Abstract
Alkaloids are the most diversified nitrogen-containing secondary metabolites, having antioxidant and antimicrobial properties, and are extensively used in pharmaceuticals to treat different types of cancer. Nicotiana serves as a reservoir of anti-cancer alkaloids and is also used as a model plant for the de novo synthesis of various anti-cancer molecules through genetic engineering. Up to 4% of the total dry weight of Nicotiana was found to be composed of alkaloids, where nicotine, nornicotine, anatabine, and anabasine are reported as the dominant alkaloids. Additionally, among the alkaloids present in Nicotiana, β-carboline (Harmane and Norharmane) and Kynurenines are found to show anti-tumor effects, especially in the cases of colon and breast cancers. Creating new or shunting of existing biosynthesis pathways in different species of Nicotiana resulted in de novo or increased synthesis of different anti-tumor molecules or their derivatives or precursors including Taxadiane (~22.5 µg/g), Artemisinin (~120 μg/g), Parthenolide (~2.05 ng/g), Costunolide (~60 ng/g), Etoposide (~1 mg/g), Crocin (~400 µg/g), Catharanthine (~60 ng/g), Tabersonine (~10 ng/g), Strictosidine (~0.23 mg/g), etc. Enriching the precursor pool, especially Dimethylallyl Diphosphate (DMAPP), down-regulating other bi-product pathways, compartmentalization or metabolic shunting, or organelle-specific reconstitution of the precursor pool, might trigger the enhanced accumulation of the targeted anti-cancer alkaloid in Nicotiana.
1. Introduction
Tobacco (Nicotiana spp.), a series of seventy-six naturally occurring species belonging to the Solanaceae family is cultivated around the world [1,2]. Several Nicotiana species are used for curing types of diseases and for recreation as they serve as a reservoir of a wide range of secondary metabolites viz. alkaloids, aromatic compounds, flavonoids, volatile compounds, acyclic hydroxygeranyllinalool, diterpene glycosides, etc. [3,4]. Therefore, plants play a vital role in the medicinal and agricultural industries. However, N. tabacum is the most popular and important species, which provides around two thousand five hundred characterized metabolites chemicals, so far [5]. Alkaloids, a major group of secondary metabolites are present in tobacco and are mostly responsible for its biological properties. Alkaloids are structured with nitrogen atoms with a ring structure, where the nitrogen atom is located inside the heterocyclic ring [6].
Tobacco plants contain 2–4% alkaloids of their total dry weight, where nicotine shares approximately 90% of total alkaloids [7]. Nornicotine, anatabine, and anabasine are the other structurally related alkaloids present in tobacco. Piperidine or pyrrolidine rings, with a positively charged nitrogen atom present in pyridine alkaloids, play the core role in toxicity against herbivores [8]. Furthermore, pyridine alkaloids and other nicotine analogs also have a toxicity effect and hence, are used to treat anxiety, different types of cancers, depression, pain, etc. [9,10,11].
Cancer, a grave threat to human beings is responsible for around ten million deaths in the world because of rare and expensive treatment [12]. Natural alkaloids including vinblastine, camptothecin, terpenoids (farnesol, geraniol, paclitaxel), anthranilic acid derivatives (tranilast), polyphenolic compounds (gossypol), lignans (podophyllotoxin), etc. commonly have the properties to act against tumor cells [5]. Tobacco plants contain a variety of alkaloids or other secondary metabolites and are considered a major source for the effective treatment of tumor cells. Cembranoidtype diterpenes (CBDs), which originated in tobacco, showed potential properties in neuroprotective functions and treating cancer cells [13]. Because of limited availability with high synthetic production costs, research around the world is focusing on the search for a new source of potential alkaloids or other strategies, which can offer an easier and less expensive means of cancer treatment. Plant suspension culture or metabolic engineering of short-cycled plants with the desired gene could offer reliable platforms for large-scale production of the targeted anti-tumor alkaloid [14]. In addition to the contribution to the fields of traditional agricultural and pharmaceutical industries, several species of tobacco including N. benthamiana, N. attenuata, etc. are considered an ideal model plant system for the production of the valuable alkaloid [15]. Tobacco can grow in a short space, has a short life cycle, can manipulate the gene easily and has high disease susceptibility [16]. Successful production of anti-tumor alkaloids or alkaloid precursors has already been achieved by the genetic engineering approaches in Nicotiana plants [17]. In this review, recent advances in the context of anti-cancer alkaloids and their de novo synthesis in tobacco plants are summarized. Finally, how genetic engineering could be useful for the genetic manipulation of tobacco has also been highlighted.
2. Plant Secondary Metabolites
Plant secondary metabolites, also known as idolizes, are chemical compounds derived from plant cells through a variety of metabolic pathways [18]. Likewise, primary metabolites and secondary metabolites do not directly participate in the growth and development of the plant. A variety of biological properties including antimicrobial, anti-tumor, etc. provides the scientific base for the use of secondary metabolites obtained from diversified herbs. To date, about fifty thousand secondary metabolites have been identified in the plants and the mode of action of many of them is yet to be explored [19]. Plant secondary metabolites are categorized into four major classes: alkaloids, phenolic compounds, sulfur-containing compounds and terpenoids [20]. However, we focus on the anti-tumor alkaloids present in or produced in Nicotiana through genetic engineering.
3. Alkaloids: The Major Plant Secondary Metabolites
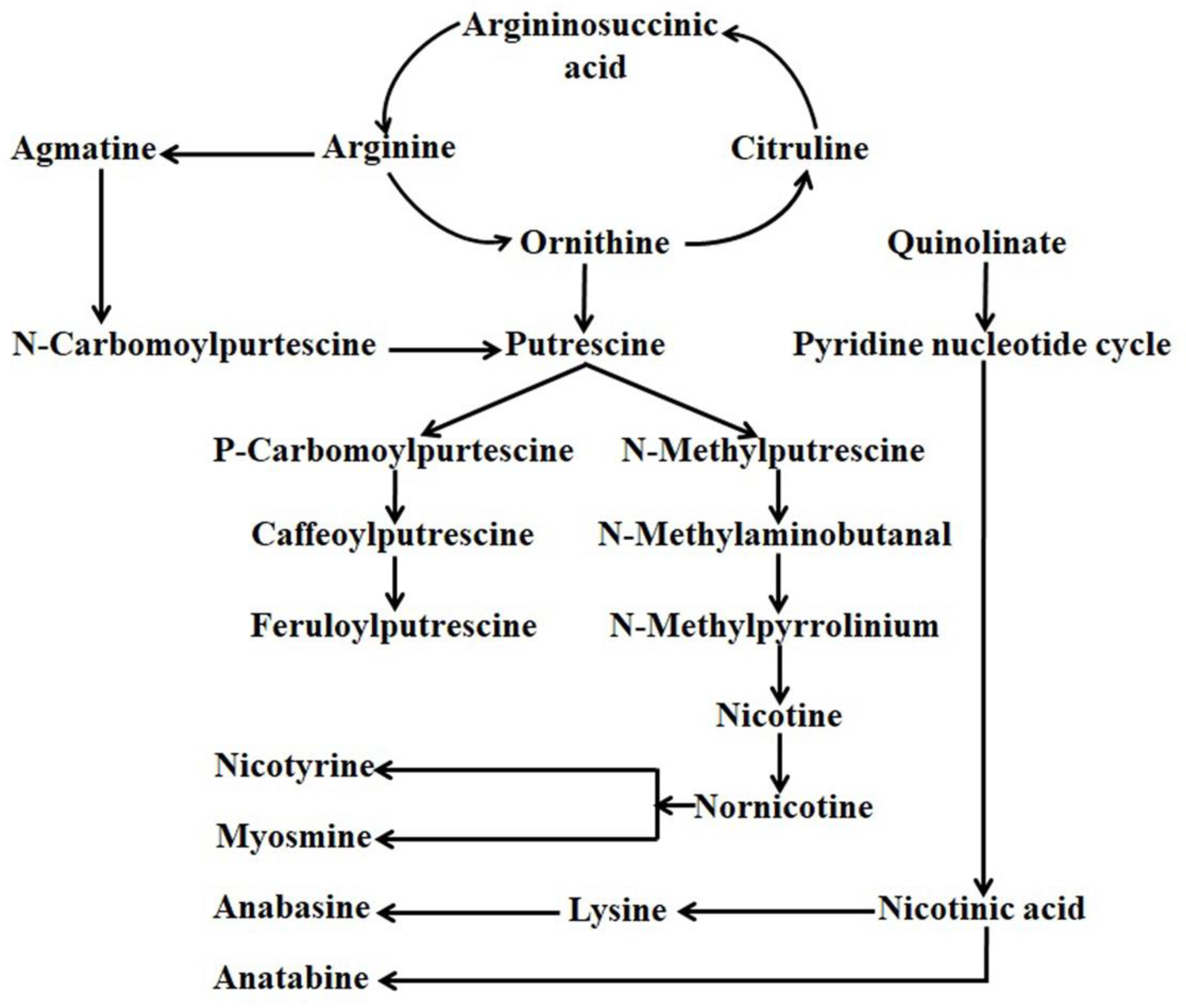
Alkaloids are the more diversified nitrogen-containing compounds composed of around twenty thousand members. Among the identified alkaloids, approximately six hundred members are found to have antioxidant and antimicrobial properties, serve as a drug, have anti-cancer properties, and can stimulate the animal nervous system [21,22,23,24]. Based on molecular structure and biosynthetic pathway, alkaloids can be divided into three major groups viz. (a) true alkaloids (heterocyclic), (b) proto alkaloids (nonheterocyclic), and (c) pseudo alkaloids. The chemical composition of Nicotiana leaves is exceptionally complex, where nicotine is found to be the utmost distinctive member of the alkaloid family [25]. In alkaloids, a pyridine ring and a pyrrolidine ring composed the structure of nicotine, whereas a pyridine ring and a piperidine ring composed the structure of anabasine [8]. Decarboxylation of ornithine-by-ornithine decarboxylase or arginine-by-arginine decarboxylase produced putrescine, which finally served as the precursor for the derivation of the pyrrolidine ring. 4-amino butanol is produced from Nmethylation (by N-methyltransferase) and oxidatively deamination (by N-methyl putrescine oxidase) spontaneously cyclized to create 1-methyl-Δ1-pyridinium cation followed by coupling with a pyridine ring originated from nicotinic acid to synthesize nicotine. In contrast, decarboxylations of lysine (decarboxylase) produce cadaverine, the precursor for the generation of the piperidine ring for the formation of anabasine. The oxidation of cadaverine by amine oxidase and subsequent cyclization produces a Δ1-piperidine ring, which couples with the pyridine ring just in the same manner for the derivation of nicotine to synthesize anabasine (Figure 1) [8].
