Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring
Abstract
1. Introduction
2. Material and Methods
2.1. Plant and Insect Material
2.2. Damage Induction
2.3. Volatiles Collection System
2.4. Gas Chromatography Coupled to Mass Spectrometer Analysis
2.5. Statistical Analysis
3. Results
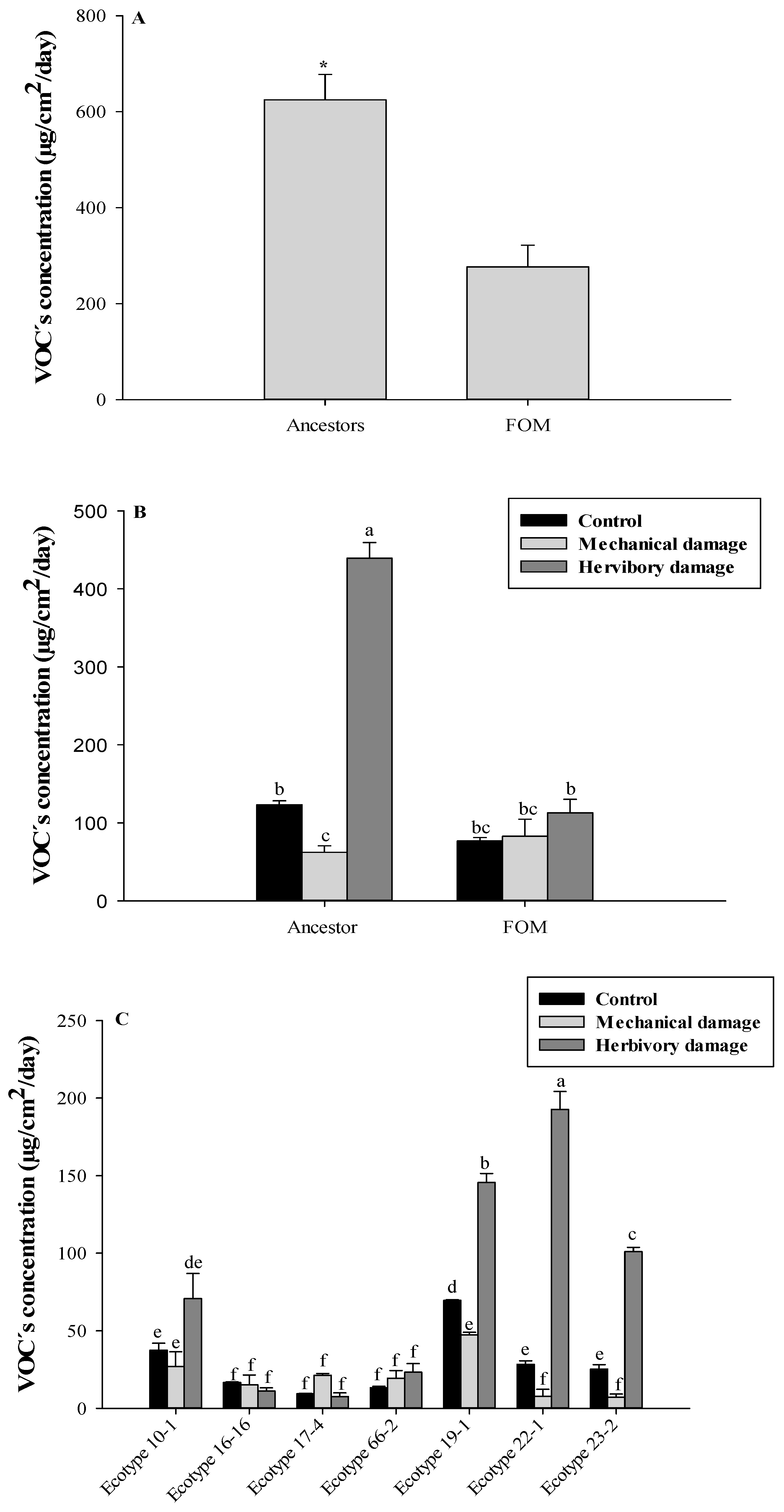
3.1. Total VOCs Identification
3.2. VOCs Identification Related to the Domestication Degree
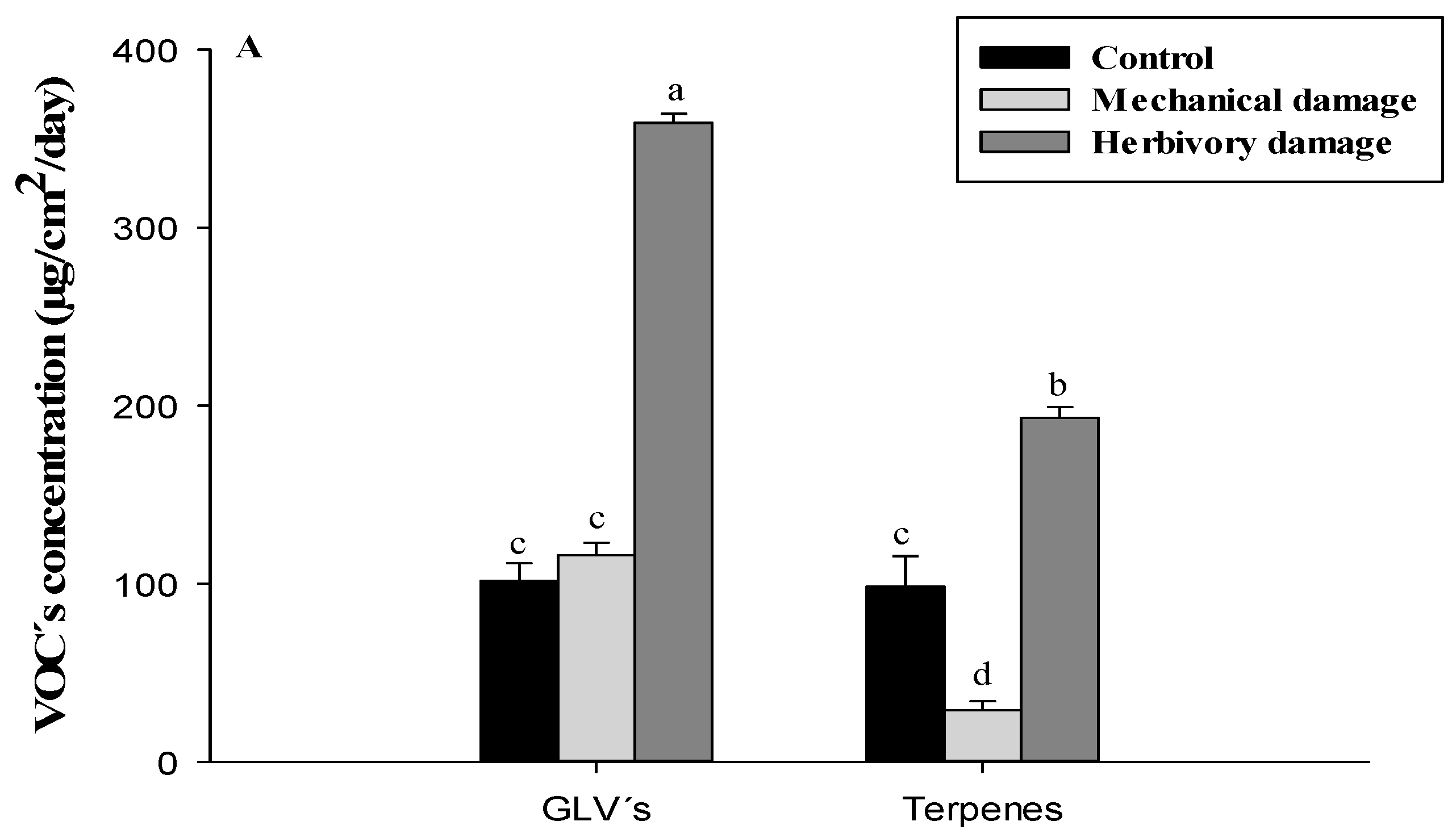
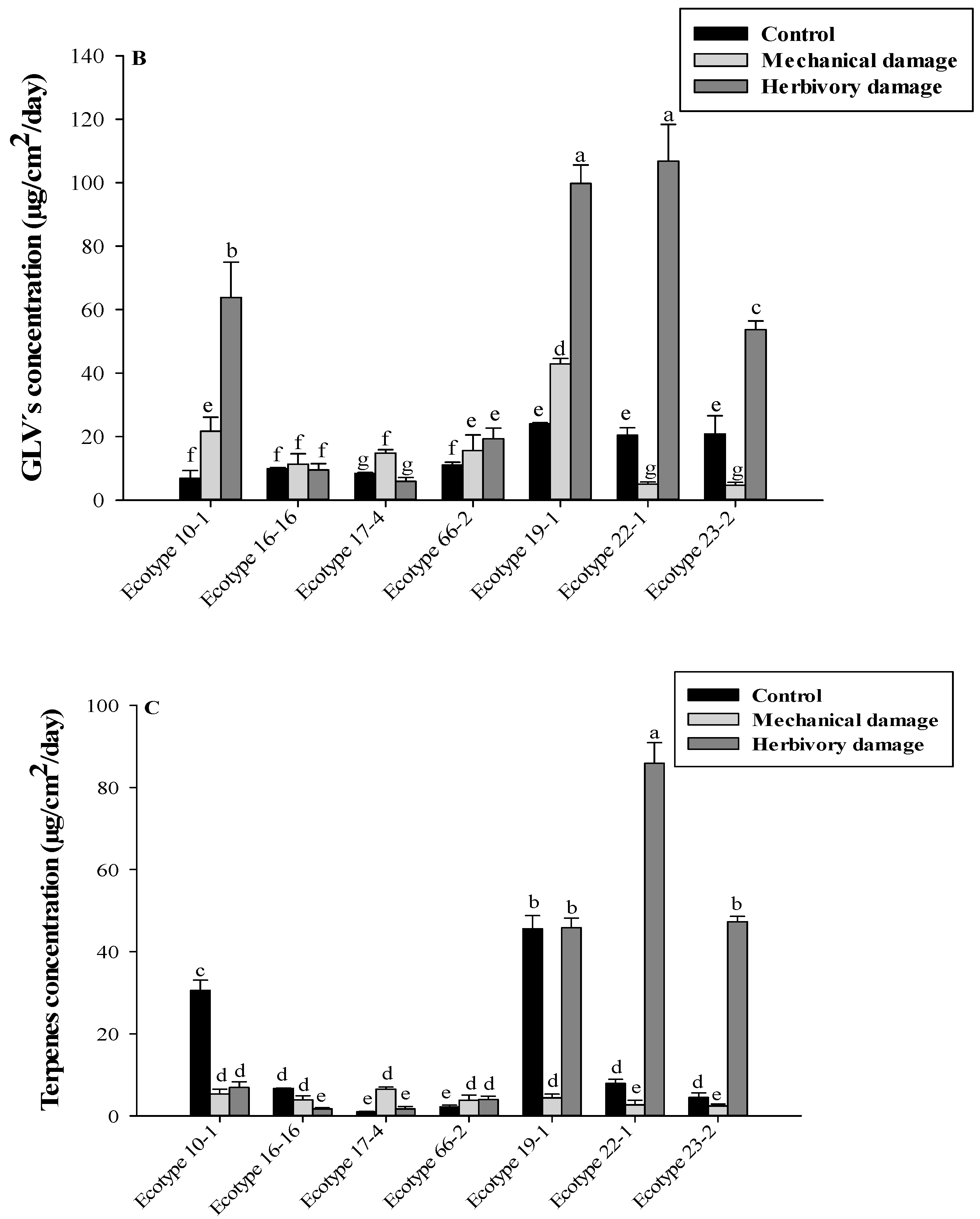
3.3. GLVs Related to the Domestication Degree
3.4. Terpenes Related to the Domestication Degree
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montenegro, G. Chile Nuestra Flora Útil: Guía de Plantas de Uso Apícola, en Medicina Folklorica, Artesanal y Ornamental; Ediciones Universidad Católica de Chile: Santiago de Chile, Chile, 2000; p. 267. [Google Scholar]
- Seguel, I.; Peñaloza, E.; Gaete, N. Colecta y caracterización molecular de germoplasma de murta (Ugni molinae Turcz.) en Chile. Agro Sur 2000, 28, 32–41. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutiérrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-Glucosidase/α-Amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Avello, M.; Pastene, E. Actividad antioxidante de infusos de Ugni molinae Turcz (“Murtilla”). BLACPMA 2005, 4, 33–39. [Google Scholar]
- Junqueira-Gonçalves, M.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.; Zúñiga, G. Isolation and characterization of phenolic compounds and anthocyanins from murta (Ugni molinae Turcz.) fruits: Assessment of antioxidant and antibacterial activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Vega-Gálvez, A.; Rodríguez, A.; Uribe, E.; Bilbao-Sainz, C. Murta (Ugni molinae Turcz.): A review on chemical composition, functional components and biological activities of leaves and fruits. Chil. J. Agric. Anim. Sci. 2018, 34, 43–56. [Google Scholar] [CrossRef]
- Peña-Cerda, M.; Arancibia-Radich, J.; Valenzuela-Bustamante, P.; Perez-Arancibia, R.; Barriga, A.; Seguel, I.; Garcia, L.; Delporte, C. Phenolic composition and antioxidant capacity of Ugni molinae Turcz leaves of different genotypes. Food Chem. 2017, 217, 219–227. [Google Scholar] [CrossRef]
- Shene, C.; Reyes, A.; Villarroel, M.; Sineiro, J.; Pinelo, M.; Rubilar, M. Plant location and extraction procedure strongly alter the antimicrobial activity of murta extracts. Eur. food Res. Technol. 2009, 228, 467–475. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Parra, L.; Rodríguez-Saona, C.; Seguel, I.; Ceballos, R.; Quiroz, A. Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 2015, 44, 627–637. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Lizama, M.; Parra, L.; Seguel, I.; Quiroz, A. Insect diversity, community composition and damage index on wild and domesticated murtilla. Cienc. Investig. Agrar. 2016, 43, 57–67. [Google Scholar]
