Metabolic Modeling of Hermetia illucens Larvae Resource Allocation for High-Value Fatty Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Larvae-Rearing Experiments
2.2. Larvae Wet Weight Measurements
2.3. Experimental Chemical Analytics and Analysis Methods
2.3.1. GC-MS Measurements
2.3.2. Fatty Acid Profile
2.3.3. Sugars (Glucose, Fructose, Sucrose, and Maltose) and Glycerol
2.3.4. Starch
2.3.5. Fat
2.4. Metabolic Network Reconstruction of Hermetia Illucens Larvae
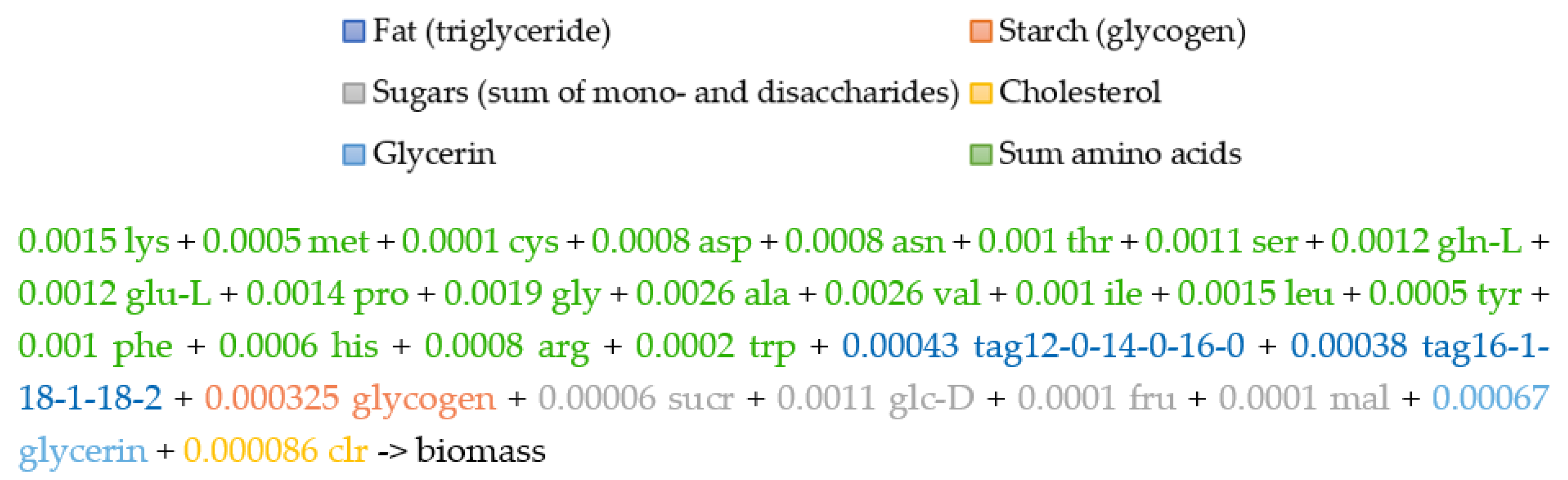
2.5. The Biomass Metabolic Reaction
2.6. Lipid Metabolism
- Fatty acid biosynthesis initiation (type I), PWY-5966;
- Palmitate biosynthesis I (type I fatty acid synthase), PWY-5994;
- Tetradecanoate biosynthesis (mitochondria), PWY66-430;
- Stearate biosynthesis I (animals), PWY-5972;
- Oleate biosynthesis II (animals and fungi), PWY-5996;
- Linoleate biosynthesis II (animals), PWY-6001.