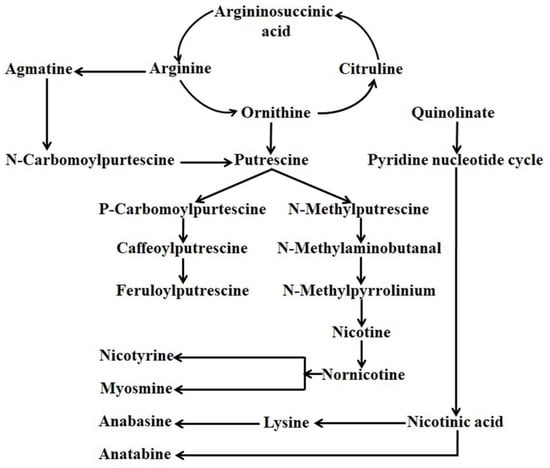
Figure 1.
General alkaloids bio-synthesis pathway in Nicotiana.
4. Alkaloid Present in Nicotiana
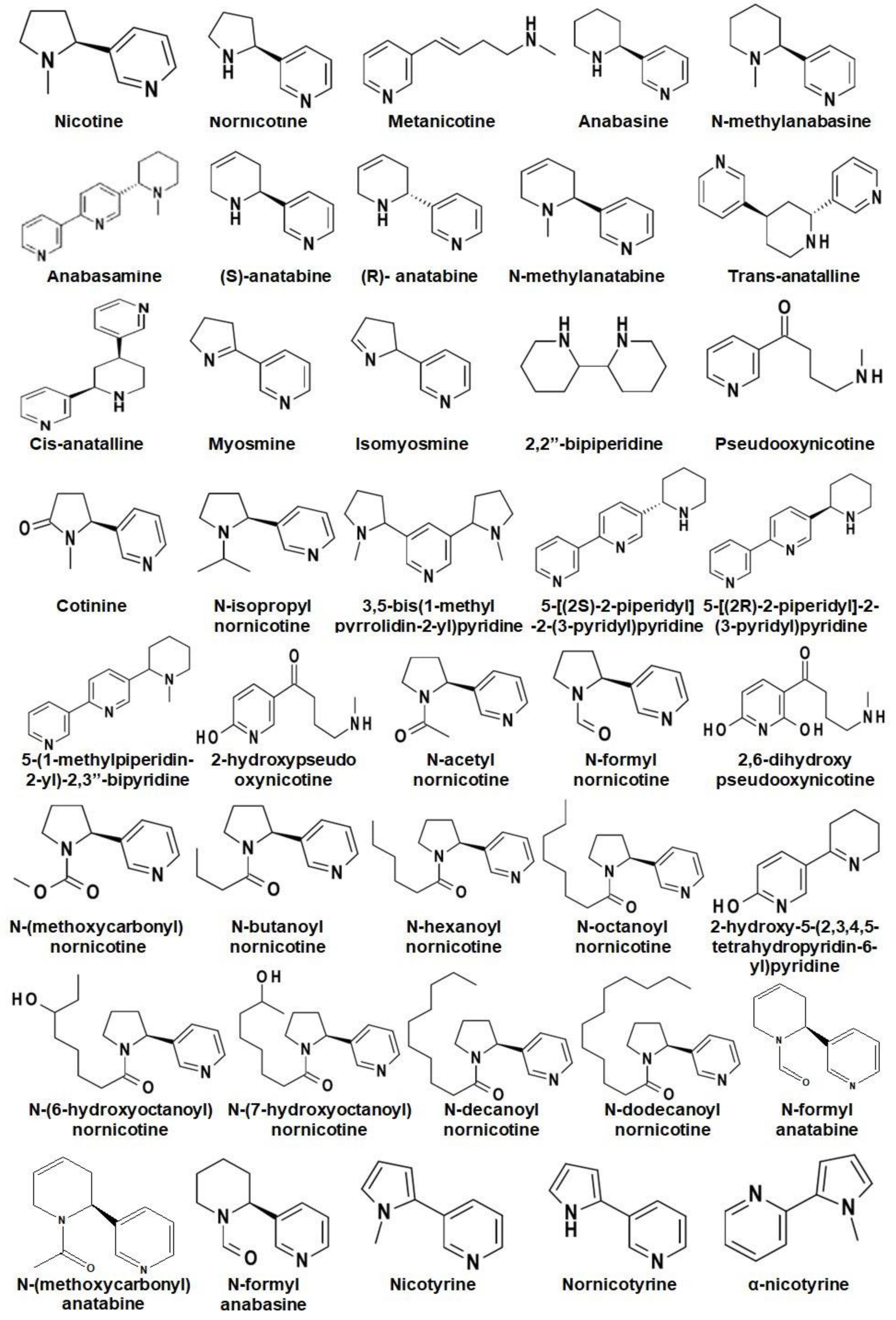
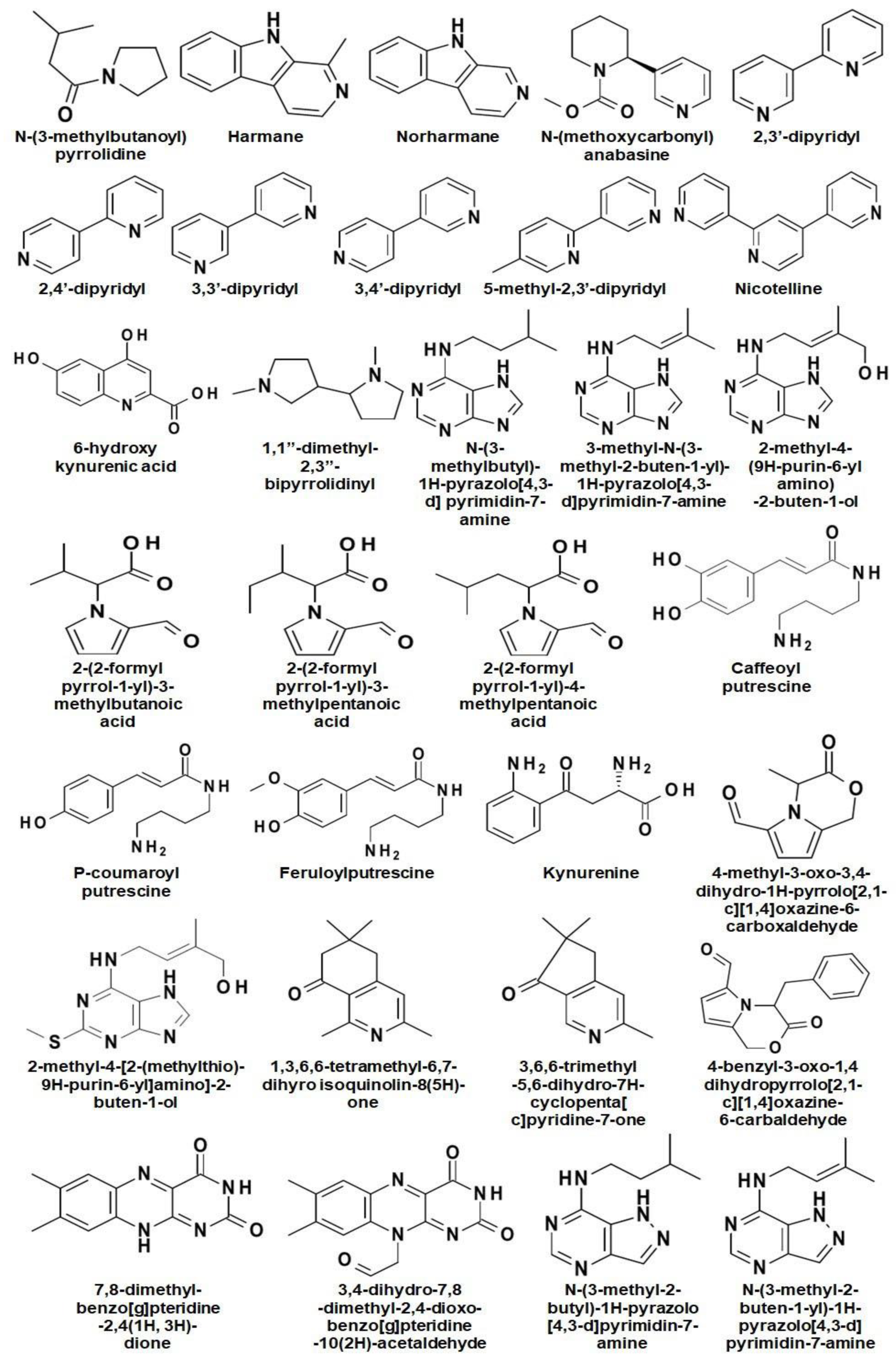
Alkaloids are the major secondary metabolites (up to 4% of DW) found in Nicotiana and their occurrence varies from species to species [7]. To date, around seventy-one alkaloids have been reported in Nicotiana spp. where, nicotine, nornicotine, anabasine, and anatabine are reported as the most abundant alkaloids (Figure 2) [26,27].