- Chacón-Fuentes, M.; Parra, L.; Lizama, M.; Seguel, I.; Urzúa, A.; Quiroz, A. Plant flavonoid content modified by domestication. Environ. Entomol. 2017, 46, 1080–1089. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Bardehle, L.; Lizama, M.; Seguel, I.; Quiroz, A. Restoration of flavonols and isoflavonoids in Ugni molinae subjected to a reciprocal transplant experiment in a domestication framework. Chem. Ecol. 2019, 35, 115–127. [Google Scholar] [CrossRef]
- Augusto, T.R.; Salinas, E.S.S.; Alencar, S.M.; D’arce, M.A.B.R.; de Camargo, A.C.; de Souza Vieira, T.M.F. Phenolic compounds and antioxidant activity of hydroalcoholic extract of wild and domesticated murtilla (Ugni molinae Turcz). Food Sci. Technol. 2014, 34, 667–673. [Google Scholar] [CrossRef]
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.; Hormazabal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinae Turcz) during storage. J. Food Sci. Agric. 2008, 88, 485–492. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Bardehle, L.; Seguel, I.; Medina, C.; Quiroz, A. Volatiles induction in response to mechanical damage is reduced by domestication in murtilla. BLACPMA 2019, 18, 435–443. [Google Scholar]
- Conboy, N.J.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Tosh, C.R. Volatile organic compounds as insect repellents and plant elicitors: An integrated pest management (IPM) strategy for glasshouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104. [Google Scholar] [CrossRef]
- Gharaei, A.M.; Ziaaddini, M.; Frérot, B.; Ebrahimi, S.N.; Jalali, M.A.; Reddy, G.V. Identification and evaluation of four cucurbitaceous host plant volatiles attractive to Diaphania indica (Saunders) (Lep.: Pyralidae). Chemoecology 2020, 30, 173–182. [Google Scholar] [CrossRef]
- Kafle, B.D.; Morawo, T.; Fadamiro, H. Host-Induced plant volatiles mediate ability of the parasitoid Microplitis croceipes to discriminate between unparasitized and parasitized Heliothis virescens larvae and avoid superparasitism. J. Chem. Ecol. 2020, 46, 967–977. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Du, X.B.; Newman, J.; Ren, Y. Behavioural responses of the parasitoid Aphytis melinus to volatiles organic compounds (VOCs) from Aonidiella aurantii on its host fruit Tahitian lime fruit Citrus latifolia. Biol. Control 2019, 133, 103–109. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of d-Limonene and β-Ocimene as attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. The costs of green leaf Volatile-Induced defense priming: Temporal diversity in growth responses to mechanical wounding and insect herbivory. Plants 2019, 8, 23. [Google Scholar] [CrossRef]
