Abstract
There are over 500 species of the genus Artemisia in the Asteraceae family distributed over the globe, with varying potentials to treat different ailments. Following the isolation of artemisinin (a potent anti-malarial compound with a sesquiterpene backbone) from Artemisia annua, the phytochemical composition of this species has been of interest over recent decades. Additionally, the number of phytochemical investigations of other species, including those of Artemisia afra in a search for new molecules with pharmacological potentials, has increased in recent years. This has led to the isolation of several compounds from both species, including a majority of monoterpenes, sesquiterpenes, and polyphenols with varying pharmacological activities. This review aims to discuss the most important compounds present in both plant species with anti-malarial properties, anti-inflammatory potentials, and immunomodulating properties, with an emphasis on their pharmacokinetics and pharmacodynamics properties. Additionally, the toxicity of both plants and their anti-malaria properties, including those of other species in the genus Artemisia, is discussed. As such, data were collected via a thorough literature search in web databases, such as ResearchGate, ScienceDirect, Google scholar, PubMed, Phytochemical and Ethnobotanical databases, up to 2022. A distinction was made between compounds involved in a direct anti-plasmodial activity and those expressing anti-inflammatory and immunomodulating activities or anti-fever properties. For pharmacokinetics activities, a distinction was made between compounds influencing bioavailability (CYP effect or P-Glycoprotein effect) and those affecting the stability of pharmacodynamic active components.
1. Introduction
If one were to nominate the most infectious parasitic diseases in the world, malaria would be at the top of the list, as it is a disease of public health importance with serious socio-economic and human consequences. Malaria is a devastating disease, affecting over 241 million people worldwide annually, with 627,000 deaths [1] There were about 14 million more cases of malaria and 69,000 more deaths in 2020 than there were in 2019, most of which were in Sub-Saharan African countries. Approximately 95% of the world’s malaria burden is concentrated in 11 countries, with 10 of them being in Sub-Saharan Africa with 96% of the death rate; over 80% of these are among children under the age of five and pregnant women [1] This is due to increasing anti-malarial drug resistance to the chemotherapy employed at present. The situation is further aggravated by not only Histidine-Rich Protein-2 gene (HRP2 gene) deletion, making diagnosis problematic, but also by mosquito resistance to insecticides and invasive vector species (Anopheles stephensi) in the Horn of Africa [1]. The fight against this life-threatening disease is one of the goals of the World Health Organization’s (WHO) Global Technical Strategy to reduce its transmission and help create a world that is free of malaria by 2030. The terrestrial ecosystem has been a source of many lead compounds that have undergone chemical derivatization and drug development to produce anti-microbial molecules, including those used to fight malaria [2]. Numerous studies have been conducted on natural products such as crude extracts or pure compounds isolated from medicinal plants. Ethnobotanical and ethnomedical alternatives for the management of health problems, including malaria, have been practiced for many years and are used by 80% of the global population in primary health care [3,4]. These alternative remedies have provided leads for the development of drugs that are useful in therapeutics such as those in used in Western medicine. A well-known example of the seminal contribution of ethnomedicine to the treatment of malaria in the modern medicinal way includes artemisinin, which is isolated from A. annua [5]. Following the isolation of this well-known anti-malarial molecule, intensive research on the phytochemistry of this species has resulted in the isolation of almost 600 secondary metabolites in recent decades [6]. The emergence of resistance to all current malaria drugs including artemisinin has increased the used of A. annua and A. afra by the population, especially in areas of high endemicity. A. annua has been used in China since ancient times as a herbal product taken in the form of tea infusions or decoction for the alleviation of intermittent fevers associated with malaria, amongst other diseases [7]. A. annua is documented in the Pharmacopeia of the People’s Republic of China and is now domesticated in other parts of Asia, Europe, Australia, and America, where it is used to fight other ailments [7,8,9,10]. However, the WHO has cautioned against the use of non-pharmaceutical sources of artemisinin because of the risk of delivering sub-therapeutic doses of artemisinin that could potentiate anti-malarial drug resistance [11]. Hence, the emergence of A. afra which contains negligible or no artemisinin content but kills malaria parasites [12] including the gametocytes [13,14]. A. afra has a long history of use as a herbal product in South Africa for the treatment of ailments, including malaria [15] and it is cultivated across the African continent for the management of malaria and other diseases [9,12]. About 400 secondary metabolites have been isolated from A. afra with a wide range of biological activities against various diseases, including malaria [9]. Being a member of the Asteraceae family, the genus Artemisia is one of the largest and most dispersed genera, with over 500 species, which are predominantly located in different geographical regions due to their ability to thrive, survive and persist in almost all habitat types [11]. The ethnobotanical and ethnomedicinal importance, potential phytotherapeutic application and traditional use of this plant genus have been reviewed by Parada et al. [16] and Wright et al. [17].
In this review, the main objective is to discuss the main secondary metabolites isolated from A. afra and A. annua and their anti-malarial and anti-inflammatory potentials, with an emphasis on their pharmacokinetics and pharmacodynamic importance.
2. Methodology
Literature Search
A thorough review of the literature in web databases relating to secondary metabolites isolated from A. annua and A. afra and their anti-malaria, anti-inflammatory and immunomodulating properties, with a focus on their pharmacokinetics and pharmacodynamics properties, was performed. A literature search of the research in web databases was conducted, and data up to 2022 were collected from published journal articles in international scientific databases, including Ethnomedicinal, ResearchGate, ScienceDirect, Google scholar, PubMed, Phytochemical and Ethnobotanical, etc., reporting on the traditional use, phytochemistry, anti-malaria, anti-inflammatory, immunomodulating, pharmacokinetics and pharmacodynamics of A. annua and A. afra and their secondary metabolites. The following key terms were employed for the literature search: Malaria/burden/A. annua/A. afra, botanical description, geographical distribution/Secondary metabolites/Asteraceae family/Anti-malarial, anti-inflammatory/Immunomodulation/Indigenous use/Herbal Remedies/Ethnobotany/Ethnopharmacology/Ethnomedicine/Ethnomedicinal/Ethnopharmaceutical/phytochemistry/Cytochrome/Cytochrome P450/Pharmacology/pharmacokinetics, pharmacodynamics, bioavailability/Resistance/Artemisinin Combination Therapy. About 200 published research articles were studied, and chemical structures of bioactive compounds were drawn using a scientifically accepted program ChemDraw.
3. Results
3.1. Botanical Description and Distribution
Artemisia annua L. is also referred to as sweet wormwood, sweet annie, and annual wormwood (English) or qinghao (Chinese). According to the Pharmacopeia of the People’s Republic of China [18], a plant containing artemisinin in the Asteraceae family is termed quinghao, meaning “the dry” above-ground parts. A. annua is a large, annually grown, highly aromatic herbaceous herb that can grow up to 2 m tall, with a single stem covered with fine grey-green hairs. It has aromatic leaves which are deeply dissected.
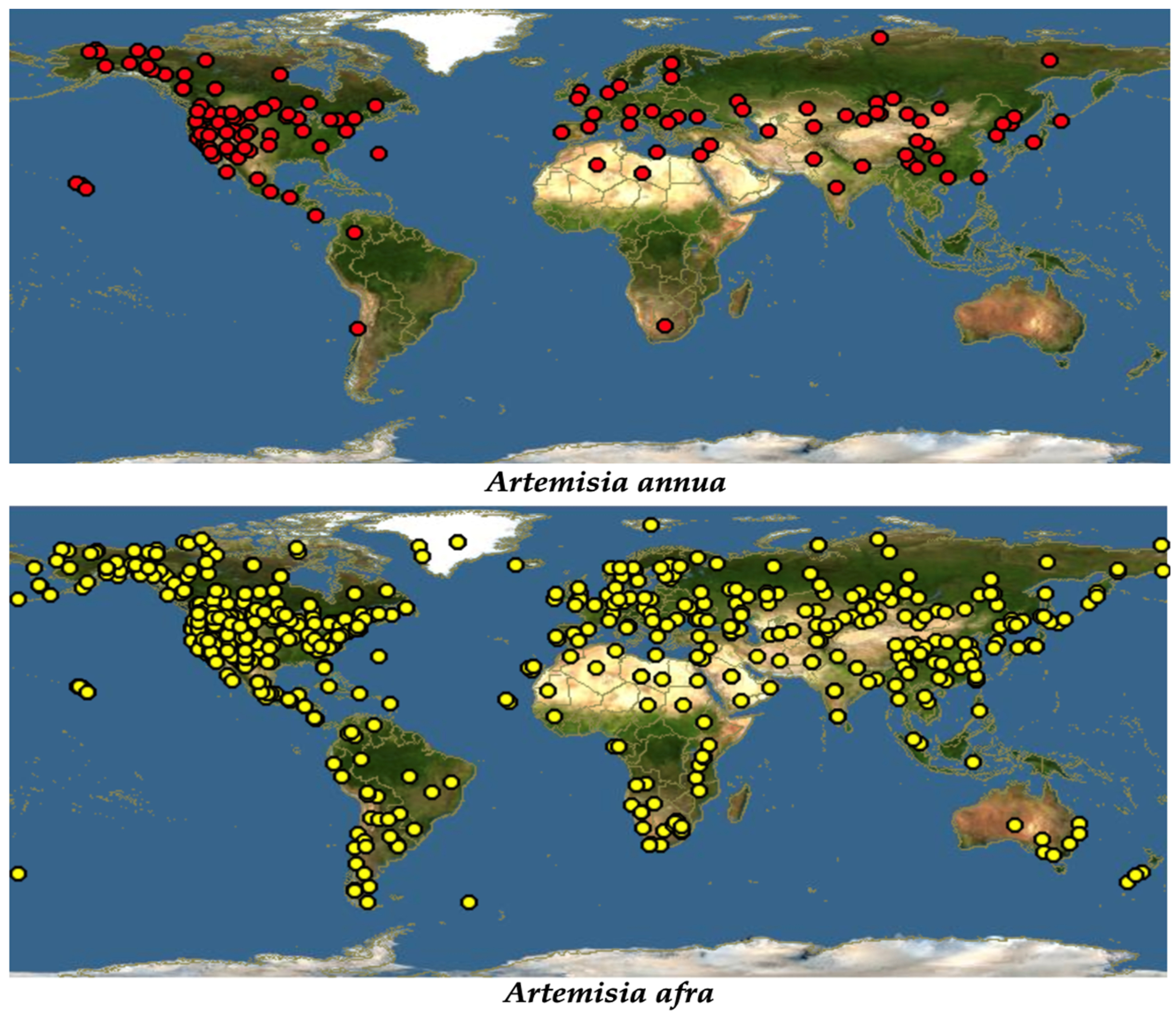
Despite it being widely distributed throughout the world due to its ability to treat a wide range of diseases, traditionally, A. annua originated in China [19]. Just like most of the members of the Asteraceae family, A. annua is highly adaptable, growing in almost all hostile environments, such as forest margins, roadsides, hillsides, dry river valleys, forest meadows, rocky slopes, semiarid climates, and grasslands. However, it was reported that the best growing condition for this plant is in a humid and subtropical monsoon climate with average temperatures of 17.6–28.4 °C [20]. A. annua grows in most parts of the temperate and subtropical parts of the central, southern, and eastern parts of Europe and Asia. Extending to the south-eastern part of Asia, this plant is also seen growing across the Mediterranean and northern parts of Africa. It has also been spotted in the United States, North America, and Canada [19], Figure 1.
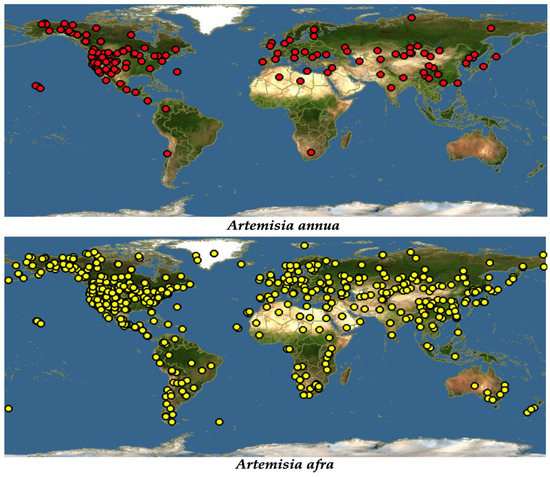
Figure 1.
Global distribution of A. annua and A. afra [21].