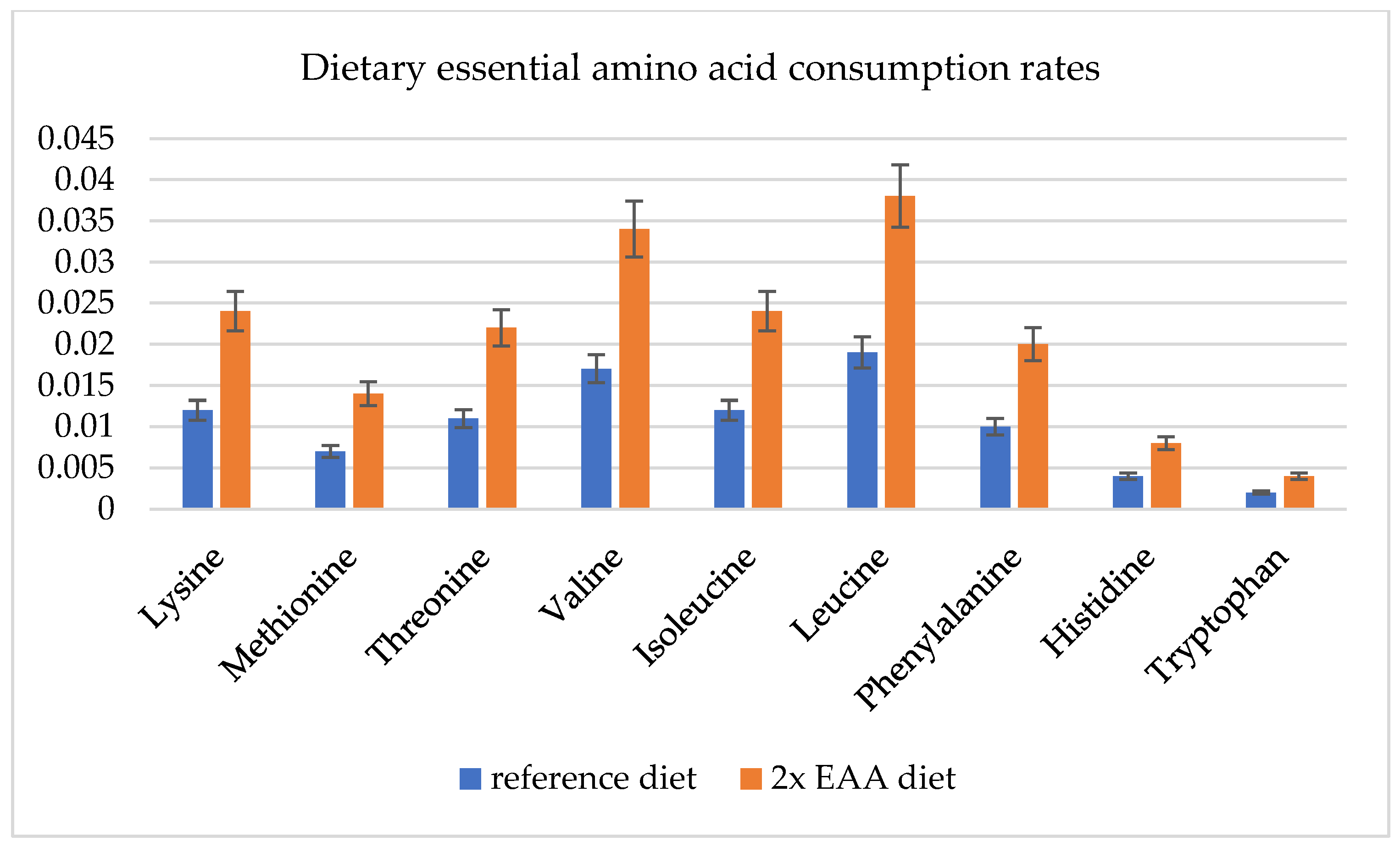
2.7. Approximation of Food Intake and Transport Reaction Constraints
2.8. Data Analysis
3. Results
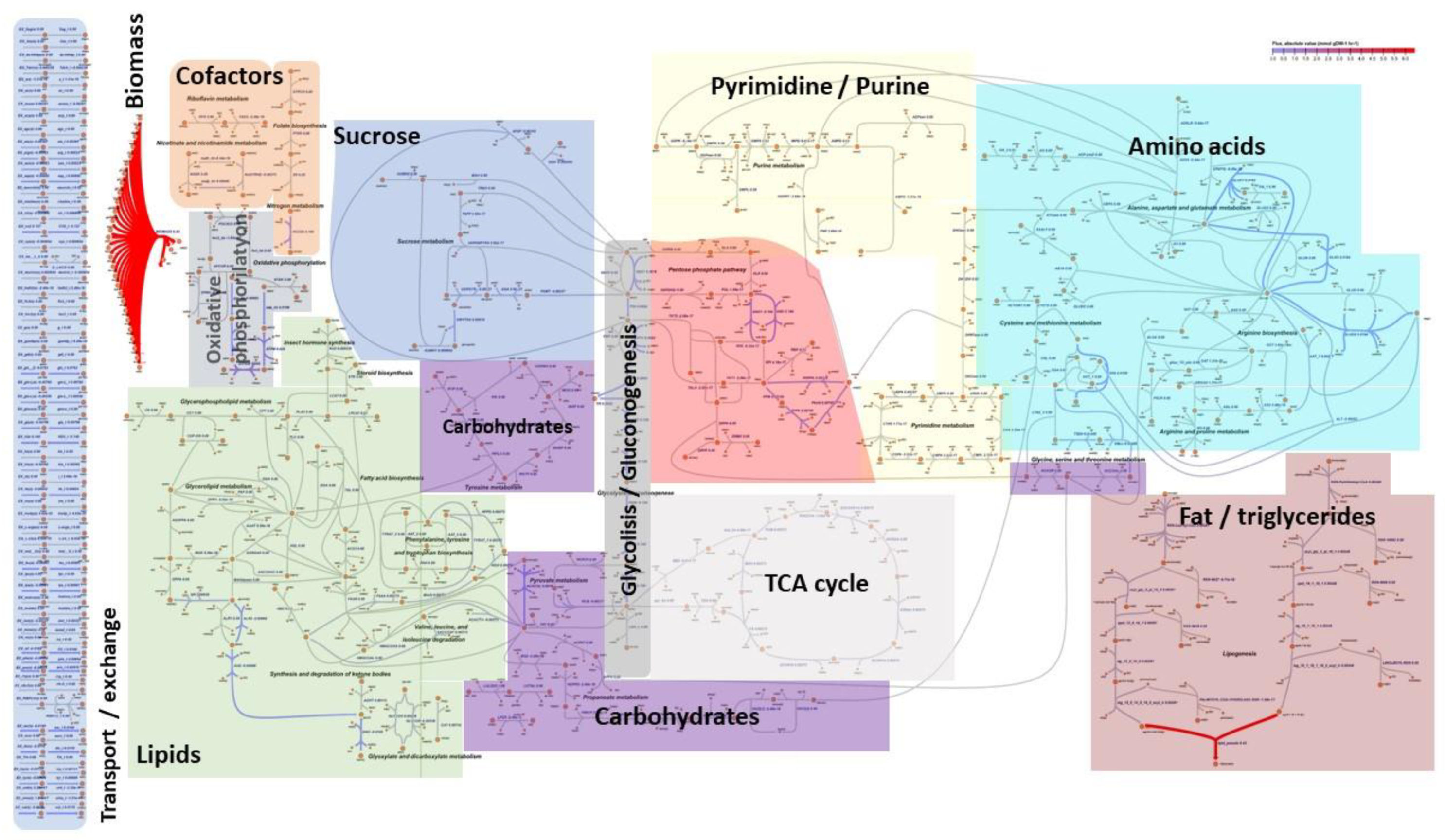
3.1. The First Medium-Scale Metabolic Model for Hermetia Illucens
3.2. Biomass Composition and Reaction
3.3. Model Validation and Evaluation
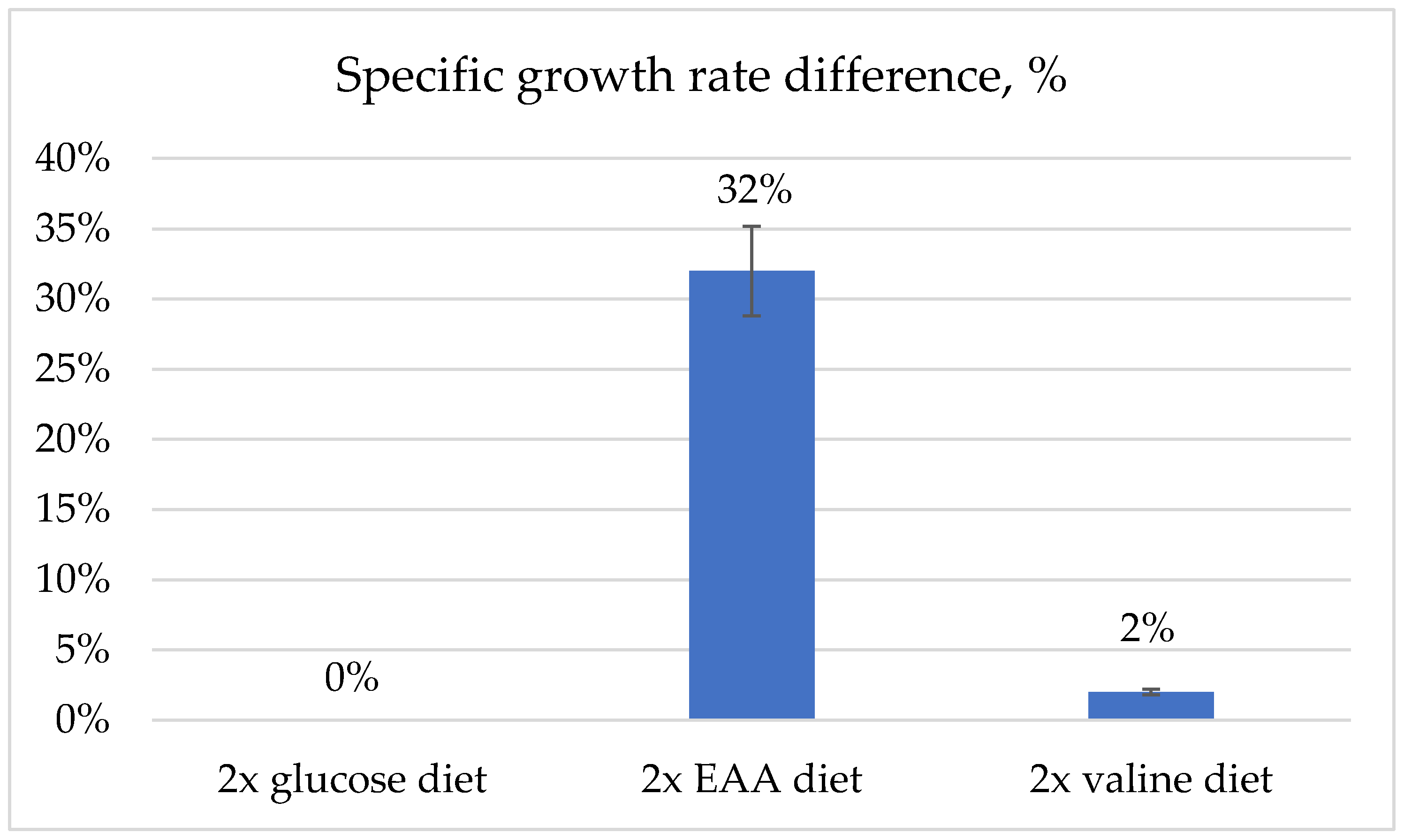
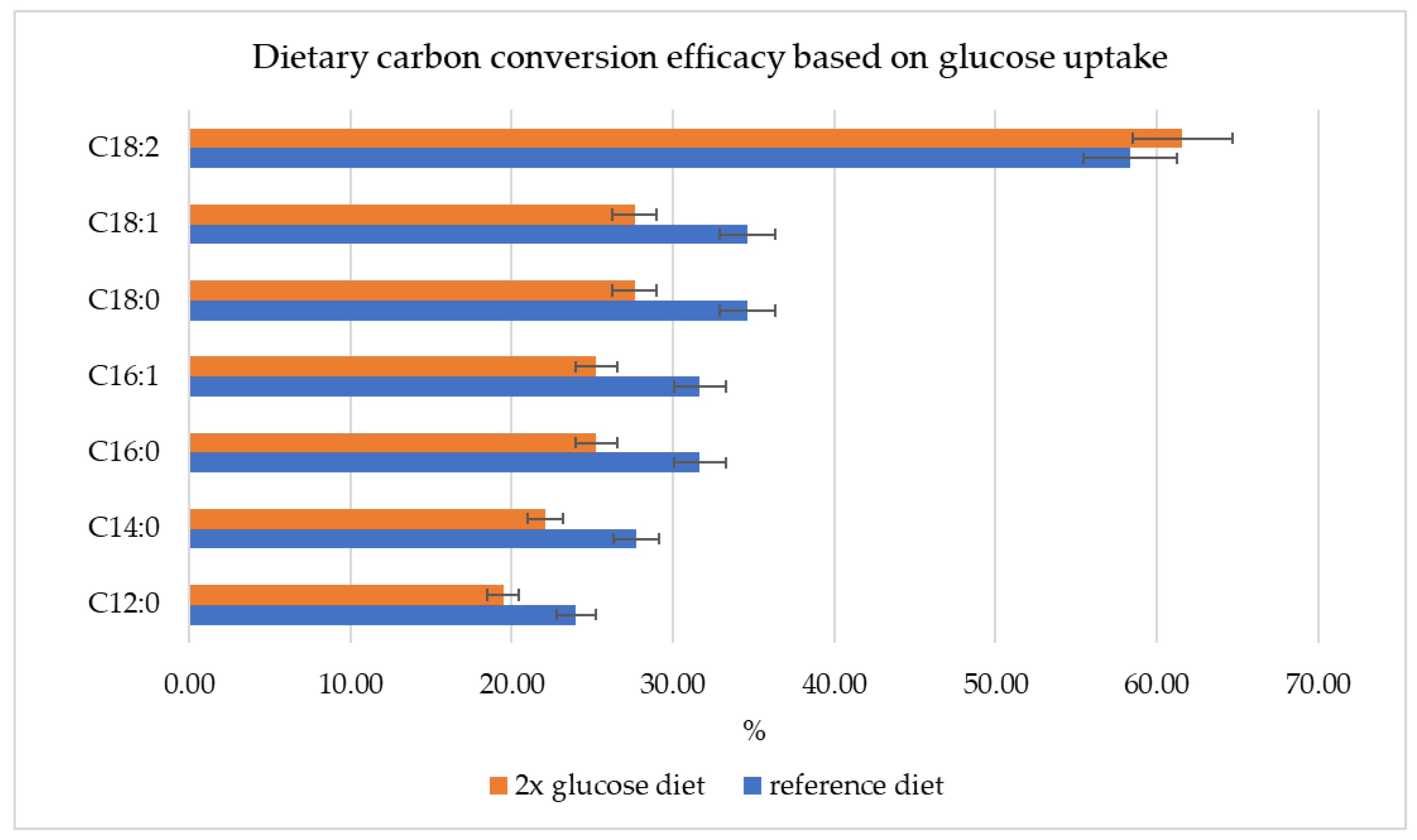
3.4. Metabolic Flux Potential as Predicted by Flux Variability Analysis (Resource Allocation)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Sundermann, E.M.; Lercher, M.J.; Heckmann, D. Modeling Photosynthetic Resource Allocation Connects Physiology with Evolutionary Environments. Sci. Rep. 2021, 11, 15979. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Cai, Y.; Yue, L.; Zhang, W. Effects of Different Nutritional Conditions on the Growth and Reproduction of Nilaparvata Lugens (Stål). Front. Physiol. 2022, 12, 794721. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, X.-P.; Li, C.-R.; Xia, Z.-Z.; Li, S.-X. Effect of Dietary Protein and Carbohydrates on Survival and Growth in Larvae of the Henosepilachna Vigintioctopunctata (F.) (Coleoptera: Coccinellidae). J. Insect Sci. 2018, 18, 3. [Google Scholar] [CrossRef]
- Berzins, K.; Muiznieks, R.; Baumanis, M.R.; Strazdina, I.; Shvirksts, K.; Prikule, S.; Galvanauskas, V.; Pleissner, D.; Pentjuss, A.; Grube, M.; et al. Kinetic and Stoichiometric Modeling-Based Analysis of Docosahexaenoic Acid (DHA) Production Potential by Crypthecodinium Cohnii from Glycerol, Glucose and Ethanol. Mar. Drugs 2022, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of Insect Use for Feed and Food: Life Cycle Assessment Perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable Use of Hermetia illucens Insect Biomass for Feed and Food: Attributional and Consequential Life Cycle Assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Cammack, J.; Tomberlin, J. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of The Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Gligorescu, A.; Toft, S.; Hauggaard-Nielsen, H.; Axelsen, J.A.; Nielsen, S.A. Development, Metabolism and Nutrient Composition of Black Soldier Fly Larvae (Hermetia illucens; Diptera: Stratiomyidae) in Relation to Temperature and Diet. J. Insects Food Feed 2018, 4, 123–133. