The Influence of Metabolic Factors in Patients with Chronic Viral Hepatitis C Who Received Oral Antiviral Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
- In patients with mild fibrosis
- In patients with severe fibrosis
- Patients with mild steatosis:
- Patients with severe steatosis:
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meryem, J.; Bisma, R.; Harunor, R.; Thao, L.; Shafquat, R. Update on global epidemiology of viral hepatitis and preventive strategies. World J. Clin. Cases 2018, 6, 589–599. [Google Scholar]
- Kamp, W.M.; Sellers, C.M.; Stein, S.; Lim, J.K.; Kim, H.S. Impact of Direct Acting Antivirals on Survival in Patients with Chronic Hepatitis C and Hepatocellular Carcinoma. Sci. Rep. 2019, 19, 17081. [Google Scholar] [CrossRef]
- Rich, N.E.; Singal, A.G. Direct-Acting Antiviral Therapy and Hepatocellular Carcinoma. Clin. Liver Dis. 2021, 17, 414–417. [Google Scholar] [CrossRef]
- Trotter, J. Liver transplantation around the world. Liver Transpl. 2017, 22, 123–127. [Google Scholar] [CrossRef]
- Gavril, O.I.; Arhire, L.I.; Gavril, R.S.; Zota, M.I.; Gherasim, A.; Nita, O.; Drugescu, A.; Oprescu, A.C.; Esanu, I.M.; Mitu, F.; et al. Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients. Medicina 2021, 15, 1249. [Google Scholar] [CrossRef]
- Byrne, C.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, 47–64. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Micco, L.; Conti, F.; Andreone, P.; Bernardi, M. Alcohol and viral hepatitis A mini-review. Dig. Liver Dis. 2009, 41, 67–70. [Google Scholar] [CrossRef]
- Bochud, P.Y.; Cai, T.; Overbeck, K. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J. Hepatol. 2009, 51, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Kralj, D.; Jukic, L.V.; Stojsavljevic, S.; Duvnjak, M.; Smolic, M.; Curcic, I.B. Hepatitis C Virus, Insulin Resistance, and Steatosis. J. Clin. Translational Hepatol. 2016, 4, 66–75. [Google Scholar]
- Seronello, S.; Ito, C.; Wakita, T.; Choi, J. Ethanol enhances hepatitis C virus replication through lipid metabolism and elevated NADH/NAD+. J. Biol. Chem. 2010, 285, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Gambardella, M.; Andreana, A. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001, 33, 1358–1364. [Google Scholar] [CrossRef]
- Allison, M.E.; Wreghitt, T.; Palmer, C.R. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J. Hepatol. 1994, 21, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Sirli, R.; Bota, S. Is ARFI elastography reliable for predicting fibrosisseverity in chronic HCV hepatitis? World J. Radiol. 2011, 3, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 4. [Google Scholar] [CrossRef]
