Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications
Abstract
1. Introduction
2. The Concept of Fever
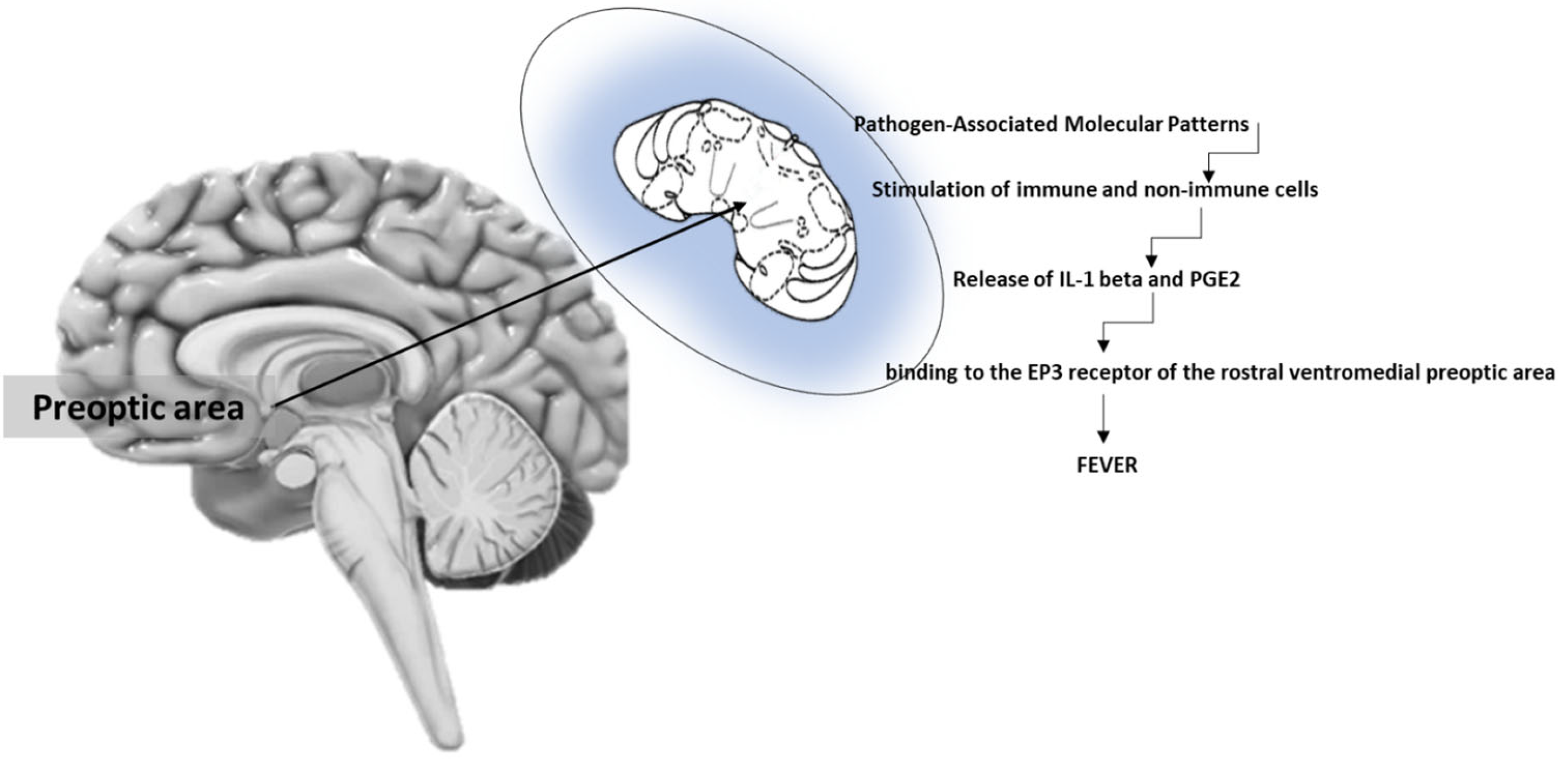
3. Brain Regulation of Fever
4. Receptors of Fever
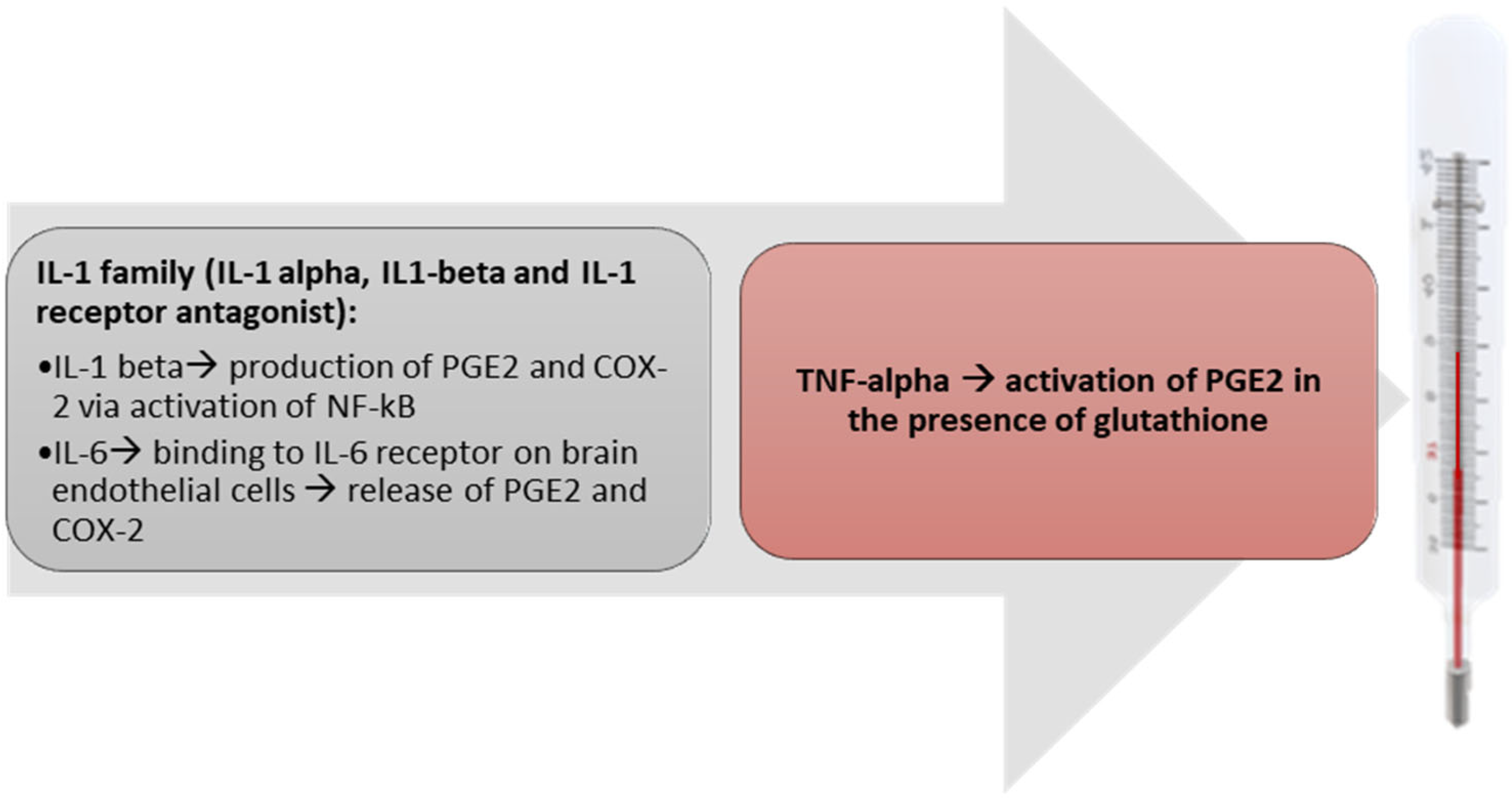
5. Pyrogenic Cytokines
6. Mediators of Fever
7. Fever of Microbial Origin
8. Fever of Unknown Origin (FUO) and Inflammation of Unknown Origin (IUO)
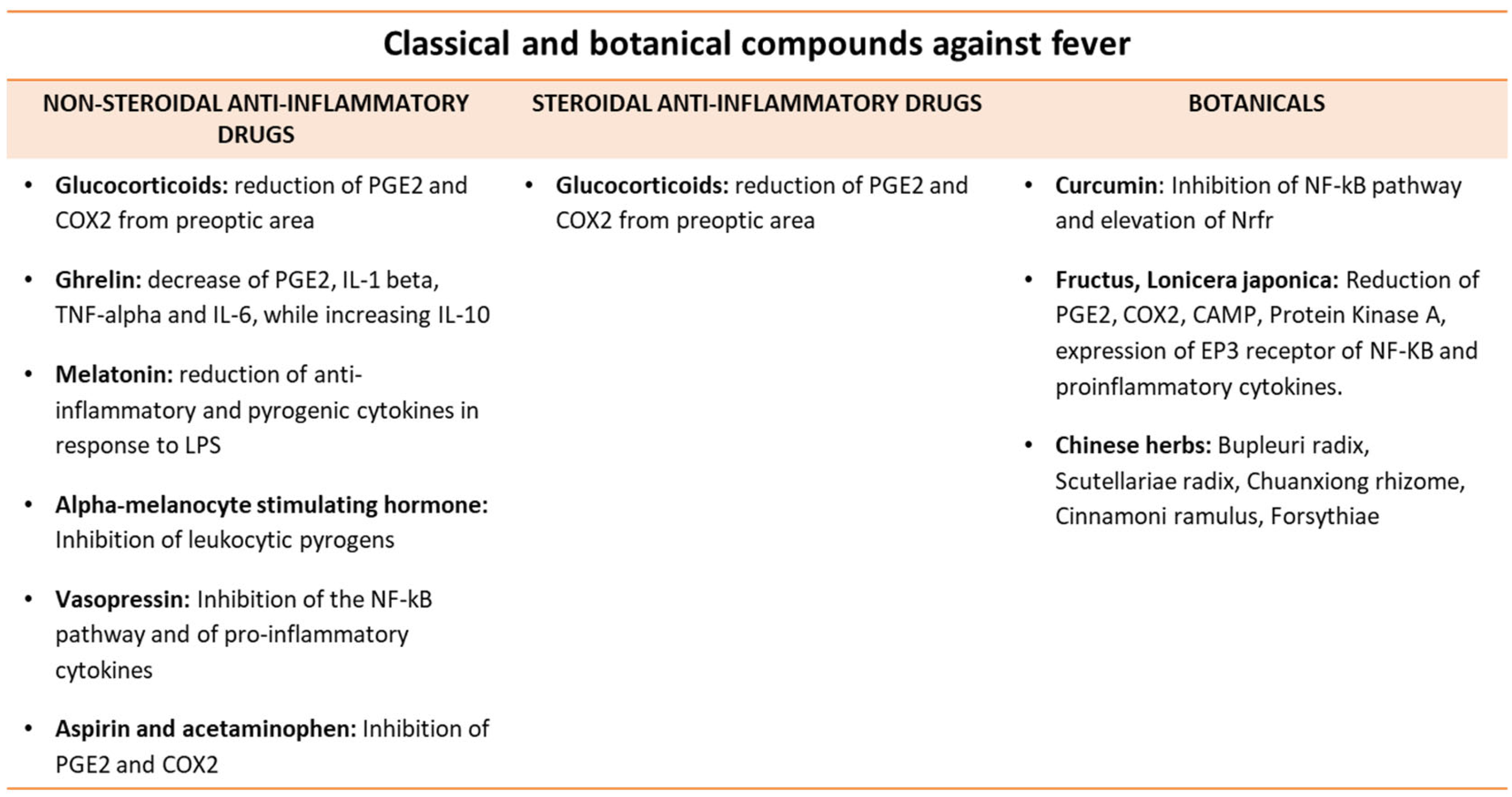
9. Antipyretic Molecules
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood Brain Barrier |
| cAMP | Cyclic AMP |
| COX-2 | Cycloxygenase 2 |
| CVO | Circumventricular Organ |
| EP | PGE2 receptor |
| IFN | Interferon |
| IL | Interleukin |
| IRF | IFN Regulatory Factors |
| IL-1RA | IL-1 Receptor Antagonist |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic Acid |
| MDA5 | Melanoma Differentiation-Associated Protein 5 |
| MSH | Melanocyte Stimulating Hormone |
| NLR | Nucleotide Binding Domain Leucine-Rich Protein Receptor |
| NO | Nitric Oxide |
| Nrfr | Nuclear Factor- Erythroid 2 Related Factor |
| NSCs | Neuronal Stem Cells |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PGE2 | Prostaglandin E2 |
| PGN | Peptidoglycan |
| POA | Preoptic area |
| PYD | Pyrin Domain |
| OVLT | Organ Vascular Lamina Terminalis |
| RIG-1 | Retinoic Acid-Inducible Gene-1 |
| ROS | Reactive Oxygen Species |
| rvmPOA | Rostral Ventromedial POA |
| SSA | Saikosaponin |
| TLRs | Toll-Like Receptors |
| TNF | Tumor Necrosis Factor |
References
- McCusker, R.H.; Kelley, K.W. Immune–neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Zhang, W.-W.; Wu, C.-H.; Wang, S.-S.; Smith, F.G.; Jin, S.-W.; Zhang, P.-H. The Novel Role of Metabolism-Associated Molecular Patterns in Sepsis. Front. Cell. Infect. Microbiol. 2022, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, T.; Morimoto, A.; Yamaguchi, K.; Watanabe, T.; Long, N.C.; Murakami, N. Organum vasculosum laminae terminalis (OVLT) is a brain site to produce interleukin-1β during fever. Brain Res. 1993, 618, 155–159. [Google Scholar] [CrossRef]
- Upton, B.A.; D’Souza, S.P.; Lang, R.A. QPLOT Neurons—Converging on a Thermoregulatory Preoptic Neuronal Population. Front. Neurosci. 2021, 15, 665762. [Google Scholar] [CrossRef]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047, Correction in: Proc. Natl. Acad. Sci. USA 2017, 114, E1755. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, J.K.; Scammell, T.E.; Jacobson, C.D.; Saper, C.B. Distribution of fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp. Neurol. 1996, 371, 85–103. [Google Scholar] [CrossRef]
- Brito, H.O.; Radulski, D.; Wilhelms, D.B.; Stojakovic, A.; Brito, L.M.O.; Gil da Costa, R.M.; Trindade, E.; Engblom, D.; Franco, C.R.C.; Zampronio, A.R. Immune-mediated febrile response in female rats: Role of central hypothalamic mediators. Sci. Rep. 2020, 10, 4073. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Yoshida, K.; Coppari, R.; Bass, C.; Mochizuki, T.; Lowell, B.B.; Saper, C.B. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007, 10, 1131–1133. [Google Scholar] [CrossRef]
- Machado, N.L.; Bandaru, S.S.; Abbott, S.B.; Saper, C.B. EP3R-Expressing Glutamatergic Preoptic Neurons Mediate Inflammatory Fever. J. Neurosci. 2020, 40, 2573–2588. [Google Scholar] [CrossRef]
- The Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission). Glossary of terms for thermal physiology, second edition. Pflug. Arch. 1987, 410, 567–587. [Google Scholar] [CrossRef]
- Santacroce, L.; Bottalico, L.; Charitos, I.A. Greek Medicine Practice at Ancient Rome: The Physician Molecularist Asclepiades. Medicines 2017, 4, 92. [Google Scholar] [CrossRef]
- Dinarello, C.A. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J. Endotoxin Res. 2004, 10, 201–222. [Google Scholar] [CrossRef]