Additionally, referred to as African wormwood, A. afra (Jac. ex Willd) is described as having different common names in different societies [22]. Being an indigenous herb to Africa, A. afra grows across the African continent in areas extending from Cederberg Mountains in the Cape to tropical East Africa and continuing through to the north, including Ethiopia [22]. Described as a condiment [18], it has been spotted in almost every comer in the world due to its high medicinal potential (Figure 1). A. afra grows up to a height of at least 2 m. It is a woody perennial herb with finely divided oval-shaped leaves, and it has an aromatic smell, and alternately arranged, silver-grey leaves in the adaxial region and light green leaves in the axial region [15].
3.2. Phytochemistry of A. afra and A. annua
For decades, the phytochemical investigation of A. annua has been at the forefront of research due to the isolation of artemisinin, a potent anti-malarial compound belonging to the sesquiterpene lactone class [5]. Further research has led to the isolation of almost six hundred secondary metabolites, the majority of which are terpenoids, polyphenols and coumarins, as summarized in the Supplementary Materials (Supplementary Tables S1–S11). There have been several publications on the phytochemistry of A. annua over the previous decade. Table 1 summarizes the secondary metabolites isolated from both plant species over the previous decade. As such, several new secondary metabolites have been identified and characterized, with most of them belonging to the monoterpenes and sesquiterpenes classes and a few polyphenols.

Table 1.
Secondary metabolites isolated from A. annua and A. afra in the previous decade.
For instance, Qin et al. [26]. isolated five new sesquiterpene compounds from A. annua: Arteannoid A, which is a sesquiterpenoid dimer composed of two cadinene sesquiterpenoid units; Arteannoids B and C, which are rearranged heterodimers of the cadinene sesquiterpene and one phenylpropanoid unit and two new rearranged cadinene sesquiterpenoids (Arteannoides D and E). In addition, thirteen new sesquiterpenoid compounds (Arteannoides F–R) were isolated from the aerial part of A. annua [39]. Arteannoid H is a new eudesmane-type sesquiterpenoid [26]. A comparative phytochemical investigation of two chemotypes of A. annua (high- and low-artemisinin-producing chemotypes, HAP and LAP, respectively) via UPLC, GC-MS and NMR also lead to the isolation and characterization of twenty-six novel compounds belonging to monoterpenes and sesquiterpenes classes. Nineteen of these secondary metabolites were highly oxygenated amorphane sesquiterpenes, which are the most diverse and abundant subclass [23]. De Magalhães et al. [34]. also confirmed the presence of phenolic acid (Rosmarinic acid) in the leaves of A. annua. Mouton and Van der Kooy [32] isolated two compounds (melilotosides) with anti-typhoid potentials. Goel et al. [40] also characterized nine new aliphatic compounds from the aerial part of A. annua.
As it is a member of the same genus, A. afra shares almost the same pattern of secondary metabolites, which is dominated by terpenoids, polyphenols and coumarins, as summarized in the Supplementary Materials (Supplementary Tables S1–S6). While A. annua cultivars have been cultivated and selected for their high artemisinin content, A. afra is devoid of this potent sesquiterpene lactone [41]. However, other studies have shown that there are cultivars of A. afra with negligible contents of artemisinin [13]. Research on the phytochemistry of A. afra has led to the isolation of almost five hundred secondary metabolites comprising monoterpenes, diterpenes, triterpenes, sesquiterpenes, guaianolides, glaucolides, coumarins and polyphenols (Supplementary Tables S1–S11). Surprisingly, a very limited amount of work on phytochemistry has been conducted on A. afra over the previous decade despite its multi-biological activity against infections [15]. However, Emmanuel et al. [36] isolated five new sesquiterpenes from this species and two other compounds (p-hydroxy acetophenone and 2,4-dihydroxy-6 methoxy acetophenone). Sotenjwa et al. [24] also isolated two new compounds, a flavonoid (rutin) and a coumarin (scopolin), from this species.
3.3. Anti-Malarial Properties, Anti-Inflammatory and Immunomodulating Effects of A. annua and A. afra
According to the Chinese culture, Artemisia annua has been known to alleviate of fevers since the second century [7,9]. It is a highly efficacious medicinal plant against malaria due to the presence of artemisinin and is traditionally used in infusions or decoctions for the treatment of malaria in China [37]. Ethnopharmacological studies for the use of A. annua in Traditional Chinese Medicine (TCM) prompted the isolation and characterization of artemisinin [5], which is a sesquiterpene with remarkable efficacy against the erythrocytic stages of the malaria parasite. Several in vitro and in vivo studies on the crude plant material and/or tea infusions have been reported, showing good anti-plasmodial activities [42,43]. For example, Diawara et al. [43] confirmed the in vitro anti-malarial activity of aqueous and hydroalcoholic crude extracts of A. annua, with an IC50 of 4.95 nM for chloroquine resistance against P. falciparum. Recent investigations on the effect of tea infusions of A. annua on pre-erythrocytic and erythrocytic stages showed growth inhibition of various Plasmodium species in a dose-dependent manner [9]. In addition, Snider et al. [41] confirmed the anti-parasitic activity of a tea infusion with A. annua against the early and late stages of P. falciparum gametocytes, as well as against the asexual forms. Table 2 summarizes the reports on anti-malarial properties.

Table 2.
Artemisia spp. with reported anti-malarial properties either in vitro, in vivo or in clinical trials.
Several clinical trials of A. annua either in tea infusions or as a whole-plant powder taken in the form of a capsule have also been reported, showing the effectiveness of this plant as a traditional remedy against malaria [12,43]. For example, a clinical study was conducted in the Democratic Republic of Congo using an infusion of high artemisinin (0.5–0.75%) cultivar (A. annua cv Artemi) at two doses (5 g of herb in 1 L of water and 9 g/L) in separate groups, and it indicated 77% and 70% cure rates, respectively, after seven days of treatment [64]. However, there were high levels of recrudescence [64]. Blanke et al. [65], also reported a 70% cure rate with A. annua tea following a 7-day treatment, with a high level of recrudescence after day 28 in a small, randomized, double-blinded trial. The high level of recrudescence could be due to the rapid clearance of artemisinin, as it is metabolized by cytochrome P450 (CYPs) enzymes.
Cytochrome P450 (CYPs) is a superfamily of hemeprotein enzymes present in the livers of humans, whose function is to metabolize xenobiotics. These enzymes are involved in the first-pass effect of many drugs, including artemisinin, which is actively metabolized by CYP2B6 and CYP3A4 enzymes [52]. Medicinal plants and or pure molecules with safety profiles that can inhibit cytochrome P450 enzymes, especially CYP2B6 and CYP3A4, which are responsible for the metabolism of artemisinin, can be utilized as a traditional remedy or as modern therapy in combination with artemisinin, respectively, to fight malaria. In an in vitro investigation of the effect of an infusion of A. afra and A. annua on human hepatic CYP2B6 and CYP3A4 enzymes, both plant extracts inhibited the activity of both enzymes [52]. It was also shown that A. annua is a potent inhibitor of CYP3A4 [23]. This could be due to the presence of molecules other than artemisinin within the plant matrix.
Despite it being devoid of artemisinin or having a negligible artemisinin content [13,66]. A. afra has equally demonstrated good anti-plasmodial properties both when it is used in infusions or in crude extracts in vitro, in vivo and in clinical studies (Table 2). A. afra is native to South Africa and has a long history of use as a traditional remedy against coughs, colds, fever, and malaria [15]. Tea infusions of A. afra are used to treat malaria traditionally [66]. Crude extracts of A. afra (ethanol, hexane, and dichloromethane extracts) collected from 5 different countries in Africa demonstrated anti-plasmodial activity against chloroquine-resistant P. falciparum strains [47]. Report on the antimalarial activities of A. afra both in vitro, and in vivo as well as clinical studies has been reviewed by du Toi and van der Kooy [67]. However, there are recent investigations on the anti-plasmodial activities of tea infusions of A. afra in vitro. For example, Tea infusions of A. afra cultivars with traces of artemisinin (19 nM artemisinin) and without detected levels of artemisinin demonstrated activity against the early-stage gametocytes associated with altered morphology of the gametes [41]. This level of activity was shown to be higher in cultivars containing traces of artemisinin [41]. In the same study, activity against the asexual forms of P. falciparum was reported [41]. Furthermore, the A. afra tea infusion demonstrated anti-plasmodial activity against the pre-erythrocytic and erythrocytic stages of P. falciparum in vitro in a dose-dependent manner [9].
Attention has been paid to A. afra and A. annua either in infusions or crude extracts for the investigation of anti-plasmodial properties over the years. However, there are several other Artemisia spp. for which a potential anti-malarial activity has been reported, either in vitro or in vivo. These include Artemisia abyssinica, A. apiacea, A. gorgonum, A. absinthium, A. vulgaris, A. lancea, A. nilagirica, A. abrotanum, A. japonica, A. nilegarica, A. ciniformis, A. bienis, A. turanica, A. indica and A. maciverae. An overview is given in Table 2.
During a malaria attack, xanthin oxidase (XO) is upregulated, leading to the production of reactive oxygen species (ROS), which trigger inflammation [68,69,70]. Ty et al. [71] showed a correlation between elevated plasma-level activities of XO and cytokine production in malaria patients. Pro-inflammatory cytokines together with other cell mediators are responsible for the pathogenesis of inflammatory diseases. Medicinal plants and pure molecules that can inhibit the production of these pro-inflammatory cytokines can be utilized as a traditional remedy or as a modern therapy for the supportive treatment of these diseases. In addition to their anti-malarial properties, A. annua and A. afra have also been reported to exhibit anti-inflammatory properties. For instance, hot water extracts of A. annua prepared in a method similar to traditional ways demonstrated anti-inflammatory properties by modulating pro- and anti-inflammatory cytokines [34,72]. At the molecular level, Abate et al. [73] confirm the anti-inflammatory potential of leaf extracts of A. annua with hydro-ethanol for the inhibition of TNF-α gene expression. Additionally, the anti-inflammatory potential of A. afra has been shown to significantly inhibit the production of nitric oxide (NO) and interleukin 6 (IL-6) in a dose-dependent fashion in several investigations [74].
There are other species of medicinal plants (Figure 2) that belong to the genus Artemisia with reported anti-inflammatory potentials, including Artemisia vulgaris, A. absinthium, A. apiacea, A. dracunculus, A. campestris, A. vestila, A. inculta, A. montana, A. scoporia, A. vulgaris, A. sieversiana, A. frigida, A. copa, A. argyi and A. princeps.


Figure 2.
Field pictures of Artemisia spp. [75] with reported anti-plasmodial properties: (a) A. annua, (b) A. afra, (c) A. absinthium, (d) A. vulgaris, (e) A. indica, (f) A. lancea, (g) A. japonica, (h) A. abrotanum nilagirica, (i) A. bienis, (j) A. nilagirica and (k) A. gorgonum.
3.4. Anti-Malarial Properties, Anti-Inflammatory and Immunomodulating Effects of Secondary Metabolites
Following the isolation of almost 600 and 400 secondary metabolites from A. annua [6] and A. afra [66] respectively, both plant species have served as a reservoir for bioactive molecules against different ailments, including malaria. Some of the isolated metabolites have been shown to have a direct anti-malarial property, while others act by increasing the bioavailability of active components against malaria. Artemisinin is one of the potent molecules with direct anti-malarial activity. However, following the emergence of resistance by the parasite against artemisinin, recent investigations on the biological activity of other secondary metabolites against malaria in the quest for the discovery of new molecules have gained attention in recent decades. The presence of these metabolites could potentialize artemisinin or reduce the risk of developing resistance.