- Munteanu, M.; Pais, R.; Peta, V.; Deckmyn, O.; Moussalli, J.; Ngo, Y.; Rudler, M.; Lebray, P.; Charlotte, F.; Thibault, V.; et al. Long-term prognostic value of the FibroTest in patients with non-alcoholic fatty liver disease, compared to chronic hepatitis C, B, and alcoholic liver disease. Aliment. Pharmacol. Ther. 2018, 48, 10. [Google Scholar] [CrossRef]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Castille, J.M.; Poynard, T.; Soldevila-Pico, C.; Clark, V.C.; Firpi-Morell, R.J.; Lai, J.; Cusi, K. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J. Investig. Med. 2018, 67, 303–311. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Charlotte, F.; Perazzo, H.; Ngo, Y.; Deckmyn, O.; Pais, R.; Merrouche, W.; De Lédinghen, V.; Mathurin, P.; et al. Diagnostic performance of a new noninvasive test for nonalcoholic steatohepatitis using a simplified histological reference. Eur. J. Gastroenterol. Hepatol. 2018, 30, 5. [Google Scholar] [CrossRef]
- Nahon, P.; Bourcier, V.; Layese, R. Eradication of Hepatitis C Virus Infection in Patients with Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2017, 152, 142–156. [Google Scholar] [CrossRef]
- Lonardo, A.; Loria, P.; Adinolfi, L.E.; Carulli, N.; Ruggiero, G. Hepatitis C and steatosis: A reappraisal. J. Viral Hepatitis. 2006, 13, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Bao, F.; Du, E.; Skillin, C.; Frenette, C.T.; Waalen, J.; Alaparthi, L.; Goodman, Z.D.; Pockros, P.J. Morphometry Confirms Fibrosis Regression From Sustained Virologic Response to Direct-Acting Antivirals for Hepatitis C. Hepatol. Commun. 2018, 21, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Rao, H.; Yang, M.; Gao, Y.; Wang, J.; Jin, Q.; Ma, D.; Wei, L. Noninvasive Measurements Predict Liver Fibrosis Well in Hepatitis C Virus Patients After Direct-Acting Antiviral Therapy. Dig. Dis. Sci. 2020, 65, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Siggaard, C.B.; Kazankov, K.; Sandahl, T.D.; Møller, H.J.; Tarp, B.; Kristensen, L.H.; Laursen, A.L.; Leutscher, P.; Grønbæk, H. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J. Viral Hepat. 2020, 27, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Caviglia, G.P.; Younes, R.; Ribaldone, D.G.; Fagoonee, S.; Pellicano, R.; Bugianesi, E. Molecular mechanisms of hepatic fibrosis in chronic liver diseases. Minerva Biotecnol. 2020, 32, 121–127. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Tsui, J.I.; Williams, E.C.; Green, P.K.; Berry, K.; Su, F.; Ioannou, G.N. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug. Alcohol. Depend. 2016, 1, 101–109. [Google Scholar] [CrossRef]
- IaOkwor, C.; Petrosyan, Y.; Lee, C.; Cooper, C. History of alcohol use does not predict HCV direct acting antiviral treatment outcomes. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2022, 27, 233–241. [Google Scholar] [CrossRef]