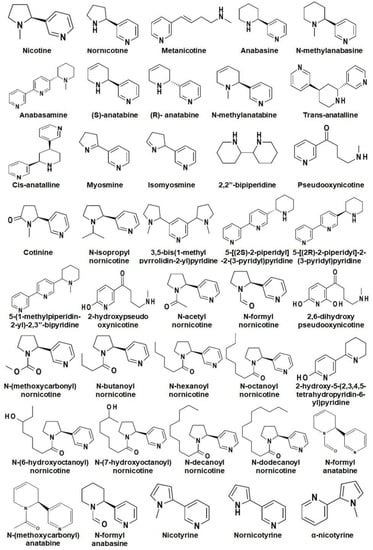
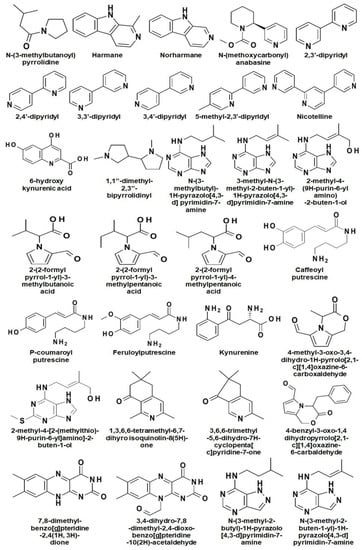
Figure 2.
Chemical structures of different alkaloids reported in Nicotiana.
5. Anti-Cancer Alkaloids Present in Nicotiana
5.1. β-Carboline
β-carboline are the bioactive alkaloids naturally produced in plants, foods, cigarette smoke, and mammalian tissues as well as in the human brain exhibiting antimicrobial, neuroactive activities and used to treat cancer [28,29,30]. Harmane and norharmane belonging to β-carboline are present in Nicotiana (up to 20 μg/g), which also possess antimicrobial, anticonvulsant, neuroprotective, and anti-tumor properties [31,32]. They act as mutagenic agents by inhibiting different enzymes’ functions including histone deacetylase, receptors of the central nervous system [33]. Norharmane consists of benzene, pyrrole, and pyridine ring where C-1, C-3, and N-9 nucleus positions serve as active sites used to develop new molecules with anti-tumor activity [34,35]. Derivatives of nonharmane, namely, harmane and harmine and other norharmane salicylic conjugate amides can depolarize the mitochondria and are also used to treat liver and colon cancer [34]. Tryptophan amino acid consists of carbon skeleton and nitrogen atoms and serve as the precursors of β-carboline. Amine derivatives or indolethylamino acid undergo an enzymatic reaction (Pictet–Spengler) and can react with an aldehyde acid or a keto acid to produce a Schiff base intermediate precursor form, from which tetrahydro β-carboline is derived through a cyclization process [36]. After that, tetrahydro β-carboline (1-methyl-1,2,3,4-tetrahydro-b-carboline-3-carboxylic acid and 1,2,3,4-tetrahydro-b-carboline-3-carboxylic acid) oxidizes by heme peroxidases and produce β-carbolines [36].
5.2. Kynurenines
Kynurenine (6-hydroxykyrunenine) and some of its derivatives isolated from leaves of Nicotiana tabacum help to relax the arterial vessels, control blood pressure, and boost immunity in response to inflammation [37,38,39]. The compound is derived from tryptophan and acts as the precursor for the derivation of kynurenic acid, anthranilic acid, and 3-hydroxykynurenine [27,40]. In the course of the Kynurenine pathway, tryptophan is catalyzed by indole-2,3-dioxygenase and tryptophan-2,3-dioxygenase to generate N-formyl-kynurenine. Further, kynurenine formylase catalyzes N-formyl kynurenine to L-kynurenine. Finally, kynurenine 3-monooxygenase or kynureninase directs kynurenine aminotransferase for the conversion of kynurenine into Kynurenic acid or 3-hydroxyanthranillic acid derived [41,42,43].
5.3. Nicotine and Nornicotine
Nicotine is the major (0.6~3.0% of the dry weight) alkaloid present in Nicotiana spp. [44,45]. Nicotine does not initiate cancer but can affect cancer development through activation and binding with the acetylcholine receptors to synthase tobacco-specific N-nitrosamines or inhibition of immune response by affecting dendritic cells [46]. Nornicotine (2-pyridin-3-ylpyrrolidine-1-carbaldehyde) is a chemical analog to nicotine without methyl group (3–5% of total alkaloid) and the synthesis of carcinogen N-nitrosonornicotine during the curing and processing of tobacco acts as a precursor [47,48]. A known type 1 carcinogen N-nitrosonornicotine is formed in human saliva for its action [49]. Interestingly, estrogen biosynthesis (for cancer development) was found to reduce using aromatase inhibitors like nornicotine in the case of breast cancer-infected and smoking people. Nornicotine and anabasine acyl derivatives such as N-(4-hydroxyundecanoyl) and N-n-octanoylnornicotine also can hinder estrogen synthesis in cancer cells [50]. Though carbaldehyde compounds are effectively used for cancer treatment, nornicotine has also been claimed for therapeutic and medical purposes because of its carbaldehydne nature and potential antimicrobial, anti-inflammatory, antioxidant, and anti-cancer properties “https://www.benchchem.com/product/b014642 (accessed on 2 April 2023)” [51]. A defensin-type protein (NAD1) in the flower of Nicotiana alata also showed anti-cancer efficacy “https://theconversation.com/tobacco-plants-may-contain-cure-for-cancer-a-new-twist-in-protein-lipid-interactions-25271 (accessed on 8 April 2023)”. However, still there is a debate whether nicotine or its derivative act as an anti-cancer agent or not. Before, making any conclusion regarding the anti-tumor properties of those compounds, more in-depth research with proper proof needs to be explored [46].
6. Metabolic Engineering of Nicotiana for Anti-Cancer Compound
For ease of genetic modification and cultivation, different species of Nicotiana are widely used in biosynthetic pathway reconstitutions of various valuable anti-cancer alkaloids [52,53].
