GC/EI/MS and 1H NMR Metabolomics Reveal the Effect of an Olive Tree Endophytic Bacillus sp. Lipopeptide Extract on the Metabolism of Colletotrichum acutatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Biological Material, Growth Conditions
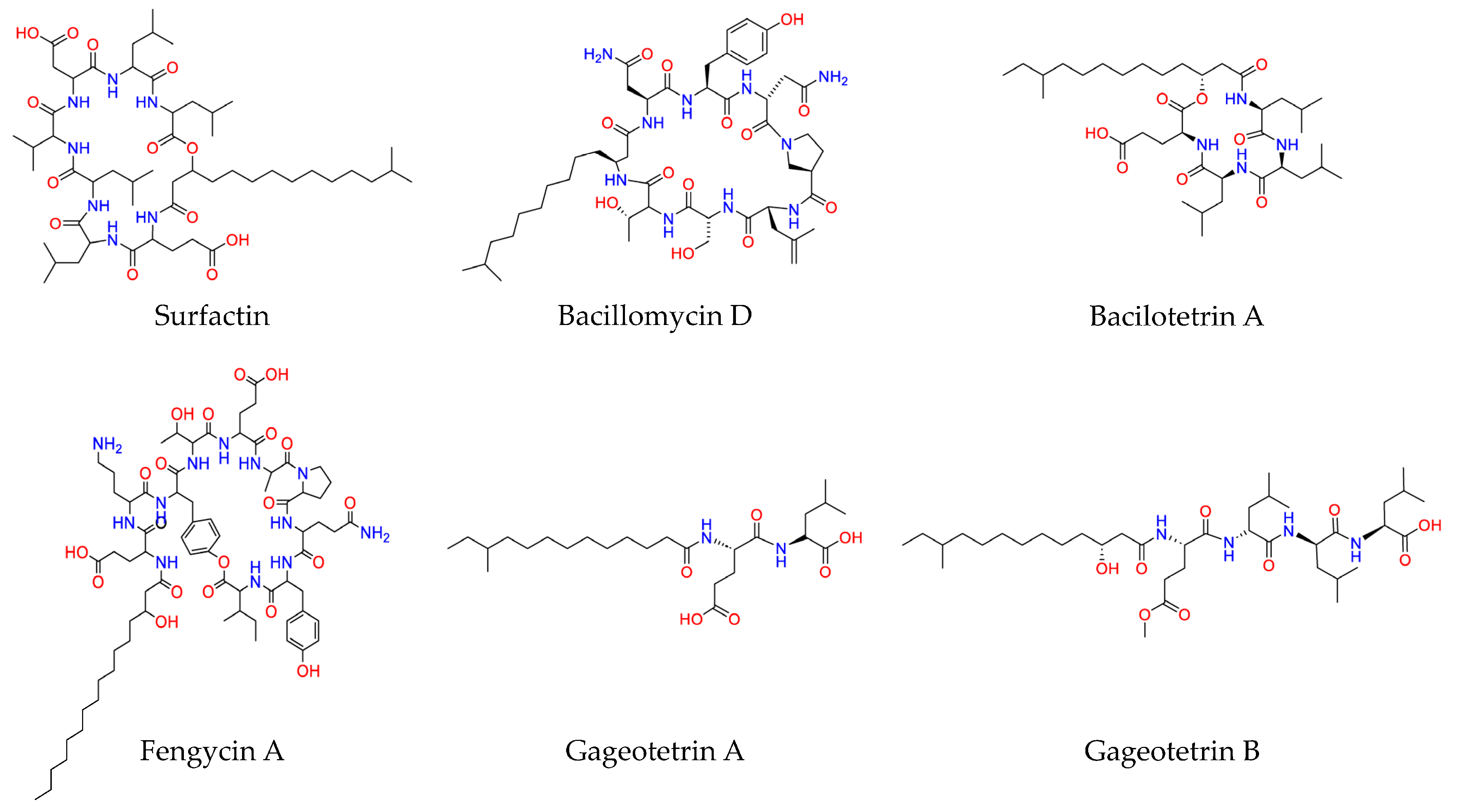
2.3. Extraction and Annotation of the Bacillus PTA13 Lipopeptides (LP)
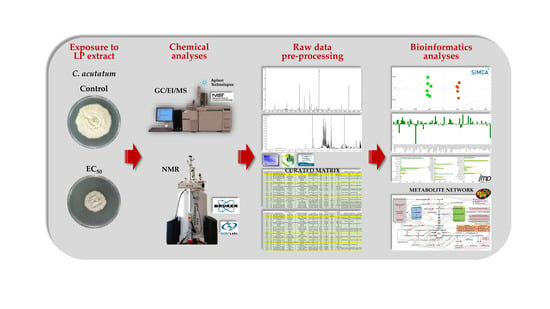
2.4. Dissecting the Toxicity of the Bacillus sp. PTA13 Lipopeptide Extract on the Metabolism of Colletotrichum acutatum by GC/EI/MS and 1H NMR Metabolomics
2.4.1. Experimental Design
2.4.2. Sampling of Colletotrichum acutatum Cultures, Metabolome Extraction, and Sample Preparation for GC/EI/MS and 1H NMR Metabolomics Analyses