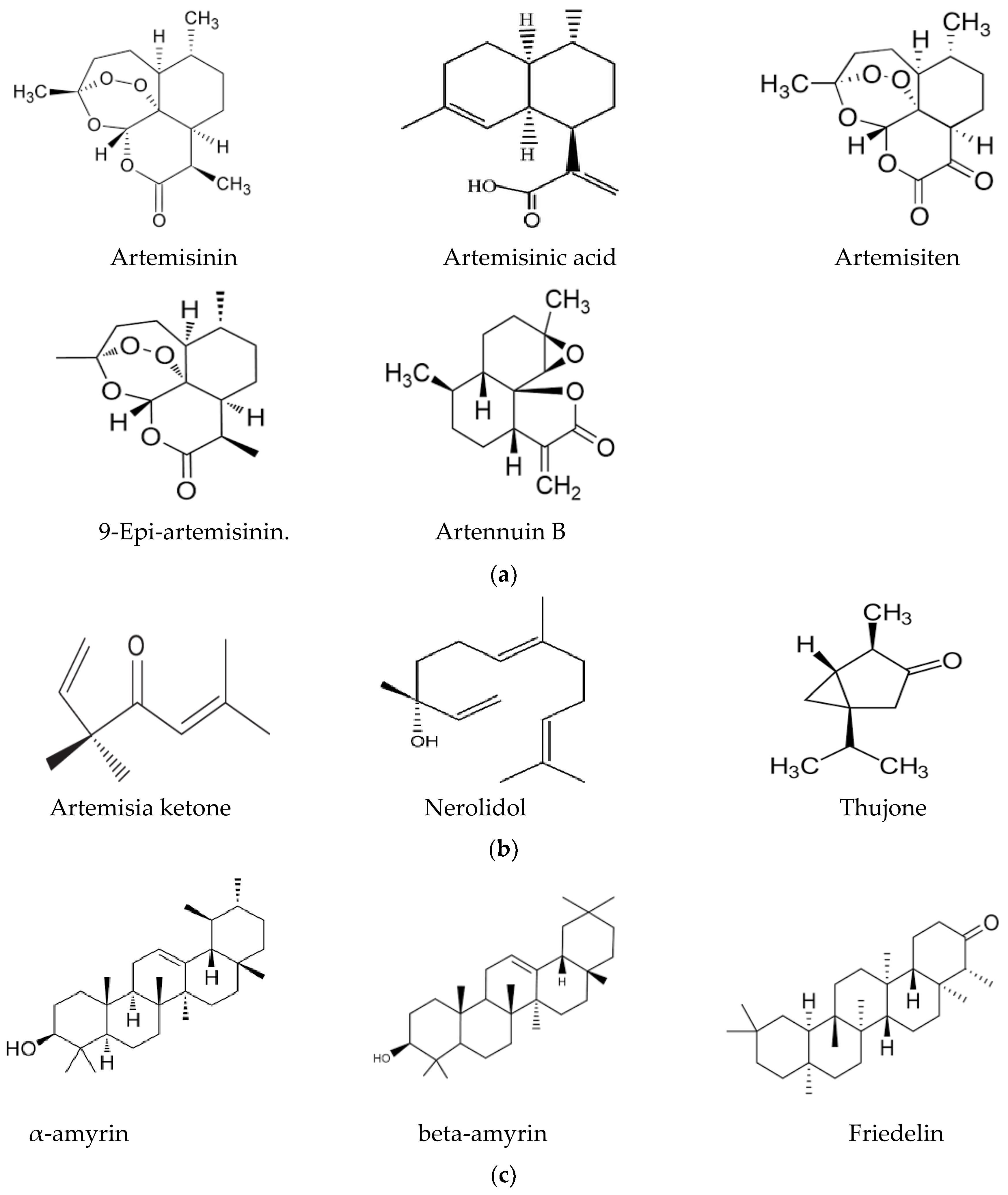
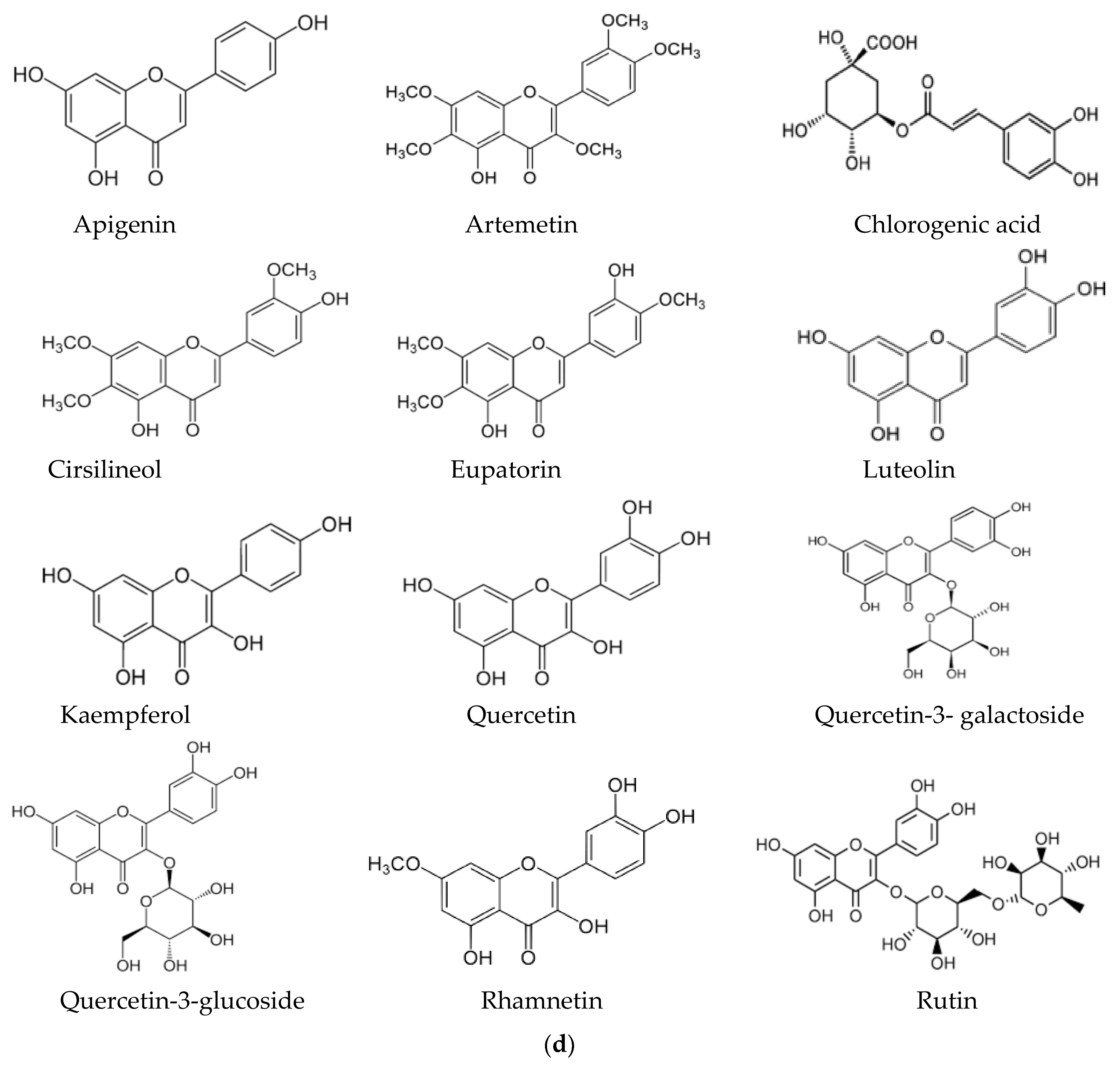
In this context, the presence of flavonoids may be a relevant key factor, broadening the pharmacological properties of Artemisia-based preparations. For instance, in an in vitro investigation of quercetin against malaria parasites, the authors showed that quercetin has moderate, direct anti-plasmodial activity, with an IC50 value of 19.31 ± 1.26 μM, and a high selectivity index [76]. In the same study, anti-malarial properties of quercetin in P. berghei-infected mice demonstrated that it performs chemo-suppressive activity in a dose-dependent manner. Jansen et al. [77], further confirmed the moderate anti-plasmodial properties of quercetin in vitro, with an IC50 value of 9.5 μg/mL (31.4 μM). Additionally, three flavonoids; rutin, rhamnetin and quercetin, alongside derivatives of quercetin, inhibited the growth of laboratory strains and field isolates of P. falciparum in an in vitro investigation [78]. Using an experimental set-up to determine the anti-malarial activity of flavonoids from A. annua, Willcox, et al. [79] also showed that chrysosplenetin, chrysosplenol-D, cirsilineol and artemetin have direct anti-plasmodial activities in vitro (with an IC50 range of 26–65 μmol/L) and that these molecules could also potentiate the effect of artemisinin on P. falciparum. Artennuic acid and artennuin B were also shown to have weak direct anti-malarial activity [80]. In an in vivo study conducted to assess the anti-malarial properties of apigenin, a flavonoid common to both A. afra and A. annua, this compound was shown to significantly suppress the growth of P. berghei in infected mice in a dose-dependent way [81]. In addition, Fallatah and Georges [82] reported that active efflux of glutathione from mature erythrocytes was induced by apigenin through an erythrocytes ATP-binding cassette subfamily C member 1 (ABCC1)-mediated mechanism. This effect may lead to oxidative stress in P. falciparum-infected erythrocytes, and therefore, the inhibition of parasite proliferation, as confirmed in an in vivo study by Amiri et al. [81]. Additionally, kaempferol (a flavonoid) was shown to inhibit the growth of P. berghei in a dose-dependent manner [83]. These researchers could show that kaempferol has a curative, prophylactic and chemo-suppressive effect on malaria in vivo. However, Jansen et al. [77], reported a lack of anti-plasmodial properties of kaempferol in vitro, although the researchers isolated the molecule from medicinal plants other than those of the Asteraceae family. Kaempferol also acts as an inhibitor of glycogen synthase kinase-3β (GSK3β) of the malaria parasite [84]; hence, it blocks erythrocyte invasion by the parasite. Barlianna et al. [85] confirmed the moderate anti-plasmodial properties of a derivative of kaempferol (kaempferol-3-O-rhamnoside) in P. falciparum, with a reported IC50 value of 106 μM. Figure 3 summarizes structures of compounds with direct or indirect anti-malaria properties.
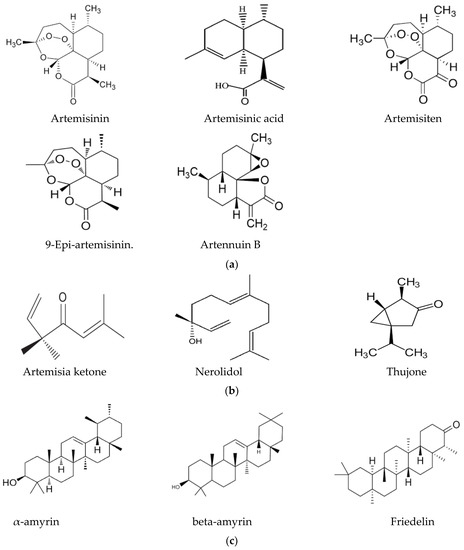
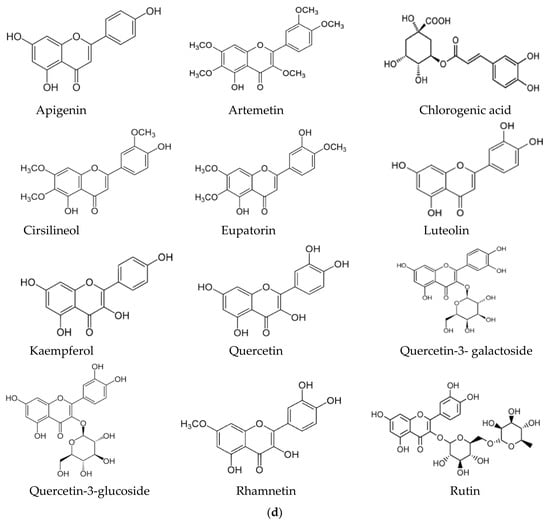
Figure 3.
(a) Sesquiterpenes with direct anti-plasmodial activity. (b) Monoterpenes with direct anti-plasmodial activity. (c) Triterpenes with direct anti-plasmodial activity. (d) Polyphenols with direct anti-plasmodial activity.
While some components show a direct anti-malarial activity, others may act indirectly by synergistically increasing the bioavailability of active compounds. One mechanism by which this may be achieved is through the inhibition of cytochrome P450 enzymes, which are involved in the rapid metabolism of active compounds and have an immunomodulating effect. In an in vivo study carried out by Li et al. [86] to investigate the effect of the interaction between artemisinin, artennuin B, artennuic acid and scopoletin on P. yoelii-infected mice, the researchers showed an increase in the level of exposure of artemisinin, which enhanced the mean plasma concentration and led to improved anti-malarial activity. In their findings, the potency of their combination (four-compound therapy) was almost four times higher compared to that of pure artemisinin. Artennuin B, artennuic acid and scopoletin were believed to have improved the bioavailability of artemisinin [87], which is highly metabolized by CYP450 enzymes, including CYP3A4 [87]. Additionally, artennuin B has been demonstrated to be a potent inhibitor of CYP3A4 [88], and this effect could enhance the mean plasma concentration of artemisinin, leading to improved bioavailability, and therefore, an increase in activity. Thujone, norilodol and artemisia ketone, on the other hand, have been reported to be potent inhibitors of CYP2B6 [88,89].
