Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cell Lines and Chemical Reagents
2.3. Metabolites Extraction for HPLC-MS/MS
2.4. Metabolites Extraction for Bioactivity Analysis
2.5. HPLC-MS/MS Analysis
2.6. Standards Database of novoDB
2.7. Metabolites Identification by Multiple Reaction Monitoring
2.8. Metabolites Quantification
2.9. Metabolites Annotation
2.10. Cell Survival Assay under H2O2
2.11. Detection of ROS Levels
2.12. Cell Lysis
2.13. Detection of Total Proteins
2.14. Detection of SOD Enzyme Activities
2.15. Detection of CAT Enzyme Activities
2.16. Detection of Total Soluble Saccharides
2.17. Detection of Total Phenols
2.18. Statistics Analysis
3. Results
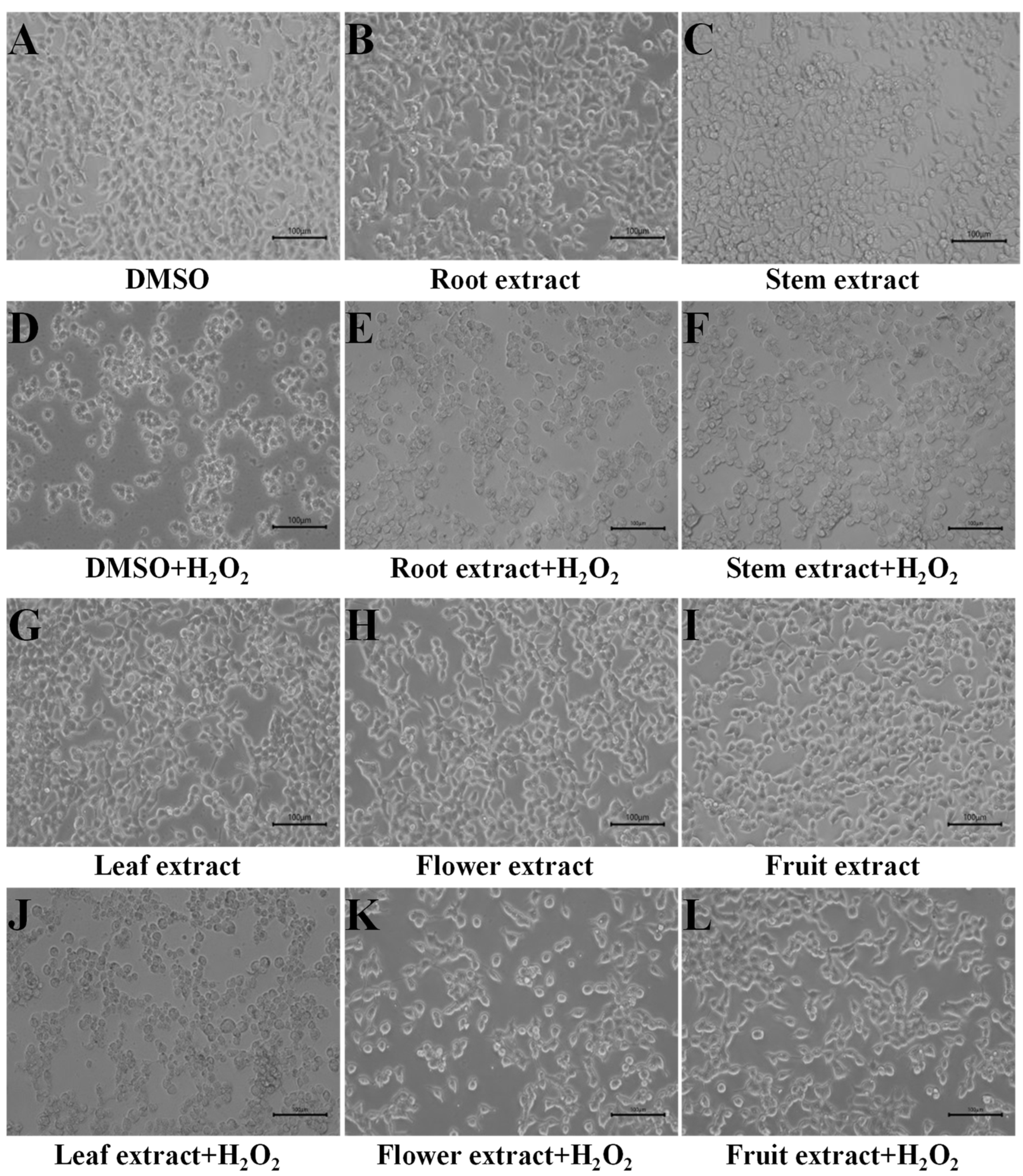
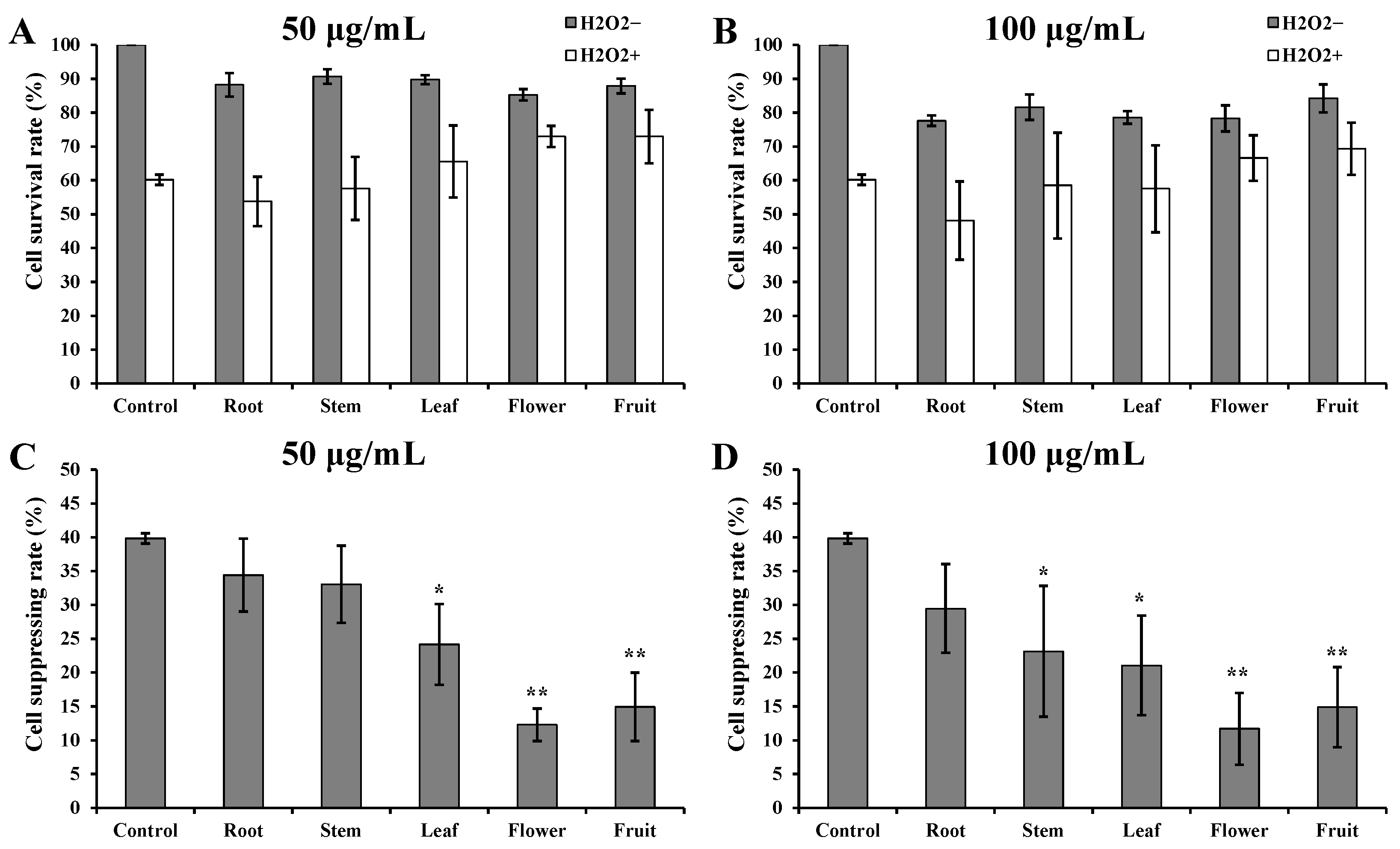
3.1. Fine Capacity to H2O2 Induction in Flower and Fruit Extracts of D. nobile
3.2. Good Intracellular ROS Scavenging Effects of Flower and Fruit Extracts
3.3. Improved CAT and SOD Activities by Flower and Fruit Extracts
3.4. Evaluation of the Stability and Reliability in HPLC-MS/MS
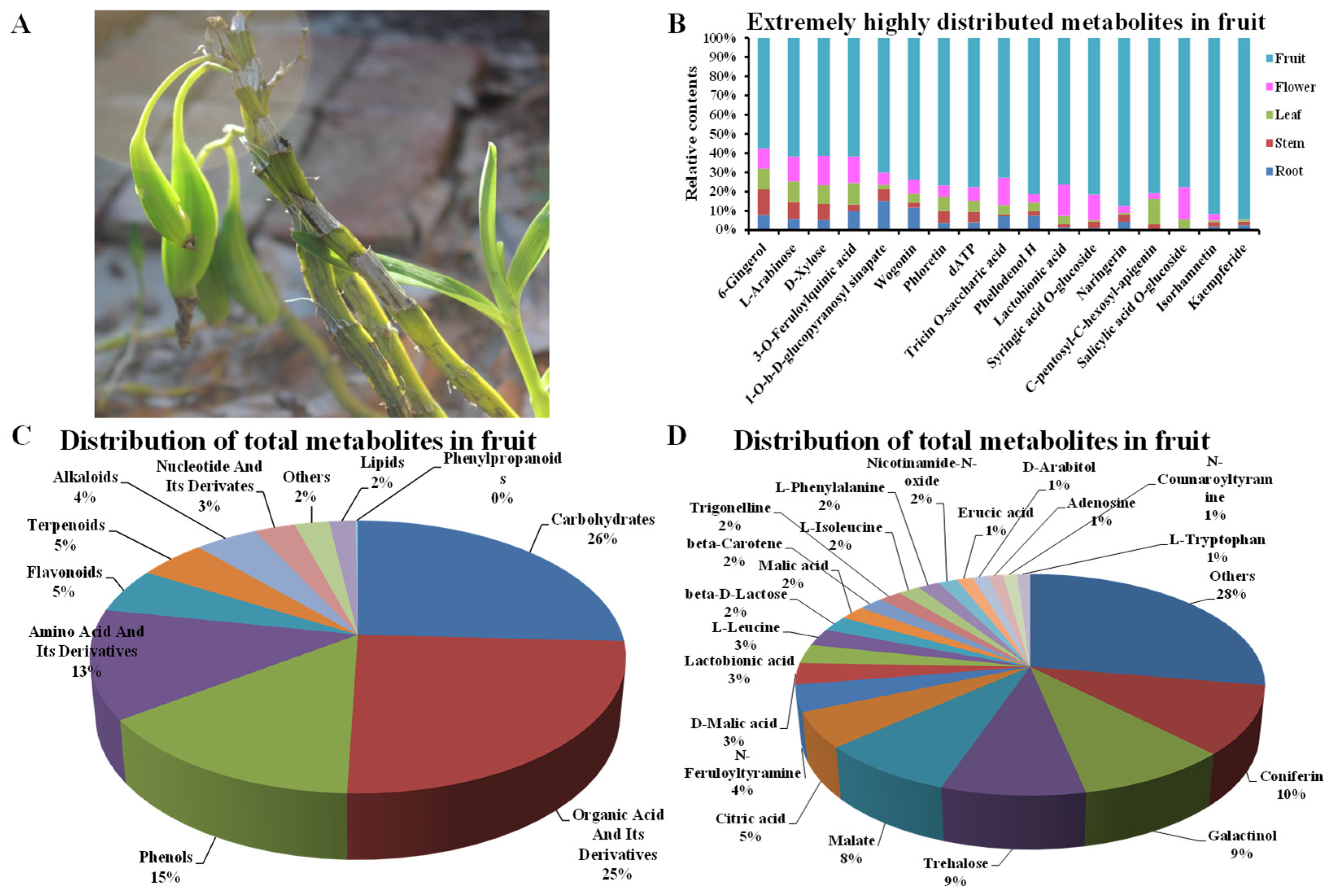
3.5. Distribution of Metabolites in D. nobile Fruits by HPLC-MS/MS
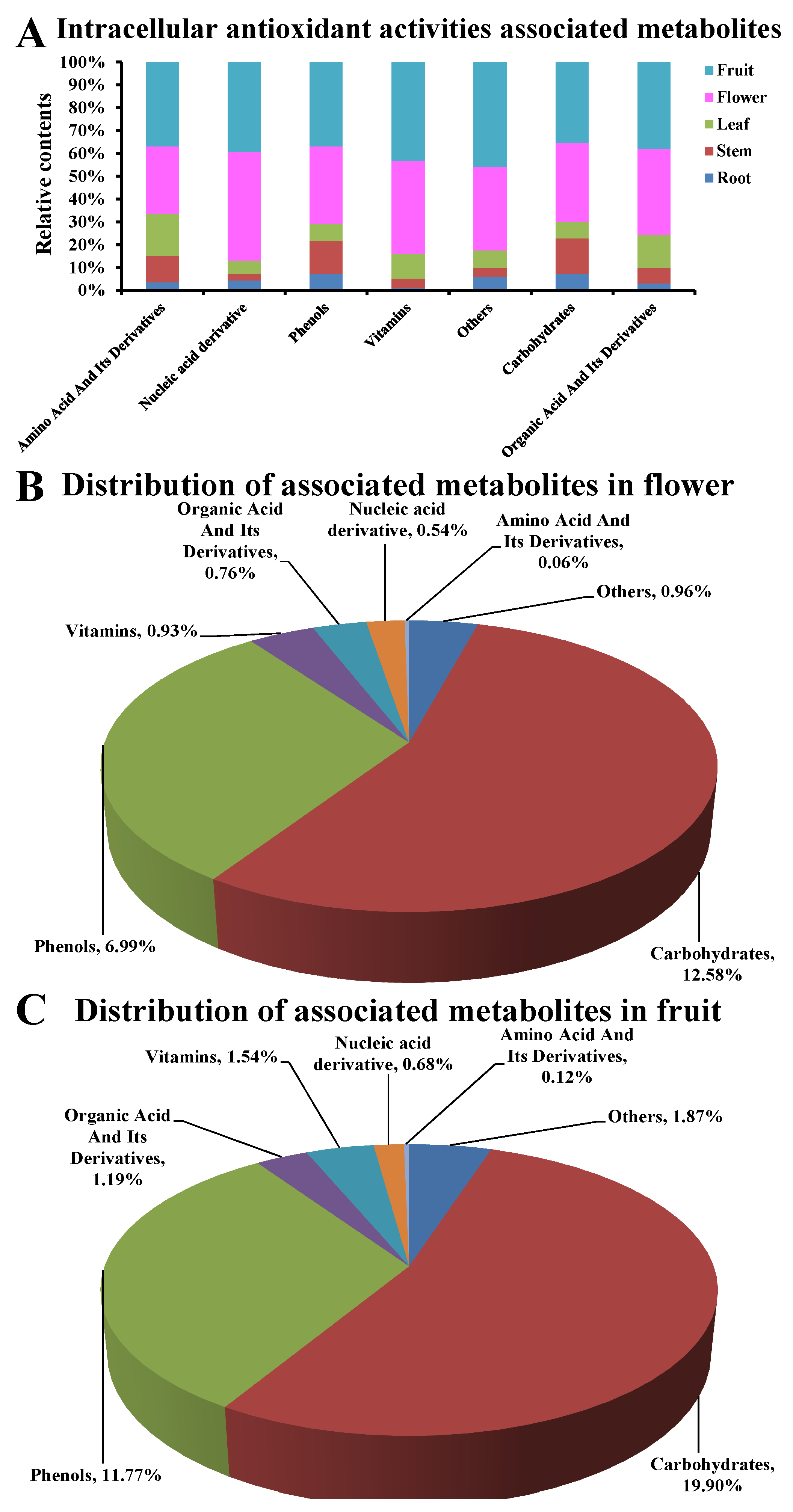
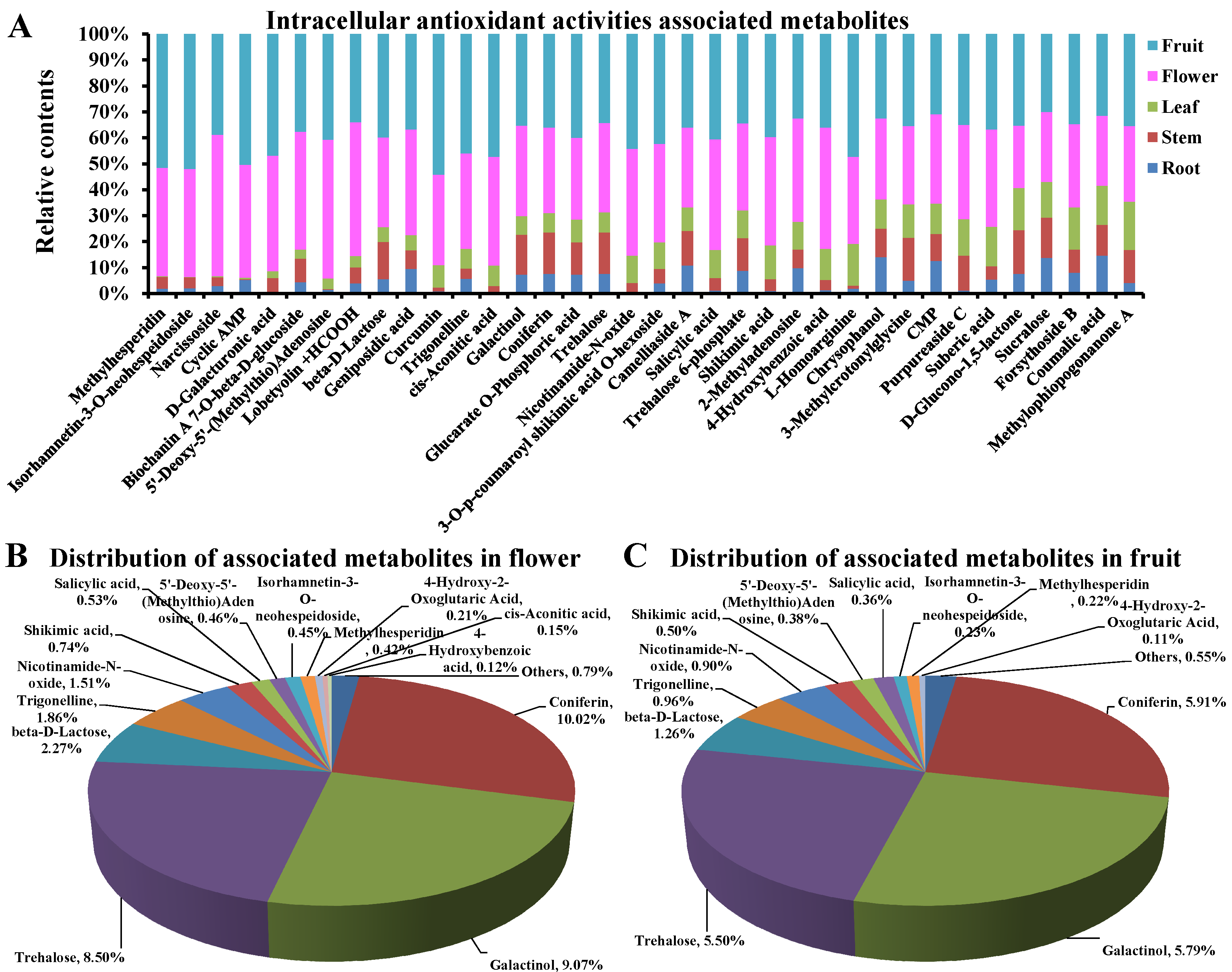
3.6. Intracellular Antioxidant Activities Associated Metabolites
3.7. The Main Intracellular Antioxidant Basis in Flowers and Fruits of D. nobile
3.8. Differences between In Vitro and Intracellular Antioxidants
3.9. Verification of the HPLC-MS/MS Results
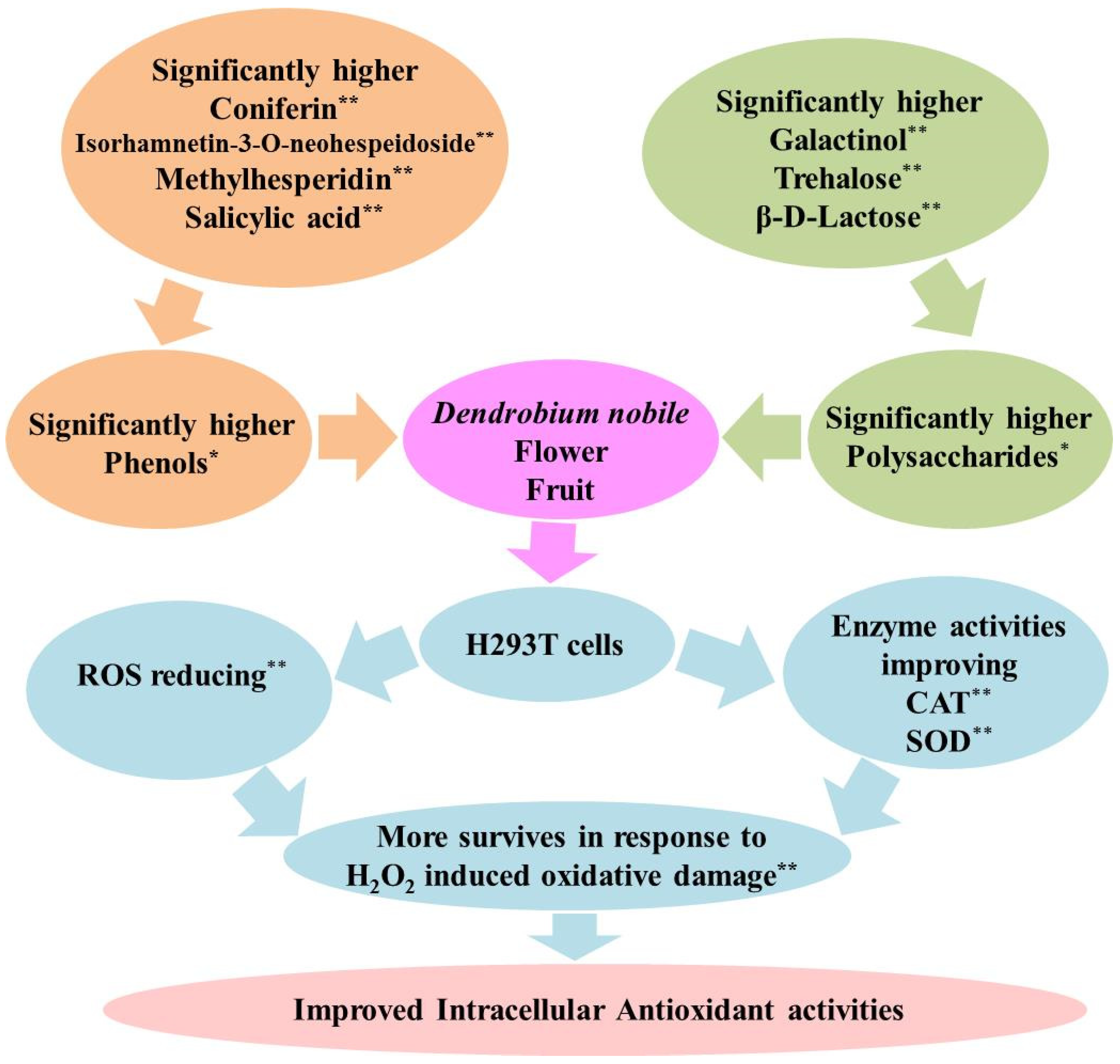
4. Discussions
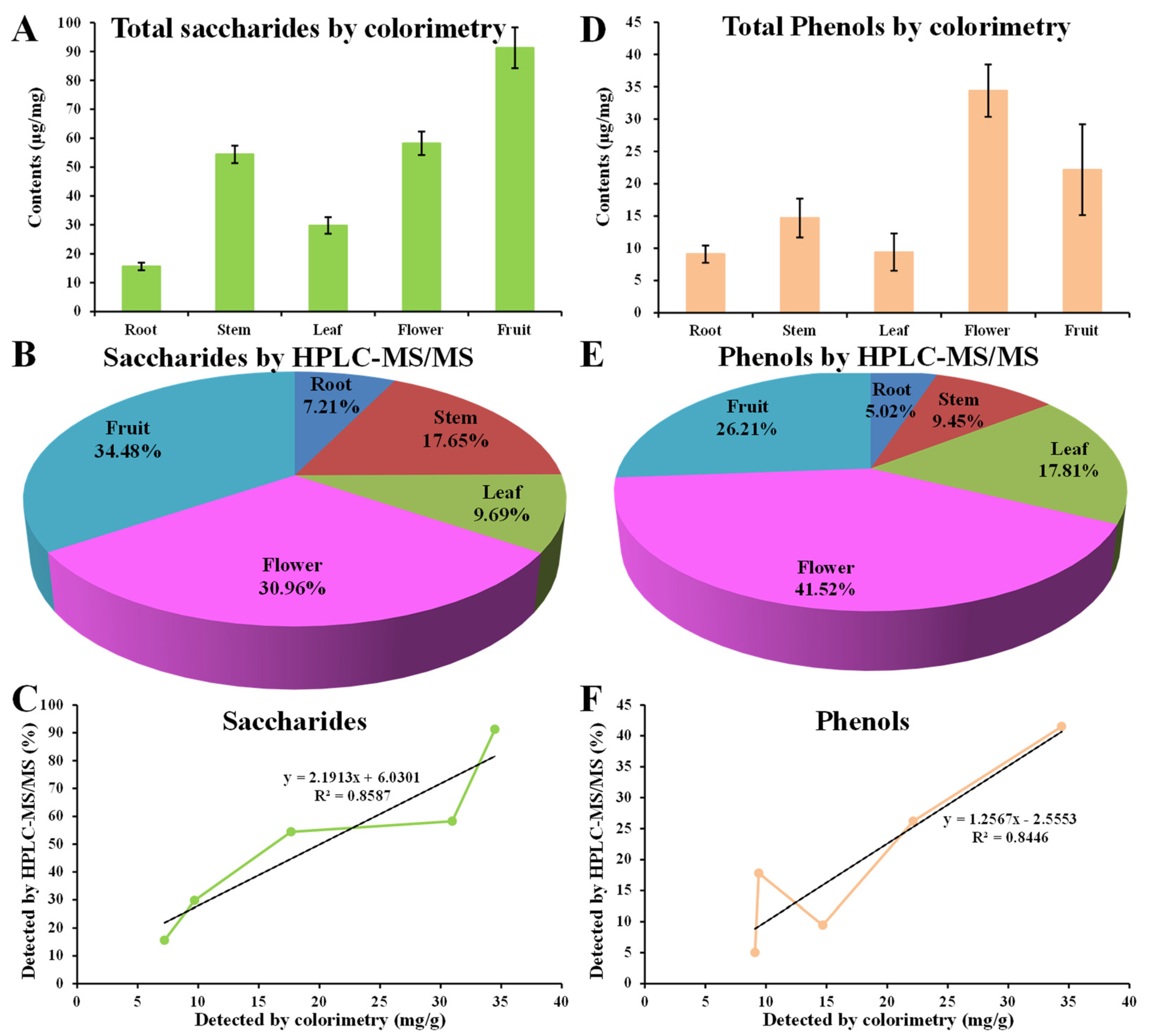
4.1. Accumulation of Saccharides and Phenols Resulted in Fine Intracellular Antioxidant Activities in D. nobile Flowers and Fruits
4.2. Intracellular Antioxidants Showed Different Characteristics from In Vitro Antioxidants in D. nobile
4.3. The Newly Identified Intracellular Antioxidants Will Further Enrich the Prospects of Dendrobium on Pharmaceuticals and Health-Care Products
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Asif, H.M.; Shaheen, G.; Ghauri, A.O.; Rajpoot, S.R.; Tasleem, M.W.; Shamim, T.; Hadi, F.; Noor, R.; Ali, T.; et al. A comprehensive review on medicinal plants possessing antioxidant potential. Clin. Exp. Pharmacol. Physiol. 2023, 50, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Periera da Silva, A.; Rocha, R.; Silva, C.M.; Mira, L.; Duarte, M.F.; Florêncio, M.H. Antioxidants in medicinal plant extracts. A research study of the antioxidant capacity of Crataegus, Hamamelis and Hydrastis. Phytother. Res. 2000, 14, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Inayatullah, S.; Prenzler, P.D.; Obied, H.K.; Rehman, A.U.; Mirza, B. Bioprospecting traditional Pakistani medicinal plants for potent antioxidants. Food Chem. 2012, 132, 222–229. [Google Scholar] [CrossRef]
- Swain, S.; Rautray, T.R. Estimation of trace elements, antioxidants, and antibacterial agents of regularly consumed indian medicinal plants. Biol. Trace Elem. Res. 2021, 199, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dou, M.; Zhang, Z.; Zhang, D.; Huang, C. Protective effect of Dendrobium officinale polysaccharides on H2O2-induced injury in H9c2 cardiomyocytes. Biomed. Pharmacother. 2017, 94, 72–78. [Google Scholar] [CrossRef]