- Bernheim, H.A.; Block, L.H.; Atkins, E. Fever: Pathogenesis, Pathophysiology, and Purpose. Ann. Intern. Med. 1979, 91, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Topi, S.; Haxhirexha, K.; Hidri, S.; Charitos, I.A.; Bottalico, L. Medicine and Healing in the Pre-Socratic Thought—A Brief Analysis of Magic and Rationalism in Ancient Herbal Therapy. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Raffaella, T.; Fiore, F.; Fabrizia, M.; Francesco, P.; Arcangela, I.; Salvatore, S.; Luigi, S.; Nicola, B. Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sci. 2012, 91, 237–243. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. Sepsis: From Historical Aspects to Novel Vistas. Pathogenic and Therapeutic Considerations. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Garrana, R.; Mohangi, G.; Maló, P.; Nobre, M. Leakage of Microbial Endotoxin through the Implant-Abutment Interface in Oral Implants: An In Vitro Study. BioMed Res. Int. 2016, 2016, 9219071. [Google Scholar] [CrossRef] [PubMed]
- Niven, D.J.; Laupland, K.B.; Tabah, A.; Vesin, A.; Rello, J.; Koulenti, D.; Dimopoulos, G.; de Waele, J.; Timsit, J.-F.; the EUROBACT Investigators. Diagnosis and management of temperature abnormality in ICUs: A EUROBACT investigators’ survey. Crit. Care 2013, 17, R289. [Google Scholar] [CrossRef]
- DeWitt, S.; Chavez, S.A.; Perkins, J.; Long, B.; Koyfman, A. Evaluation of fever in the emergency department. Am. J. Emerg. Med. 2017, 35, 1755–1758. [Google Scholar] [CrossRef]
- Santacroce, L.; Cazzolla, A.P.; Lovero, R.; Brescia, V.; Ciavarella, D.; Spirito, F.; Colella, M.; Bilancia, M.; Muzio, L.L.; Di Serio, F. Neurosensory alterations and Interleukins Cascade in SARS-CoV-2 Infection—Results from a Retrospective Cohort of COVID-19 Inpatients. Endocr. Metab. Immune Disord. Drug Targets 2023. ahead of print. [Google Scholar] [CrossRef]
- Osaka, T. The EP3 and EP4 Receptor Subtypes both Mediate the Fever-producing Effects of Prostaglandin E2 in the Rostral Ventromedial Preoptic Area of the Hypothalamus in Rats. Neuroscience 2022, 494, 25–37. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Scammell, T.E.; Lee, C.; Kelly, J.F.; Nantel, F.; Elmquist, J.K.; Saper, C.B. Relationship of EP1-4 prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J. Comp. Neurol. 2000, 428, 20–32. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Kobayashi, T.; Sugimoto, Y.; Ichikawa, A.; Ushikubi, F.; Narumiya, S.; Saper, C.B. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J. Physiol. 2003, 551, 945–954. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Saper, C.B. Contrasting effects of E type prostaglandin (EP) receptor agonists on core body temperature in rats. Brain Res. 2003, 968, 256–262. [Google Scholar] [CrossRef]
- Oka, K.; Oka, T.; Hori, T. Prostaglandin E2 may induce hyperthermia through EP1 receptor in the anterior wall of the third ventricle and neighboring preoptic regions. Brain Res. 1997, 767, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rivest, S. Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur. J. Neurosci. 1999, 11, 2651–2668. [Google Scholar] [CrossRef] [PubMed]
- Osterhout, J.A.; Kapoor, V.; Eichhorn, S.W.; Vaughn, E.; Moore, J.D.; Liu, D.; Lee, D.; DeNardo, L.A.; Luo, L.; Zhuang, X.; et al. A preoptic neuronal population controls fever and appetite during sickness. Nature 2022, 606, 937–944. [Google Scholar] [CrossRef]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Comprehensive biology of antipyretic pathways. Cytokine 2019, 116, 120–127. [Google Scholar] [CrossRef]
- Hampton, T. Fever Induces a Molecular Homing Response in Immune Cells During Infection. JAMA 2019, 321, 1657–1658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef]
- Salvi, V.; Vaira, X.; Gianello, V.; Vermi, W.; Bugatti, M.; Sozzani, S.; Bosisio, D. TLR Signalling Pathways Diverge in Their Ability to Induce PGE2. Mediat. Inflamm. 2016, 2016, 5678046. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.A.; Chakravarty, S.; Rudaya, A.Y.; Herkenham, M.; Romanovsky, A.A. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood 2006, 107, 4000–4002. [Google Scholar] [CrossRef]