- Mitra, P.; Das, S.; Debnath, R.; Mobarak, S.H.; Barik, A. Identification of Lathyrus sativus plant volatiles causing behavioral preference of Aphis craccivora. Pest Manag. Sci. 2020, 77, 285–299. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.S.; Farnier, K.; Gaudillat, B.; Turlings, T.C. Comparing the attraction of two parasitoids to herbivore-induced volatiles of maize and its wild ancestors, the teosintes. Chemoecology 2016, 26, 33–44. [Google Scholar] [CrossRef]
- Gols, R.; Bullock, J.M.; Dicke, M.; Bukovinszky, T.; Harvey, J.A. Smelling the wood from the trees: Non-linear parasitoid responses to volatile attractants produced by wild and cultivated cabbage. J. Chem. Ecol. 2011, 37, 795. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.; Mutyambai, D.M.; Charles, M.A.; Otieno, S.A.; Nyaga, T.M.; Niassy, S.; Khan, Z.R. Responses of stemborer Chilo partellus to volatiles emitted by maize landraces exposed to signal grass (Brachiaria brizantha). J. Plant. Interact. 2020, 15, 345–357. [Google Scholar] [CrossRef]
- Paudel, S.; Lin, P.A.; Foolad, M.R.; Ali, J.G.; Rajotte, E.G.; Felton, G.W. Induced plant defenses against herbivory in cultivated and wild tomato. J. Chem. Ecol. 2019, 45, 693–707. [Google Scholar] [CrossRef]
- Naranjo-Guevara, N.; Peñaflor, M.F.G.V.; Silva, D.B.; Bento, J.M.S. A comparison of the direct and indirect defence abilities of cultivated maize versus perennial and annual teosintes. Chemoecology 2021, 31, 63–74. [Google Scholar] [CrossRef]
- Meyer, R.; Purugganan, M. Evolution of crop species: Genetics of domestication and diversification. Nature 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Gepts, P. The contribution of genetic and genomic approaches to plant domestication studies. Curr. Opin. Plant. Biol. 2014, 18, 51–59. [Google Scholar] [CrossRef]
- Rodríguez-Saona, C.; Vorsa, N.; Singh, P.; Johnson-Cicalese, J.; Szendrei, Z.; Mescher, M.; Frost, C. Tracing the history of plant traits under domestication in cranberries: Potential consequences on anti-herbivore defences. J. Exp. Bot. 2011, 62, 2633–2644. [Google Scholar] [CrossRef]
- Hernández-Cumplido, J.; Giusti, M.; Yucheng, Z.; Vera, K.; Chen, Y.; Rodríguez, C. Testing the ‘plant domestication-reduced defense’ hypothesis in blueberries: The role of herbivore identity. Arthropod-Plant Interact. 2018, 12, 483–493. [Google Scholar] [CrossRef]