- Chan, C.F.; Wu, C.T.; Huang, W.Y.; Lin, W.S.; Wu, H.W.; Huang, T.K.; Chang, M.Y.; Lin, Y.S. Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef] [PubMed]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Antioxidant and cytotoxic activities of Dendrobium moniliforme extracts and the detection of related compounds by GC-MS. BMC Complement. Altern. Med. 2018, 18, 134. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Gao, B.; Qian, Z.; Liu, J.; Wu, S.; Si, J. Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Res. Int. 2019, 123, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant sctivities and protective effects of dendropachol, a new bisbibenzyl compound from Dendrobium pachyglossum, on hydrogen peroxide-induced oxidative stress in HaCaT keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Zheng, S.; Chun, Z.; Hu, Y. Dendrobium liquor eliminates free radicals and suppresses cellular proteins expression disorder to protect cells from oxidant damage. J. Food Biochem. 2020, 44, e13509. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Hu, Y.D.; Zhao, R.X.; Li, H.J.; Chun, Z.; Zheng, S.G. Quantitative identification of antioxidant basis for Dendrobium nobile flower by high performance liquid chromatography-tandem mass spectrometry. Int. J. Anal. Chem. 2022, 2022, 9510598. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zou, S.; Lin, J.; Zhai, L.; Zhang, Y.; Fu, X.; Chen, S.; Niu, H.; Liu, F.; Wu, C.; et al. Antioxidant and anti-inflammatory activity of constituents isolated from Dendrobium nobile (Lindl.). Front. Chem. 2022, 10, 988459. [Google Scholar] [CrossRef]
- Long, Y.; Wang, W.; Zhang, Y.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Dendrobium nobile Lindl polysaccharides attenuate UVB-induced photodamage by regulating oxidative stress, inflammation and MMPs expression in mice model. Photochem. Photobiol. 2023, online. [Google Scholar] [CrossRef]
- Zheng, S.G.; Hu, Y.D.; Zhao, R.X.; Yan, S.; Zhang, X.Q.; Zhao, T.M.; Chun, Z. Genome-wide researches and applications on Dendrobium. Planta 2018, 248, 769–784. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, H.P.; He, Y.Y.; Chen, W.L.; Chen, J.W.; Gao, L.; Hu, H.Y.; Wang, J. Effects of rich-polyphenols extract of Dendrobium loddigesii on anti-diabetic, anti-inflammatory, anti-oxidant, and gut microbiota modulation in db/db mice. Molecules 2018, 23, 3245. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, Y.; Zhao, R.; Zhao, T.; Li, H.; Rao, D.; Chun, Z. Quantitative assessment of secondary metabolites and cancer cell inhibiting activity by high performance liquid chromatography fingerprinting in Dendrobium nobile. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1140, 122017. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, J.; Li, G.; Wang, J.; Liu, D.; Chen, L.; Song, X.; Cui, L.; Sun, Y. Application and prospect of quasi-targeted metabolomics in age-related hearing loss. Hear Res. 2022, 424, 108604. [Google Scholar] [CrossRef]
- Gao, J.N.; Li, Y.; Liang, J.; Chai, J.H.; Kuang, H.X.; Xia, Y.G. Direct acetylation for full analysis of polysaccharides in edible plants and fungi using reverse phase liquid chromatography-multiple reaction monitoring mass spectrometry. J. Pharm. Biomed. Anal. 2023, 222, 115083. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, L.; Nie, Q.; Huang, M.; Luo, C.; Sun, Y.; Ma, Y.; Yu, J.; Du, F. HPLC-based metabolomics of Dendrobium officinale revealing its antioxidant ability. Front. Plant. Sci. 2023, 14, 1060242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zheng, S.; Li, Y.; Zhang, X.; Rao, D.; Chun, Z.; Hu, Y. As a novel anticancer candidate, ether extract of Dendrobium nobile overstimulates cellular protein biosynthesis to induce cell stress and autophagy. J. Appl. Biomed. 2022, 21, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Gai, F.; Janiak, M.A.; Sulewska, K.; Peiretti, P.G.; Karamać, M. Phenolic compound profile and antioxidant capacity of flax (Linum usitatissimum L.) harvested at different growth stages. Molecules 2023, 28, 1807. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased oxidative stress in the prefrontal cortex as a shared feature of depressive- and PTSD-like syndromes: Effects of a standardized herbal antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef]