Several of these compounds with direct or indirect anti-malarial properties have also shown anti-inflammatory properties. In addition to its anti-malarial properties described above, kaempferol also has anti-inflammatory properties [90]. In an investigation carried out by Kim et al. [91] on the anti-inflammatory properties of artemisinin extracted from A. annua using different solvents, artemisinin was also found to have an inhibitory effect on lipopolysaccharide-induced nitric oxide (NO), prostaglandins and proinflammatory cytokine production, such as IL-1β, IL-6 and IL-10. Additionally, artemisinin was found to downregulate pro-inflammatory cytokines, and hence, ameliorate inflammation [92] in an in vivo investigation. Furthermore, artemisinin was found to be an inhibitor of TNF-α expression [89]; hence, this demonstrates its anti-inflammatory potential at the molecular scale. Additionally, artennuin B isolated from A. annua has a strong inhibitory effect on the production of cytokines, such as IL-1β, IL-6 and TNF-α [93]. This could further explain the anti-inflammatory potential of A. annua. Table 3 summarizes compounds isolated from A. annua and A. afra with direct or indirect anti-plasmodial and anti-inflammatory properties.

Table 3.
Secondary metabolites with direct and indirect-antimalaria properties.
Quercetin showed a GSK3β-mediated cytokine-modulating effect by regulating pro- and anti-inflammatory cytokine levels in P. berghei-infected mice [76]. In this investigation, quercetin was also found to significantly reduce the levels of TNF-α and IFN-Y and raise the levels of IL-10 and IL-4 [76]. In a study carried out by Min et al. [98] to determine the anti-inflammatory effect of eupatilin and jasceosidin on carrageenan-induced inflammation in mice, both molecules were found to inhibit the expression and activation of cyclooxygenase (COX)-2 and nuclear factor kappa β, respectively. The same authors further reported a reduction in TNF-α and IL-1 β, as well as prostaglandin E2 (PGE2), levels in mice by eupatilin and jasceosidin. The researchers isolated these molecules from Artemisia princeps; however, these molecules have also been isolated from A. annua and A. afra, and they are also present in other Artemisia species. Qin et al. [26], also reported the anti-inflammatory properties of two newly rearranged heterodimers of cadinene sesquiterpene and one phenylpropanoid unit (Arteanoids B and C).
3.5. Pharmacokinetics and Bioavailability of Secondary Metabolites of A. annua and A. afra
While pharmacokinetics describes the absorption, distribution, metabolism, and elimination of a drug or pharmakon, bioavailability, on the other hand, describes the fraction of the absorbed drug that reaches the systemic circulation, and it varies between compounds, both of which influence the observed therapeutic or biological responses [101]. The pharmacokinetics and bioavailability of artemisinin and derivatives have been more extensively studied either in vitro, in vivo or in clinical studies than other metabolites in this plant species have. This is probably due to its excellent anti-malarial properties, and it is also a drug that is used in therapy. Several pharmacokinetic studies have been conducted on artemisinin administered as a single compound or in a plant matrix, such as in whole-plant powder or as an infusion, both of which may influence the pharmacokinetics of artemisinin. For example, in a pharmacokinetic study of artemisinin following the oral administration of a traditional preparation of A. annua, it was found that artemisinin is absorbed faster from herbal tea preparations than it is from capsules (from 30 min to 2.3 h) [102]. However, both formulations had a similar bioavailability. Desrosiers et al. [103] reported increases in the serum levels of artemisinin when it is administered as a whole plant as compared to that when the capsule of artemisinin is administered, indicating that the plant matrix has a positive impact on the bioavailability of artemisinin. In a multi-component assessment of the pharmacokinetics parameters of A. annua in rats, Fu et al. [104] showed that scopoline, scopoletin, rutin, chrysosplenol D, casticin and three sesquiterpenes (arteannuin B, dihydroartemisinic acid and artemisinic acid) were detected in rat’s plasma post-oral administration. In the same study, the authors concluded that chrysosplenol D and casticin were rapidly absorbed with shorter half-lives (t1/2, 2.68 ± 3.62 h and 0.33 ± 0.07 h, respectively) as compared to the speed of the absorption of scopoletin, which has a longer half-life (t1/2, 6.53 ± 1.84 h). The long half-life of scopoletin in vivo could explain its effect on the modulation of cytochrome P450 enzymes and the modulation of pro- and anti-inflammatory cytokines. In the same study, scopoletin, casticin, artemisinin and chrysosplenol D were metabolized by phase II enzymes. Following the intravenous administration of eupatilin in mice, it was found that eupatilin was poorly adsorbed due to its low systemic exposure [105]. Eupatilin was rapidly metabolized by phase II enzyme into eupatilin-7-glucuronide (E-7-G), and both molecules were distributed in the intestine, liver, and kidneys. It was confirmed by the authors that eupatilin has a shorter half-life (t1/2, 0.29 h) as compared to that of its metabolite, E-7-G, which has a longer half-life (t1/2, 4.15 h) and might be responsible for the potency of eupatilin in vivo. Poor absorption and a high biliary elimination rate limit the bioavailability of quercetin. However, the absorption of quercetin depends on the chemical structure of the molecule. Quercetin aglycon is mainly absorbed in the small intestine and stomach [106], while quercetin rutinoside is absorbed in the colon [92]. Erlund et al. [107] also confirmed the absorption of quercetin aglycon in the gastrointestinal tract in clinical studies. The results from an in vitro incubation of human small microsomes with quercetin aglycon show that it is highly metabolized by phase II enzymes in the small intestine [108]. Apigenin, a natural flavonoid common in the Asteraceae family and found in other plant species, has a poor systemic availability due to its low solubility in water and lipids. After a single oral administration in rats, apigenin was found widely distributed throughout the body (liver, kidneys, RBCs, urine, and intestine) within 10 days post-administration, and it was excreted mainly in urine [109]. Apigenin is extensively metabolized by phase I and phase II enzymes, making its bioavailability very low [109,110]. The results of the oral and intravenous administration (400 mg/kg and 50 mg/kg, respectively) of casticin in rats showed rapid distribution and elimination with a longer half-life (t1/2, 36.48 min ± 7.24) for the oral administration as compared to those of intravenous administration (t1/2, 20.86 min ± 2.02) [111]. The absolute bioavailability (45.5 ± 11.0%) was high, which is an important factor for its clinical application [111]. Barve et al. [112] reported a low bioavailability (approximately 2%) of kaempferol due to its extensive first pass-effect metabolism in rats after oral administration. In the same study, the plasma concentration of kaempferol indicated a rapid absorption (tmax,1–2 h) with a half-life of 3–4 h and with a large distribution (8–12 L/h/kg) and a high rate of clearance (approximately 3 L/h/kg). Zabela et al. [113] also reported the rapid clearance (4.40–6.44 L/h/kg) and extremely short half-life (t1/2, 2.93–3.79 min) of kaempferol after intravenous administration in rats. In a study carried out to determine the effect of plant matrix on the uptake of luteolin from aqueous solutions of A. afra, luteolin aglycone and luteolin-7-0-glucoside were more rapidly absorbed than when they were administered as pure solutions in Caco-2 cells after 1 h exposure. These results suggest that plant matrix may have a positive effect on the bioavailability of the flavonoid and hence greater in vivo potency [114].
3.6. Toxicity of Crude Extracts of A. annua and A. afra and Their Secondary Metabolites
Pre-clinical toxicity is often required for new substances and new medicinal products, which contain herbs with no traditional history of use. According to the WHO, traditional use refers to documentary evidence that a substance has been used for a specific health-related or medicinal purpose over three or more generations. The guidelines of the WHO state that if a herbal product has a long history of traditional use without signs of toxicity (harmful effects), it should not be restricted, and they maintains it stand that no pre-clinical toxicity investigation is required [115]. Artemisia annua and Artemisia afra have a long history of use as a traditional remedy in Southeast Asia and South Africa, respectively, for the alleviation of fevers related to malaria, amongst other diseases, for generations. However, several toxicity studies (cytotoxicity, acute and chronic toxicity) have been conducted to determine the safety of A. annua and A. afra, including a couple of their secondary metabolites. For example, Motshudi et al. [116] showed that crude extracts of A. afra prepared using chloroform, ethanol and water were relatively non-toxic to vero cells, with LC50 values > 400 g/mL, as compared to that of the positive control (doxorubicin), which was cytotoxic, with an LC50 value of 57.83 ± 3.02 mg/mL. Loggenberg et al. [117] also confirmed the non-cytotoxic effect of aqueous extract of A. afra, with IC50 > 350 μg/mL. In the same study, A. annua was confirmed to be non-cytotoxic, with IC50 > 550 μg/mL, to vero cells. In addition to in vitro toxicity assays used to assess the safety profiles of A. annua and A. afra, several in vivo studies (acute and chronic studies) have been conducted to confirm the claims observed in, in vitro studies. For instance, in an investigation carried out by Mekenon et al. [118] to evaluate the toxicity of aqueous extracts of A. afra leaves on brain, heart and suprarenal glands in Swiss albino mice via an acute and sub-acute test, the researchers showed that leaves extract of A. afra is relatively safe for mice, with an LD50 > 5000 mg/kg body weight. In the sub-acute study, the microscopic examination of brain, heart and suprarenal glands showed no signs of toxicity in all the treatment groups. Kane et al. [119] also confirmed the safety of A. afra leave extracts in vivo by determining its pharmaco-toxicological effects after acute oral administration in mice at doses of 1000, 2000 and 2500 mg/kg as per body weight. An LD50 > 2500 mg/kg body weight, no signs of toxicity and no effect on the levels of alanine transaminase (ALT) and aspartate transaminase (AST) were reported and compared to those of the control in the study. Additionally, following the oral administration of hydro-ethanolic plant extract of A. annua by Swiss mice, the results showed no lethality or toxic reactions, with LD50 > 5000 mg/kg body weight [120]. The absence of toxicity symptoms suggests that A. annua is non-toxic and is well tolerated [120]. According to the classification of Loomis and Haye, substances with LD50 values between 500 and 5000 and between 5000 and 15,000 mg/kg body weight are regarded as being slightly toxic and practically non-toxic, respectively. The safety profiles of A. afra was further confirmed by Mukinda et al. [121] by using an aqueous extract of A. afra in acute and chronic tests on mice and rats, respectively. The pharmaco-toxicological effects of the extract were determined after intraperitoneal and oral administration. The researchers reported LD50 values of 2450 and 8960 mg/kg body weight for intraperitoneal and oral administration, respectively. Both methods of administration were safe as the LD50 values fell within the cut-off mark, following the guidelines of Loomis and Haye [122]. After a chronic investigation of rats administered oral doses of A. afra extract (0.1 or 1 g/kg/day), the rats survived 3 months of dosing, and LD50 > 1000 mg/kg body weight was reported. There were no significant changes in their general behavior and hematological and biochemical parameters experienced, except for a transient decrease in AST activity level. No significant changes were observed in organ weights, and the histopathological results showed a normal profile, suggesting no morphological alterations. The above findings clearly support the use of A. annua and A. afra as a traditional remedy in the fight against infections.
Several compounds isolated from A. annua and A. afra have demonstrated good biological activities against different ailments, including malaria, with several toxicity studies having been conducted to determine their safety profiles. For example, in an investigation to determine the cytotoxic effect of dietary flavones on normal trout liver cell lines, the authors of [123] showed that while methylated flavones (5,7-Dimethoxyflavone and 3′,4′-dime-thoxyflavone at concentration 25 μM) are non-cytotoxic even at higher concentrations after 24 h of exposure, the hydroxylated flavones (Chrysin, quercetin, apigenin and luteolin), especially chrysin, were cytotoxic, with a reported IC50 value of 2 μM. However, in a sub-chronic toxicity study carried out by Yao et al. [124], following the daily oral administration of chrysin (1000 mg/kg), the compound significantly decrease body weight, liver weight was increased, there were significant alteration in the hematology, and an LD50 value of 4350 mg/kg was reported. The results from a sub-chronic toxicity study of quercetin examined in male and female CD2F1 mice administered at 62, 125 and 250 mg/kg indicated that quercetin is safe to CD2F1 mice, as they showed no signs of toxicity, and no changes in hematological and histopathological parameters were observed [125]. Lucida et al. [126] also confirmed the safety of quercetin in an acute test, with an LD50 value > 1600 mg/kg. Several studies have been conducted to investigate the toxicity of volatile components of A. annua and A. afra. For instance, in an investigation to evaluate the toxic effects of α-terpinene (a monoterpene common to both species) in the liver tissue of rats intraperitoneally administered at doses of 0.5, 0.75 and 1.0 mL/kg for 10 days, Baldissera et al. [127] showed that α-terpinene induces oxidative stress and has cytotoxic and genotoxic effects on the liver tissue, involving caspase activation. This was due to increased levels of reactive oxygen species, alanine aminotransferase, aspartate aminotransferase increased and glutathione S-transferase. There are several reviews that have detailed the toxicity of volatile components of both plants [89,128,129].