- Whitehead, S.; Turcotte, M.; Poveda, K. Domestication impacts on plant-herbivore interactions: A meta-analysis. Philos. Trans. R. Soc. 2016, 372, 20160034. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Saona, C.; Mescher, M.; De Moraes, C. The role of volatiles in plant–plant interactions. Signal. Commun. Plants 2013, 55, 393–412. [Google Scholar]
- Barrios-San Martín, J.; Quiroz, A.; Verdugo, J.A.; Parra, L.; Hormazábal, E.; Astudillo, L.A.; Rojas-Herrera, M.; Ramírez, C.C. Host selection and probing behavior of the poplar aphid Chaitophorus leucomelas (Sternorrhyncha: Aphididae) on two poplar hybrids with contrasting susceptibility to aphids. J. Econom. Entomol. 2014, 107, 268–276. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Reed, J.J.; Zenkevich, I.G.; Brown, R.L.; Mallard, W.G.; Stein, S.E. Development of a database of gas chromatographic retention properties of organic compounds. J. Chromatogr. 2007, 1157, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ilavarasan, R.; Jayachandran, T.; Decaraman, M.; Aravindhan, P.; Padmanabhan, N.; Krishnan, M. Phytochemicals investigation on a tropical plant, Syzygium cumini from Kattuppalayam, Erode District, Tamil Nadu, South India. Pak. J. Nutr. 2009, 8, 83–85. [Google Scholar] [CrossRef]
- Boulanger, R.; Chassagne, D.; Crouzet, J. Free and bound flavour components of amazonian fruits. Flavour. Frag. J. 1999, 14, 303–311. [Google Scholar] [CrossRef]
- Isidorov, V.; Purzynska, A.; Modzelewska, A.; Serowiecka, M. Distribution coefficients of aliphatic alcohols, carbonyl compounds and esters between air and Carboxen/polydimethylsiloxane fiber coating. Anal. Chim. Acta 2006, 560, 103–109. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Lazarevic, J.; Radulovic, N.; Palic, R.; Zlatkovic, B. Chemical analysis of volatile constituents of Berula erecta (Hudson) Coville subsp. erecta (Apiaceae) from Serbia. J. Essent. Oil Res. 2010, 22, 153–156. [Google Scholar]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar]
- Gkinis, G.; Tzakou, O.; Iliopoulou, D.; Roussis, V. Chemical composition and biological activity of Nepeta parnassica oils and isolated nepetalactones. Z. Nat. C 2003, 58, 681–686. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghassemi, N. Volatile constituents of Nepeta glomerulosa Boiss. subsp. Carmanica. Flavour. Frag. J. 1999, 14, 265–267. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Zhao, C.-X.; Liang, Y.-Z.; Yang, H.; Fang, H.Z.; Yi, L.Z.; Zeng, Z.D. Comparative analysis of volatile components from Clematis species growing in China. Anal. Chim. Acta 2007, 595, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Lopes, M.C.; Limberger, R.P.; Apel, M.A.; Henriques, A.T.; Moreno, P.R. Analysis of the volatile oil from Pilocarpus pennatifolius Lemmaire (Rutaceae) leaves by GC-MS. Flavour. Frag. J. 2004, 19, 325–326. [Google Scholar] [CrossRef]