- Bastin, A.R.; Nazari-Robati, M.; Sadeghi, H.; Doustimotlagh, A.H.; Sadeghi, A. Trehalose and N-acetyl cysteine alleviate inflammatory vytokine production and oxidative stress in LPS-stimulated human peripheral blood mononuclear cells. Immunol. Investig. 2022, 51, 963–979. [Google Scholar] [CrossRef]
- Gao, W.; Yu, T.; Li, G.; Shu, W.; Jin, Y.; Zhang, M.; Yu, X. Antioxidant activity and anti-apoptotic effect of the small molecule procyanidin B1 in early mouse embryonic development produced by somatic cell nuclear transfer. Molecules 2021, 26, 6150. [Google Scholar] [CrossRef]
- Xiaojin, Y.; Caiyan, L.; Lianrong, Y.; Guoliang, X.; Zhengqing, L.; Shizhe, C.; Xiaodi, Y.; Hua, H. Study on the antioxidant and anticancer activities of Sorbus pohuashanensis (hance) Hedl flavonoids in vitro and its screen of small molecule active components. Nutr. Cancer 2022, 74, 2243–2253. [Google Scholar] [CrossRef]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.N.; López-Camarillo, C.; Salas, E. Phenolipids, amphipilic phenolic antioxidants with modified properties and their spectrum of applications in development: A review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef]
- Shin, H.K.; Kim, T.W.; Kim, Y.J.; Park, S.R.; Seo, C.S.; Ha, H.; Jung, J.Y. Protective effects of Dendrobium nobile against cisplatin nephrotoxicity both in-vitro and in-vivo. Iran. J. Pharm. Res. 2017, 16, 197–206. [Google Scholar]
- Tanveer, M.A.; Rashid, H.; Nazir, L.A.; Archoo, S.; Shahid, N.H.; Ragni, G.; Umar, S.A.; Tasduq, S.A. Trigonelline, a plant derived alkaloid prevents ultraviolet-B-induced oxidative DNA damage in primary human dermal fibroblasts and BALB/c mice via modulation of phosphoinositide 3-kinase-Akt-Nrf2 signalling axis. Exp. Gerontol. 2023, 171, 112028. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L. Shikimic acid from Artemisia absinthium inhibits protein glycation in diabetic rats. Int. J. Biol. Macromol. 2019, 122, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.P.; Garcia, E.F.; de Oliveira, M.A.; Candido, L.C.M.; Coelho, F.M.; Costa, V.V.; Batista, N.V.; Queiroz-Junior, C.M.; Brito, L.F.; Sousa, L.P.; et al. cis-Aconitic Acid, a constituent of Echinodorus grandiflorus leaves, inhibits antigen-induced arthritis and gout in mice. Planta Med. 2022, 88, 1123–1131. [Google Scholar] [CrossRef] [PubMed]



| Compound ID | Name | Formula | MW (Da) | RT (min) | Q1 (m/z) | Class | Peak Areas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flower1 | Flower2 | Flower3 | Fruit1 | Fruit2 | Fruit3 | QC1 | QC2 | QC3 | |||||||
| Com_405_pos | (−)-trans-Carveol | C10H16O | 152.233 | 1.09 | 153.13 | Lipids | 124,600 | 107,900 | 92,890 | 103,500 | 121,200 | 90,420 | 89,580 | 82,780 | 101,400 |
| Com_245_neg | (+)-Dihydrojasmonic acid | C12H20O3 | 212.285 | 13.03 | 211.10 | Organic Acid And Its Derivatives | 36,260 | 26,710 | 22,320 | 27,540 | 36,160 | 30,830 | 28,420 | 20,820 | 17,560 |
| Com_131_neg | 13-HOTrE | C18H30O3 | 294.000 | 12.70 | 293.00 | Lipids | 24,070 | 22,970 | 32,880 | 26,400 | 29,030 | 28,130 | 18,910 | 20,200 | 25,650 |
| Com_558_pos | 2-Methyladenosine | C11H15N5O4 | 281.268 | 0.77 | 282.10 | Nucleotide And Its Derivates | 4,340,000 | 1,754,000 | 1,430,000 | 2,165,000 | 1,921,000 | 2,051,000 | 1,351,000 | 1,199,000 | 1,256,000 |
| Com_437_pos | 3-Hydroxy-3-methylpentane-1,5-dioic acid | C6H10O5 | 162.141 | 4.23 | 163.00 | Amino Acid And Its Derivatives | 1,626,000 | 683,500 | 1,504,000 | 1,670,000 | 1,635,000 | 1,518,000 | 508,700 | 453,400 | 506,600 |