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Influence of Larval Density and Dietary Nutrient Concentration on Performance, Body Protein, and Fat Contents of Black Soldier Fly Larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Perdana, M.P.; Bara, M.A.; Anggraeni, L.W.; Putra, R.E. Effects of Aeration Rate and Feed on Growth, Productivity and Nutrient Composition of Black Soldier Fly (Hermetia illucens L.) Larvae. J. Asia. Pac. Entomol. 2022, 25, 101902. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Fowles, T.M.; Nansen, C. Insect-Based Bioconversion: Value from Food Waste. In Food Waste Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 321–346. [Google Scholar]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Caparros Megido, R. About Lipid Metabolism in Hermetia illucens (L. 1758): On the Origin of Fatty Acids in Prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Mass Production of the Black Soldier Fly, Hermetia illucens (L.), (Diptera: Stratiomyidae) Reared on Three Manure Types. Animals 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Bellezza Oddon, S.; Biasato, I.; Resconi, A.; Gasco, L. Determination of Lipid Requirements in Black Soldier Fly through Semi-Purified Diets. Sci. Rep. 2022, 12, 10922. [Google Scholar] [CrossRef]
- Monk, J.M.; Lloyd, C.J.; Brunk, E.; Mih, N.; Sastry, A.; King, Z.; Takeuchi, R.; Nomura, W.; Zhang, Z.; Mori, H.; et al. IML1515, a Knowledgebase That Computes Escherichia Coli Traits. Nat. Biotechnol. 2017, 35, 904–908. [Google Scholar] [CrossRef]
- Lu, H.; Li, F.; Sánchez, B.J.; Zhu, Z.; Li, G.; Domenzain, I.; Marcišauskas, S.; Anton, P.M.; Lappa, D.; Lieven, C.; et al. A Consensus S. Cerevisiae Metabolic Model Yeast8 and Its Ecosystem for Comprehensively Probing Cellular Metabolism. Nat. Commun. 2019, 10, 3586. [Google Scholar] [CrossRef]
- Stalidzans, E.; Seiman, A.; Peebo, K.; Komasilovs, V.; Pentjuss, A. Model-Based Metabolism Design: Constraints for Kinetic and Stoichiometric Models. Biochem. Soc. Trans. 2018, 46, 261–267. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Boren, J.; Smith, U.; Uhlen, M.; Nielsen, J. Systems Biology in Hepatology: Approaches and Applications. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 365–377. [Google Scholar] [CrossRef]