- Dávila-Flores, A.M.; DeWitt, T.J.; Bernal, J.S. Facilitated by nature and agriculture: Performance of a specialist herbivore improves with host-plant life history evolution, domestication, and breeding. Oecologia 2013, 173, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Moreira, X.; Abdala-Roberts, L.; Gols, R.; Francisco, M. Plant domestication decreases both constitutive and induced chemical defences by direct selection against defensive traits. Sci. Rep. 2018, 218, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, Y.; Xue, J.; Yang, X.; Gong, S. Response of a predatory insect, Chrysopa sinica, toward the volatiles of persimmon trees infested with the herbivore, Japanese wax scale. Int. J. Ecol. 2012, 1, 653869. [Google Scholar] [CrossRef]
- Harmel, N.; Almohamad, R.; Fauconnier, M.; Jardin, P.D.; Verheggen, F.; Marlier, M.; Haubruge, E.; Francis, F. Role of terpenes from aphid-infested potato en searching and oviposition behavior of Episyrphus balteatus. Insect Sci. 2007, 14, 57–63. [Google Scholar] [CrossRef]
- Salamanca, J.; Pareja, M.; Rodríguez-Saona, C.; Resende, A.; Souza, B. Behavioral responses of adult lacewings, Chrysoperla externa, to a rose-aphid-coriander complex. Biol. Control. 2015, 80, 103–112. [Google Scholar] [CrossRef]
- Gouinguené, S.; Alborn, H.; Turlings, T.C. Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J. Chem. Ecol. 2001, 29, 145–162. [Google Scholar] [CrossRef]
- Alborn, H.; Jones, T.; Stenhagen, G.; Tumlinson, J. Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J. Chem. Ecol. 2000, 26, 203–220. [Google Scholar] [CrossRef]
- Cherqui, A.; Tjallingii, F. Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J. Insect Physiol. 2000, 46, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
| First Reciprocal Crosses (FOM) | Wild Relatives |
|---|---|
| Ecotype 10-1 | Ecotypes 22-1 × ecotype 19-1 |
| Ecotype 16-16 | Ecotypes 19-1 × ecotype 22-1 |
| Ecotype 17-4 | Ecotypes 23-2 × ecotype 22-1 |
| Ecotype 66-2 | Ecotypes 23-2 × ecotype 19-1 |
| RT (min) | Group | Compound | KIexp | KILib | Reference |
|---|---|---|---|---|---|
| 4.37 | Ketones | 2-Hexanone | - | 750 | [37] * |
| 4.44 | 3-Hexanone | - | 786 | [38] * | |
| 4.71 | Alcohols | 3-Hexanol | - | 797 | [39] * |
| 4.83 | 2-Hexanol | - | 803 | [40] * | |
| 8.28 | Monoterpenes | Pinene | 929 | 931 | [41] * |
| 9.40 | Sabinene | 969 | 969 | [41] * | |
| 9.94 | β-Myrcene | 986 | 988 | [41] * | |