| Com_375_pos | 3-Methylcrotonylglycine | C7H11NO3 | 157.167 | 0.61 | 158.10 | Organic Acid And Its Derivatives | 154,600 | 131,500 | 110,400 | 154,400 | 150,700 | 159,300 | 120,400 | 94,650 | 67,130 |
| Com_271_neg | 3-O-p-Coumaroyl shikimic acid O-hexoside | C22H26O12 | 482.100 | 5.30 | 481.10 | Organic Acid And Its Derivatives | 244,000 | 33,630 | 7233 | 110,400 | 104,900 | 104,000 | 44,740 | 43,480 | 43,210 |
| Com_247_neg | 4-Hydroxy-2-oxoglutaric acid | C5H6O6 | 162.098 | 0.68 | 161.00 | Organic Acid And Its Derivatives | 5,392,000 | 3,086,000 | 3,504,000 | 4,633,000 | 5,496,000 | 4,981,000 | 4,075,000 | 3,752,000 | 3,866,000 |
| Com_195_neg | 4-Hydroxybenzoic acid | C7H6O3 | 138.121 | 0.78 | 137.02 | Phenols | 4,686,000 | 3,329,000 | 2,630,000 | 2,804,000 | 2,826,000 | 2,589,000 | 1,798,000 | 1,737,000 | 1,761,000 |
| Com_647_pos | 4-Nitrophenol | C6H5NO3 | 139.109 | 29.43 | 140.00 | Phenols | 1,210,000 | 1,138,000 | 1,205,000 | 1,245,000 | 1,166,000 | 1,228,000 | 698,600 | 1,059,000 | 1,281,000 |
| Com_485_pos | 5′-Deoxy-5′-(methylthio)adenosine | C11H15N5O3S | 297.333 | 2.57 | 298.00 | Nucleotide And Its Derivates | 2,370,000 | 22,660,000 | 16,880,000 | 11,170,000 | 10,400,000 | 10,500,000 | 4,789,000 | 4,347,000 | 4,120,000 |
| Com_300_neg | beta-D-Lactose | C12H22O11 | 342.297 | 0.74 | 341.11 | Carbohydrates | 37,570,000 | 44,770,000 | 54,900,000 | 54,530,000 | 52,990,000 | 51,770,000 | 31,150,000 | 33,200,000 | 35,770,000 |
| Com_487_pos | Biochanin A 7-O-beta-D-glucoside | C22H22O10 | 446.404 | 5.46 | 447.20 | Flavonoids | 1,057,000 | 74,550 | 72,300 | 325,800 | 352,000 | 323,100 | 132,500 | 220,300 | 177,900 |
| Com_368_pos | Biotin | C10H16N2O3S | 244.311 | 9.67 | 245.10 | Terpenoids | 563,500 | 363,600 | 393,000 | 402,600 | 388,600 | 456,800 | 274,300 | 390,400 | 416,400 |
| Com_728_pos | Camelliaside A | C33H40O20 | 756.668 | 4.21 | 757.22 | Flavonoids | 47,800 | 67,980 | 40,690 | 55,470 | 50,270 | 77,070 | 14,590 | 23,090 | 15,770 |
| Com_417_pos | Chrysophanol | C15H10O4 | 254.238 | 8.17 | 255.06 | Phenols | 131,998 | 22,440 | 6,259 | 56,540 | 44,750 | 66,440 | 45,470 | 53,540 | 51,910 |
| Com_178_neg | cis-Aconitic acid | C6H6O6 | 174.108 | 0.60 | 173.00 | Organic Acid And Its Derivatives | 4,248,000 | 2,009,000 | 3,113,000 | 3,624,000 | 3,708,000 | 3,272,000 | 1,294,000 | 1,589,000 | 1,520,000 |
| Com_672_pos | Cytidine monophosphate | C9H14N3O8P | 323.197 | 0.74 | 324.00 | Nucleotide And Its Derivates | 246,900 | 253,000 | 226,900 | 218,300 | 227,000 | 206,500 | 108,300 | 126,100 | 124,600 |
| Com_253_neg | Coniferin | C16H22O8 | 342.341 | 0.87 | 341.00 | Phenols | 169,400,000 | 223,900,000 | 251,000,000 | 240,200,000 | 223,600,000 | 240,500,000 | 149,200,000 | 160,000,000 | 170,700,000 |
| Com_277_neg | Coumalic acid | C6H4O4 | 140.094 | 1.15 | 141.02 | Organic Acid And Its Derivatives | 98,560 | 90,310 | 69,600 | 77,940 | 114,800 | 110,700 | 56,040 | 70,340 | 62,880 |
| Com_32_neg | Curcumin | C21H20O6 | 368.380 | 4.53 | 367.12 | Phenols | 118,100 | 49,710 | 60,470 | 126,100 | 108,100 | 120,400 | 30,700 | 36,390 | 33,940 |
| Com_134_neg | Cyclic adenosine monophosphate | C10H12N5O6P | 329.206 | 3.60 | 328.10 | Nucleotide And Its Derivates | 478,100 | 2,589,000 | 2,549,000 | 2,181,000 | 2,054,000 | 2,283,000 | 870,500 | 880,800 | 980,400 |
| Com_29_neg | D-Galacturonic acid | C6H10O7 | 194.139 | 0.71 | 193.04 | Carbohydrates | 137,000 | 118,200 | 342,900 | 180,000 | 226,200 | 225,000 | 92,630 | 87,140 | 103,400 |
| Com_27_neg | D-Glucono-1,5-lactone | C6H10O6 | 178.140 | 0.80 | 177.14 | Amino Acid And Its Derivatives | 254,700 | 128,600 | 83,170 | 230,200 | 222,400 | 235,300 | 136,800 | 166,100 | 145,300 |