6.1. Taxol
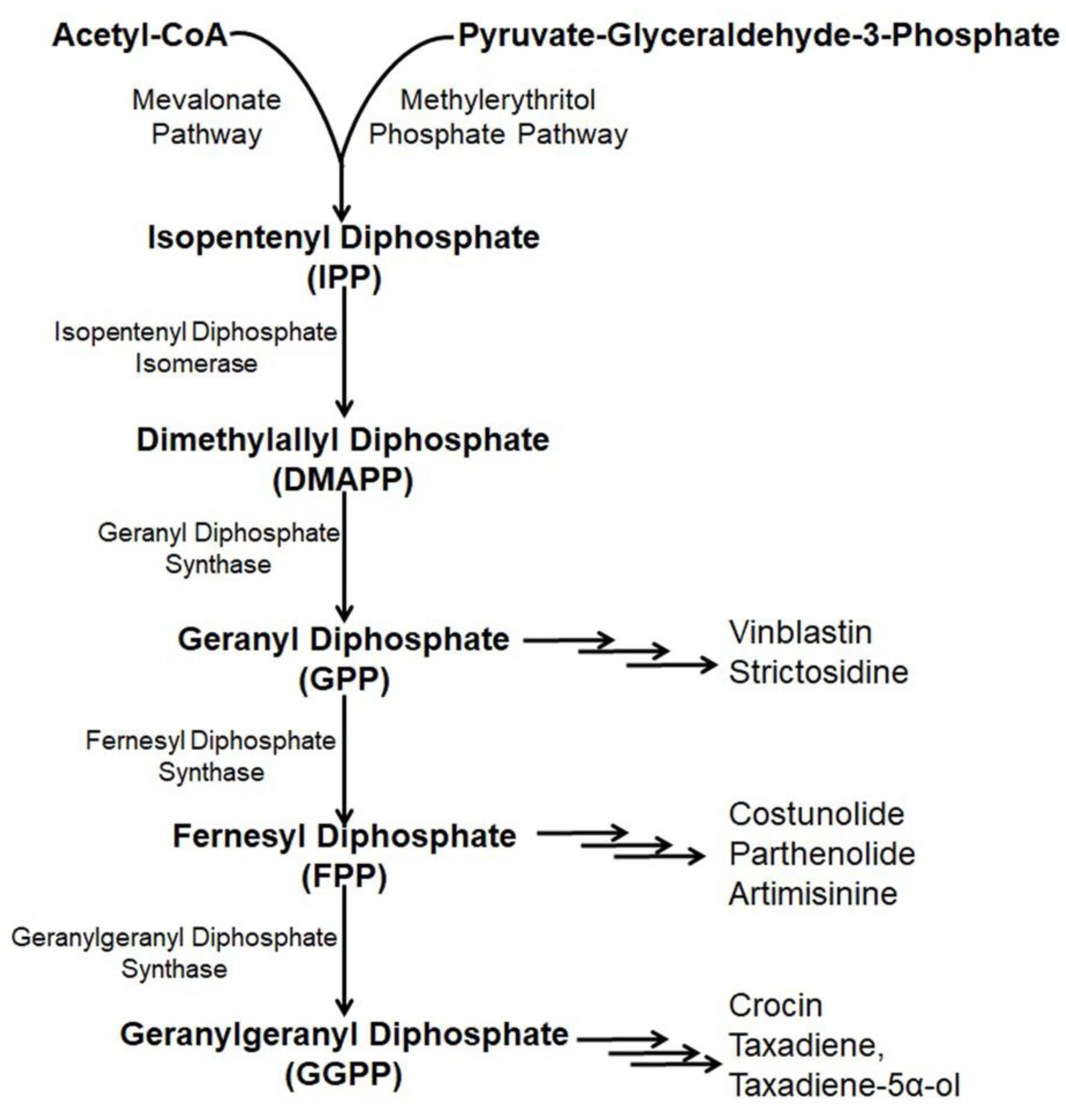
Paclitaxel (Taxol) is a natural alkaloid that was isolated from the bark of Taxus brevifolia at a very low concentration (0.01%) and was found to be very effective to treat various malignancies like ovarian cancer, lung cancer, breast cancer, kidney failure, restenosis, rheumatoid arthritis, etc. [54,55,56]. The biosynthetic pathway of taxol has nineteen steps from GGPP (geranylgeranyl pyrophosphate) [57] including several cytochrome P450 (CYP) mediated modifications [58], hence, its enzymatic production is very high [59,60,61]. Nicotiana benthamina was used to produce taxadiene, the core skeleton of taxol through the successful introduction and integration of the taxadiene synthase gene (TS gene) [15]. The transformed N. benthaniana plants with TS genes containing CaMV 35S promoter leads to the de novo synthesis of taxadiene in the leaves (11–27 µg taxadiene/g dw) and the roots (14.6–22.5 µg/g) [15,62]. Along with the de novo synthesis of taxadiene, in N. benthamina, taxadiene-5α-ol was also produced through the compartmentalization of cytochrome P450 reductase, T5αH, and TS in the chloroplast coupled with the elicited pool of isoprenoid precursor [63]. In the genetically engineered N. benthamiana, silencing or shunting of the existing metabolic pathway directed by the phytoene synthase gene demonstrated a 1.4- or 1.9- fold increase in the synthesis of taxadiene [15]. Suppressing carotenoid synthesis by shunting the phytoene synthase in the TS- transformed N. benthamiana increased the taxadiene synthesis by 1.9-fold, whereas silencing of the phytoene desaturase gene, the second devoted step, failed to redirect the GGPP pool for increased taxadiene production because of the interference of a newly formed biosynthetic pathway or some unknown reasons (Figure 3) [15,64].
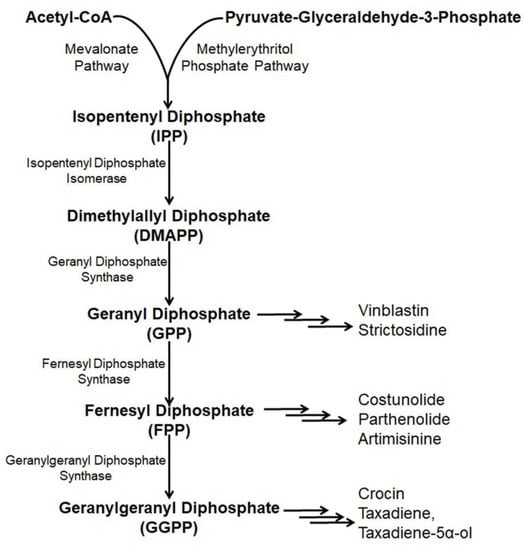
Figure 3.
Existing and/or genetically engineered bio-synthesis pathway of different anti-cancer alkaloids in Nicotiana.
6.2. Artemisinin
Artemisinin, a sesquiterpene alkaloid present in the aerial parts of Artemisia annua with anti-cancer properties is effectively used in pharmaceutical industries [65,66]. Artemisinin is derived through a general terpenoid biosynthesis pathway, where farnesyl diphosphate synthase (FPPS/FPS) helps to unite isopentenyl diphosphate (IPP) with dimethylallyl diphosphate (DMAPP) for the synthesis of farnesyl diphosphate (FPP, farnesyl pyrophosphate) [67,68]. Through carbocation formation and cyclization, FPP is transformed to amorpha-4, 11-diene catalyzed by amorpha-4, 11-diene synthase (ADS), which is further hydroxylased into artemisinic alcohol and then oxidized by amorphadiene monooxygenase (CYP71AV1) to artemisinic aldehyde [69,70,71]. Artemisinic aldehyde 11(13) reductase (DBR2) further reduced artemisinic aldehyde into dihydro artemisinic aldehyde, which is then oxidized by aldehyde dehydrogenase (ALDH1) to dihydroartemisinic acid [72,73]. The accumulation of artemisinin is affected because of the transformation of dihydro artemisinic aldehyde to dihydro artemisinic alcohol incited by dihydro artemisinic aldehyde reductase (RED1) [72]. Finally, a spontaneous light-depended non-enzymatic reaction yielded artemisinin from dihydroartemisinic acid [74].
Incorporating the genes or their transient expression related in the heterologous plants yielded artemisinin [75,76,77]. Nicotiana spp. has also been used in artemisinin research for its availability, flexibility to accept foreign genes with swift growth and high biomass. N. tabacum modified with the diverse genes of MVA results in enhancing the IPP pool, which increases production of artemisinin up to 0.8 mg/g dw [75,78]. A higher accumulation of amorpha-4,11-diene, the initial product in the synthesis of artemisinin was also achieved in Nicotiana tabacum through the expression of ADS [79]. However, the accumulation of amorpha-4,11-diene increased up to 4 mg/g fresh weight after the simultaneous incorporation of CYP71AV1, DBR2, and ALDH1 with ADS [76]. Other than N. tabacum, artemisinic acid or glycosylated artemisinin precursors were also produced in N. benthamiana through the transient expression of ADS, HMGR, CYP71AV1, and FPS or artemisinin genes [77,80].
Introduction of the artemisinin pathway through the transformation of the plastid genome in the chloroplasts of N. tabacum overcame the problem and resulted in higher artemisinic acid accumulation (120 µg/g) [20,81]. The introduction of six genes from the mevalonate pathway targeting the chloroplast accompanied by artemisinin pathway genes insertion into nuclear genome of N. tabacum through chloroplast transit peptide produced a higher amount of artemisinin (∼0.8 mg/g dry weight) [78]. Yet the significant production of the compound is not possible because of the biosynthesis pathway and multifaceted behavior of gene expression along with the composite glycosylation process (Figure 3) [82].