- Erman, A.; Krahn, M.D.; Hansen, T. Estimation of fibrosis progression rates for chronic hepatitis C: A systematic review and meta-analysis update. BMJ Open 2019, 9, 27491. [Google Scholar] [CrossRef]
- Patton, H.M.; Patel, K.; Behling, C. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C. patients. J. Hepatol. 2004, 40, 484–490. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Fabris, P.; Paganin, S. Steatosis a ffects chronic hepatitis C progression in a genotype speci fic way. Gut 2004, 53, 406–412. [Google Scholar] [CrossRef]
- Mihalache, L.; Graur, L.I.; Popescu, D.S.; Nita, O.; Graur, M. Anthropometric parameters—Predictive factors for cardio-metabolic diseases. Rev. Med. Chir. Soc. Med. Nat. Lasi 2012, 116, 794–798. [Google Scholar]
- Ortiz, V.; Berenguer, M.; Rayon, J.M.; Carrasco, D.; Berenguer, J. Contribution of obesity to hepatitis C-related fibrosis progression. Am. J. Gastroenterol. 2002, 97, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, N.A.; Fox, R.K. Hepatitis C Virus Cure and Obesity: Watch the Weight. J. Gen. Intern. Med. 2020, 35, 2836–2837. [Google Scholar] [CrossRef]
- Nkwocha, C.L.; Carter, P.S.; Blair, S.; Blackwell, J.M.; Fasanmi, E.O. Understanding the effect of direct-acting antiviral therapy on weight in patients with chronic hepatitis C. Antivir. Ther. 2022, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Sirinawasatien, A.; Techasirioangkun, T. The Prevalence and Determinants of Hepatic Steatosis Assessed by Controlled Attenuation Parameter in Thai Chronic Hepatitis C Patients. Gastroenterol. Res. Pract. 2020, 2020, 8814135. [Google Scholar] [CrossRef]
- Hurjui, D.M.; Niţă, O.; Graur, L.I.; Mihalache, L.; Popescu, D.S.; Graur, M. The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev. Med. Chir. Soc. Med. Nat. Lasi 2012, 116, 425–431. [Google Scholar]
- Hurjui, D.M.; Niţă, O.; Graur, L.I.; Mihalache, L.; Popescu, D.S.; Huţanaşu, I.C.; Ungureanu, D.; Graur, M. Non-alcoholic fatty liver disease is associated with cardiovascular risk factors of metabolic syndrome. Rev. Med. Chir. Soc. Med. Nat. Lasi 2012, 116, 692–699. [Google Scholar]
- Shina, G.; Soliman, R.; Mikhail, N.; Ibrahim, A.; Serwah, A.H.; Khattab, M. Changes in hepatic fibrosis stages after achieving SVR following direct-acting anti-viral treatment: A prospective stud. GastroHep 2020, 18, 39–48. [Google Scholar]
- Lee, C.H.; Lui, D.T.; Lam, K.S. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J. Diabetes Investig. 2022, 13, 930–940. [Google Scholar] [CrossRef]
- Rhee, E.J. Nonalcoholic Fatty Liver Disease and Diabetes: An Epidemiological Perspective. Endocrinol. Metab. 2019, 34, 226–233. [Google Scholar] [CrossRef]
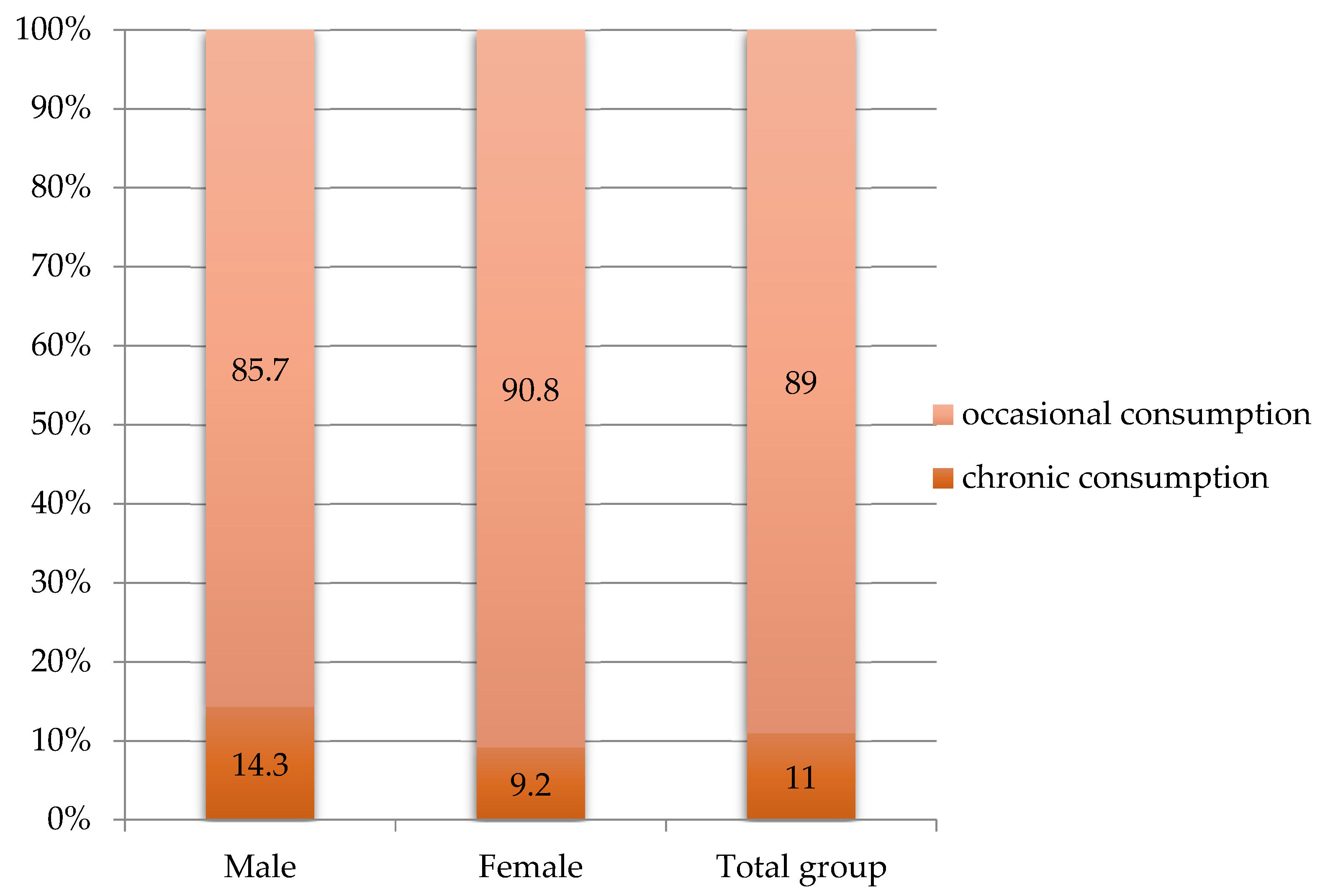