| 10.87 | 1,8 Cineole | 1018 | 1015 | [42] * | |
| 10.99 | Limonene | 1022 | 1025 | [43] * | |
| 17.29 | Ester | 2,4-Dimethyl acetophenone | 1240 | - | [44] * |
| 21.79 | Sesquiterpenes | Caryophyllene | 1409 | 1414 | [45] * |
| 22.68 | α-Caryophyllene | 1446 | 1442 | [46] * |
| Groups | Control | Mechanical Damage | Herbivory Damage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds/ Ecotypes | 19-1 | 10-1 | 16-16 | 66-2 | 19-1 | 10-1 | 16-16 | 66-2 | 19-1 | 10-1 | 16-16 | 66-2 | |
| Alcohols and cetones | 2-Hexanone | 0.4 ± 0.0 b | 0.8 ± 0.1 a | 0.3 ± 0.0 b | 0.8 ± 0.1 a | 3.9 ± 0.6 a | 1.7 ± 0.2 b | 0.7 ± 0.0 c | 2.1 ± 0.3 b | 2.3 ± 0.3 a | 2.1 ± 0.3 a | 1.7 ± 0.6 a | 1.9 ± 0.2 a |
| 3-Hexanone | 0.2 ± 0.0 b | 0.4 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.1 a | 4.2 ± 0.1 a | 2.4 ± 0.4 b | 0.4 ± 0.0 c | 2.5 ± 0.1 b | 3.0 ± 0.5 b | 1.8 ± 0.2 c | 2.7 ± 0.1 b | 9.7 ± 1.8 a | |
| 3-Hexanol | 0.1 ± 0.0 c | 0.3 ± 0.0 b | 0.5 ± 0.0 a | 0.6 ± 0.0 a | 6.0 ± 0.6 a | 1.9 ± 0.2 b | 1.1 ± 0.1 c | 2.1 ± 0.4 b | 5.9 ± 0.7 a | 3.2 ± 0.4 b | 1.7 ± 0.3 c | 3.6 ± 0.7 b | |
| 2-Hexanol | 0.2 ± 0.0 c | 0.9 ± 0.1 b | 1.2 ± 0.1 b | 0.8 ± 0.0 b | 7.7 ± 1.9 a | 4.3 ± 0.7 b | 1.1 ± 0.1 d | 2.8 ± 0.5 c | 6.2 ± 0.6 a | 3.2 ± 0.3 b | 3.4 ± 0.0 b | 3.9 ± 0.6 b | |
| Monoterpenes | Pinene | 15.9 ± 2.4 a | 10.2 ± 1.1 b | 3.4 ± 0.3 c | 1.1 ± 0.2 d | 1.6 ± 0.0 a | 1.0 ± 0.1 b | 1.5 ± 0.2 a | 0.9 ± 0.1 b | 12.6 ± 1.2 a | 2.3 ± 0.4 c | 0.9 ± 0.1 d | 3.3 ± 0.1 b |
| Sabinene | 4.7 ± 0.2 b | 7.0 ± 0.4 a | 0.2 ± 0.0 c | 0.3 ± 0.0 c | 0.6 ± 0.0 b | 1.5 ± 0.4 a | 1.5 ± 0.2 a | 0.5 ± 0.0 b | 15.3 ± 0.2 a | 3.4 ± 0.9 b | 0.1 ± 0.0 c | 0.1 ± 0.0 c | |
| β-Myrcene | 2.8 ± 0.3 b | 5.3 ± 0.3 a | 1.0 ± 0.1 c | 0.2 ± 0.0 d | ND | 0.4 ± 0.0 a | ND | 0.5 ± 0.0 a | 7.5 ± 0.0 a | ND | 0.1 ± 0.0 b | 0.1 ± 0.0 b | |
| 1,8 Cineole | 7.9 ± 0.5 a | 2.6 ± 0.6 b | 0.3 ± 0.0 c | 0.2 ± 0.0 c | 0.2 ± 0.0 b | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.4 ± 0.0 a | 5.1 ± 0.0 a | 0.1 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.0 b | |
| Limonene | 13.7 ± 1.4 a | 10.0 ± 1.1 a | 0.5 ± 0.0 b | 0.2 ± 0.0 c | 0.1 ± 0.0 d | 1.4 ± 0.2 a | 0.6 ± 0.0 c | 0.9 ± 0.0 b | 5.3 ± 0.3 a | 0.7 ± 0.0 b | 0.4 ± 0.0 c | 0.2 ± 0.0 d | |
| Esters | 2,4-Dimethyl acetophenone | 23.1 ± 2.7 a | 4.5 ± 0.3 c | 7.4 ± 1.2 b | 8.4 ± 1.4 b | 21.1 ± 2.1 a | 11.4 ± 1.7 b | 8.0 ± 1.1 c | 6.1 ± 0.6 c | 82.5 ± 2.2 a | 53.5 ± 8.4 b | ND | 0.2 ± 0.0 c |
| Sesquiterpenes | Caryophyllene | 0.6 ± 0.0 b | 0.4 ± 0.0 b | 1.2 ± 0.1 a | 0.2 ± 0.0 c | 1.9 ± 0.2 b | 0.6 ± 0.0 a | ND | 0.5 ± 0.0 a | ND | 0.2 ± 0.0 a | ND | 0.1 ± 0.0 a |