6.3. Parthenolide
Parthenolide mostly obtained in the feverfew plant (Tanacetum parthenium) is a sesquiterpene lactone that serves as a drug, especially for the treatment of colon cancer [83]. Structural parthenolide biosynthetic pathway genes including germacrene A oxidase (TpGAO), germacrene A synthase (TpGAS), parthenolide synthase (TpPTS), and costunolide synthase (TpCOS) were isolated from the feverfew plant [84]. A transient heterologous gene expression of TpGAO, TpGAS, TpPTS, and TpCOS coding sequences was cloned into pBIN binary expression vector under the Rubisco promoter control and introduced into the N. benthamiana plants. The reconstituted pathway did not result in any free parthenolide in the leaf of transformed N. benthamiana, however, a minor amount of parthenolide (2.05 ng/g FW) was produced when FDP precursor supply was boosted through the addition of AtHMGR. Interestingly, some parthenolide conjugates, namely, cysteine and glutathione were also produced along with parthenolide (1.4 μg/g) (Figure 3) [85].
6.4. Costunolide
Costunolide is a well-known sesquiterpene lactone present in several medicinal plants including Magnolia grandiflora and Tanacetu parthenium [86]. Costunolide is used to treat different types of cancers including leukemias, breast cancer, liver cancer, etc. [87,88,89]. Transient expression with feverfew germacrene A synthase (TpGAS), chicory germacrene A oxidase (CiGAO), and chicory costunolide synthase (CiCOS) in N. benthamiana produce costunolide up to 60 ng/g FW. The costunolide precursor germacrene A increases with mitochondrial TpGAS steering as compared to the cytosol targeting. However, when the leaf is infiltrated with the CiGAO and TpGAS, germacrene A disappeared due to the effect of CiGAO. This happened due to the CiGAO enzyme, which converts germacrene A into germacra-1(10), 4, 11(13)-trien-12-oic acid (Figure 3) [89].
6.5. Etoposide and Related Anti-Cancer Molecules
Etoposide obtained from the mandrake plant (Podophyllum peltatum) is an alkaloid used for the treatment of gastric cancer, testicular cancer, germ cell tumors, breast cancer, Hodgkin’s and non-Hodgkin’s lymphomas as well as lung cancer by preventing DNA unwinding through the inhibition of the function of topoisomerase II [90,91]. In N. benthamiana, the etoposide production pathway was reprogrammed by Agrobacterium- based transient expression using a single lignin-associated transcription factor and MYB85, which resulted in increased etoposide aglycone (EA) production by two times (up to 1 mg/g, DW), deoxypodophyllotoxin (DPT), the last biosynthetic anti-cancer precursor of the etoposide aglycone (EA) production pathway by eight times (35 mg/g DW) and epipodophyllotoxin (3.5 mg/g DW) [92]. Coniferyl alcohol (CA), a monolignol produced from the L-phenylalanine in the Podophyllum spp. acted as a building block to produce lignin compounds, which also acted as the precursor for the synthesis of etoposide. Agrobacterium containing the DPT pathway genes were infiltrated along with coniferyl alcohol (CA) resulting in a thirteen-fold increase in DPT production as paralleled to no infiltration of CA. Transient expression of N. banthamiana with sixteen genes includes coniferyl alcohol and enzymes of the etoposide production pathway resulting in 4.3 mg/g DW DPT production in the leaves. [93]. On the other hand, agro-infiltration of eight genes of the DPT pathway without coniferyl alcohol genes into the N. banthamiana also resulted in increased synthesis of DPT. Along with the mentioned genes, the addition of (+) pinoresinol resulted in the eight-fold elicited production of DPT in the same heterologous plant system [94]. Increased production of DPT through genetic manipulation using various pathway-related genes including enzymes responsible for etoposide aglycone and coniferyl alcohol (CA), proved a significant way to increase etoposide production (Figure 3) [91,93].
6.6. Crocin
Crocin (crocetin digentiobiose ester) is the alkaloid present in saffron (Crocus sativus) and is used to treat cancer as it inhibits the mitotic cell division, triggering apoptosis and proliferation of cells [95,96]. The enzyme carotenoid cleavage dioxygenase 2L (CsCCD2L) plays a vital role in the crocin biosynthesis pathway. Agrobacterium-mediated genetic transformation of N. tabacum and N. glauca using the orange mutant gene of Arabidopsis thaliana (AtOrMut) and β carotene hydroxylase (BrCrtZ), CsCCD2L through Arabidopsis AtUBQ10, tobacco polyubiquitin Ubi.U4 and promoter CaMV35S with a marker of hygromycin gene, resulted in ten times increased synthesis of crocin in N. glauca (400 µg/g DW) as compared to N. tabacum (36 µg/g DW) (Figure 3) [97].
6.7. Vinblastine
Vinblastine is a pharmaceutical agent used to treat various types of cancer derived from Catharanthus roseus [21]. The vinblastine production pathway is composed of thirty-one enzymes from geranyl pyrophosphate, where strictosidine monoterpene indole alkaloid is used as the precursor [98]. Agrobacterium-mediated transient expression of N. banthamiana with six stemmadenine acetate biosynthesis genes, namely, strictosidine glucosidase (SGD), geissoschizine synthase (GS), redox1, redox2, geissoschizine oxidase (GO) and stemmadenine acetyltransferase (SAT) from C. reseus, was carried under the controlling of SIUbq10 promoter by using the Golden Braid assembly system along with a P19 silencing suppressor to escape RNA silencing deleterious effects [99]. Further infiltration of the infiltrated leaves with strictosidine substrate resulted in no synthesis of stemmadenine acetate rather than the synthesis of stemmadenine acetate oxidized compound, namely, precondylocarpine acetate. Further, reconstitution of catharanthiane and tabersonine pathways by co-infiltration using precondylocarpine acetate synthase (PAS), dihydroprecondylocarpine synthase (DPAS), and catharanthine synthase (CS) or tabersonine synthase (TS) genes under the transcription control of a SIUbq10 promoter demonstrated increased accumulation of the precursor of vinblastine, namely, tabersonine and catharanthine (Figure 3) [98].
6.8. Strictosidine
Strictosidine is the last core skeleton biosynthetic precursor, first isolated from Rhazya stricta [100,101,102]. Strictosidine is produced from the amino acid tryptophan decarboxylation product tryptamine and the monoterpene precursor loganin, through the production of secologanin [103]. A higher level of strictosidine (0.23 mg/g DW) was produced in N. banthamiana through the reconstituted pathway genes including GPPS (Geranyl Diphosphate Synthase) and MLPL (Major Latex Protein-like enzyme) [17]. In the previous concept to maximize the synthesis, a thirteen step biosynthesis pathway needed to be reprogrammed following two phases where the second phase was considered for the synthesis of an intermediate substrate (iridotrial) [104]. Co-expression of 8-hydroxygeraniol oxidoreductase (CrGOR), geraniol 8-oxidase (CrG8H) and iridoid synthase (CrISY) resulting in elicited accumulation of nepetalactol, which directly facilitates the production of a higher level of strictosidine without adding any metabolite intermediates or precursors [17]. However, major latex protein-like enzyme (MLPL) from Nepeta (catmint) with an early step in chloroplast and subsequent steps in cytosol play a crucial role in the maximum production of strictosidine in N. benthamiana (Figure 3) [17].
7. Challenges and Future Prospects
Nicotiana contains approximately seventy-five species made up of twelve chromosomes, as in the majority of Solanaceae crops. In the last couple of decades, the plant became one of the key platforms for the genetic engineering program with novel biological achievements including tissue culture, hybridization, genetic transformation, transient expression, gene silencing, etc. Nicotine and other minor alkaloids present in tobacco possess pharmacological properties due to their binding ability with various nicotinic acetylcholine receptors.