- Turrin, N.P.; Rivest, S. Unraveling the Molecular Details Involved in the Intimate Link between the Immune and Neuroendocrine Systems. Exp. Biol. Med. 2004, 229, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 Forms an IL-1β-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Khare, S.; Dorfleutner, A.; Bryan, N.B.; Yun, C.; Radian, A.D.; de Almeida, L.; Rojanasakul, Y.; Stehlik, C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity 2012, 36, 464–476. [Google Scholar] [CrossRef]
- Kinoshita, T.; Wang, Y.; Hasegawa, M.; Imamura, R.; Suda, T. PYPAF3, a PYRIN-containing APAF-1-like Protein, Is a Feedback Regulator of Caspase-1-dependent Interleukin-1β Secretion. J. Biol. Chem. 2005, 280, 21720–21725. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Shi, J.; Shao, F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl. Acad. Sci. USA 2013, 110, 14408–14413. [Google Scholar] [CrossRef]
- Qu, Y.; Misaghi, S.; Izrael-Tomasevic, A.; Newton, K.; Gilmour, L.L.; Lamkanfi, M.; Louie, S.; Kayagaki, N.; Liu, J.; Kömüves, L.; et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 2012, 490, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Richards, N.; Schaner, P.; Diaz, A.; Stuckey, J.; Shelden, E.; Wadhwa, A.; Gumucio, D.L. Interaction between Pyrin and the Apoptotic Speck Protein (ASC) Modulates ASC-induced Apoptosis. J. Biol. Chem. 2001, 276, 39320–39329. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Lovero, R.; D’Agostino, D.; Nisi, L.; Miragliotta, G.; Contino, R.; Man, A.; Ciccone, M.M.; Santacroce, L. Evaluation of procalcitonin, vitamin d and C-reactive protein levels in septic patients, with positive emocoltures. Our preliminary experience. Acta Med. Mediterr. 2016, 32, 1911–1914. [Google Scholar] [CrossRef]
- Equils, O.; Kellogg, C.; McGregor, J.; Gravett, M.; Neal-Perry, G.; Gabay, C. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complications†. Biol. Reprod. 2020, 103, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Hopkins, S.; Luheshi, G.N. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br. J. Pharmacol. 1997, 120, 1274–1279. [Google Scholar] [CrossRef]
- Ching, S.; Zhang, H.; Belevych, N.; He, L.; Lai, W.; Pu, X.-A.; Jaeger, L.B.; Chen, Q.; Quan, N. Endothelial-Specific Knockdown of Interleukin-1 (IL-1) Type 1 Receptor Differentially Alters CNS Responses to IL-1 Depending on Its Route of Administration. J. Neurosci. 2007, 27, 10476–10486. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1β (IL-1β) Processing Pathway. Sci. Signal. 2010, 3, cm2. [Google Scholar] [CrossRef]
- Nadjar, A.; Tridon, V.; May, M.J.; Ghosh, S.; Dantzer, R.; Amédée, T.; Parnet, P. NFκB Activates in vivo the Synthesis of Inducible Cox-2 in the Brain. J. Cereb. Blood Flow Metab. 2005, 25, 1047–1059. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Nilsberth, C.; Elander, L.; Hamzic, N.; Norell, M.; Lönn, J.; Engströ;m, L.; Blomqvist, A. The Role of Interleukin-6 in Lipopolysaccharide-Induced Fever by Mechanisms Independent of Prostaglandin E2. Endocrinology 2009, 150, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Harden, L.M.; du Plessis, I.; Poole, S.; Laburn, H.P. Interleukin (IL)-6 and IL-1β act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav. Immun. 2008, 22, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, A.; Mirrasekhian, E.; Dufour, S.; Schwaninger, M.; Engblom, D.; Blomqvist, A. Immune-Induced Fever Is Mediated by IL-6 Receptors on Brain Endothelial Cells Coupled to STAT3-Dependent Induction of Brain Endothelial Prostaglandin Synthesis. J. Neurosci. 2014, 34, 15957–15961. [Google Scholar] [CrossRef]