| Characteristics | Values |
|---|---|
| Sex | |
| Female | 65.0% |
| Male | 35.0% |
| Age | |
| Average ± SD | 60.74 ± 8.58 |
| Min-max/median/Skewness test values | 35–77/61/−0.512 |
| Fibrosis (FibroTest) | |
| Average ± SD | 0.65 ± 0.18 |
| Min-max/median/Skewness test values | 35–77/61/−0.512 |
| Degree of liver fibrosis | |
| F4 | 43.0% |
| F3 | 21.0% |
| F2 | 36.0% |
| Steatosis (SteatoTest) | |
| Average ± SD | 0.50 ± 0.18 |
| Min-max/median/Skewness test values | 0.11–0.89/0.49/−0.015 |
| Degree of liver steatosis | |
| S3 | 19.0% |
| S2 | 37.0% |
| S1 | 32.0% |
| S0 | 12.0% |
| KERRYPNX | T0 | T3 | Paired Samples Statistics |
|---|---|---|---|
| Fibrosis (FibroTest)- AUC | 0.66 ± 0.18 | 0.55 ± 0.18 | 0.001 |
| F2 | 0.45 ± 0.07 | 0.43 ± 0.13 | 0.205 |
| F3 | 0.65 ± 0.04 | 0.51 ± 0.09 | 0.001 |
| F4 | 0.82 ± 0.08 | 0.67 ± 0.16 | 0.001 |
| Steatosis (SteatoTest)- AUC | 0.50 ± 0.18 | 0.34 ± 0.14 | 0.001 |
| S0 | 0.33 ± 0.14 | 0.30 ± 0.14 | 0.481 |
| S1 | 0.34 ± 0.08 | 0.25 ± 0.11 | 0.001 |
| S2 | 0.58 ± 0.06 | 0.40 ± 0.12 | 0.001 |
| S3 | 0.76 ± 0.05 | 0.45 ± 0.11 | 0.001 |
| Parameter | T0 | T3 | Difference from the Average Period | p for Paired Sample T Test |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| mild fibrosis severe fibrosis | 25.96 ± 3.14 27.92 ± 5.14 | 27.18 ± 4.19 29.76 ± 4.66 | +1.08 +1.64 | 0.410 0.183 |
| p for FANOVA test | 0.043 | 0.034 | - | |
| Fasting blood glucose (mg/dL) | ||||
| mild fibrosis severe fibrosis | 106.58 ± 37.23 116.72 ± 48.66 | 102.81 ± 27.43 113.52 ± 38.28 | −3.78 −3.20 | 0.331 0.583 |
| p for FANOVA test | 0.281 | 0.143 | ||
| Triglyceride (mg/dL) | ||||
| mild fibrosis severe fibrosis | 105.28 ± 58.80 103.80 ± 35.74 | 126.17 ± 55.85 106.33 ± 39.23 | +20.58 +2.53 | 0.013 0.617 |
| p for FANOVA test | 0.833 | 0.039 | ||
| GGT (U/L) | ||||
| mild fibrosis severe fibrosis | 38.18 ± 22.83 87.22 ± 77.35 | 22.47 ± 9.50 34.28 ± 25.34 | −15.71 −52.94 | 0.001 0.001 |
| p for FANOVA test | 0.001 | 0.010 | ||
| ALT (U/L) | ||||
| mild fibrosis severe fibrosis | 74.24 ± 44.60 109.52 ± 75.46 | 26.64 ± 12.46 25.59 ± 15.05 | −47.60 −83.93 | 0.001 0.001 |
| p for FANOVA test | 0.012 | 0.724 | ||
| AST (U/L) | ||||
| mild fibrosis severe fibrosis | 51.81 ± 28.64 86.81 ± 50.69 | 22.28 ± 6.79 24.63 ± 8.32 | −29.53 −62.18 | 0.001 0.001 |
| p for FANOVA test | 0.001 | 0.154 | ||
| LDL cholesterol (mg/dL) | ||||
| mild fibrosis severe fibrosis | 123.83 ± 56.22 97.58 ± 32.49 | 125.67 ± 37.30 119.85 ± 38.66 | +26.75 +16.30 | 0.175 0.504 |
| p for FANOVA test | 0.269 | 0.585 | - | |
| Parameter | T0 | T3 | Difference from the Average Period | p for Paired Sample T Test |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| mild steatosissevere steatosis | 25.39 ± 4.11 28.71 ± 4.49 | 29.62 ± 5.17 28.08 ± 4.05 | +3.94 −0.84 | 0.004 0.457 |