2.4.3. Analytical Conditions for the Recording of the Colletotrichum acutatum Metabolite Profile
2.4.4. Data Pre-Processing, Trend, and Biomarker Discovery
3. Results and Discussion
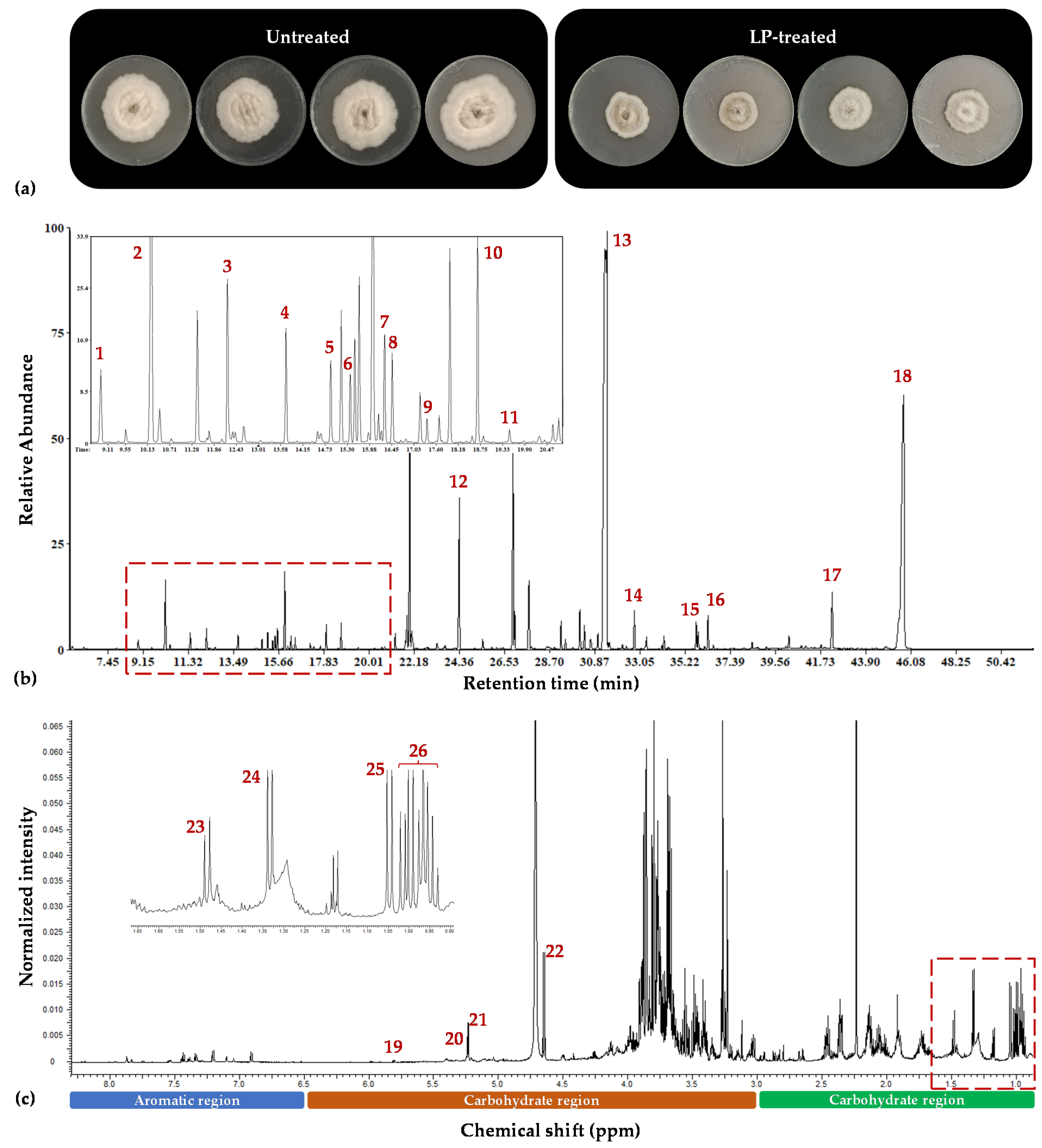
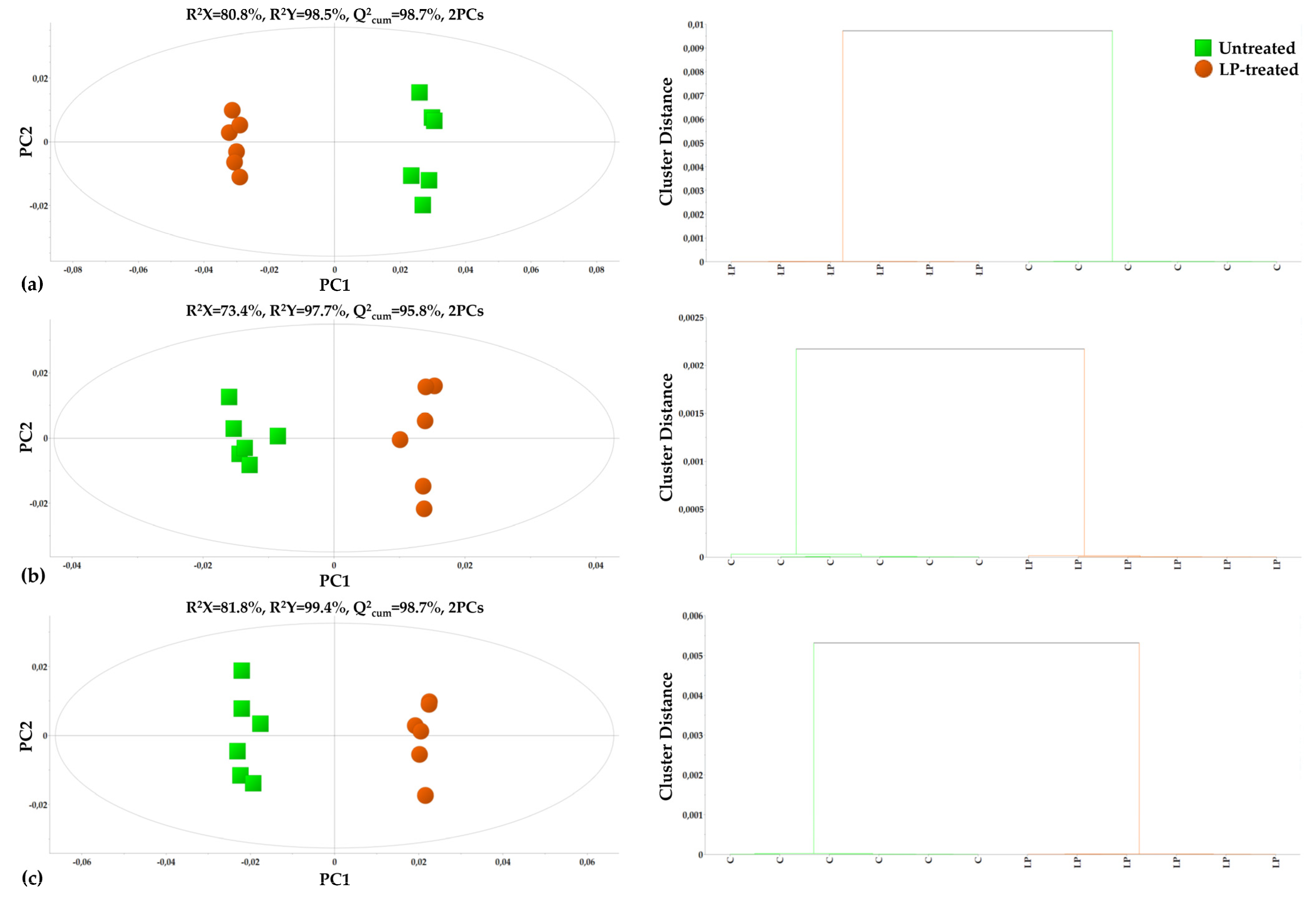
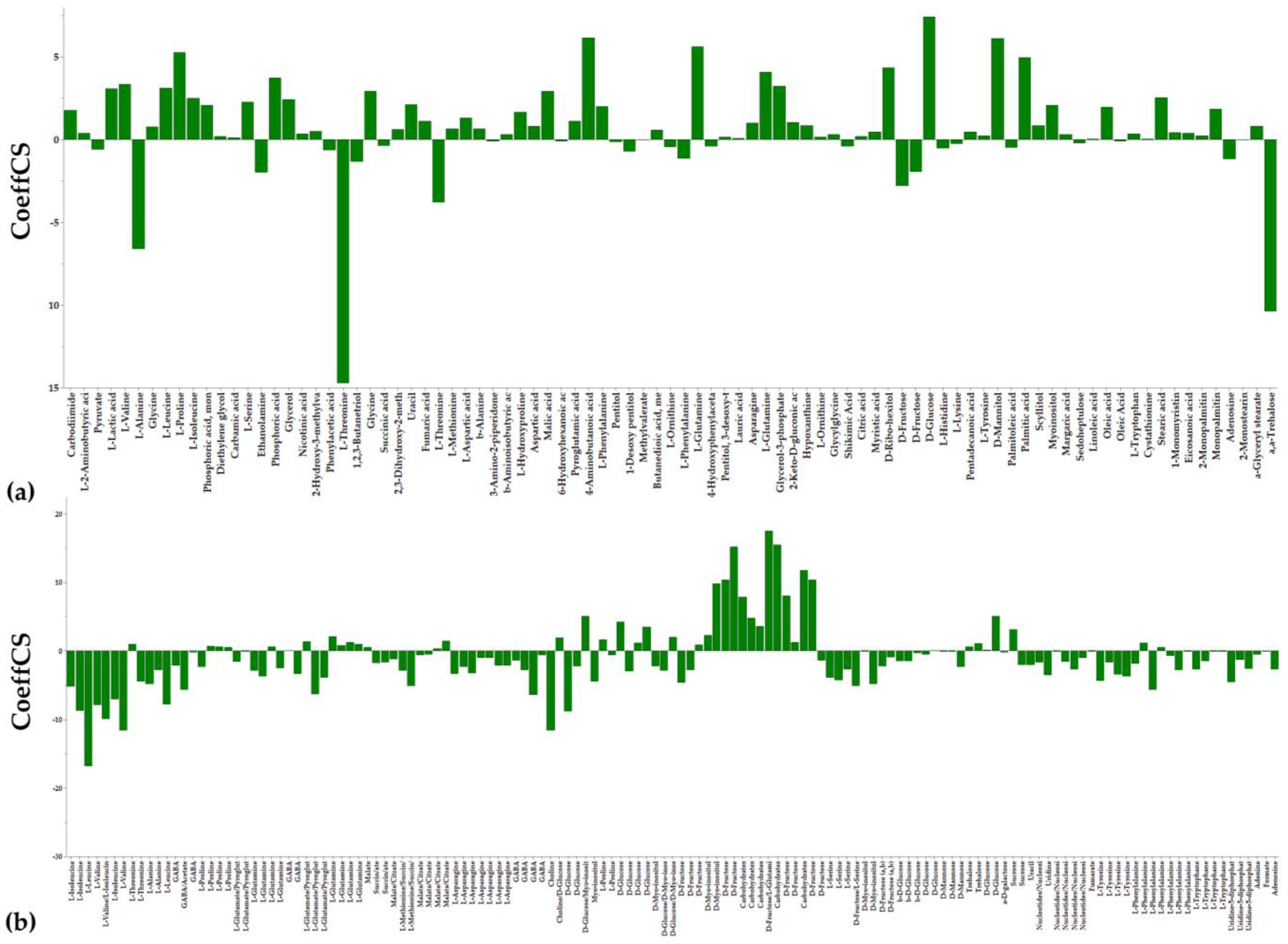
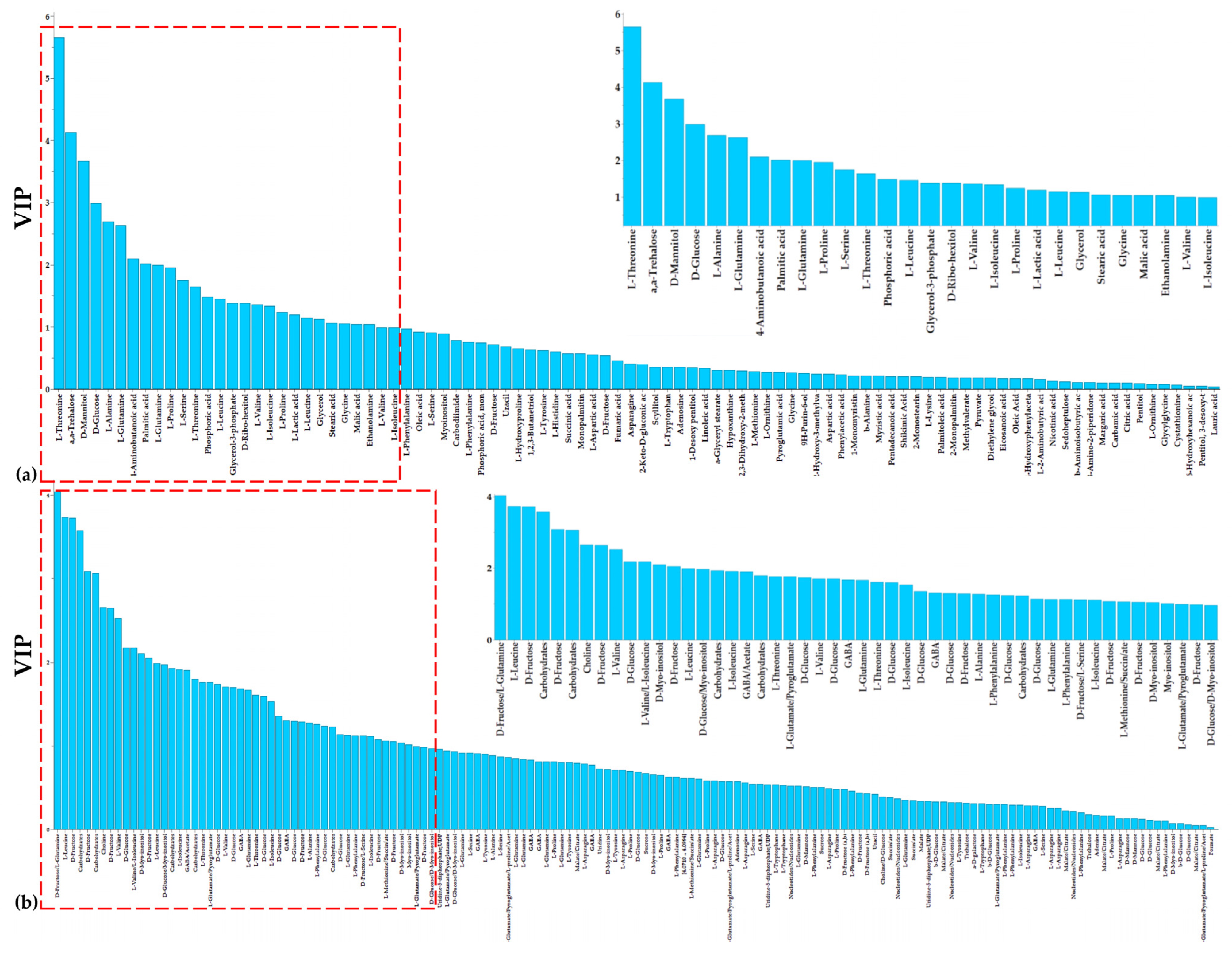
3.1. Overview of the Metabolomics Analysis
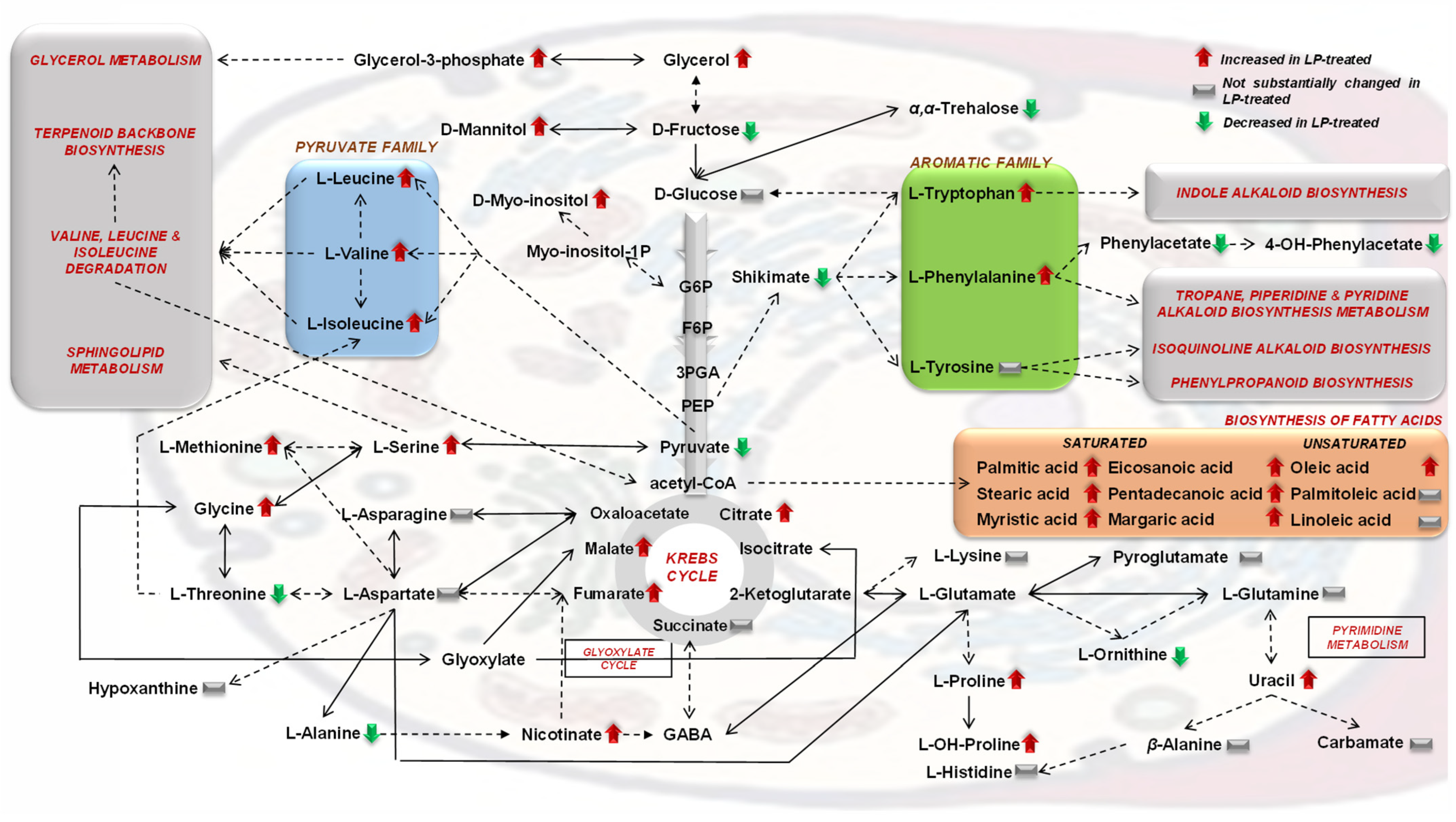
3.2. The Aromatic Amino Acid Metabolism of Colletotrichum acutatum Is Substantially Affected by the Bacillus sp. PTA13 Lipopeptide Extract
3.3. Effect of the Bacillus sp. PTA13 Lipopeptide Extract on the Fatty Acid Composition and Energy Equilibrium of Colletotrichum acutatum