| Com_4_neg | Echinacoside | C35H46O20 | 786.737 | 7.70 | 685.22 | Phenols | 275,200 | 311,500 | 448,500 | 365,900 | 334,300 | 344,500 | 171,900 | 154,800 | 117,000 |
| Com_194_neg | Forsythoside B | C34H44O19 | 756.702 | 7.52 | 755.24 | Phenylpropanoids | 446,900 | 549,000 | 325,000 | 486,400 | 465,100 | 469,100 | 183,700 | 190,800 | 194,700 |
| Com_238_neg | Galactinol | C12H22O11 | 342.116 | 0.87 | 341.10 | Carbohydrates | 192,900,000 | 203,400,000 | 234,800,000 | 208,300,000 | 191,200,000 | 238,000,000 | 151,100,000 | 148,800,000 | 156,700,000 |
| Com_79_neg | Geniposidic acid | C16H22O10 | 374.340 | 0.75 | 373.11 | Terpenoids | 104,700 | 43,100 | 36,340 | 65,210 | 45,800 | 56,160 | 33,990 | 36,810 | 28,020 |
| Com_273_neg | Glucarate O-phosphoric acid | C6H11PO11 | 290.100 | 0.63 | 289.10 | Carbohydrates | 280,000 | 47,750 | 48,560 | 164,600 | 145,500 | 167,800 | 79,100 | 80,190 | 102,700 |
| Com_707_pos | Homovanillic Acid | C9H10O4 | 182.173 | 9.37 | 183.10 | Organic Acid And Its Derivatives | 90,000 | 5,783 | 4,315 | 25,880 | 24,000 | 24,810 | 12,050 | 14,070 | 18,100 |
| Com_588_pos | iP9G | C16H23N5O5 | 365.384 | 0.77 | 366.20 | Others | 810,100 | 906,900 | 1,022,000 | 911,800 | 893,000 | 955,300 | 651,900 | 691,700 | 753,500 |
| Com_111_neg | Isorhamnetin-3-O-neohespeidoside | C28H32O16 | 624.544 | 7.93 | 623.16 | Flavonoids | 4,094,000 | 10,180,000 | 10,700,000 | 10,560,000 | 9,969,000 | 10,810,000 | 3,783,000 | 4,058,000 | 4,088,000 |
| Com_213_neg | Jionoside A1 | C36H48O20 | 800.760 | 8.14 | 799.27 | Others | 50,711 | 14,330 | 17,309 | 40,330 | 32,340 | 45,130 | 11,840 | 11,080 | 9100 |
| Com_522_pos | L-Homoarginine | C7H16N4O2 | 188.227 | 2.59 | 189.00 | Amino Acid And Its Derivatives | 176,900 | 833,600 | 723,000 | 888,500 | 785,800 | 780,400 | 332,100 | 317,700 | 304,100 |
| Com_182_neg | L-Homocitrulline | C7H15N3O3 | 189.212 | 0.82 | 188.10 | Amino Acid And Its Derivatives | 9204 | 7947 | 6433 | 8819 | 6806 | 10,430 | 7105 | 7172 | 3215 |
| Com_164_neg | Lobetyolin +HCOOH | C21H30O10 | 442.460 | 0.81 | 441.18 | Others | 291,200 | 16,640 | 18,360 | 73,710 | 70,290 | 71,450 | 65,130 | 61,160 | 34,790 |
| Com_301_neg | LysoPA 18:0 | C21H35O7P | 438.270 | 0.80 | 437.27 | Lipids | 25,280 | 19,130 | 29,190 | 27,660 | 22,070 | 23,370 | 23,600 | 21,710 | 17,100 |
| Com_320_neg | Methylhesperidin | C29H36O15 | 624.587 | 7.95 | 623.20 | Flavonoids | 3,760,000 | 9,394,000 | 10,600,000 | 10,530,000 | 9,403,000 | 9,479,000 | 3,518,000 | 3,835,000 | 3,961,000 |
| Com_47_neg | Methylophiopogonanone A | C19H18O6 | 342.343 | 0.76 | 341.10 | Flavonoids | 39,480 | 67,950 | 78,550 | 75,730 | 75,180 | 75,100 | 49,290 | 35,960 | 53,470 |
| Com_103_neg | N,N-Dimethylglycine | C4H9NO2 | 103.120 | 0.64 | 102.06 | Amino Acid And Its Derivatives | 754,700 | 222,600 | 124,000 | 297,800 | 361,300 | 377,700 | 257,600 | 287,000 | 124,000 |
| Com_600_pos | Narcissoside | C28H32O16 | 624.544 | 5.92 | 625.18 | Flavonoids | 1,213,000 | 3,106,000 | 4,647,000 | 2,422,000 | 1,918,000 | 2,037,000 | 983,100 | 1,251,000 | 1,191,000 |
| Com_51_neg | Nicotinamide-N-oxide | C6H6N2O2 | 138.043 | 0.70 | 137.00 | Terpenoids | 32,870,000 | 33,780,000 | 31,940,000 | 34,720,000 | 38,290,000 | 33,330,000 | 20,920,000 | 18,380,000 | 19,710,000 |
| Com_446_pos | Nicotinic acid-hexoside | 1.09 | 286.00 | Phenols | 144,700 | 104,500 | 130,100 | 120,400 | 104,000 | 84,530 | 65,870 | 67,680 | 70,900 | ||
| Com_171_neg | Palmitaldehyde | C16H32O | 240.425 | 14.03 | 239.00 | Organic Acid And Its Derivatives | 63,100 | 32,180 | 16,110 | 33,370 | 51,830 | 32,360 | 46,760 | 35,500 | 19,610 |