| p for FANOVA test | 0.001 | 0.198 | - | |
| Fasting blood glucose (mg/dL) | ||||
| mild steatosissevere steatosis | 102.91 ± 32.02 115.58 ± 20.45 | 100.59 ± 26.80 118.68 ± 25.11 | −2.32 +3.11 | 0.497 0.584 |
| p for FANOVA test | 0.045 | 0.021 | ||
| Triglyceride (mg/dL) | ||||
| mild steatosissevere steatosis | 88.89 ± 35.38 115.16 ± 44.45 | 106.00 ± 45.11 114.58 ± 46.11 | +17.41 −0.58 | 0.001 0.950 |
| p for FANOVA test | 0.001 | 0.148 | ||
| GGT(U/L) | ||||
| mild steatosissevere steatosis | 40.20 ± 23.77 105.21 ± 95.67 | 24.02 ± 15.77 31.95 ± 17.51 | −16.18 −73.26 | 0.005 0.002 |
| p for FANOVA test | 0.001 | 0.011 | ||
| ALT(U/L) | ||||
| mild steatosissevere steatosis | 58.64 ± 29.58 143.76 ± 69.84 | 23.27 ± 13.83 25.11 ± 9.22 | −35.36 −118.66 | 0.001 0.001 |
| p for FANOVA test | 0.001 | 0.090 | ||
| AST(U/L) | ||||
| mild steatosissevere steatosis | 47.64 ± 17.07 108.46 ± 50.09 | 23.67 ± 10.14 23.26 ± 6.05 | −23.98 −85.20 | 0.001 0.001 |
| p for FANOVA test | 0.001 | 0.154 | ||
| LDL cholesterol(mg/dL) | ||||
| mild steatosissevere steatosis | 98.75 ± 45.57 111.47 ± 44.89 | 119.48 ± 38.71 124.57 ± 37.65 | +44.67 +5.48 | 0.064 0.692 |
| p for FANOVA test | 0.636 | 0.623 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavril, O.I.; Gavril, R.S.; Mitu, F.; Gavrilescu, O.; Popa, I.V.; Tatarciuc, D.; Drugescu, A.; Oprescu, A.C.; Gherasim, A.; Mihalache, L.; et al. The Influence of Metabolic Factors in Patients with Chronic Viral Hepatitis C Who Received Oral Antiviral Treatment. Metabolites 2023, 13, 571. https://doi.org/10.3390/metabo13040571
Gavril OI, Gavril RS, Mitu F, Gavrilescu O, Popa IV, Tatarciuc D, Drugescu A, Oprescu AC, Gherasim A, Mihalache L, et al. The Influence of Metabolic Factors in Patients with Chronic Viral Hepatitis C Who Received Oral Antiviral Treatment. Metabolites. 2023; 13(4):571. https://doi.org/10.3390/metabo13040571
Chicago/Turabian StyleGavril, Oana Irina, Radu Sebastian Gavril, Florin Mitu, Otilia Gavrilescu, Iolanda Valentina Popa, Diana Tatarciuc, Andrei Drugescu, Andrei Catalin Oprescu, Andreea Gherasim, Laura Mihalache, and et al. 2023. "The Influence of Metabolic Factors in Patients with Chronic Viral Hepatitis C Who Received Oral Antiviral Treatment" Metabolites 13, no. 4: 571. https://doi.org/10.3390/metabo13040571
APA StyleGavril, O. I., Gavril, R. S., Mitu, F., Gavrilescu, O., Popa, I. V., Tatarciuc, D., Drugescu, A., Oprescu, A. C., Gherasim, A., Mihalache, L., & Esanu, I. M. (2023). The Influence of Metabolic Factors in Patients with Chronic Viral Hepatitis C Who Received Oral Antiviral Treatment. Metabolites, 13(4), 571. https://doi.org/10.3390/metabo13040571