3.7. Resistance to ACTs and Broad-Spectrum Pharmacology of Artemisia-Based Samples
With the persistence of malaria as a devastating disease with high burden and mortality rates, the WHO recommends “Artemisinin Combination Therapy (ACTs)” as the first line of treatment (artemether + lumefantrine, artesunate + amodiaquine, artesunate + mefloquine, dihydroartemisinin + piperaquine or artesunate + sulfadoxine–pyrimethamine) [1]. However, there have been reported cases of resistance to ACTs, leading to treatment failures. Artemisinin resistance was documented in 2008 on the Thailand–Cambodia border after artesunate monotherapy [130]. However, retrospective analysis indicated that artemisinin resistance likely emerged in 2001, before the widespread deployment of ACTs in Cambodia [131]. The mechanism of resistance to artemisinin drugs is associated with point mutations in the Kelch 13 (K13) propeller gene. In Cameroon and other African countries (Central African Republic, Chad, Kenya, Madagascar, Malawi, Mali, Rwanda, etc.), non-synonymous K13 mutations have been reported, and the most frequent allele observed in Africa is A578S [132]. Further studies revealed that four mutations (Y493H, R539T, I543T and C580Y) of the K13-propeller gene in P. falciparum are associated with artemisinin resistance [132].
The emergence of resistance to ACTs has prompted the search for new strategies to fight against the disease. The optimal therapy for the management of the disease would consist of one that will target both gametocidal and clinical forms of the parasite. This will enable them to break the transmission cycle, and hence, it’s possible eradication. The isolation of gametocidal compounds from A. afra [14] and in vitro and in vivo investigations of the anti-plasmodial activity against the erythrocytic forms of malaria parasite [41,64], [133,134] as well as clinical results from trial studies, indicate that A. afra may be an important contributor in the fight against malaria. The presence of the plant matrix, as reported, may play an important part, as it improves the pharmacokinetics of molecules with direct anti-plasmodial properties and those with indirect anti-plasmodial properties [114]. This could be explained by the fact that other molecules present in the plant may limit the rate of the metabolism of compounds with direct anti-plasmodial properties by inhibiting the action of cytochrome P450 enzymes responsible for the metabolism of these compounds. This further demonstrates the potential of A. afra in the fight against malaria. Additionally, new drug combinations with molecules that could enhance the bioavailability of artemisinin, together with compounds that have direct anti-malarial properties, including those showing gametocidal effects, could function best in eliminating the disease. As an example, a drug combination or a standardized plant extract containing artemisinin (a potent anti-malarial molecule), artennuin B (a CYP3A4 inhibitor, but also showing a direct anti-malarial activity), quercetin (with direct anti-malarial activity and an inhibitor of thioredoxin reductase and anti-inflammatory), yomogiartemin (a gametocidal molecule) and thujone (has an direct anti-malarial effect and a CYP2A6 and CYP2B6 inhibitor) could be of potential interest in treating malaria.
4. Conclusions
Based on in vitro and/or in vivo studies and limited clinical trials, there are more than twenty different species of Artemisia with the anti-malarial/anti-inflammatory properties investigated. Research over the years in the search for new molecules showing anti-malarial activities has been focused on erythrocytic forms of the malaria parasite, with limited work having been conducted on the pre-erythrocytic and gametocidal forms.
In addition to artemisinin, several secondary metabolites present in different Artemisia spp. show direct anti-malarial activity. Other metabolites act indirectly by increasing the bioavailability of active components or by affecting other metabolic pathways that are important in sustaining the patient when they are fighting a malaria infection. A few of the isolated secondary metabolites with anti-plasmodial properties and immunomodulating effects, as well as anti-inflammatory properties, have been studied for their pharmacokinetics and pharmacodynamics properties in humans and their toxicological profiles. It would, therefore, be interesting to broaden the knowledge of Artemisia-based preparations by investigating their total pharmacological effectiveness in clinical trials in relation to their chemical composition. The focus should, therefore, be put on investigating the presence of molecules in addition to artemisinin, resulting in a broad pharmacological activity and effectiveness during different stages of the malaria parasite, especially the gametocidal forms. This would enable the reduction of the link between human and insect vectors, and hence, reduce malaria transmission.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13050613/s1. Table S1: Monoterpenes isolated from A. annua and A. afra. Table S2: Sesquiterpenes from A. annua and A. afra. Table S3: Polyphenols from A. annua and A. afra. Table S4: Triterpenes from A. annua and A. afra. Table S5: Coumarins from A. annua and A. afra. Table S6: Diterpenes from A. annua and A. afra. Table S7: Guaianolides from A. annua and A. afra. Table S8: Simple Aryl Ketones from A. annua and A. afra. Table S9: Glaucolides from A. annua and A. afra. Table S10: Long chain alkanes from A. annua and A. afra. Table S11: Other compounds from A. annua and A. afra. References cited in supplementary materials are [135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197].
Author Contributions
Conceptualization, M.F., K.D. and L.M.S.; methodology and data curation, M.F., K.D. and L.M.S.; original draft preparation, L.M.S.; review and editing, O.J., G.E.L., L.M., A.R., A.L., S.F.N. and S.N.A.; grant seeking and management, S.M.G. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funds from Académie de Recherche et d’Enseignement Supérieur (ARES-CCD), Rue Royale 180,1000 Bruxelles, Belgium. Grant number: PRD-PFS 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. World Malaria Report 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 31 October 2022).
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2010, 49, 101–109. [Google Scholar] [CrossRef]
- Milani, B.; Scholten, W. The World Medicines Situation 2011: Access to Controlled Medicines, 3rd ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Jeremić, D.; Jokić, A.; Behbud, A.; Stefanović, M. A new type of sesqiterpene lactone isolated from Artemisia annua L. arteannuin B. Tetrahedron Lett. 1973, 14, 3039–3042. [Google Scholar] [CrossRef]
- Brown, G.D. The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef]
- Klayman, D.L. Qinghaosu (Artemisinin): An Antimalarial Drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crop. Prod. 2011, 37, 479–485. [Google Scholar] [CrossRef]
- Mueller, M.; Karhagomba, I.; Hirt, H.; Wemakor, E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: Agricultural, chemical and clinical aspects. J. Ethnopharmacol. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crop. Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Price, R.; Nosten, F.; Luxemburger, C.; ter Kuile, F.; Paiphun, L.; Chongsuphajaisiddhi, T.; White, N. Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996, 347, 1654–1658. [Google Scholar] [CrossRef]
- Weathers, P.J.; Towler, M.; Hassanali, A.; Lutgen, P.; Engeu, P.O. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries? World J. Pharmacol. 2014, 3, 39–55. [Google Scholar] [CrossRef]
- Karl, S.; Gurarie, D.; Zimmerman, P.A.; King, C.H.; Pierre, T.G.S.; Davis, T.M.E. A Sub-Microscopic Gametocyte Reservoir Can Sustain Malaria Transmission. PLoS ONE 2011, 6, e20805. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Parada, M.; Carrió, E.; Bonet, M.; Vallès, J. Ethnobotany of the Alt Empordà region (Catalonia, Iberian Peninsula): Plants used in human traditional medicine. J. Ethnopharmacol. 2009, 124, 609–618. [Google Scholar] [CrossRef]
- Rajendra, S.B.; Dharam, C.J.; Ram, P.S. Phytochemistry of Artemisia annua and The Development of Artemisinin-Derived Antimalarial Agents. In Artemisia; CRC Press: Boca Raton, FL, USA, 2001; pp. 184–213. [Google Scholar] [CrossRef]
- Pharmacopoeia. Chinese Pharmacopoeia Commission. In Pharmacopoeia of the People’s Republic of China 2010; China Medical Science and Technology Press: Beijing, China, 2010; Available online: https://www.scirp.org (accessed on 31 October 2022).
- Ma, J.; Clemants, S. A history and overview of the Flora Reipublicae Popularis Sinicae (FRPS, Flora of China, Chinese edition, 1959–2004). Taxon 2006, 55, 451–460. [Google Scholar] [CrossRef]
- Ferreira, J.; Janick, J. Production and Detection of Artemisinin from Artemisia annua. Acta Hortic. 1995, 390, 41–50. [Google Scholar] [CrossRef]
- Discover Life. Annual Mugwort. Available online: https://www.discoverlife.org/20/q?search=Artemi (accessed on 10 April 2023).
- Roberts, M. Indigenous Healing Plants by Roberts, Margaret: Very Good Hardcover, 1st ed.; Southern Book Publishers: Capetown, South Africa, 1990; Available online: https://www.abebooks.com/first-edition/Indigenous-Healing-Plants-Roberts-Margaret-Southern/22732613283/bd (accessed on 31 October 2022).
- Czechowski, T.; Larson, T.R.; Catania, T.M.; Harvey, D.; Wei, C.; Essome, M.; Brown, G.D.; Graham, I.A. Detailed Phytochemical Analysis of High- and Low Artemisinin-Producing Chemotypes of Artemisia annua. Front. Plant Sci. 2018, 9, 641. [Google Scholar] [CrossRef]
- Sotenjwa, V.Z.; Chen, W.; Veale, C.G.; Anokwuru, C.P.; Tankeu, S.Y.; Combrinck, S.; Kamatou, G.P.; Viljoen, A.M. Chemotypic variation of non-volatile constituents of Artemisia afra (African wormwood) from South Africa. Fitoterapia 2020, 147, 104740. [Google Scholar] [CrossRef]
- Qin, D.-P.; Li, T.; Shao, J.-R.; He, X.-Q.; Shi, D.-F.; Wang, Z.-Z.; Xiao, W.; Yao, X.-S.; Li, H.-B.; Yu, Y. Arteannoides U–Z: Six undescribed sesquiterpenoids with anti-inflammatory activities from the aerial parts of Artemisia annua (Qinghao). Fitoterapia 2021, 154, 105002. [Google Scholar] [CrossRef]
- Qin, D.-P.; Pan, D.-B.; Xiao, W.; Li, H.-B.; Yang, B.; Yao, X.-J.; Dai, Y.; Yu, Y.; Yao, X.-S. Dimeric Cadinane Sesquiterpenoid Derivatives from Artemisia annua. Org. Lett. 2018, 20, 453–456. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, G.M.; Li, D.Y.; Li, Z.L. A new coumarin glycoside isolated from Artemisia annua. Chin. Tradit. Herb. Drugs 2018, 49, 2953–2958. [Google Scholar] [CrossRef]
- Takenaka, Y.; Seki, S.; Nishi, T.; Tanahashi, T. Two new sesquiterpenes from Artemisia annua L. J. Nat. Med. 2020, 74, 811–818. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-R.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Goel, D.; Singh, V.; Ali, M.; Mir, S.R.; Ali, C.M.; Sultana, S. New Phytoconstituents from the shoots of Artemisia annua L. Cultivar. J. Med. Plants Stud. 2017, 5, 136–139. [Google Scholar]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Mouton, J.; van de Kooy, F. View of Identification of cis- and trans- Melilotoside within an Artemisia annua Tea Infusion. Eur. J. Med. Plants 2013, 4, 52–63. Available online: https://journalejmp.com/index.php/EJMP/article/view/585/1173 (accessed on 31 October 2022). [CrossRef]
- Hu, J.; Li, K.-M.; Dong, X.; Ma, Y.-N.; Wu, Z.-H.; Yan, Y.-M.; Cheng, Y.-X. Terpenoids and flavonoids from Artemisia species. Planta Med. 2000, 66, 391–393. [Google Scholar]
- de Magalhães, P.M.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef]
- Youyou, T.; Muyun, N.; Yurong, Z.; Lanna, L.; Shulian, G.; Muqun, Z.; Xiuzhen, W.; Xiaotian, L. Studies on the constit-uents of Artemisia annua L. Pharm. Sin. 2015, 50, 366–370. (In Chinese) [Google Scholar]
- Gakuba, B.E. Isolation and Characterisation of Secondary Metabolites of Two Asteraceae Species, Artemisia afra and Elytropappus Rhinocerotis. Available online: https://researchspace.ukzn.ac.za/handle/10413/639 (accessed on 8 November 2022).