| Com_460_pos | Palmitic acid | C16H32O2 | 256.424 | 18.32 | 257.25 | Lipids | 17,060 | 17,170 | 13,510 | 15,500 | 15,020 | 17,780 | 13,480 | 15,510 | 15,230 |
| Com_304_neg | Purpureaside C | C35H46O20 | 786.728 | 7.61 | 785.25 | Phenylpropanoids | 242,900 | 239,600 | 345,200 | 275,900 | 278,200 | 239,000 | 120,500 | 112,200 | 109,800 |
| Com_196_neg | Salicylic acid | C7H6O3 | 138.121 | 0.67 | 137.10 | Organic Acid And Its Derivatives | 18,580,000 | 9,875,000 | 10,580,000 | 12,990,000 | 12,950,000 | 11,250,000 | 7,783,000 | 7,140,000 | 7,478,000 |
| Com_218_neg | Shikimic acid | C7H10O5 | 174.151 | 0.57 | 173.05 | Organic Acid And Its Derivatives | 23,910,000 | 15,040,000 | 15,710,000 | 16,810,000 | 17,570,000 | 17,540,000 | 11,030,000 | 10,600,000 | 10,850,000 |
| Com_65_neg | Suberic acid | C8H14O4 | 174.194 | 0.63 | 173.10 | Organic Acid And Its Derivatives | 2,458,000 | 1,542,000 | 1,552,000 | 1,737,000 | 1,788,000 | 1,937,000 | 1,051,000 | 1,212,000 | 1,131,000 |
| Com_311_neg | Sucralose | C12H19Cl3O8 | 397.634 | 0.63 | 395.01 | Carbohydrates | 317,600 | 314,900 | 204,600 | 294,400 | 315,100 | 316,600 | 250,000 | 225,000 | 195,300 |
| Com_38_neg | trans-Ferulic acid | C10H10O4 | 194.184 | 2.87 | 193.00 | Phenols | 628,200 | 94,470 | 155,500 | 348,700 | 242,900 | 271,700 | 295,000 | 166,100 | 138,000 |
| Com_262_neg | Trehalose | C12H22O11 | 342.297 | 0.74 | 341.11 | Carbohydrates | 175,400,000 | 207,700,000 | 216,200,000 | 209,200,000 | 192,200,000 | 196,300,000 | 137,600,000 | 147,400,000 | 162,400,000 |
| Com_263_neg | Trehalose 6-phosphate | C12H23O14P | 422.276 | 0.51 | 421.08 | Carbohydrates | 740,600 | 528,700 | 634,900 | 673,200 | 668,300 | 615,900 | 328,800 | 325,700 | 397,700 |
| Com_340_pos | Trigonelline | C7H7NO2 | 137.136 | 0.73 | 138.06 | Alkaloids | 38,200,000 | 29,560,000 | 36,390,000 | 45,160,000 | 43,450,000 | 41,950,000 | 17,400,000 | 17,590,000 | 17,930,000 |
| Com_268_neg | Vitamin D3 | C27H44O | 384.339 | 0.75 | 383.00 | Terpenoids | 576,800 | 175,500 | 249,600 | 235,800 | 227,100 | 242,000 | 145,600 | 209,500 | 229,500 |
| Names | Flower1 | Flower2 | Flower3 | Fruit1 | Fruit2 | Fruit3 | QC1 | QC2 | QC3 |
|---|---|---|---|---|---|---|---|---|---|
| Flower1 | 1.000 | 0.985 ** | 0.983 ** | 0.729 | 0.724 | 0.729 | 0.539 | 0.556 | 0.562 |
| Flower2 | 0.985 ** | 1.000 | 0.986 ** | 0.711 | 0.710 | 0.713 | 0.542 | 0.559 | 0.567 |
| Flower3 | 0.983 ** | 0.986 ** | 1.000 | 0.665 | 0.667 | 0.668 | 0.542 | 0.561 | 0.568 |
| Fruit1 | 0.729 | 0.711 | 0.665 | 1.000 | 0.987 ** | 0.986 ** | 0.543 | 0.570 | 0.542 |
| Fruit2 | 0.724 | 0.710 | 0.667 | 0.987 ** | 1.000 | 0.986 ** | 0.550 | 0.577 | 0.543 |
| Fruit3 | 0.729 | 0.713 | 0.668 | 0.986 ** | 0.986 ** | 1.000 | 0.547 | 0.570 | 0.538 |
| QC1 | 0.539 | 0.542 | 0.542 | 0.543 | 0.550 | 0.547 | 1.000 | 0.982 ** | 0.986 ** |
| QC2 | 0.556 | 0.559 | 0.561 | 0.570 | 0.577 | 0.570 | 0.982 ** | 1.000 | 0.989 ** |
| QC3 | 0.562 | 0.567 | 0.568 | 0.542 | 0.543 | 0.538 | 0.986 ** | 0.989 ** | 1.000 |
| NO. | Name | Cell Survival Rate | Cell Suppressing Rate | ROS1 | ROS2 | CAT1 | CAT2 | SOD1 | SOD2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 50 | 100 | ||||||||
| 1 | (-)-trans-Carveol | 0.996 ** | 0.940 * | −0.984 ** | −0.982 ** | −0.980 ** | −0.929 * | 0.976 ** | −0.897 * | 0.993 ** | −0.916 * |
| 2 | (+)-Dihydrojasmonic acid | 0.913 * | 0.861 | −0.839 | −0.817 | −0.871 | −0.816 | 0.933 * | −0.899 * | 0.921 * | −0.877 |
| 3 | 13-HOTrE | 0.976 ** | 0.932 * | −0.975 ** | −0.935 * | −0.999 ** | −0.990 ** | 0.921 * | −0.892 * | 0.938 * | −0.899 * |
| 4 | 2-Methyladenosine | 0.876 | 0.816 | −0.935 * | −0.892 * | −0.927 * | −0.937* | 0.789 | −0.714 | 0.855 | −0.752 |