- Komasilovs, V.; Pentjuss, A.; Elsts, A.; Stalidzans, E. Total Enzyme Activity Constraint and Homeostatic Constraint Impact on the Optimization Potential of a Kinetic Model. Biosystems 2017, 162, 128–134. [Google Scholar] [CrossRef]
- Elsts, A.; Pentjuss, A.; Stalidzans, E. SpaceScanner: COPASI Wrapper for Automated Management of Global Stochastic Optimization Experiments. Bioinformatics 2017, 33, 2966–2967. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Song, C.W.; Shin, J.H.; Lee, S.Y. Biorefineries for the Production of Top Building Block Chemicals and Their Derivatives. Metab. Eng. 2015, 28, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Kalnenieks, U.; Balodite, E.; Strähler, S.; Strazdina, I.; Rex, J.; Pentjuss, A.; Fuchino, K.; Bruheim, P.; Rutkis, R.; Pappas, K.M.; et al. Improvement of Acetaldehyde Production in Zymomonas Mobilis by Engineering of Its Aerobic Metabolism. Front. Microbiol. 2019, 10, 2533. [Google Scholar] [CrossRef] [PubMed]
- Kalnenieks, U.; Pentjuss, A.; Rutkis, R.; Stalidzans, E.; Fell, D.A. Modeling of Zymomonas Mobilis Central Metabolism for Novel Metabolic Engineering Strategies. Front. Microbiol. 2014, 5, 42. [Google Scholar] [CrossRef]
- McNally, C.P.; Borenstein, E. Metabolic Model-Based Analysis of the Emergence of Bacterial Cross-Feeding via Extensive Gene Loss. BMC Syst. Biol. 2018, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Moutinho, J.L.; Golan, R.; Yu, T.; Ladva, C.N.; Niedzwiecki, M.; Walker, D.I.; Sarnat, S.E.; Chang, H.H.; Greenwald, R.; et al. Use of High-Resolution Metabolomics for the Identification of Metabolic Signals Associated with Traffic-Related Air Pollution. Environ. Int. 2018, 120, 145–154. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Schirmeier, S. Insect Models of Central Nervous System Energy Metabolism and Its Links to Behavior. Glia 2018, 66, 1160–1175. [Google Scholar] [CrossRef]
- Ankrah, N.Y.D.; Chouaia, B.; Douglas, A.E. The Cost of Metabolic Interactions in Symbioses between Insects and Bacteria with Reduced Genomes. mBio 2018, 9, e01433-18. [Google Scholar] [CrossRef]