3.4. The Bacillus sp. PTA13 Lipopeptide Extract Substantially Affects the Biosynthesis of Colletotrichum acutatum Metabolites That Play Key Roles in Its Physiology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topping, C.J.; Aldrich, A.; Berny, P. Overhaul environmental risk assessment for pesticides. Science 2020, 367, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Angelis, A.; Antoniadi, L.; Aliferis, K.A.; Skaltsounis, A.-L. Discovering the Next-Generation Plant Protection Products: A Proof-of-Concept via the Isolation and Bioactivity Assessment of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides. Metabolites 2021, 11, 833. [Google Scholar] [CrossRef]
- Kolainis, S.; Koletti, A.; Lykogianni, M.; Karamanou, D.; Gkizi, D.; Tjamos, S.E.; Paraskeuopoulos, A.; Aliferis, K.A. An integrated approach to improve plant protection against olive anthracnose caused by the Colletotrichum acutatum species complex. PLoS ONE 2020, 15, e0233916. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Giaki, K.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics 2022, 10, 494. [Google Scholar] [CrossRef]
- ChemDraw. Available online: https://perkinelmerinformatics.com/products/research/chemdraw (accessed on 13 March 2023).
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [PubMed]
- Penha, R.O.; Vandenberghe, L.P.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef]
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215. [Google Scholar] [CrossRef]