| 5 | 3-Hydroxy-3-methylpentane- 1,5-dioic acid | 0.966 ** | 0.965 ** | −0.924 * | −0.923 * | −0.967 ** | −0.935 * | 0.937 * | −0.964 ** | 0.943 * | −0.952 * |
| 6 | 3-Methylcrotonylglycine | 0.895 * | 0.981 ** | −0.883 * | −0.922 * | −0.945 * | −0.941 * | 0.817 | −0.949 * | 0.898 * | −0.882 * |
| 7 | 3-O-p-coumaroyl shikimic- acid O-hexoside | 0.924 * | 0.906 * | −0.946 * | −0.904 * | −0.978 ** | −0.995 ** | 0.839 | −0.848 | 0.885 * | −0.824 |
| 8 | 4-Hydroxy-2-oxoglutaric acid | 0.955 * | 0.930 * | −0.899 * | −0.888 * | −0.937 * | −0.895 * | 0.947 * | −0.946 * | 0.926 * | −0.950 * |
| 9 | 4-Hydroxybenzoic acid | 0.937 * | 0.873 | −0.975 ** | −0.952 * | −0.960 ** | −0.943 * | 0.874 | −0.782 | 0.929* | −0.847 |
| 10 | 4-Nitrophenol | 0.973 ** | 0.952 * | −0.926 * | −0.953 * | −0.940 * | −0.873 | 0.975 ** | −0.944 * | 0.979 ** | −0.894 * |
| 11 | 5′-Deoxy-5′-(methylthio)- adenosine | 0.883 * | 0.837 | −0.937 * | −0.907 * | −0.932 * | −0.937 * | 0.796 | −0.736 | 0.869 | −0.772 |
| 12 | beta-D-Lactose | 0.827 | 0.917 * | −0.845 | −0.864 | −0.910 * | −0.938 * | 0.717 | −0.863 | 0.818 | −0.772 |
| 13 | Biochanin A-7-O-beta- D-glucoside | 0.846 | 0.863 | −0.896 * | −0.899 * | −0.912 * | −0.924 * | 0.746 | −0.768 | 0.847 | −0.761 |
| 14 | Biotin | 0.938 * | 0.867 | −0.934 * | −0.974 ** | −0.889 * | −0.800 | 0.945 * | −0.801 | 0.978 ** | −0.945 * |
| 15 | Camelliaside A | 0.822 | 0.877 | −0.851 | −0.832 | −0.912 * | −0.955 * | 0.705 | −0.819 | 0.790 | −0.728 |
| 16 | Chrysophanol | 0.832 | 0.815 | −0.884 * | −0.823 | −0.916 * | −0.961 ** | 0.719 | −0.734 | 0.784 | −0.693 |
| 17 | cis-Aconitic acid | 0.923 * | 0.909 * | −0.945 * | −0.906 * | −0.978 ** | −0.994 ** | 0.838 | −0.851 | 0.886 * | −0.826 |
| 18 | Cytidylic acid | 0.856 | 0.818 | −0.915 * | −0.870 | −0.922 * | −0.946 * | 0.757 | −0.720 | 0.828 | −0.728 |
| 19 | Coniferin | 0.816 | 0.912 * | −0.837 | −0.866 | −0.899 * | −0.924 * | 0.705 | −0.853 | 0.815 | −0.766 |
| 20 | Coumalic acid | 0.870 | 0.836 | −0.900 * | −0.817 | −0.943 * | −0.987 ** | 0.772 | −0.784 | 0.801 | −0.733 |
| 21 | Curcumin | 0.891 * | 0.888 * | −0.894 * | −0.833 | −0.955 * | −0.988 ** | 0.803 | −0.861 | 0.827 | −0.789 |
| 22 | Cyclic adenylic acid | 0.839 | 0.834 | −0.882 * | −0.825 | −0.924 * | −0.970 ** | 0.726 | −0.763 | 0.789 | −0.708 |
| 23 | D-Galacturonic acid | 0.880 * | 0.900 * | −0.911 * | −0.895 * | −0.949 * | −0.971 ** | 0.779 | −0.831 | 0.858 | −0.790 |
| 24 | D-Glucono-1,5-lactone | 0.863 | 0.954 * | −0.820 | −0.837 | −0.910 * | −0.916 * | 0.797 | −0.968 ** | 0.837 | −0.857 |
| 25 | Echinacoside | 0.989 ** | 0.966 ** | −0.969 ** | −0.982 ** | −0.980 ** | −0.931 * | 0.965 ** | −0.932 * | 0.991 ** | −0.975 ** |
| 26 | Ferulic acid | 0.984 ** | 0.890 * | −0.996 ** | −0.942 * | −0.991 ** | −0.971 ** | 0.941 * | −0.833 | 0.948 * | −0.892 * |
| 27 | Forsythoside B | 0.962 ** | 0.908 * | −0.976 ** | −0.924 * | −0.994 ** | −0.995 ** | 0.897 * | −0.855 | 0.920 * | −0.865 |
| 28 | Galactinol | 0.825 | 0.908 * | −0.853 | −0.883 * | −0.903 * | −0.922 * | 0.717 | −0.838 | 0.831 | −0.772 |
| 29 | Geniposidic acid | 0.834 | 0.819 | −0.893* | −0.855 | −0.910 * | −0.941 * | 0.726 | −0.724 | 0.808 | −0.710 |
| 30 | Glucarate O-phosphoric acid | 0.853 | 0.912 * | −0.866 | −0.853 | −0.933 * | −0.967 ** | 0.748 | −0.869 | 0.821 | −0.777 |
| 31 | Homovanillic acid | 0.966 ** | 0.867 | −0.978 ** | −0.975 ** | −0.937 * | −0.874 | 0.954 * | −0.793 | 0.978 ** | −0.923 * |
| 32 | N6-Isopentenyl adenine- 9-glucoside | 0.849 | 0.906 * | −0.880 * | −0.891 * | −0.924 * | −0.944 * | 0.744 | −0.834 | 0.844 | −0.780 |
| 33 | Isorhamnetin-3-O- neohespeidoside | 0.845 | 0.878 | −0.874 | −0.841 | −0.930 * | −0.971 ** | 0.734 | −0.821 | 0.806 | −0.743 |