- Zhang, Z.; La Placa, D.; Nguyen, T.; Kujawski, M.; Le, K.; Li, L.; Shively, J.E. CEACAM1 regulates the IL-6 mediated fever response to LPS through the RP105 receptor in murine monocytes. BMC Immunol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Charitos, I.A.; Castellaneta, F.; Santacroce, L.; Bottalico, L. Historical Anecdotes and Breakthroughs of Histamine: From Discovery to Date. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 801–814. [Google Scholar] [CrossRef]
- Michie, H.R.; Spriggs, D.R.; Manogue, K.R.; Sherman, M.L.; Revhaug, A.; O’Dwyer, S.T.; Arthur, K.; Dinarello, C.A.; Cerami, A.; Wolff, S.M. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery 1988, 104, 280–286. [Google Scholar] [PubMed]
- Roth, J.; Martin, D.; Störr, B.; Zeisberger, E. Neutralization of pyrogen-induced tumour necrosis factor by its type 1 soluble receptor in guinea-pigs: Effects on fever and interleukin-6 release. J. Physiol. 1998, 509, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Engström, L.; Mackerlova, L.; Jakobsson, P.-J.; Blomqvist, A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R1100–R1107. [Google Scholar] [CrossRef]
- Thorén, S.; Jakobsson, P. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Eur. J. Biochem. 2000, 267, 6428–6434. [Google Scholar] [CrossRef]
- Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Montagnani, M.; Santacroce, L. Cardiac Involvement in COVID-19 Patients: A Contemporary Review. Infect. Dis. Rep. 2021, 13, 494–517. [Google Scholar] [CrossRef]
- Blatteis, C.M. Endotoxic fever: New concepts of its regulation suggest new approaches to its management. Pharmacol. Ther. 2006, 111, 194–223. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wetmore, L.; Sorensen, C.M.; Greenberg, A.H.; Nance, D.M. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 1994, 34, 7–14. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanisms of Fever Production and Lysis: Lessons from Experimental LPS Fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar] [CrossRef]
- Perlik, V.; Li, Z.; Goorha, S.; Ballou, L.R.; Blatteis, C.M. LPS-activated complement, not LPS per se, triggers the early release of PGE2 by Kupffer cells. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R332–R339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schieferdecker, H.L.; Schlaf, G.; Jungermann, K.; Götze, O. Functions of anaphylatoxin C5a in rat liver: Direct and indirect actions on nonparenchymal and parenchymal cells. Int. Immunopharmacol. 2001, 1, 469–481. [Google Scholar] [CrossRef]
- Wilhelms, D.B.; Kirilov, M.; Mirrasekhian, E.; Eskilsson, A.; Kugelberg, U.; Klar, C.; Ridder, D.A.; Herschman, H.R.; Schwaninger, M.; Blomqvist, A.; et al. Deletion of Prostaglandin E2 Synthesizing Enzymes in Brain Endothelial Cells Attenuates Inflammatory Fever. J. Neurosci. 2014, 34, 11684–11690. [Google Scholar] [CrossRef]
- Steiner, A.; Ivanov, A.I.; Serrats, J.; Hosokawa, H.; Phayre, A.N.; Robbins, J.; Roberts, J.L.; Kobayashi, S.; Matsumura, K.; Sawchenko, P.E.; et al. Cellular and Molecular Bases of the Initiation of Fever. PLoS Biol. 2006, 4, e284. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-T.; Lin, M.-T.; Chang, C.-P. An NMDA receptor-dependent hydroxyl radical pathway in the rabbit hypothalamus may mediate lipopolysaccharide fever. Neuropharmacology 2006, 50, 504–511. [Google Scholar] [CrossRef]
- Suffredini, A.F.; Noveck, R.J. Human Endotoxin Administration as an Experimental Model in Drug Development. Clin. Pharmacol. Ther. 2014, 96, 418–422. [Google Scholar] [CrossRef]
- Kyvelidou, C.; Sotiriou, D.; Zerva, I.; Athanassakis, I. Protection Against Lipopolysaccharide-Induced Immunosuppression by IgG and IgM. Shock 2018, 49, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Engler, H.; Wegner, A.; Rebernik, L.; Spreitzer, I.; Schedlowski, M.; Elsenbruch, S. What Makes You Feel Sick After Inflammation? Predictors of Acute and Persisting Physical Sickness Symptoms Induced by Experimental Endotoxemia. Clin. Pharmacol. Ther. 2017, 102, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Rudaya, A.Y.; Steiner, A.; Robbins, J.; Dragic, A.S.; Romanovsky, A.A. Thermoregulatory responses to lipopolysaccharide in the mouse: Dependence on the dose and ambient temperature. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R1244–R1252. [Google Scholar] [CrossRef] [PubMed]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; Wildenberg, J.V.D.; Sweep, F.C.G.J.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef] [PubMed]