- Braünlich, P.M.; Inngjerdingen, K.T.; Inngjerdingen, M.; Johnson, Q.; Paulsen, B.S.; Mabusela, W. Polysaccharides from the South African medicinal plant Artemisia afra: Structure and activity studies. Fitoterapia 2018, 124, 182–187. [Google Scholar] [CrossRef]
- Han, X.; Chai, Y.; Lv, C.; Chen, Q.; Liu, J.; Wang, Y.; Chou, G. Sesquiterpenes from Artemisia annua and Their Cytotoxic Activities. Molecules 2022, 27, 5079. [Google Scholar] [CrossRef]
- Qin, D.-P.; Li, H.-B.; Pang, Q.-Q.; Huang, Y.-X.; Pan, D.-B.; Su, Z.-Z.; Yao, X.-J.; Yao, X.-S.; Xiao, W.; Yu, Y. Structurally diverse sesquiterpenoids from the aerial parts of Artemisia annua (Qinghao) and their striking systemically anti-inflammatory activities. Bioorganic Chem. 2020, 103, 104221. [Google Scholar] [CrossRef]
- Goel, D.; Singh, V.; Ali, M.; Mir, S.R.; Ali, C.M.; Sultana, S.L. Composition of the essential oil from the root of Artemisia annua. J. Nat. Med. 2007, 61, 458–461. [Google Scholar] [CrossRef]
- Snider, D.; Weathers, P.J. In vitro reduction of Plasmodium falciparum gametocytes: Artemisia spp. tea infusions vs. artemisinin. J. Ethnopharmacol. 2020, 268, 113638. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, S.; van der Kooy, F.; Verpoorte, R. Artemisia annua as a self-reliant treatment for malaria in developing countries. J. Ethnopharmacol. 2008, 120, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, B.M.; Weathers, P.J. In vitro analyses of Artemisia extracts on Plasmodium falciparum suggest a complex antimalarial effect. PLoS ONE 2021, 16, e0240874. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.K.; Verma, G.; Agarwal, D.D.; Kaiser, M.; Brun, R. Antiprotozoal activities of traditional medicinal plants from the Garhwal region of Northwest Himalaya, India. J. Ethnopharmacol. 2011, 136, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Seddek, A.-L.S.; Abdelmageed, N.; Badry, M.O.; Nishikawa, Y. Wild Egyptian medicinal plants show in vitro and in vivo cytotoxicity and antimalarial activities. BMC Complement. Med. Ther. 2022, 22, 130. [Google Scholar] [CrossRef]
- Tasdemir, D.; Tierney, M.; Sen, R.; Bergonzi, M.C.; Demirci, B.; Bilia, A.R.; Baser, K.H.C.; Brun, R.; Chatterjee, M. Antiprotozoal Effect of Artemisia indica Extracts and Essential Oil. Planta Med. 2015, 81, 1029–1037. [Google Scholar] [CrossRef]
- Kane, N.; Kyama, M.; Nganga, J.; Hassanali, A.; Diallo, M.; Kimani, F. Comparison of phytochemical profiles and antimalarial activities of Artemisia afra plant collected from five countries in Africa. South Afr. J. Bot. 2019, 125, 126–133. [Google Scholar] [CrossRef]
- Ogbole, E.A.; Ogbole, Y.; Peter, J.Y.; Builders, M.I.; Aguiyi, J.C. Phytochemical Screening and In vivo Antiplasmodial Sensitivity Study of Locally Cultivated Artemisia annua Leaf Extract Against Plasmodium berghei. Am. J. Ethnomedicine 2014, 1. [Google Scholar]
- Mojarrab, M.; Shiravand, A.; Delazar, A.; Afshar, F.H. Evaluation of In Vitro Antimalarial Activity of Different Extracts of Artemisia aucheri Boiss. and A. armeniaca Lam. and Fractions of the Most Potent Extracts. Sci. World J. 2014, 2014, 825370. [Google Scholar] [CrossRef]
- Sankhuan, D.; Niramolyanun, G.; Kangwanrangsan, N.; Nakano, M.; Supaibulwatana, K. Variation in terpenoids in leaves of Artemisia annua grown under different LED spectra resulting in diverse antimalarial activities against Plasmodium falciparum. BMC Plant Biol. 2022, 22, 128. [Google Scholar] [CrossRef]
- Afshar, F.H.; Delazar, A.; Janneh, O.; Nazemiyeh, H.; Pasdaran, A.; Nahar, L.; Sarker, S.D. Evaluation of antimalarial, free-radical-scavenging and insecticidal activities of Artemisia scoparia and A. Spicigera, Asteraceae. Rev. Bras. Farm. 2011, 21, 986–990. [Google Scholar] [CrossRef]
- Kane, N.F.; Kiani, B.H.; Desrosiers, M.R.; Towler, M.J.; and Weathers, P.J. Artemisia extracts differ from artemisinin effects on human hepatic CYP450s 2B6 and 3A4 in vitro. J. Ethnopharmacol. 2022, 298, 115587. [Google Scholar] [CrossRef]
- Oma, G.P.E.; Mouhamadou, D.; Bineta, D.A.; Sadikh, B.A.; Mare, D.D.; Ambroise, A.; Ndiay, A.A.; Thérése, D.; Pierre, L.; Souleymane, M.; et al. Tea Artemisia annua inhibits Plasmodium falciparum isolates collected in Pikine, Senegal. Afr. J. Biochem. Res. 2013, 7, 107–113. [Google Scholar] [CrossRef]
- De Donno, A.; Grassi, T.; Idolo, A.; Guido, M.; Papadia, P.; Caccioppola, A.; Villanova, L.; Merendino, A.; Bagordo, F.; Fanizzi, F.P. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 696–700. [Google Scholar] [CrossRef]
- Weathers, P.J.; Elfawal, M.A.; Towler, M.J.; Acquaah-Mensah, G.; Rich, S.M. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J. Ethnopharmacol. 2014, 153, 732–736. [Google Scholar] [CrossRef]
- Ortet, R.; Thomas, O.P.; Regalado, E.L.; Pino, J.A.; Filippi, J.-J.; Fernández, M.D. Composition and Biological Properties of the Volatile Oil of Artemisia gorgonum Webb. Chem. Biodivers. 2010, 7, 1325–1332. [Google Scholar] [CrossRef]
- Mojarrab, M.; Emami, S.A.; Gheibi, S.; Taleb, A.M.; Afshar, F.H. Evaluation of Anti-Malarial Activity of Artemisia Turcomanica and A. Kopetdaghensis by Cell-Free β-Hematin formation Assay. Res. J. Pharmacogn. 2016, 3, 59–65. [Google Scholar]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M.; Larijani, K. A new antimalarial agent; effects of extracts of Artemisia diffusa against Plasmodium berghei. Planta Med. 2007, 73, 219. [Google Scholar] [CrossRef]
- Panda, S.; Rout, J.R.; Pati, P.; Ranjit, M.; Sahoo, S.L. Antimalarial activity of Artemisia nilagirica against Plasmodium falciparum. J. Parasit. Dis. 2017, 42, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Mojarrab, M.; Naderi, R.; Afshar, F.H. Screening of Different Extracts from Artemisia Species for Their Potential Antimalarial Activity. Iran. J. Pharm. Res. 2015, 14, 603–608. [Google Scholar]
- Adugna, M.; Feyera, T.; Taddese, W.; Admasu, P. In vivo Antimalarial Activity of Crude Extract of Aerial Part of Artemisia abyssinica Against Plasmodium berghei in Mice. Glob. J. Pharmacol. 2014, 8, 460–468. [Google Scholar] [CrossRef]
- Bamunuarachchi, G.S.; Ratnasooriya, W.D.; Premakumara, S.; Udagama, P.V. Antimalarial properties of Artemisia vulgaris L. ethanolic leaf extract in a Plasmodium berghei murine malaria model. J. Vector Borne Dis. 2013, 50, 278–284. [Google Scholar] [PubMed]
- Udagama, P.; Ratnasooriya, W.; Kodippili, K.; Premakumara, S. An investigation of the antimalarial activity of Artemisia vulgaris leaf extract in a rodent malaria model. Int. J. Green Pharm. 2011, 5, 276. [Google Scholar] [CrossRef]
- Mueller, M.S.; Runyambo, N.; Wagner, I.; Borrmann, S.; Dietz, K.; Heide, L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 318–321. [Google Scholar] [CrossRef]
- Blanke, C.H.; Naisabha, G.B.; Balema, M.B.; Mbaruku, G.M.; Heide, L.; Müller, M.S. Herba Artemisiae annuae tea preparation compared to sulfadoxine-pyrimethamine in the treatment of uncomplicated falciparum malaria in adults: A randomized double-blind clinical trial. Trop. Dr. 2008, 38, 113–116. [Google Scholar] [CrossRef]
- Liu, N.; Van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? South Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef]
- du Toit, A.; van der Kooy, F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnopharmacol. 2019, 244, 112127. [Google Scholar] [CrossRef]
- Narsaria, N.; Mohanty, C.; Das, B.K.; Mishra, S.P.; Prasad, R. Oxidative Stress in Children with Severe Malaria. J. Trop. Pediatr. 2011, 58, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Iwalokun, B.A.; Bamiro, S.B.; Ogunledun, A. Levels and interactions of plasma xanthine oxidase, catalase and liver function parameters in Nigerian children with Plasmodium falciparum infection. Apmis 2006, 114, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Helft, J.; Claser, C.; Deroubaix, S.; Karanje, H.; Gazumyan, A.; Darrasse-Jeze, G.; Telerman, S.B.; Breton, G.; Schreiber, H.A.; et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat. Med. 2013, 19, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Ty, M.C.; Zuniga, M.; Götz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 2019, 11, e9903. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, Y.; Wang, L.; Ao, C. Ethanol Extract of Artemisia annua Prevents LPS-Induced Inflammation and Blood–Milk Barrier Disruption in Bovine Mammary Epithelial Cells. Animals 2022, 12, 1228. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Muleya, E.; Ahmed, A.S.; Sipamla, A.; Mtunzi, F.; Mutatu, W. Evaluation of Anti-Microbial, Anti-Inflammatory and Anti-Oxidative Properties Artemisia afra, Gunnera perpensa and Eucomis autumnalis. J. Nutr. Food Sci. 2014, 4, 6. [Google Scholar] [CrossRef]
- Stratosphere. 11 Different Types of Artemisia Flowers. Available online: https://www.homestratosphere.com/types-of-artemisia-flowers/ (accessed on 31 October 2022).
- Ali, A.H.; Sudi, S.; Shi-Jing, N.; Hassan, W.R.M.; Basir, R.; Agustar, H.K.; Embi, N.; Sidek, H.M.; Latip, J. Dual Anti-Malarial and GSK3β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals 2021, 14, 248. [Google Scholar] [CrossRef]
- Jansen, O.; Tchinda, A.T.; Loua, J.; Esters, V.; Cieckiewicz, E.; Ledoux, A.; Toukam, P.D.; Angenot, L.; Tits, M.; Balde, A.M.; et al. Antiplasmodial activity of Mezoneuron benthamianum leaves and identification of its active constituents. J. Ethnopharmacol. 2017, 203, 20–26. [Google Scholar] [CrossRef]
- Ganesh, D.; Fuehrer, H.-P.; Starzengrüber, P.; Swoboda, P.; Khan, W.A.; Reismann, J.A.B.; Mueller, M.S.K.; Chiba, P.; Noedl, H. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: Evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol. Res. 2012, 110, 2289–2295. [Google Scholar] [CrossRef]
- Willcox, M. Artemisia Species: From Traditional Medicines to Modern Antimalarials—And Back Again. J. Altern. Complement. Med. 2009, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Gong, F.Y.-H.; Chang, T.-L. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J. Ethnopharmacol. 2008, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Nourian, A.; Khoshkam, M.; Ramazani, A. Apigenin inhibits growth of the Plasmodium berghei and disrupts some metabolic pathways in mice. Phytother. Res. 2018, 32, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, O.; Georges, E. Apigenin-induced ABCC1-mediated efflux of glutathione from mature erythrocytes inhibits the proliferation of Plasmodium falciparum. Int. J. Antimicrob. Agents 2017, 50, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Somsak, V.; Damkaew, A.; Onrak, P. Antimalarial Activity of Kaempferol and Its Combination with Chloroquine in Plasmodium berghei Infection in Mice. J. Pathog. 2018, 2018, 3912090. [Google Scholar] [CrossRef]
- Sok, K.W.; Jann, M.L.S.; Sudi, S.; Hassan, W.R.M.; Lee, P.C.; Embi, N.; Sidek, H.M.; Wong, S.K. Anti-malarial and Anti-inflammatory Effects of Gynura procumbens are Mediated by Kaempferol via Inhibition of Glycogen Synthase Kinase-3β (GSK3β). Sains Malays. 2015, 44, 1489–1500. [Google Scholar] [CrossRef]
- Barliana, M.I.; Suradji, E.W.; Abdulah, R.; Diantini, A.; Hatabu, T.; Nakajima-Shimada, J.; Subarnas, A.; Koyama, H. Antiplasmodial properties of kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii against chloroquine-resistant Plasmodium falciparum. Biomed. Rep. 2014, 2, 579–583. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Gong, M.; Wang, M. Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria: Pharmacodynamic and pharmacokinetic studies. Phytother. Res. 2018, 32, 1415–1420. [Google Scholar] [CrossRef]
- Chen, N.; LaCrue, A.N.; Teuscher, F.; Waters, N.C.; Gatton, M.L.; Kyle, D.E.; Cheng, Q. Fatty Acid Synthesis and Pyruvate Metabolism Pathways Remain Active in Dihydroartemisinin-Induced Dormant Ring Stages of Plasmodium falciparum. Antimicrob. Agents Chemother. 2014, 58, 4773–4781. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Abass, K.; Reponen, P.; Mattila, S.; Pelkonen, O. Metabolism of α-thujone in human hepatic preparations in vitro. Xenobiotica 2010, 41, 101–111. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 21. [Google Scholar] [CrossRef]
- Kim, W.-S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.-S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. 2014, 19, 21–27. [Google Scholar] [CrossRef]
- Huai, M.; Zeng, J.; Ge, W. Artemisinin ameliorates intestinal inflammation by skewing macrophages to the M2 phenotype and inhibiting epithelial–mesenchymal transition. Int. Immunopharmacol. 2020, 91, 107284. [Google Scholar] [CrossRef]
- Cai, T.-Y.; Zhang, Y.-R.; Ji, J.-B.; Xing, J. Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J. Ethnopharmacol. 2017, 207, 86–91. [Google Scholar] [CrossRef]
- Kriel, Y. The Immune-Modulating Activity of Artemisia afra. Available online: https://etd.uwc.ac.za/xmlui/handle/11394/2020 (accessed on 21 October 2022).