- Hall, R.J.; Thorpe, S.; Thomas, G.H.; Wood, A.J. Simulating the Evolutionary Trajectories of Metabolic Pathways for Insect Symbionts in the Genus Sodalis. Microb. Genom. 2020, 6, mgen000378. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. ‘Insects as Food and Feed: If You Can’t Beat Them, Eat Them!’—To the Magnificent Seven and Beyond. J. Insect Sci. 2021, 21, 9. [Google Scholar] [CrossRef]
- Reimers, A.-M.; Reimers, A.C. The Steady-State Assumption in Oscillating and Growing Systems. J. Theor. Biol. 2016, 406, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What Is Flux Balance Analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Marcišauskas, S.; Sánchez, B.J.; Domenzain, I.; Hermansson, D.; Agren, R.; Nielsen, J.; Kerkhoven, E.J. RAVEN 2.0: A Versatile Toolbox for Metabolic Network Reconstruction and a Case Study on Streptomyces Coelicolor. PLoS Comput. Biol. 2018, 14, e1006541. [Google Scholar] [CrossRef]
- Karp, P.D.; Billington, R.; Caspi, R.; Fulcher, C.A.; Latendresse, M.; Kothari, A.; Keseler, I.M.; Krummenacker, M.; Midford, P.E.; Ong, Q.; et al. The BioCyc Collection of Microbial Genomes and Metabolic Pathways. Brief. Bioinform. 2019, 20, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.; Park, S.; Kim, S.; Lee, J. Inhibition of Polymicrobial Biofilm Formation by Saw Palmetto Oil, Lauric Acid and Myristic Acid. Microb. Biotechnol. 2022, 15, 590–602. [Google Scholar] [CrossRef]
- Degtyarenko, K.; de Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcantara, R.; Darsow, M.; Guedj, M.; Ashburner, M. ChEBI: A Database and Ontology for Chemical Entities of Biological Interest. Nucleic Acids Res. 2007, 36, D344–D350. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Palsson, B.Ø.; Fleming, R.M.T. Reconstruction and Use of Microbial Metabolic Networks: The Core Escherichia Coli Metabolic Model as an Educational Guide. EcoSal Plus 2010, 4. [Google Scholar] [CrossRef]
- Petrovs, R.; Stalidzans, E.; Pentjuss, A. IMFLer: A Web Application for Interactive Metabolic Flux Analysis and Visualization. J. Comput. Biol. 2021, 28, 1021–1032. [Google Scholar] [CrossRef]