| α-Caryophyllene | ND b | ND b | 0.1 ± 0.0 a | ND b | ND | 0.1 ± 0.0 a | ND | 0.1 ± 0.0 a | ND | 0.2 ± 0.0 a | ND | 0.1 ± 0.0 a | |
| Groups | Control | Mechanical Damage | Herbivory Damage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds/ Ecotypes | 22-1 | 10-1 | 16-16 | 17-4 | 22-1 | 10-1 | 16-16 | 17-4 | 22-1 | 10-1 | 16-16 | 17-4 | |
| Alcohols and cetones | 2-Hexanone | 0.4 ± 0.0 b | 0.8 ± 0.1 a | 0.3 ± 0.0 c | ND | 0.4 ± 0.3 c | 1.7 ± 0.2 a | 0.7 ± 0.0 b | 2.1 ± 0.6 a | 7.0 ± 1.1 a | 2.1 ± 0.3 b | 1.7 ± 0.6 c | 0.5 ± 0.2 d |
| 3-Hexanone | 0.8 ± 0.5 a | 0.4 ± 0.0 c | 0.5 ± 0.0 b | 0.2 ± 0.2 d | 0.9 ± 0.6 b | 2.4 ± 0.4 a | 0.4 ± 0.0 c | 2.8 ± 1.3 a | 7.4 ± 1.7 a | 1.8 ± 0.2 c | 2.7 ± 0.1 b | 0.4 ± 0.2 d | |
| 3-Hexanol | 0.7 ± 0.1 a | 0.3 ± 0.0 c | 0.5 ± 0.0 b | ND | 0.8 ± 0.5 d | 1.9 ± 0.2 b | 1.1 ± 0.1 c | 3.7 ± 1.3 a | 6.4 ± 0.7 a | 3.2 ± 0.4 b | 1.7 ± 0.3 c | 1.0 ± 0.6 c | |
| 2-Hexanol | 0.8 ± 0.1 c | 0.9 ± 0.1 b | 1.2 ± 0.1 a | 0.3 ± 0.3 d | 1.9 ± 0.9 c | 4.3 ± 0.7 b | 1.1 ± 0.1 d | 6.2 ± 2.2 a | 4.4 ± 0.2 a | 3.2 ± 0.3 b | 3.4 ± 0.0 b | 0.6 ± 0.3 c | |
| Monoterpenes | Pinene | 3.9 ± 0.5 b | 10.2 ± 1.1 a | 3.4 ± 0.3 b | 0.7 ± 0.3 c | 0.3 ± 0.2 d | 1.0 ± 0.1 c | 1.5 ± 0.2 b | 3.7 ± 3.0 a | 29.9 ± 6.6 a | 2.3 ± 0.4 b | 0.9 ± 0.1 c | 1.2 ± 0.7 c |
| Sabinene | 0.5 ± 0.3 b | 7.0 ± 0.4 a | 0.2 ± 0.0 c | 0.1 ± 0.0 c | 0.2 ± 0.2 b | 1.5 ± 0.4 a | 1.5 ± 0.2 a | 0.5 ± 0.4 b | 13.0 ± 1.8 a | 3.4 ± 0.9 b | 0.1 ± 0.0 c | 0.1 ± 0.1 c | |
| β-Myrcene | 1.2 ± 0.7 b | 5.3 ± 0.3 a | 1.0 ± 0.1 b | ND | 0.1 ± 0.0 c | 0.4 ± 0.0 b | ND | 0.9 ± 0.6 a | 13.2 ± 2.0 a | ND | 0.1 ± 0.0 b | ND | |
| 1,8 Cineole | 0.4 ± 0.3 b | 2.6 ± 0.6 a | 0.3 ± 0.0 b | 0.1 ± 0.1 b | 0.1 ± 0.0 b | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.1 ± 0.1 b | 15.3 ± 0.2 a | 0.1 ± 0.0 b | 0.2 ± 0.0 b | ND | |
| Limonene | 0.7 ± 0.4 b | 10.0 ± 1.1 a | 0.5 ± 0.0 b | 0.1 ± 0.1 c | 1.0 ± 0.9 b | 1.4 ± 0.2 a | 0.6 ± 0.0 c | 0.4 ± 0.2 c | 12.2 ± 1.9 a | 0.7 ± 0.0 b | 0.4 ± 0.0 c | 0.3 ± 0.2 c | |
| Esters | 2,4-Dimethyl acetophenone | 17.7 ± 3.0 a | 4.5 ± 0.3 c | 7.4 ± 1.2 b | 7.9 ± 9.1 b | 5.8 ± 3.2 c | 11.4 ± 1.7 a | 8.0 ± 1.1 b | 13.3 ± 9.2 a | 81.6 ± 1.3 a | 53.5 ± 8.4 b | ND | 3.4 ± 2.1 c |
| Sesquiterpenes | Caryophyllene | 1.2 ± 0.8 a | 0.4 ± 0.0 b | 1.2 ± 0.1 a | ND | 0.9 ± 0.8 a | 0.6 ± 0.0 b | ND | 0.9 ± 0.8 a | 1.8 ± 1.2 a | 0.2 ± 0.0 b | ND | ND |
| α-Caryophyllene | ND | ND | 0.1 ± 0.0 d | ND | 0.1 ± 0.1 a | 0.1 ± 0.0 a | ND | ND | 0.5 ± 0.3 a | 0.2 ± 0.0 b | ND | 0.1 ± 0.1 c | |
| Groups | Control | Mechanical Damage | Herbivory Damage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds/Ecotypes | 23-2 | 17-4 | 66-2 | 23-2 | 17-4 | 66-2 | 23-2 | 17-4 | 66-2 | |