| 34 | Jionoside A1 | 0.946 * | 0.893 * | −0.917 * | −0.852 | −0.960 ** | −0.956 * | 0.909 * | −0.894 * | 0.882 * | −0.875 |
| 35 | L-Homoarginine | 0.942 * | 0.885 * | −0.938 * | −0.862 | −0.976 ** | −0.989 ** | 0.881 * | −0.861 | 0.874 | −0.839 |
| 36 | L-Homocitrulline | 0.845 | 0.986 ** | −0.803 | −0.910 * | −0.869 | −0.831 | 0.794 | −0.975 ** | 0.889 * | −0.911 * |
| 37 | Lobetyolin +HCOOH | 0.837 | 0.810 | −0.900 * | −0.897 * | −0.885 * | −0.884 * | 0.749 | −0.696 | 0.846 | −0.743 |
| 38 | LysoPA 18:0 | 0.957 * | 0.941 * | −0.907 * | −0.956 * | −0.911 * | −0.828 | 0.969 ** | −0.930 * | 0.981 ** | −0.856 |
| 39 | Methylhesperidin | 0.846 | 0.880 * | −0.875 | −0.845 | −0.930 * | −0.970 ** | 0.735 | −0.821 | 0.808 | −0.745 |
| 40 | Methylophiopogonanone A | 0.971 ** | 0.971 ** | −0.943 * | −0.940 * | −0.983 ** | −0.958 * | 0.931 * | −0.957 * | 0.950 * | −0.945 * |
| 41 | N,N-Dimethylglycine | 0.866 | 0.975 ** | −0.850 | −0.952 * | −0.889 * | −0.847 | 0.811 | −0.932 * | 0.919 * | −0.910 * |
| 42 | Narcissoside | 0.832 | 0.812 | −0.896 * | −0.883 * | −0.892 * | −0.903 * | 0.735 | −0.702 | 0.830 | −0.727 |
| 43 | Nicotinamide-N-oxide | 0.944 * | 0.914 * | −0.963 ** | −0.925 * | −0.988 ** | −0.994 ** | 0.868 | −0.856 | 0.910 * | −0.850 |
| 44 | Nicotinic acid-hexoside | 0.971 ** | 0.836 | −0.969 ** | −0.946 * | −0.922 * | −0.849 | 0.982 ** | −0.779 | 0.970 ** | −0.929 * |
| 45 | Palmitaldehyde | 0.962 ** | 0.992 ** | −0.935 * | −0.966 ** | −0.973 ** | −0.938 * | 0.920 * | −0.968 ** | 0.965 ** | −0.901 * |
| 46 | Palmitic acid | 0.971 ** | 0.952 * | −0.974 ** | −0.959 ** | −0.997 ** | −0.983 ** | 0.912 * | −0.903 * | 0.951 * | −0.910 * |
| 47 | Purpureaside C | 0.949 * | 0.974 ** | −0.948 * | −0.982 ** | −0.971 ** | −0.942 * | 0.892 * | −0.921 * | 0.962 ** | −0.929 * |
| 48 | Salicylic acid | 0.944 * | 0.912 * | −0.970 ** | −0.944 * | −0.983 ** | −0.980 ** | 0.871 | −0.841 | 0.923 * | −0.856 |
| 49 | Shikimic acid | 0.960 ** | 0.913 * | −0.982 ** | −0.951 * | −0.989 ** | −0.980 ** | 0.896 * | −0.845 | 0.938 * | −0.874 |
| 50 | Suberic acid | 0.961 ** | 0.887 * | −0.985 ** | −0.931 * | −0.989 ** | −0.985 ** | 0.898 * | −0.821 | 0.923 * | −0.856 |
| 51 | Sucralose | 0.857 | 0.898 * | −0.882 * | −0.863 | −0.937 * | −0.970 ** | 0.749 | −0.841 | 0.827 | −0.768 |
| 52 | Trehalose | 0.823 | 0.910 * | −0.850 | −0.886 * | −0.899 * | −0.916 * | 0.716 | −0.839 | 0.832 | −0.775 |
| 53 | Trehalose 6-phosphate | 0.884 * | 0.913 * | −0.913 * | −0.906 * | −0.951 * | −0.968 ** | 0.787 | −0.844 | 0.869 | −0.805 |
| 54 | Trigonelline | 0.880 * | 0.871 | −0.906 * | −0.848 | −0.953 * | −0.990 ** | 0.780 | −0.818 | 0.826 | −0.762 |
| 55 | Vitamin D3 | 0.841 | 0.866 | −0.881 * | −0.938 * | −0.872 | −0.842 | 0.769 | −0.763 | 0.886 * | −0.811 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, D.; Zhao, R.; Hu, Y.; Li, H.; Chun, Z.; Zheng, S. Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Metabolites 2023, 13, 702. https://doi.org/10.3390/metabo13060702
Rao D, Zhao R, Hu Y, Li H, Chun Z, Zheng S. Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Metabolites. 2023; 13(6):702. https://doi.org/10.3390/metabo13060702
Chicago/Turabian StyleRao, Dan, Ruoxi Zhao, Yadong Hu, Hongjie Li, Ze Chun, and Shigang Zheng. 2023. "Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry" Metabolites 13, no. 6: 702. https://doi.org/10.3390/metabo13060702
APA StyleRao, D., Zhao, R., Hu, Y., Li, H., Chun, Z., & Zheng, S. (2023). Revealing of Intracellular Antioxidants in Dendrobium nobile by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Metabolites, 13(6), 702. https://doi.org/10.3390/metabo13060702