- Ataman, M.; Hernandez Gardiol, D.F.; Fengos, G.; Hatzimanikatis, V. RedGEM: Systematic Reduction and Analysis of Genome-Scale Metabolic Reconstructions for Development of Consistent Core Metabolic Models. PLoS Comput. Biol. 2017, 13, e1005444. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and Analysis of Biochemical Constraint-Based Models Using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef]
- Ceccotti, C.; Bruno, D.; Tettamanti, G.; Branduardi, P.; Bertacchi, S.; Labra, M.; Rimoldi, S.; Terova, G. New Value from Food and Industrial Wastes—Bioaccumulation of Omega-3 Fatty Acids from an Oleaginous Microbial Biomass Paired with a Brewery by-Product Using Black Soldier Fly (Hermetia illucens) Larvae. Waste Manag. 2022, 143, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Seyedalmoosavi, M.M.; Mielenz, M.; Veldkamp, T.; Daş, G.; Metges, C.C. Growth Efficiency, Intestinal Biology, and Nutrient Utilization and Requirements of Black Soldier Fly (Hermetia illucens) Larvae Compared to Monogastric Livestock Species: A Review. J. Anim. Sci. Biotechnol. 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.B. Introduction. In Functional Dietary Lipids; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty Acids and Related Phase Change Materials for Reliable Thermal Energy Storage at Moderate Temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef]
- Feist, A.M.; Palsson, B.O. The Biomass Objective Function. Curr. Opin. Microbiol. 2010, 13, 344–349. [Google Scholar] [CrossRef]
- Schönborn, J.W.; Jehrke, L.; Mettler-Altmann, T.; Beller, M. FlySilico: Flux Balance Modeling of Drosophila Larval Growth and Resource Allocation. Sci. Rep. 2019, 9, 17156. [Google Scholar] [CrossRef]
- Thiele, C.; Spandl, J. Cell Biology of Lipid Droplets. Curr. Opin. Cell Biol. 2008, 20, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D. The Biogenesis and Functions of Lipid Bodies in Animals, Plants and Microorganisms. Prog. Lipid Res. 2001, 40, 325–438. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A.; Gould, A.P. Lipid Droplet Functions beyond Energy Storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Thiele, I. Computationally Efficient Flux Variability Analysis. BMC Bioinform. 