| Alcohols and cetones | 2-Hexanone | 0.1 ± 0.0 b | ND | 0.8 ± 0.1 a | 0.5 ± 0.5 b | 2.1 ± 0.6 a | 2.1 ± 0.3 a | 5.3 ± 0.1 a | 0.5 ± 0.2 c | 1.9 ± 0.2 b |
| 3-Hexanone | 1.9 ± 0.2 a | 0.2 ± 0.2 c | 0.5 ± 0.1 b | 0.7 ± 0.5 b | 2.8 ± 1.3 a | 2.5 ± 0.1 a | 5.7 ± 0.3 b | 0.4 ± 0.2 c | 9.7 ± 1.8 a | |
| 3-Hexanol | 0.7 ± 0.1 a | ND | 0.6 ± 0.0 a | 0.5 ± 0.3 b | 3.7 ± 1.3 a | 2.1 ± 0.4 a | 4.4 ± 0.2 a | 1.0 ± 0.6 c | 3.6 ± 0.7 b | |
| 2-Hexanol | 1.9 ± 0.7 a | 0.3 ± 0.3 c | 0.8 ± 0.0 b | 1.0 ± 0.5 b | 6.2 ± 2.2 a | 2.8 ± 0.5 b | 20.2 ± 19.0 a | 0.6 ± 0.3 c | 3.9 ± 0.6 b | |
| Monoterpenes | Pinene | 1.2 ± 0.8 a | 0.7 ± 0.3 b | 1.1 ± 0.2 a | 0.1 ± 0.1 c | 3.7 ± 3.0 a | 0.9 ± 0.1 b | 13.8 ± 3.3 a | 1.2 ± 0.7 c | 3.3 ± 0.1 b |
| Sabinene | 1.3 ± 0.9 a | 0.1 ± 0.0 b | 0.3 ± 0.0 b | 0.1 ± 0.1 b | 0.5 ± 0.4 a | 0.5 ± 0.0 a | 10.6 ± 0.5 a | 0.1 ± 0.1 b | 0.1 ± 0.0 b | |
| β-Myrcene | 0.6 ± 0.5 a | ND | 0.2 ± 0.0 b | 0.1 ± 0.1 c | 0.9 ± 0.6 a | 0.5 ± 0.0 b | 15.2 ± 0.1 a | ND | 0.1 ± 0.0 b | |
| 1,8 Cineole | 0.3 ± 0.2 a | 0.1 ± 0.1 a | 0.2 ± 0.0 a | 0.1 ± 0.1 b | 0.1 ± 0.1 b | 0.4 ± 0.0 a | 7.5 ± 0.0 a | ND | 0.1 ± 0.0 b | |
| Limonene | 1.1 ± 0.8 a | 0.1 ± 0.1 b | 0.2 ± 0.0 b | ND | 0.4 ± 0.2 b | 0.9 ± 0.0 a | 0.1 ± 0.1 a | 0.3 ± 0.2 a | 0.2 ± 0.0 a | |
| Esters | 2,4-Dimethyl acetophenone | 16.2 ± 3.0 a | 7.9 ± 9.1 c | 8.4 ± 1.4 b | 2.3 ± 1.6 c | 13.3 ± 9.2 a | 6.1 ± 0.6 b | 18.1 ± 2.8 a | 3.4 ± 2.1 b | 0.2 ± 0.0 c |
| Sesquiterpenes | Caryophyllene | ND | ND | 0.2 ± 0.0 d | 1.7 ± 0.7 a | 0.9 ± 0.8 b | 0.5 ± 0.0 b | ND | ND | 0.1 ± 0.0 a |
| α-Caryophyllene | ND | ND | ND | 0.3 ± 0.1 a | ND | 0.1 ± 0.0 b | 0.1 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Fuentes, M.; Bardehle, L.; Seguel, I.; Espinoza, J.; Lizama, M.; Quiroz, A. Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring. Metabolites 2023, 13, 616. https://doi.org/10.3390/metabo13050616
Chacón-Fuentes M, Bardehle L, Seguel I, Espinoza J, Lizama M, Quiroz A. Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring. Metabolites. 2023; 13(5):616. https://doi.org/10.3390/metabo13050616
Chicago/Turabian StyleChacón-Fuentes, Manuel, Leonardo Bardehle, Ivette Seguel, Javier Espinoza, Marcelo Lizama, and Andrés Quiroz. 2023. "Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring" Metabolites 13, no. 5: 616. https://doi.org/10.3390/metabo13050616
APA StyleChacón-Fuentes, M., Bardehle, L., Seguel, I., Espinoza, J., Lizama, M., & Quiroz, A. (2023). Herbivory Damage Increased VOCs in Wild Relatives of Murtilla Plants Compared to Their First Offspring. Metabolites, 13(5), 616. https://doi.org/10.3390/metabo13050616