- Degré, M. Cytokines and bacterial infections. Biotherapy 1996, 8, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Garimella, R.; Halye, J.L.; Harrison, W.; Klebba, P.E.; Rice, C.V. Conformation of the Phosphate d-Alanine Zwitterion in Bacterial Teichoic Acid from Nuclear Magnetic Resonance Spectroscopy. Biochemistry 2009, 48, 9242–9249. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Zeng, Z.; Surewaard, B.G.; Wong, C.H.; Geoghegan, J.A.; Jenne, C.N.; Kubes, P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe 2016, 20, 99–106. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef]
- Szentirmai, É.; Massie, A.R.; Kapás, L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav. Immun. 2021, 92, 184–192. [Google Scholar] [CrossRef]
- Gottwein, E.; Cullen, B.R. Viral and Cellular MicroRNAs as Determinants of Viral Pathogenesis and Immunity. Cell Host Microbe 2008, 3, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mittal, S.; Tripathi, L.P.; Nussinov, R.; Ahmad, S. Host-pathogen protein-nucleic acid interactions: A comprehensive review. Comput. Struct. Biotechnol. J. 2022, 20, 4415–4436. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef]
- Ashman, R.F.; Goeken, J.A.; Latz, E.; Lenert, P. Optimal oligonucleotide sequences for TLR9 inhibitory activity in human cells: Lack of correlation with TLR9 binding. Int. Immunol. 2011, 23, 203–214. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Mikamo-Satoh, E.; Hirai, R.; Kawai, T.; Matsushita, K.; Hiiragi, A.; Dermody, T.S.; Fujita, T.; Akira, S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid–inducible gene-I and melanoma differentiation–associated gene 5. J. Exp. Med. 2008, 205, 1601–1610. [Google Scholar] [CrossRef]
- Miyake, K.; Shibata, T.; Ohto, U.; Shimizu, T.; Saitoh, S.-I.; Fukui, R.; Murakami, Y. Mechanisms controlling nucleic acid-sensing Toll-like receptors. Int. Immunol. 2018, 30, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Akira, S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef]
- Kim-Hellmuth, S.; Kaiser, V.; Beier, E.; Bechheim, M.; Guenthner-Biller, M.; Ablasser, A.; Berger, M.; Endres, S.; Hartmann, G.; Hornung, V. Self-priming determines high type I IFN production by plasmacytoid dendritic cells. Eur. J. Immunol. 2014, 44, 807–818. [Google Scholar] [CrossRef]
- Vanderschueren, S.; Knockaert, D.; Adriaenssens, T.; Demey, W.; Durnez, A.; Blockmans, D.; Bobbaers, H. From Prolonged Febrile Illness to Fever of Unknown Origin. Arch. Intern. Med. 2003, 163, 1033–1041. [Google Scholar] [CrossRef]
- Wright, W.F.; Mulders-Manders, C.M.; Auwaerter, P.G.; Bleeker-Rovers, C.P. Fever of Unknown Origin (FUO)—A Call for New Research Standards and Updated Clinical Management. Am. J. Med. 2022, 135, 173–178. [Google Scholar] [CrossRef] [PubMed]
- De Kleijn, E.M.H.A.; Vandenbroucke, J.P.; Van Der Meer, J.W.M.; The Netherlands FUO Study Group. Fever of Unknown Origin (FUO): I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. Medicine 1997, 76, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.F.E.; Rodrigues, M.S.; Silva, M.P.A.; Brito, R.J.V.C.; Nicacio, J.M.; Duarte, R.M.S.C.; Gomes, O.V. Fever of unknown origin in special groups. Rev. Assoc. Med. Bras. 2019, 65, 1308–1313. [Google Scholar] [CrossRef]