- Suberu, J.O.; Gorka, A.P.; Jacobs, L.; Roepe, P.D.; Sullivan, N.; Barker, G.C.; Lapkin, A.A. Anti-Plasmodial Polyvalent Interactions in Artemisia annua L. Aqueous Extract—Possible Synergistic and Resistance Mechanisms. PLoS ONE 2013, 8, e80790, Corrigendum in PLoS ONE 2013, 8, e80790. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, G.; Zhu, C.; Cheng, B.; Zhang, C.; Ma, Y.; Dong, Y. Artemisinin, an anti-malarial agent, inhibits rat cardiac hypertrophy via inhibition of NF-κB signaling. Eur. J. Pharmacol. 2010, 649, 277–284. [Google Scholar] [CrossRef]
- Cheng, A.-S.; Cheng, Y.-H.; Chang, T.-L. Scopoletin attenuates allergy by inhibiting Th2 cytokines production in EL-4 T cells. Food Funct. 2012, 3, 886–890. [Google Scholar] [CrossRef]
- Min, S.-W.; Kim, N.-J.; Baek, N.-I.; Kim, D.-H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J. Ethnopharmacol. 2009, 125, 497–500. [Google Scholar] [CrossRef]
- Ferreira, J.; Laughlin, J.C.; Delabays, N.; de Magalhães, P. Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin. Plant Genet. Resour. Charact. Util. 2005, 3, 206–229. [Google Scholar] [CrossRef]
- Malik, A.; Kushnoor, A.; Saini, V.; Singhal, S.; Kumar, S.; Yadav, Y. In vitro antioxidant properties of Scopoletin. J. Chem. Pharm. Res. 2011, 3, 659–665. [Google Scholar]
- Ritter, J.M.; Rod, J.F.; Henderson, G.; Loke, Y.K.; MacEwan, D.; Rang, H.P. Rang and Dale’s Pharmacology, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 808. [Google Scholar]
- Räth, K.; Heide, L.; Gleiter, C.H.; Taxis, K.; Li, S.-M.; Walz, G. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (Annual wormwood). Am. J. Trop. Med. Hyg. 2004, 70, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.R.; Mittleman, A.; Weathers, P.J. Dried Leaf Artemisia annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, K.; Wang, M.; Qiu, F. Multi-component pharmacokinetics assessment of Artemisia annua L. in rats based on LC-ESI-MS/MS quantification combined with molecular docking. Arab. J. Chem. 2022, 15, 2920–2925. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Zhu, S.; Ren, G.; Wang, L.; Chen, X.; Qiu, Z.; Zhang, C. Pharmacokinetics and tissue distribution of eupatilin and its metabolite in rats by an HPLC-MS/MS method. J. Pharm. Biomed. Anal. 2018, 159, 113–118. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not Its Glycosides, Is Absorbed from the Rat Stomach. J. Agric. Food Chem. 2001, 50, 618–621. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- van der Woude, H.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M.C.M. Identification of 14 Quercetin Phase II Mono- and Mixed Conjugates and Their Formation by Rat and Human Phase II in Vitro Model Systems. Chem. Res. Toxicol. 2004, 17, 1520–1530. [Google Scholar] [CrossRef]
- Gradolatto, A.; Canivenc-Lavier, M.-C.; Basly, J.-P.; Siess, M.-H.; Teyssier, C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab. Dispos. 2004, 32, 58–65. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, J.; Yang, C.-H.; Xia, B.-J.; Hu, M.; Liu, Z.-Q. Systematic Studies of Sulfation and Glucuronidation of 12 Flavonoids in the Mouse Liver S9 Fraction Reveal both Unique and Shared Positional Preferences. J. Agric. Food Chem. 2012, 60, 3223–3233. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.; Zhao, L.; Wang, Y.; Xue, L.; Han, T.; Zheng, C.; Qin, L. Quantitative determination and pharmacokinetic study of casticin in rat plasma by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2012, 61, 242–246. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.-L.; Kong, A.-N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Mukinda, J.T.; Syce, J.A.; Fisher, D.; Meyer, M. Effect of the Plant Matrix on the Uptake of Luteolin Derivatives-containing Artemisia afra Aqueous-extract in Caco-2 cells. J. Ethnopharmacol. 2010, 130, 439–449. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. No Toxicity Detected for Artemisia annua or Artemisia afra. Available online: https://www.malariaworld.org (accessed on 13 April 2023).
- Motshudi, M.; Olaokun, O.; Mkolo, N. Evaluation of GC × GC-TOF-MS untargeted metabolomics, cytotoxicity and antimicrobial activity of leaf extracts of Artemisia afra (Jacq.) purchased from three local vendors. J. King Saud Univ. Sci. 2021, 33, 101422. [Google Scholar] [CrossRef]
- van Loggenberg, S.; Willers, C.; van der Kooy, F.; Gouws, C.; Hamman, J.H.; Steyn, J.D. Evaluating in vitro cytotoxic effects of Artemisia afra and Artemisia annua infusions against selected lung cancer cell lines. South Afr. J. Bot. 2022, 150, 404–411. [Google Scholar] [CrossRef]
- Mekonen, K.; Afework, M.; Makonnen, E.; Debela, A.; Ergete, W.; Tolessa, T. Evaluation of Acute and Sub-Acute Toxicity of Aqueous Extracts of Artemisia afra Leaves on Brain, Heart and Suprarenal Glands in Swiss Albino Mice. Ethiop. J. Health Sci. 2020, 30, 981–990. [Google Scholar] [CrossRef]
- Kane, N.F.; Kyama, M.C.; Nganga, J.K.; Hassanali, A.; Diallo, M.; Kimani, F.T. Acute Toxicity Effect of Artemisia afra Plant Extracts on the Liver, Kidney, Spleen and in Vivo Antimalarial Assay on Swiss Albino Mice. Adv. Biosci. Bioeng. 2019, 7, 64. [Google Scholar] [CrossRef]
- Siddiqui, M.; Waghmare, S.; Hajare, S.; Deshmukh, R.S.I.; Chepte, S.; Ali, S.A. Phytochemical analysis and acute toxicity studies of Artemisia annua in Swiss albino mice. J. Pharmacogn. Phyto-Chem. 2018, 7, 1893–1895. [Google Scholar]
- Mukinda, J.T.; Syce, J.A. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. 2017, 112, 138–144. [Google Scholar] [CrossRef]
- Loomis, T.A.; Hayes, A.W. Loomis’s Essential of Toxicology, 4th ed.; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Tsuji, P.; Walle, T. Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem. Interactions 2008, 171, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Cheng, J.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L.; Lu, G. Toxicological evaluation of a flavonoid, chrysin: Morphological, behavioral, biochemical and histopathological assessments in rats. Drug Chem. Toxicol. 2019, 44, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; Patton, E.; Vanderveen, B.N.; Unger, C.; Aladhami, A.; Enos, R.T.; Madero, S.; Chatzistamou, I.; Fan, D.; Murphy, E.A.; et al. Sub-chronic oral toxicity screening of quercetin in mice. BMC Comple. Ment. Med. 2022, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Lucida, H.; Primadini, Y.; Suhatri. A Study on the acute toxicity of quercetin solid dispersion as a potential nephron-protector. Rasayan J. Chem. 2019, 12, 727–732. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Dolci, G.S.; Grando, T.H.; Sagrillo, M.R.; Vaucher, R.A.; da Luz, S.C.; Silveira, S.O.; Duarte, M.M.; Duarte, T.; et al. Monoterpene alpha-terpinene induced hepatic oxidative, cytotoxic and genotoxic damage is associated to caspase activation in rats. J. Appl. Biomed. 2017, 15, 187–195. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Alker, A.P.; Lim, P.; Sem, R.; Shah, N.K.; Yi, P.; Bouth, D.M.; Tsuyuoka, R.; Maguire, J.D.; Fandeur, T.; Ariey, F.; et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am. J. Trop. Med. Hyg. 2007, 76, 641–647. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Isozumi, R.; Uemura, H.; Kimata, I.; Ichinose, Y.; Logedi, J.; Omar, A.H.; Kaneko, A. Novel Mutations in K13 Propeller Gene of Artemisinin-Resistant Plasmodium falciparum. Emerg. Infect. Dis. 2015, 21, 490–492. [Google Scholar] [CrossRef]
- Zime-Diawara, H.; Ganfon, H.; Gbaguidi, F.; Yemoa, A.; Bero, J.; Jansen, O.; Evrard, B.; Moudachirou, M.; Frederich, M.; Quetin-Leclercq, J. The antimalarial action of aqueous and hydro alcoholic extracts of Artemisia annua L. cultivated in Benin: In vitro and in vivo studies. J. Chem. Pharm. Res. 2015, 7, 817–823. [Google Scholar]
- Liu, N.Q.; Cao, M.; Frédérich, M.; Choi, Y.H.; Verpoorte, R.; van der Kooy, F. Metabolomic investigation of the ethnopharmacological use of Artemisia afra with NMR spectroscopy and multivariate data analysis. J. Ethnopharmacol. 2010, 128, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. 13C-2H correlation NMR spectroscopy studies of the in vivo transformations of natural products from Artemisia annua. Phytochem. Rev. 2003, 2, 45–59. [Google Scholar] [CrossRef]
- Tian, J.; Feng, W.; He, B. Study on volatile constituents of Artemisia annua and its preparation by GC-MS. Shizen Guoyi Guoyao 2007, 18, 1840–1842. [Google Scholar]
- Goel, D.; Singh, G.; Ali, M.; Mallavarupu, G.R.; Kumar, S. Essential oils of petal, leaf and stem of the antimalarial plant Artemisia annua. J. Nat. Med. 2006, 61, 187–191. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Yao, J.; Yang, Y.-L.; Wang, Y.-F.; Zeng, J.-Y. Studies on the chemical constituents of the essential oil of Artemisia tournefortiana Reichb. Sichuan Daxue Xuebao (Ziran Kexueban) 2004, 41, 1287–1289. [Google Scholar]
- Viljoen, A.M.; van Vuuren, S.F.; Gwebu, T.; Demirci, B.; Başer, K.H.C. The Geographical Variation and Antimicrobial Activity of African Wormwood (Artemisia afra Jacq.) Essential Oil. J. Essent. Oil Res. 2006, 18, 19–25. [Google Scholar] [CrossRef]
- Bilia, A.R.; Flamini, G.; Morgenni, F.; Isacchi, B.; FrancescoVincieri, F. GC MS Analysis of the Volatile Constituents of Essential Oil and Aromatic Waters of Artemisia annua L. at Different Developmental Stages. Nat. Prod. Commun. 2008, 3, 2075–2078. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Lu, X.; Li, H.; Liu, B.; Xu, G. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1150, 50–53. [Google Scholar] [CrossRef]
- Yakobashvili, N.Z.; Kuchukhidze, N.M.; Chikvanya, V.E. Chemical Profile Characterization of Artemisia annua L. Essential Oils from South India Through GC-FID and GC-MS Analyses. J. Essent. Oil-Bear. Plants JEOP 2014, 17, 1249–1256. [Google Scholar] [CrossRef]
- Georgiev, E.; Gevov, N.; Khristov, N. Yield and Quality Changes in the Essential Oil of Annual Wormwood (Artemisia annua L.) During Vegetation. Plant Sci. 1981, 18, 95–102. [Google Scholar]