2010, 11, 489. [Google Scholar] [CrossRef]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The Environmental Sustainability of Insects as Food and Feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and Characterization of Myristic Acid/Expanded Graphite Composite Phase Change Materials for Thermal Energy Storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Ceccon Lanes, C.F.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) Larvae and Prepupae: Biomass Production, Fatty Acid Profile and Expression of Key Genes Involved in Lipid Metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef]
- Opatovsky, I.; Vitenberg, T.; Jonas-Levi, A.; Gutman, R. Does Consumption of Baker’s Yeast (Saccharomyces Cerevisiae) by Black Soldier Fly (Diptera: Stratiomyidae) Larvae Affect Their Fatty Acid Composition? J. Insect Sci. 2021, 21, 5. [Google Scholar] [CrossRef]

| Ingredients | % | g/100 g | Total Diet, g | Left in Residue, g |
|---|---|---|---|---|
| Glucose | 10 | 10.58 | 846 | 0 |
| Egg White Protein | 20 | 23.38 | 1870 | 1310 |
| Water | 70 | 66.05 | 5284 | 4890 |
| Amino Acid Profile | mg per 100 g Protein | Consumed Protein Weight, g | mg/larva/day | mmol/larva/day |
|---|---|---|---|---|
| Lysine | 6000 | 85.272 | 1.769 | 0.012 |
| Methionine | 3500 | 50.116 | 1.039 | 0.007 |
| Cystine | 2500 | 36.465 | 0.756 | 0.003 |
| Aspartate | 4800 | 48.62 | 1.008 | 0.008 |
| Asparagine | 4800 | 48.62 | 1.008 | 0.008 |
| Threonine | 4300 | 62.832 | 1.303 | 0.011 |
| Serine | 6400 | 93.687 | 1.943 | 0.018 |
| Glutamate | 6150 | 62.271 | 1.292 | 0.009 |
| Glutamine | 6150 | 62.271 | 1.292 | 0.009 |
| Proline | 3600 | 52.921 | 1.098 | 0.010 |
| Glycine | 3300 | 47.872 | 0.993 | 0.013 |
| Alanine | 5800 | 84.898 | 1.761 | 0.020 |
| Valine | 6500 | 95.183 | 1.974 | 0.017 |
| Isoleucine | 5100 | 74.239 | 1.540 | 0.012 |
| Leucine | 8000 | 117.062 | 2.428 | 0.019 |
| Tyrosine | 3700 | 54.604 | 1.133 | 0.006 |
| Phenylalanine | 5500 | 78.914 | 1.637 | 0.010 |
| Histidine | 2200 | 32.164 | 0.667 | 0.004 |
| Arginine | 5400 | 79.475 | 1.648 | 0.009 |
| Tryptophan | 1500 | 23.001 | 0.477 | 0.002 |
| Fatty Acid | g/100 g |