In addition to the naturally occurring anti-cancer alkaloids in Nicotiana, the plant is also used as a heterologous system for the de novo synthesis of a variety of anti-tumor alkaloids through Agrobacterium-mediated gene transfer, agroinfiltration, virus-mediated overexpression/gene silencing, and gene editing, etc. However, the de novo synthesis of various anti-tumor alkaloids, including terpenoids, is not explored at the desired level in tobacco. MEP and MVA, the two complex pathways along with a wide range of native enzymes make tobacco plants somewhat difficult to maximize de novo synthesis of the targeted compounds or their precursors. The most noticeable and reported limitation in the metabolic engineering of this plant is the supply of substrate. The limitations of the maximum supply of the substrate could be achieved through the compartmentalization of the precursor pool by working with related enzymes or engineering of flux for the formation of precursor or organelle-specific reconstitution of the precursor-synthesizing pathway [75,105]. In recent times, metabolic engineering using compartmentalization tactics has come into view as a capable and fruitful approach to overcome those limitations and has been found to increase the synthesis of terpenoids [78,81]. Silent metabolism directed by glycosylation and methylation is also a potential limiting factor in the tobacco plant for the overexpression of genes. Targeting of subcellular compartments like cytosol, mitochondria, and plastid makes possible the reconstructed pathway for a full swing mood [106]. For the elicited production of alkaloids, virus-induced gene silencing from the endogenous precursors through compartmentation of the final product can play a vital role [107,108]. Moreover, organelle-specific engineering for the metabolic catabolism of the precursor pools could be another way to boost the increased accumulation of the desired compounds. Further, metabolic flux analysis techniques, along with improved analytical abilities, optimize the carbon flux localization in metabolic networks, revealing the metabolic channel and exploring the persistent non-functional metabolic pools [109]. The tools will ultimately boost the prospective of metabolic modeling, which directs the improvement of not only the models, but also make it possible to predict the accomplishment of future genetic engineering strategies. Successful application of CRISPR/Cas9 will further help the synthesis of recombinant protein as double-knockout genes, namely dicer-like proteins 2 and 4 that accumulate maximum human fibroblast growth factor in N. benthamiana [110].
8. Conclusions
Alkaloids are basic nitrogen-containing plant secondary metabolites that naturally occur in a wide range of plants. Alkaloids containing plants including the Nicotiana species have been used since ancient times for therapeutic and recreational purposes [111]. Nicotine, anatabine, anabasine, and nornicotine are the predominant alkaloids present in tobacco where nicotine contains more than 90% of the total alkaloids pool [112]. The root is the primary synthesizing area of nicotine, the related alkaloids and reached leaves through the xylem vessel [113,114]. Amino acids play the role of a precursor for the synthesis of most alkaloids, which comprised the pyridine ring and pyrrolidine ring pathways [8]. Natural secondary metabolites, including alkaloids, are synthesized in the plant like tobacco, in trace amounts, and are broadly used to treat different types of cancers [115]. Different species of Nicotiana such as N. benthamiana, N. tabacum, and N. glauca are widely used as a potential platform for the de novo synthesis of various anti-cancer alkaloids, including strictosidine, vinblastine, crocin, etoposide, costunolide, parthenolide, artemisinin, etc. However, the demand for plant-derived anti-cancer alkaloids is increasing day by day. High versatility in the metabolic pathway and the ability to produce high biomass in a short time turned the tobacco plant into a potential chassis for the production of plant secondary metabolites, including anti-cancer alkaloids. Among different species of the tobacco plant, there can be easy replication of transient expression vector established N. benthamiana for the in vitro synthesis of small molecules and recombinant proteins, where N. tabacum is considered best for the in vivo or large-scale field production of the desired molecules [52,53]. The docility in nuclear and plastid transformation of Nicotiana plants also plays a role in its extensive use in the field of classical transgenesis-based metabolic engineering. However, the genetic makeup of the plant makes it easy for agroinfiltration, virus-induced gene silencing, or gene editing to reconstitute the synthesis pathway of endogenous metabolites and the de novo synthesis of distant metabolites. Therefore, a huge prospect prevailed for the commercial-based production of valuable alkaloids from the existing or enriched precursor’s pools, as different valued anti-cancer alkaloids have already been produced in the plant [15,75,116,117,118]. In addition, metabolic catabolism of the precursor pools with simultaneous organelle-specific genetic engineering may perhaps facilitate maximizing the yield.
Author Contributions
Conceptualization-editing-reviewing, M.M.H. and K.-H.B.; writing-original draft preparation, M.A.H.; writing-original draft preparation, M.M.I. (Md. Mobinul Islam); writing-reviewing, M.M.I. (Md. Mukul Islam). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cooperative Research Program for Agriculture Science and Technology Development (project No. PJ015726) and the Rural Development Administration (RDA), Republic of Korea.
Data Availability Statement
Data is presented within the manuscript.
Acknowledgments
We appreciate the funding from RDA (PJ015726), Republic of Korea and Institute of Research and Training (IRT), Hajee Mohammad Danesh Science and Technology University Dinajpur-5200, Bangladesh.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lemos, R.D.C.; Pádua, J.M.V.; Bruzi, A.T.; Oliveira, V.B.D.; Ramalho, M.A.P. Comparison between doubled haploid lines and lines obtained via the bulk method in tobacco. Crop. Breed. Appl. Biotechnol. 2022, 22, e42992249. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G. Genetic diversity of one cultivar and 29 genotypes of tobacco based on morphological and yield properties. J. Anim. Plant Sci. 2020, 30, 442–453. [Google Scholar]
- Leal, M.; Moreno, M.A.; Albornoz, P.L.; Mercado, M.I.; Zampini, I.C.; Isla, M.I. Nicotiana tabacum leaf waste: Morphological characterization and chemical-functional analysis of extracts obtained from powder leaves by using green solvents. Molecules 2023, 28, 1396. [Google Scholar] [CrossRef] [PubMed]
- Jaber, A.; Soukariyeh, R.; Khalil, A.; Abdel-Sater, F.; Cheble, E. Biological activities of total oligomeric flavonoids enriched extracts of Nicotiana tabacum from eight lebanese regions. Int. J. Pharm. Sci. Rev. Res. 2020, 6, 70–77. [Google Scholar]
- Coseri, S. Natural products and their analogues as efficient anticancer drugs. Mini-Rev. Med. Chem. 2009, 9, 560–571. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Chen, M.W.; Cheng, K.J.; Yu, P.Z.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef]
- Saitoh, F.; Noma, M.; Kawashima, N. The alkaloid contents of sixty Nicotiana species. Phytochemistry 1985, 24, 477–480. [Google Scholar] [CrossRef]
- Dewey, R.E.; Xie, J. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 2013, 94, 10–27. [Google Scholar] [CrossRef]
- Mishra, A.; Chaturvedi, P.; Datta, S.; Sinukumar, S.; Joshi, P.; Garg, A. Harmful effects of nicotine. Indian J. Med. Paediatr. Oncol. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Khan, H.; Patel, S.; Kamal, M.A. Pharmacological and toxicological profile of harmane-β-Carboline alkaloid: Friend or foe. Curr. Drug Metab. 2017, 18, 853–857. [Google Scholar] [CrossRef]
- Echeverria, V.; Grizzell, J.A.; Barreto, G.E. Neuroinflmmation: A therapeutic target of cotinine for the treatment of psychiatric disorders? Curr. Pharmaceut. Des. 2016, 22, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Mao, X.X.; Du, Y.M.; Yan, P.Z.; Hou, X.D.; Zhang, Z.F. Anti-tumor activity of cembranoid-type diterpenes isolated from Nicotiana tabacum L. Biomoleculars 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Pickens, L.B.; Tang, Y.; Chooi, Y.H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef]
- Hasan, M.M.; Kim, H.S.; Jeon, J.H.; Kim, S.H.; Moon, B.K.; Song, J.Y.; Shim, S.H.; Baek, K.H. Metabolic engineering of Nicotiana benthamiana for the increased production of taxadiene. Plant Cell Rep. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Rosales-Campos, A.L.; Gutiérrez-Ortega, A. Agrobacterium-mediated transformation of Nicotiana tabacum cv. Xanthi leaf explants. Bio-Protocol 2019, 101, e3150. [Google Scholar] [CrossRef]