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.M.; Pisapia, R.; Nardiello, S.; Cicala, S.D.; Gaeta, G.B.; Brancaccio, G. Fever of unknown origin (FUO): Which are the factors influencing the final diagnosis? A 2005–2015 systematic review. BMC Infect. Dis. 2019, 19, 653. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, G.B.; Fusco, F.M.; Nardiello, S. Fever of unknown origin: A systematic review of the literature for 1995–2004. Nucl. Med. Commun. 2006, 27, 205–211. [Google Scholar] [CrossRef]
- Wallace, B.I.; Kenney, B.; Malani, P.N.; Clauw, D.J.; Nallamothu, B.K.; Waljee, A.K. Prevalence of Immunosuppressive Drug Use Among Commercially Insured US Adults, 2018–2019. JAMA Netw. Open 2021, 4, e214920. [Google Scholar] [CrossRef]
- Bissuel, F.; Leport, C.; Perronne, C.; Longuet, P.; Vilde, J.-L. Fever of unknown origin in HIV-infected patients: A critical analysis of a retrospective series of 57 cases. J. Intern. Med. 1994, 236, 529–535. [Google Scholar] [CrossRef]
- Van Kempen, T.S.; Wenink, M.H.; Leijten, E.F.A.; Radstake, T.R.D.J.; Boes, M. Perception of self: Distinguishing autoimmunity from autoinflammation. Nat. Rev. Rheumatol. 2015, 11, 483–492. [Google Scholar] [CrossRef]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Haidar, G.; Dorritie, K.; Farah, R.; Bogdanovich, T.; Nguyen, M.H.; Samanta, P. Invasive Mold Infections After Chimeric Antigen Receptor–Modified T-Cell Therapy: A Case Series, Review of the Literature, and Implications for Prophylaxis. Clin. Infect. Dis. 2020, 71, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Garner, W.; Hill, J.A. Infections after anti-CD19 chimeric antigen receptor T-cell therapy for hematologic malignancies: Timeline, prevention, and uncertainties. Curr. Opin. Infect. Dis. 2020, 33, 449–457. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Singh, N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr. Opin. Infect. Dis. 2009, 22, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Bleeker-Rovers, C.P.; Vos, F.J.; de Kleijn, E.M.H.A.; Mudde, A.H.; Dofferhoff, T.S.M.; Richter, C.; Smilde, T.J.; Krabbe, P.F.M.; Oyen, W.J.G.; van der Meer, J.W.M. A Prospective Multicenter Study on Fever of Unknown Origin. Medicine 2007, 86, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.F.; Betz, J.F.; Auwaerter, P.G. Prospective Studies Comparing Structured vs Nonstructured Diagnostic Protocol Evaluations Among Patients With Fever of Unknown Origin: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2215000. [Google Scholar] [CrossRef]
- Betrains, A.; Wright, W.; Moreel, L.; Staels, F.; Blockmans, D.; Vanderschueren, S. Etiological spectrum and outcome of fever and inflammation of unknown origin. Does symptom duration matter? Eur. J. Intern. Med. 2022, 106, 103–110. [Google Scholar] [CrossRef]
- Erdem, H.; Baymakova, M.; Alkan, S.; Letaief, A.; Ben Yahia, W.; Dayyab, F.; Kolovani, E.; Grgic, S.; Cosentino, F.; Hasanoglu, I.; et al. Classical fever of unknown origin in 21 countries with different economic development: An international ID-IRI study. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 387–398. [Google Scholar] [CrossRef]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef]
- Knockaert, D.C.; Dujardin, K.S.; Bobbaers, H.J. Long-term follow-up of patients with undiagnosed fever of unknown origin. Arch. Intern. Med. 1996, 156, 618–620. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Korte, S.M.; Lentjes, E.G.; Romijn, F.; Schönbaum, E.; De Nicola, A.; De Kloet, E.R. The amount of free corticosterone is increased during lipopolysaccharide-induced fever. Life Sci. 2000, 66, 553–562. [Google Scholar] [CrossRef]
- Soriano, R.N.; Branco, L.G. Reduced stress fever is accompanied by increased glucocorticoids and reduced PGE2 in adult rats exposed to endotoxin as neonates. J. Neuroimmunol. 2010, 225, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Soriano, R.N.; Nicoli, L.G.; Carnio, E.C.; Branco, L.G. Exogenous ghrelin attenuates endotoxin fever in rats. Peptides 2011, 32, 2372–2376. [Google Scholar] [CrossRef]