|---|---|
| Caprylic acid (C8:0) | 0.13 |
| * Lauric acid (C12:0) | 29.4 |
| * Myristic acid (C14:0) | 5.75 |
| Myristoleic acid (C14:1) | 0.73 |
| * Palmitic acid (C16:0) | 10.45 |
| * Palmitoleic acid (C16:1) | 5.69 |
| Stearic acid (C18:0) | 3.5 |
| * Oleic Acid (C18:1) | 21.05 |
| * Linoleic acid (C18:2) | 19.8 |
| Linolenic acid, alpha (C18:3) | 0.48 |
| Linolenic acid, gamma (C18:3) | <0.10 |
| Eicosapentaenoic acid (C20:5) | <0.10 |
| Docosahexaenoic acid (C22:6) | <0.10 |
| Organism | Model ID | Metabolites | Reactions | Genes | % Dead-End Metabolites | % Blocked Reactions | % Unbalanced Reactions | % Exchange Reactions |
|---|---|---|---|---|---|---|---|---|
| E. coli | e_coli_core | 72 | 95 | 137 | 4.21 | 17.51 | 16.84 | 21.10 |
| H. illucens | Hermetia_01 | 326 | 407 | 471 | 2.45 | 16.71 | 19.66 | 18.43 |
| S. cerevisiae | yeastGEM_v8.6.0 | 2749 | 4069 | 1151 | 12.44 | 31.73 | 11.40 | 6.46 |
| E. coli | iWFL_1372 | 1973 | 2782 | 1372 | 9.17 | 40.08 | 12.80 | 14.06 |
| Human | Recon3D_01 | 8399 | 13543 | 3697 | 6.56 | 11.70 | 17.19 | 13.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grausa, K.; Siddiqui, S.A.; Lameyer, N.; Wiesotzki, K.; Smetana, S.; Pentjuss, A. Metabolic Modeling of Hermetia illucens Larvae Resource Allocation for High-Value Fatty Acid Production. Metabolites 2023, 13, 724. https://doi.org/10.3390/metabo13060724
Grausa K, Siddiqui SA, Lameyer N, Wiesotzki K, Smetana S, Pentjuss A. Metabolic Modeling of Hermetia illucens Larvae Resource Allocation for High-Value Fatty Acid Production. Metabolites. 2023; 13(6):724. https://doi.org/10.3390/metabo13060724
Chicago/Turabian StyleGrausa, Kristina, Shahida A. Siddiqui, Norbert Lameyer, Karin Wiesotzki, Sergiy Smetana, and Agris Pentjuss. 2023. "Metabolic Modeling of Hermetia illucens Larvae Resource Allocation for High-Value Fatty Acid Production" Metabolites 13, no. 6: 724. https://doi.org/10.3390/metabo13060724
APA StyleGrausa, K., Siddiqui, S. A., Lameyer, N., Wiesotzki, K., Smetana, S., & Pentjuss, A. (2023). Metabolic Modeling of Hermetia illucens Larvae Resource Allocation for High-Value Fatty Acid Production. Metabolites, 13(6), 724. https://doi.org/10.3390/metabo13060724