- Dudley, Q.M.; Jo, S.; Guerrero, D.A.S.; Chhetry, M.; Smedley, M.A.; Harwood, W.A.; Sherden, N.H.; O’Connor, S.E.; Caputi, L.; Patron, N.J. Reconstitution of monoterpene indole alkaloid biosynthesis in genome engineered Nicotiana benthamiana. Commun. Biol. 2022, 5, 949. [Google Scholar] [CrossRef]
- Chadwick, D.J.; Whelan, J. Secondary Metabolites: Their Function and Evolution; John Wiley & Sons: New York, NY, USA, 1992; pp. 3–4. [Google Scholar]
- Teoh, E.S. Secondary metabolites of plants. In Medicinal Orchids of Asia; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–73. ISBN 9783319242743. [Google Scholar]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Wangchuk, P. Plant alkaloids: Classification, isolation and drug development. In Medicinal Plants: Chemistry, Pharmacology and Therapeutic Applications; Swamy, M.K., Patra, J.K., Rudramurthy, G.R., Eds.; Taylor & Francis Ltd.: Boca Raton, FL, USA, 2019; pp. 131–137. [Google Scholar]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K. Cucurbita plants: From farm to industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Kurek, J. Introductory chapter: Alkaloids-Their importance in nature and for human life. In Alkaloids—Their Importance in Nature and for Human Life; Kurek, J., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Zenkner, F.F.; Margis-Pinheiro, M.; Cagliari, A. Nicotine biosynthesis in Nicotiana: A Metabolic Overview. Tobacco Sci. 2019, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alijevic, O.; McHugh, D.; Rufener, L.; Mazurov, A.; Hoeng, J.; Peitsch, M. An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human α4β2 and α7 nicotinic acetylcholine receptors. Phytochemistry 2020, 170, 1121187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, K.; Kumar, V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017, 142, 48–73. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. β-Carbolines in foods. In Bioactive Compounds in Foods; Gilbert, J., Senyuva, H.Z., Eds.; Blackwell Publishing: Oxford, UK, 2008; pp. 199–223. [Google Scholar]
- Cao, R.H.; Peng, W.L.; Wang, Z.H.; Xu, A.L. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive beta-Carbolines in food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, E.H.; Carpenter, R.D. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry 1962, 1, 215–221. [Google Scholar] [CrossRef]
- Manasa, K.L.; Yadav, S.S.; Srikanth, D.; Nagesh, N.; Alvalaa, M. Recent insights into β-Carboline alkaloids with anticancer potential. IOSR J. Pharm. Biol. Sci. 2020, 15, 01–27. [Google Scholar]
- Chen, Y.F.; Lin, Y.C.; Chen, J.P.; Chan, H.C.; Hsu, M.H.; Lin, H.Y.; Kuo, S.C.; Huang, L.J. Synthesis and biological evaluation of novel 3,9-substituted β-carboline derivatives as anticancer. Bioorg. Med. Chem. Lett. 2015, 25, 3873–3877. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. Norharmane as a potential chemical entity for development of anticancer drugs. Eur. J. Med. Chem. 2019, 162, 752–764. [Google Scholar] [CrossRef]
- Cox, E.D.; Cook, J.M. The pictet-spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef] [PubMed]
- Steiner, N.; Müller, U.; Hajek, R.; Sevcikova, S.; Borjan, B.; Jöhrer, K.; Göbel, G.; Pircher, A.; Gunsilius, E. The metabolomic plasma profile of myeloma patients is considerably different from healthy subjects and reveals potential new therapeutic targets. PLoS ONE 2018, 13, e0202045. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of metabolic profile and kynurenine metabolism in the plasma of parkinson’s disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligands of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Bartnik, M.; Kocki, T.; Turski, W.A. Distribution, synthesis, and absorption of kynurenic acid in plants. Planta Med. 2011, 77, 858–864. [Google Scholar] [CrossRef]
- Mo, Z.; Duan, L.; Pu, Y.; Tian, Z.; Ke, Y.; Luo, W.; Pi, K.; Huang, Y.; Nie, Q.; Liu, R. Proteomics and co-expression network analysis reveal the importance of hub proteins and metabolic pathways in nicotine synthesis and accumulation in tobacco (Nicotiana tabacum L.). Front. Plant Sci. 2022, 13, 860455. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Secondary metabolites. Compr. Biotechnol. 2019, 10, 131–143. [Google Scholar]
- Sanner, T.; Grimsrud, T.K. Nicotine: Carcinogenicity and effects on response to cancer treatment—A review. Front. Oncol. 2015, 5, 196. [Google Scholar] [CrossRef]
- Gavilano, L.B.; Coleman, N.P.; Burnley, L.E.; Bowman, M.L.; Kalengamaliro, N.E.; Hayes, A.; Bush, L.; Siminszky, B. Genetic engineering of Nicotiana tabacum for reduced nornicotine content. J. Agric. Food Chem. 2006, 54, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Pakdeechanuan, P.; Teoh, S.; Shoji, T.; Hashimoto, T. Non-functionalization of two CYP82E Nicotine N-demethylase genes abolishes nornicotine formation in Nicotiana langsdorffii. Plant Cell Physiol. 2012, 53, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Knezevich, A.; Muzic, J.; Hatsukami, D.K.; Hecht, S.S.; Stepanov, I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N′-nitrosonornicotine in humans. Nicotine Tob. Res. 2013, 15, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, N.; Shintani, K.; Osawa, Y. Tobacco alkaloid derivatives as inhibitors of breast cancer aromatase. Cancer Lett. 1993, 75, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Talo, M.; Yildirim, O.; Karabatak, M.; Acharya, U.R. Automated invasive ductal carcinoma detection based using deep transfer learning with whole-slide images. Pattern Recognit. Lett. 2020, 133, 232–239. [Google Scholar] [CrossRef]
- Reed, J.; Osbourn, A. Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell. Rep. 2018, 37, 1431–1441. [Google Scholar] [CrossRef]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The rise and rise of Nicotiana benthamiana: A plant for all reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M.J.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef]
- Ahn, J.H.; Eum, K.H.; Lee, M. Spry2 does not directly modulate Raf-1 kinase activity in v-Ha-ras-transformed NIH 3T3 fibroblasts. BMB Rep. 2010, 43, 205–211. [Google Scholar] [CrossRef]
- Hata, K.; Osaki, M.; Dhar, D.K.; Nakayama, K.; Fujiwaki, R.; Ito, H.; Nagasue, N.; Miyazaki, K. Evaluation of the antiangiogenic effect of taxol in a human epithelial ovarian carcinoma cell line. Cancer Chemother. Pharmacol. 2004, 53, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Ketchum, R.E.B.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Kaspera, R.; Croteau, R. Cytochrome P450 oxygenases of taxol biosynthesis. Phytochem. Rev. 2006, 5, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S.; Pullaiah, T. 6-Botany of paclitaxel producing plants. In Paclitaxel; Swamy, M.K., Pullaiah, T., Chen, Z.-S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 155–170. [Google Scholar]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; Walsh, V. The Story of Taxol; Cambridge University Press: Cambridge, UK, 2001; p. 282. [Google Scholar]
- Hasan, M.M.; Dhakal, R.; Baek, K.H. Accumulation of taxadiene by root culture of Nicotiana benthamiana domin transformed with taxadiene synthase. Bangladesh J. Bot. 2017, 46, 899–905. [Google Scholar]
- Li, S. Review and expectation of the study on quantitative analysis of steroidal alkaloid. Drug Stanoaros China 2001, 2, 8–11. [Google Scholar]
- Kovacs, K.; Zhang, L.; Linforth, R.T.; Whittaker, B.; Hayes, C.; Fray, R. Redirection of carotenoid metabolism for the efficient production of taxadiene [taxa-4(5),11(12)-diene] in transgenic tomato fruit. Transgen. Res. 2007, 16, 121–126. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H. Assessment of artemisinin contents in selected Artemisia species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as anticancer drugs: Novel therapeutic approaches, molecular mechanisms, and clinical trials. Front. Pharmacol. 2020, 11, 1608. [Google Scholar] [CrossRef]
- Brown, G.D.; Sy, L.K. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 2004, 60, 1139–1159. [Google Scholar] [CrossRef]
- Chen, R.; Bu, Y.; Ren, J.; Pelot, K.A.; Hu, X.; Diao, Y.; Zhang, L. Discovery and modulation of diterpenoid metabolism improves glandular trichome formation, artemisinin production and stress resilience in Artemisia annua. New Phytol. 2021, 230, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Olofsson, L.; Brodelius, M.; Brodelius, P.E. Expression, purification and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch. Biochem. Biophys. 2005, 436, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G.; Covello, P.S. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006, 580, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef]
- Rydén, A.-M.; Ruyter-Spira, C.; Quax, W.J.; Osada, H.; Muranaka, T.; Kayser, O.; Bouwmeester, H. The molecular cloning of dihydroartemisinic aldehyde reductase and its implication in artemisinin biosynthesis in Artemisia annua. Planta Med. 2010, 76, 1778. [Google Scholar] [CrossRef]
