10.3390/metabo13070780
Chicago/Turabian StyleStenlid, Rasmus, Hannes Manell, Rikard Seth, Sara Y. Cerenius, Azazul Chowdhury, Camilla Roa Cortés, Isabelle Nyqvist, Thomas Lundqvist, Maria Halldin, and Peter Bergsten. 2023. "Low Fasting Concentrations of Glucagon in Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency" Metabolites 13, no. 7: 780. https://doi.org/10.3390/metabo13070780
APA StyleStenlid, R., Manell, H., Seth, R., Cerenius, S. Y., Chowdhury, A., Roa Cortés, C., Nyqvist, I., Lundqvist, T., Halldin, M., & Bergsten, P. (2023). Low Fasting Concentrations of Glucagon in Patients with Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Metabolites, 13(7), 780. https://doi.org/10.3390/metabo13070780