- Chagonda, L.S.; Makanda, C.; Chalchat, J.-C. The essential oil of cultivated Artemisia afra (Jacq.) from Zimbabwe. Flavour Fragr. J. 1999, 14, 140–142. [Google Scholar] [CrossRef]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Mukhtar, H.M.; Ansari, S.; Ali, M.; Mir, S.; Abdin, M.; Singh, P. GC-MS Analysis of Volatile Oil of Aerial Parts of Artemisia annua Linn. J. Essent. Oil Bear. Plants 2007, 10, 168–171. [Google Scholar] [CrossRef]
- Jain, N.; Srivastava, S.K.; Aggarwal, K.K.; Kumar, S.; Syamasundar, K.V. Essential Oil Composition of Artemisia annua L. ‘Asha’ from the Plains of Northern India. J. Essent. Oil Res. 2002, 14, 305–307. [Google Scholar] [CrossRef]
- Misra, L.N. Arteannuin-C, a sesquiterpene lactone from Artemisia annua. Phytochemistry 1986, 25, 2892–2893. [Google Scholar] [CrossRef]
- Burits, M.; Asres, K.; Bucar, F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytother. Res. 2001, 15, 103–108. [Google Scholar] [CrossRef]
- Ali, M.; Singh, V.; Archana, M.; Sultana, S. New aliphatic constituents from the aerial parts of Artemisia annua L. Alger. J. Nat. Prod. 2017, 5, 515–523. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Ouyang, F.; Ye, H.; Li, G. Advances in artemisinin research. Prog. Chem. 1999, 11, 41. [Google Scholar]
- Ahmad, A.; Misra, L.N. Terpenoids from Artemisia annua and constituents of its essential oil. Phytochemistry 1994, 37, 183–186. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Pras, N.; Van Uden, W.; De Boer, A.; Batterman, S.; Visser, J.F.; Malingré, T.M. High Peroxidase Activity in Cell Cultures of Artemisia annua with Minute Artemisinin Contents. Nat. Prod. Lett. 1992, 1, 121–128. [Google Scholar] [CrossRef]
- Moody, J.O.; Adeleye, S.A.; Gundidza, M.G.; Wyllie, G. Analysis of the Essential oil of Artemisia afra. Pharmazie 1994, 49, 935–936. [Google Scholar]
- Nguyen, X.D.; Leclercq, P.A.; Dinh, H.K.; Nguyen, M.T. Chemical Composition of essential oil of Vietnamese Artemisia annua L. Tap. Chi. Duoc Hoc 1990, 2, 11–13. [Google Scholar]
- Asfaw, N.; Licence, P.; Novitskii, A.A.; Poliakoff, M. Green Chemistry in Ethiopia: The cleaner extraction of essential oils from Artemisia afra: A comparison of clean technology with conventional methodology. Green Chem. 2005, 7, 352–356. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D. Deoxyarteannuin B, dihydro-deoxyarteannuin B and trans-5-hydroxy-2-isopropenyl-5-methylhex-3-en-1-ol from Artemisia anuua. Phytochemistry 2001, 58, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The development of new antimalarial drugs: Qinghaosu and dihydro-qinghaosu. Chin. Med. J. 1999, 112, 976–977. [Google Scholar] [PubMed]
- Verdian-rizi, M.; Sadat-Ebrahimi, E.; Hadjiakhoondi, A.; Fazeli, M.; Hamedani, M.P. Chemical Composition and Antimicrobial Activity of Artemisia annua L. Essential Oil from Iran. J. Med. Plants 2008, 7, 58–62. [Google Scholar]
- Bertea, C.M.; Freije, J.R.; van der Woude, H.; Verstappen, F.W.A.; Perk, L.; Marquez, V.; De Kraker, J.-W.; Posthumus, M.A.; Jansen, B.J.M.; de Groot, A.; et al. Identification of Intermediates and Enzymes Involved in the Early Steps of Artemisinin Biosynthesis in Artemisia annua. Planta Med. 2005, 71, 40–47. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y. Structure and synthesis of arteannuin and related compounds. XI, Identification of epoxyarte-annuic acid. Acta Chim. Sin. 1984, 42, 596–598. [Google Scholar]
- Brown, G.D. Cadinanes from Artemisia annua that may be intermediates in the biosynthesis of artemisinin. Phytochemistry 1994, 36, 637–641. [Google Scholar] [CrossRef]
- Perazzo, F.F.; Carvalho, J.C.T.; Rehder, V.L.G. Central properties of the essential oil and the crude ethanol extract from aerial parts of Artemisia annua L. Pharmacol. Res. 2003, 48, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.R. Chemical constituents of volatile oils of Artemisia annua. Zhong Yao Tong Bao 1983, 8, 31–32. [Google Scholar] [PubMed]
- Zhai, D.D.; Jin, H.Z.; Zhong, J.J. A new sesquiterpene from hairy root culture of Artemisia annua. Chin. Chem. Lett. 2010, 21, 590–592. [Google Scholar] [CrossRef]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia afra Jacq. ex Willd. against Periodontal Pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Feng, L.-L.; Yang, X.-Q.; Yin, L.-L.; Xu, X.-L.; Zeng, Q.-P. Quantitative Transcript Profiling Reveals Down-Regulation of A Sterol Pathway Relevant Gene and Overexpression of Artemisinin Biogenetic Genes in Transgenic Artemisia annua Plants. Planta Med. 2008, 74, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.-K.; Brown, G.D. Three sesquiterpenes from Artemisia annua. Phytochemistry 1998, 48, 1207–1211. [Google Scholar] [CrossRef]
- Roth, R.J.; Acton, N. Isolation of Epi-Deoxyarteannuin B from Artemisia annua. Planta Med. 1987, 53, 576. [Google Scholar] [CrossRef]
- Picaud, S.; Olofsson, L.; Brodelius, M.; Brodelius, P.E. Expression, purification, and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch. Biochem. Biophys. 2005, 436, 215–226. [Google Scholar] [CrossRef]
- Zheng, G.-Q. Cytotoxic Terpenoids and Flavonoids from Artemisia annua. Planta Med. 1994, 60, 54–57. [Google Scholar] [CrossRef]
- Jakupovic, J.; Klemeyer, H.; Bohlmann, F.; Graven, E. Glaucolides and guaianolides from Artemisia afra. Phytochemistry 1988, 27, 1129–1133. [Google Scholar] [CrossRef]
- Bolt, A.J.N.; Cocker, W.; McMurry, T.B.H. 998. The stereochemistry of artemisin. J. Chem. Soc. 1963, 5235–5238. [Google Scholar] [CrossRef]
- Wei, Z.-X.; Pan, J.-P.; Li, Y. Artemisinin G: A Sesquiterpene from Artemisia annua. Planta Med. 1992, 58, 300. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, S.; Liu, B.; Fan, G.; Liu, J.; Xu, R. Study on antibacterial constituents of Qing Hao (Artemisia annua L.). Zhongcaoyao (Chin. Trad. Heb. Drug) 1982, 13, 54. [Google Scholar]
- Nkunya, M.M.H.; Weenen, H.; Kinabo, L.S. Constituents of Artemisia afra. Fitoterapia 1992, 63, 279–280. [Google Scholar]
- Acton, N.; Klayman, D.L. Artemisitene, a New Sesquiterpene Lactone Endoperoxide from Artemisia annua. Planta Med. 1985, 51, 441–442. [Google Scholar] [CrossRef]
- Wallaart, T.E.; van Uden, W.; Lubberink, H.G.M.; Woerdenbag, H.J.; Pras, N.; Quax, W.J. Isolation and Identification of Dihydroartemisinic Acid from Artemisia annua and Its Possible Role in the Biosynthesis of Artemisinin. J. Nat. Prod. 1999, 62, 430–433. [Google Scholar] [CrossRef]
- Brown, G. Phytene-1,2-diol from Artemisia annua. Phytochemistry 1994, 36, 1553–1554. [Google Scholar] [CrossRef]
- Venables, L.; Koekemoer, T.; Van de Venter, M.; Goosen, E. Isoalantolactone, a sesquiterpene lactone from Artemisia afra Jacq. ex Willd and its in vitro mechanism of induced cell death in HeLa cells. South Afr. J. Bot. 2016, 103, 216–221. [Google Scholar] [CrossRef]
- Goodson, J.A. The Constituents of the Flowering Tops of Artemisia afra, Jacq. Biochem. J. 1922, 16, 489–493. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in Essential Oils, 2001. Available online: www.PerfumerFlavorist.com (accessed on 3 November 2022).
- Pinhey, J.; Sternhell, S. Structure of α-hydroxysantonin and some aspects of the stereochemistry of related eudesmanolides and guaianolides. Aust. J. Chem. 1965, 18, 543–557. [Google Scholar] [CrossRef]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupovic, J.; Mavi, S.; Bienzle, U.; Eich, E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Ni, F.-Y.; Song, Y.-L.; Wang, S.-Y.; Huang, W.-Z.; Wang, Z.-Z.; Xiao, W. Chemical constituents from Artemisia annua. China J. Chin. Mater. Med. 2014, 39, 4816–4821. [Google Scholar]
- . Marco, J.A.; Sanz, J.F.; Bea, J.F.; Barbera, O. Phenolic constituents from Artemisia annua. Pharmazie 1990, 45, 382–383. [Google Scholar]
- Dube, A. The Design, Preparation and Evaluation of Artemisia afra and Placebos in Tea Bag Dosage form Suitable for Use in Clinical Trials. Master’s Thesis, University of the Western Cape, Bellville, South Africa, June 2006. [Google Scholar]
- Lai-King, S.; Brown, G.D. Novel seco-cycloartanes from Kadsura coccinea and the assisted autoxidation of a tri-substituted alkene. Tetrahedron 1999, 55, 119–132. [Google Scholar] [CrossRef]
- Shilin, Y.; Roberts, M.F.; Phillipson, J.D. Methoxylated flavones and coumarins from Artemisia annua. Phytochemistry 1989, 28, 1509–1511. [Google Scholar] [CrossRef]
- Yang, S.-L.; Roberts, M.F.; O’Neill, M.J.; Bucar, F.; Phillipson, J. Flavonoids and chromenes from Artemisia annua. Phytochemistry 1995, 38, 255–257. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Jain, D.; Bhakuni, R.; Zaim, M.; Thakur, R. Occurrence of some antiviral sterols in Artemisia annua. Plant Sci. 1991, 75, 161–165. [Google Scholar] [CrossRef]
- Takemoto, T.; Nakajima, T. Studies on the Essential Oil of Artemisia annua L. IV. Yakugaku Zasshi 1957, 77, 1344–1347. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D.; Haynes, R. A novel endoperoxide and related sesquiterpenes from Artemisia annua which are possibly derived from allylic hydroperoxides. Tetrahedron 1998, 54, 4345–4356. [Google Scholar] [CrossRef]
- Shukla, A.; Farooqi, A.; Shukla, Y. A New Adenine Derivative from Artemisia annua. J. Indian Chem. Soc. 1997, 74, 59. [Google Scholar]
- Chen, J.; Zhou, Y.; Zhang, X.; Huang, L.; Sun, W.; Wang, J. Chemical constituents of Artemisia annua L. J. Shenyang Pharm Univ. 2008, 25, 866–870. [Google Scholar]
- Chu, Y.; Wang, H.; Chen, J.; Hou, Y. New Sesquiterpene and Polymethoxy-Flavonoids from Artemisia annua L. Pharmacogn. Mag. 2014, 10, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-H.; Li, H.-B.; Huang, Y.-X.; Qin, D.-P.; Zhang, C.-F.; Wang, Z.-Z.; Yu, Y. Research on chemical constituents from Artemisia annua I. China J. Chin. Mater. Med. 2021, 46, 1160–1167. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).