- Wang, L.; Basa, N.R.; Shaikh, A.; Luckey, A.; Heber, D.; St-Pierre, D.H.; Taché, Y. LPS inhibits fasted plasma ghrelin levels in rats: Role of IL-1 and PGs and functional implications. Am. J. Physiol. Liver Physiol. 2006, 291, G611–G620. [Google Scholar] [CrossRef] [PubMed]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef]
- Yu, G.-M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Lee, K.M.; Lee, J.E. The Protective Effect of Melatonin on Neural Stem Cell against LPS-Induced Inflammation. BioMed Res. Int. 2015, 2015, 854359. [Google Scholar] [CrossRef]
- Gantz, I.; Fong, T.M. The melanocortin system. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E468–E474. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Holdeman, M.; Lipton, J.M. Analysis of the antipyretic action of alpha-melanocyte-stimulating hormone in rabbits. J. Physiol. 1985, 359, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-H.; Entwistle, M.L.; Alvaro, J.D.; Duman, R.S.; Hruby, V.J.; Tatro, J.B. Antipyretic Role of Endogenous Melanocortins Mediated by Central Melanocortin Receptors during Endotoxin-Induced Fever. J. Neurosci. 1997, 17, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-C.; Huang, C.-J. Vasopressin inhibits endotoxin-induced upregulation of inflammatory mediators in activated macrophages. Tzu Chi Med. J. 2013, 25, 150–154. [Google Scholar] [CrossRef]
- Park, J.; Eo, E.Y.; Lee, K.-H.; Park, J.S.; Lee, J.-H.; Yoo, C.-G.; Lee, C.-T.; Cho, Y.-J. The Anti-Inflammatory Effect of Arginine-Vasopressin on Lipopolysaccharide-Induced IκBα/Nuclear Factor-κB Cascade. Korean J. Crit. Care Med. 2015, 30, 151–157. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Velázquez-Martínez, C.A.; Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015, 98, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Liu, M.; Huang, Y.; Shi, J.; Dong, N.; Xu, K. Curcumin inhibits calcification of human aortic valve interstitial cells by interfering NF-κB, AKT, and ERK pathways. Phytother. Res. 2020, 34, 2074–2081. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- Kumar, P.; Sulakhiya, K.; Barua, C.C.; Mundhe, N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol. Cell. Biochem. 2017, 431, 113–122. [Google Scholar] [CrossRef]
- Hassan, F.U.; Rehman, M.S.-u.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- Reis, L.; Oliveira, M.K.; Rojas, V.C.T.; Batista, T.H.; Estevam, E.S.; Vitor-Vieira, F.; Vilela, F.C.; Giusti-Paiva, A. Curcumin attenuates LPS-induced sickness behavior and fever in rats by modulating Nrf2 activity. Neurosci. Lett. 2022, 781, 136680. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. BioMed Res. Int. 2017, 2017, 7597596. [Google Scholar] [CrossRef]
- Nagy-Bota, M.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.; Pintea-Simon, I.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control Against Enterococcus faecalis—A Preliminary Study. Appl. Sci. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef]
- Chen, E.; Chen, J.; Cao, S.-L.; Zhang, Q.-Z.; Jiang, X.-G. Preparation of nasal temperature-sensitive in situ gel of Radix Bupleuri and and evaluation of the febrile response mechanism. Drug Dev. Ind. Pharm. 2010, 36, 490–496. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, K.; Fang, X.; Lu, F.; Zhang, W.; Song, X.; Chen, L.; Sun, J.; Chen, H. Inhibition of Lipopolysaccharide-Induced Inflammatory Bone Loss by Saikosaponin D is Associated with Regulation of the RANKL/RANK Pathway. Drug Des. Dev. Ther. 2021, 15, 4741–4757. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Y.; Wang, H.; Niu, X.; Wang, J. Baicalin Weakens Staphylococcus aureus Pathogenicity by Targeting Sortase B. Front. Cell. Infect. Microbiol. 2018, 8, 418. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Lin, M.-T.; Wang, J.-J.; Liao, J.-F.; Huang, W.-T. The antipyretic effects of baicalin in lipopolysaccharide-evoked fever in rabbits. Neuropharmacology 2006, 51, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Lim, J.H.; Suh, S.-I.; Kwon, Y.-K.; Shin, S.-W.; Kim, S.C.; Choi, Y.H.; Park, J.-W.; Kwon, T.K. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPβ DNA-binding activity. Immunobiology 2006, 211, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.-S.; Hsu, J.-D.; Wang, C.-J.; Yang, S.-H.; Cheng, S.-Y.; Lee, H.-J. Polyphenols extracted from Hibiscus sabdariffa L. inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating Cyclooxygenase-2 Expression. Biosci. Biotechnol. Biochem. 2009, 73, 385–390. [Google Scholar] [CrossRef]