- Liu, M.; Shi, P.; Fu, X.; Brodelius, P.E.; Shen, Q.; Jiang, W.; He, Q.; Tang, K. Characterization of a trichome-specific promoter of the aldehyde dehydrogenase 1 (ALDH1) gene in Artemisia annua. Plant Cell Tissue Organ Cult. 2016, 126, 469–480. [Google Scholar] [CrossRef]
- Czechowski, T.; Larson, T.R.; Catania, T.M.; Harvey, D.; Brown, G.D.; Graham, I.A. Artemisia annua mutant impaired in artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 15150–15155. [Google Scholar] [CrossRef]
- Farhi, M.; Marhevka, E.; Ben-Ari, J.; Algamas-Dimantov, A.; Liang, Z.; Zeevi, V.; Edelbaum, O.; Spitzer-Rimon, B.; Abeliovich, H.; Schwartz, B.; et al. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat. Biotechnol. 2011, 29, 1072–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef]
- Ting, H.M.; Wang, B.; Rydén, A.M.; Woittiez, L.; van Herpen, T.; Verstappen, F.W.; Ruyter-Spira, C.; Beekwilder, J.; Bouwmeester, H.J.; van der Krol, A. The metabolite chemotype of Nicotiana benthamiana transiently expressing artemisinin biosynthetic pathway genes is a function of CYP71AV1 type and relative gene dosage. New Phytol. 2013, 199, 352–366. [Google Scholar] [CrossRef]
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Kalamuddin, M.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized metabolic engineering for artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant 2016, 9, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Wallaart, T.E.; Bouwmeester, H.J.; Hille, J.; Poppinga, L.; Maijers, N.C.A. Amorpha-4,11-diene synthase: Cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 2001, 212, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Van Herpen, T.W.J.M.; Cankar, K.; Nogueira, M.; Bosch, D.; Bouwmeester, H.J.; Beekwilder, J. Nicotiana benthamiana as a production platform for artemisinin precursors. PLoS ONE 2010, 5, e14222. [Google Scholar] [CrossRef]
- Fuentes, P.; Zhou, F.; Erban, A.; Karcher, D.; Kopka, J.; Bock, R. A new synthetic biology approach allows transfer of an entire metabolic pathway from a medicinal plant to a biomass crop. eLife 2016, 5, e13664. [Google Scholar] [CrossRef] [PubMed]
- Ikram, N.K.B.K.; Simonsen, H.T. A review of biotechnological artemisinin production in plants. Front. Plant Sci. 2017, 8, 1966. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Ueda-Nakamura, T.; Cortez, D.A.G.; Dias, B.P.; Morgado-Diaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum Parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef]
- Majdi, M.; Liu, Q.; Karimzadeh, G.; Malboobi, M.A.; Beekwilder, J.; Cankar, K.; De Vos, R.; Todorović, S.; Simonović, A.; Bouwmeester, H. Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz Bip.). Phytochemistry 2011, 72, 1739–1750. [Google Scholar] [CrossRef]
- Liu, Q.; Manzano, D.; Tanic, N.; Pesic, M.; Bankovic, J.; Pateraki, I.; Ricard, L.; Ferrer, A.; de Vos, R.; van de Krol, S.; et al. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab. Eng. 2014, 23, 145–153. [Google Scholar] [CrossRef]
- Rasul, A.; Parveen, S.; Ma, T. Costunolide: A novel anti-cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012, 7, 6–13. [Google Scholar] [CrossRef]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef]
- Pitchai, D.; Roy, A.; Banu, S. In vitro and in silico evaluation of NF-κB targeted costunolide action on estrogen receptor-negative breast cancer cells-a comparison with normal breast cells. Phytother. Res. 2014, 28, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.; de Vos, R.C.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Thirumaran, R.; Prendergast, G.C.; Gilman, P.B. Cytotoxic chemotherapy in clinical treatment of cancer. In Cancer Immunotherapy: Immune Suppression and Tumor Growth; Prendergast, G.C., Jaffee, E.M., Eds.; Elsevier Inc.: Burlington, VT, USA; San Diego, CA, USA; London, UK, 2007; pp. 101–116. [Google Scholar]
- Davey, S.G. Engineering etoposide. Nat. Rev. Chem. 2020, 4, 63. [Google Scholar] [CrossRef]
- Kim, S.S.; Wengier, D.L.; Ragland, C.J.; Sattely, E.S. Transcriptional reactivation of lignin biosynthesis for the heterologous production of etoposide aglycone in Nicotiana benthamian. ACS Synth. Biol. 2022, 11, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.J.; Kim, S.Y.; Lau, W.; Sattely, E.S. Total biosynthesis for milligram-scale production of etoposide intermediates in a plant chassis. J. Am. Chem. Soc. 2019, 141, 19231–19235. [Google Scholar] [CrossRef]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef]
- Ranjbar, R.; Shayanfar, P.; Maniati, M. In vitro antileishmanial effects of saffron compounds, crocin and stigmasterol, on iranian strain of Leishmania major (MHOM/IR/75/ER). Iran. J. Parasitol. 2021, 16, 151. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Zoubi, M.S.A.; Faruck, H.L.; Aljabali, A.A.A.; Rabi, F.A.; Hafiz, A.A.; Al-Batanyeh, K.M.; Al-Trad, B.; Ansari, P.; Nasef, M.M.; et al. Dietary crocin is protective in pancreatic cancer while reducing radiation-induced hepatic oxidative damage. Nutrients 2020, 12, 1901. [Google Scholar] [CrossRef]
- Ahrazem, O.; Zhu, C.; Huang, X.; Rubio-Moraga, A.; Capell, T.; Christou, P.; Gómez-Gómez, L. Metabolic engineering of crocin biosynthesis in Nicotiana species. Front. Plant Sci. 2022, 13, 861140. [Google Scholar] [CrossRef]
- Grzech, D.; Hong, B.; Caputi, L.; Sonawane, P.D.; O’Connor, S.E. Engineering the biosynthesis of late-stage vinblastine precursors precondylocarpine acetate, catharanthine, tabersonine in Nicotiana benthamiana. ACS Synth. Biol. 2023, 12, 27–34. [Google Scholar] [CrossRef]
- Sarrion-Perdigones, A.; Vazquez-Vilar, M.; Palací, J.; Castelijns, B.; Forment, J.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. GoldenBraid 2.0: A comprehensive DNA assembly framework for plant synthetic biology. Plant Physiol. 2013, 162, 1618–1631. [Google Scholar] [CrossRef]
- Stoeckigt, J.; Ruppert, L. Strictosidine, the biosynthetic key to monoterpenoid indole alkaloids. In Comprehensive Natural Products Chemisty; Kelly, J.W., Ed.; Elsevier B. V.: Amsterdam, The Netherlands, 1999; Volume 4, pp. 109–138. [Google Scholar]
- De Luca, V.; Salim, V.; Levac, D.; Atsumi, S.M.; Yu, F. Discovery and functional analysis of monoterpenoid indole alkaloid pathways in plants. Methods Enzymol. 2012, 515, 207–229. [Google Scholar] [PubMed]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.F. Rigorous biogenetic network for a group of indole alkaloids derived from strictosidine. Molecules 2008, 13, 1875–1896. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Dong, L.; Navrot, N.; Schneider, T.; Burlat, V.; Pollier, J.; Woittiez, L.; van der Krol, S.; Lugan, R.; Ilc, T.; et al. The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 2014, 5, 3606. [Google Scholar] [CrossRef]
- Kumar, S.; Hahn, F.M.; Baidoo, E.; Kahlon, T.S.; Wood, D.F.; McMahan, C.M.; Cornish, K.; Keasling, J.D.; Daniell, H.; Whalen, M.C. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab. Eng. 2012, 14, 19–28. [Google Scholar] [CrossRef]
- Wellen, K.E.; Snyder, N.W. Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 347–354. [Google Scholar] [CrossRef]
- Lynch, J.H.; Orlova, I.; Zhao, C.; Guo, L.; Jaini, R.; Maeda, H.; Akhtar, T.; Cruz-Lebron, J.; Rhodes, D.; Morgan, J. Multifaceted plant responses to circumvent Phehyperaccumulation by downregulation of flux through the shikimate pathway and by vacuolar Phe sequestration. Plant J. 2017, 92, 939–950. [Google Scholar] [CrossRef]
- Garg, A.; Sharma, S.; Srivastava, P.; Ghosh, S. Application of virus-induced gene silencing in Andrographis paniculata, an economically important medicinal plant. Protoplasma 2021, 258, 1155–1162. [Google Scholar] [CrossRef]
- Shih, M.-L.; Morgan, J.A. Metabolic flux analysis of secondary metabolism in plants. Metab. Eng. Commun. 2020, 10, e00123. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop. Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Fester, K. Plant alkaloids. Encycl. Life Sci. 2010, 11, 74–81. [Google Scholar]
- Sisson, V.; Severson, R. Alkaloid composition of the Nicotiana species. Beitr. Tabakforsch. Int. 1990, 14, 327–339. [Google Scholar] [CrossRef]
- Dawson, R.F. Nicotine synthesis in excised tobacco roots. Amer. Jour. Bot. 1942, 29, 813–815. [Google Scholar] [CrossRef]
- Saunders, J.A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979, 64, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Lu, Y.; Stegemann, S.; Agrawal, S.; Karcher, D.; Ruf, S.; Bock, R. Horizontal transfer of a synthetic metabolic pathway between plant species. Curr. Biol. 2017, 27, 3034–3041. [Google Scholar] [CrossRef]
- Demurtas, O.C.; De Brito Francisco, R.; Diretto, G.; Ferrante, P.; Frusciante, S.; Pietrella, M.; Aprea, G.; Borghi, L.; Feeney, M.; Frigerio, L.; et al. ABCC transporters mediate the vacuolar accumulation of crocins in saffron stigmas. Plant Cell 2019, 31, 2789–2804. [Google Scholar] [CrossRef]
- Sonawane, P.D.; Pollier, J.; Panda, S.; Szymanski, J.; Massalha, H.; Yona, M.; Unger, T.; Malitsky, S.; Arendt, P.; Pauwels, L.; et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 2016, 3, 16205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).