- Ran, X.; Ma, L.; Peng, C.; Zhang, H.; Qin, L.-P. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm. Biol. 2011, 49, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Huo, H.-R.; Li, C.-H.; Zhao, B.-S.; Li, L.-F.; Sui, F.; Guo, S.-Y.; Jiang, T.-L. Effects of cinnamaldehyde on PGE2 release and TRPV4 expression in mouse cerebral microvascular endothelial cells induced by interleukin-1β. Biol. Pharm. Bull. 2008, 31, 426–430. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Yuan, W.; Zhou, L.; Wang, S.-X.; Xie, Y.; Fu, Y.-J. Forsythoside A exerts an anti-endotoxin effect by blocking the LPS/TLR4 signaling pathway and inhibiting Tregs in vitro. Int. J. Mol. Med. 2017, 40, 243–250. [Google Scholar] [CrossRef]
- Serio, G.; Fortarezza, F.; Pezzuto, F.; Santacroce, L.; Nazzaro, P.; Bellitti, E.; Cavone, D.; Marzullo, A.; Vimercati, L. Legionnaires’ disease arising with hirsutism: Case report of an extremely confusing event. BMC Infect. Dis. 2021, 21, 532. [Google Scholar] [CrossRef]
- Xu, Y.; Oliverson, B.G.; Simmons, D.L. Trifunctional inhibition of COX-2 by extracts of Lonicera japonica: Direct inhibition, transcriptional and post-transcriptional down regulation. J. Ethnopharmacol. 2007, 111, 667–670. [Google Scholar] [CrossRef]
- Shuwen, H.; Kefeng, D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes 2022, 14, 2113717. [Google Scholar] [CrossRef]
- Santacroce, L.; Muzio, E.L.; Bottalico, L.; Spirito, F.; Charitos, I.A.; Passarelli, P.C.; Jirillo, E. Subversion of the Oral Microbiota and Induction of Immune-Mediated Systemic Inflammation with Special Reference to Periodontitis. Current Knowledge and Perspectives. Endocr. Metab. Immune Disord.-Drug Targets 2022, 23, 470–484. [Google Scholar] [CrossRef]
- Kao, P.H.N.; Kline, K.A. Dr. Jekyll and Mr. Hide: How Enterococcus faecalis Subverts the Host Immune Response to Cause Infection. J. Mol. Biol. 2019, 431, 2932–2945. [Google Scholar] [CrossRef]
- Haidar, G.; Singh, N. Fever of Unknown Origin. N. Engl. J. Med. 2022, 386, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Jirillo, E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-Converting Enzyme 2 as a Potential Drug Target-a Perspective. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herbal therapy: A brief history of Panax ginseng. J. Ginseng Res. 2022, in press. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, Z.; Wang, W.-X.; Huang, Y.-X.; Zhang, Q.; Li, J.-J.; Tang, Y.-P.; Yue, S.-J. Methodology of network pharmacology for research on Chinese herbal medicine against COVID-19: A review. J. Integr. Med. 2022, 20, 477–487. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. https://doi.org/10.3390/metabo13030461
Santacroce L, Colella M, Charitos IA, Di Domenico M, Palmirotta R, Jirillo E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites. 2023; 13(3):461. https://doi.org/10.3390/metabo13030461
Chicago/Turabian StyleSantacroce, Luigi, Marica Colella, Ioannis Alexandros Charitos, Marina Di Domenico, Raffaele Palmirotta, and Emilio Jirillo. 2023. "Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications" Metabolites 13, no. 3: 461. https://doi.org/10.3390/metabo13030461
APA StyleSantacroce, L., Colella, M., Charitos, I. A., Di Domenico, M., Palmirotta, R., & Jirillo, E. (2023). Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites, 13(3), 461. https://doi.org/10.3390/metabo13030461