- Baumgart, F.; Kluge, B.; Ullrich, C.; Vater, J.; Ziessow, D. Identification of amino acid substitutions in the lipopeptide surfactin using 2D NMR spectroscopy. Biochem. Biophys. Res. Commun. 1991, 177, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Kowall, M.; Vater, J.; Kluge, B.; Stein, T.; Franke, P.; Ziessow, D. Separation and characterization of Surfactin isoforms produced by Bacillus subtilis OKB 105. J. Colloid Interface Sci. 1998, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R.; Mouillon, J.-M.; Pohl, S.; Arnau, J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 2018, 42, 721–738. [Google Scholar] [CrossRef]
- Malviya, D.; Sahu, P.K.; Singh, U.B.; Paul, S.; Gupta, A.; Gupta, A.R.; Singh, S.; Kumar, M.; Paul, D.; Rai, J.P. Lesson from ecotoxicity: Revisiting the microbial lipopeptides for the management of emerging diseases for crop protection. Int. J. Environ. Res. Public Health 2020, 17, 1434. [Google Scholar] [CrossRef]
- Liu, J.-F.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int. J. Mol. Sci. 2015, 16, 4814–4837. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Silveira, E.; Pereira, B.B. Toxicity and applications of surfactin for health and environmental biotechnology. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 382–399. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, L.; Ding, J.; Wang, M.; Ge, R.; Zhao, H.; Zhang, B.; Fan, J. Natural antimicrobial lipopeptides secreted by Bacillus spp. and their application in food preservation, a critical review. Trends Food Sci. Technol. 2022, 127, 26–37. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.; Liu, W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pest. Biochem. Physiol. 2020, 163, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.B.; Meyer, T.; Arias, A.A.; Ongena, M.; Oni, F.E.; Höfte, M. Bacillus cyclic lipopeptides iturin and fengycin control rice blast caused by Pyricularia oryzae in potting and acid sulfate soils by direct antagonism and induced systemic resistance. Microorganisms 2021, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and Mechanistic Insights Into the Direct and Indirect Antimicrobial Properties of Bacillus subtilis Lipopeptides on Plant Pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Romo, T.D.; Grossfield, A. Selectivity and mechanism of fengycin, an antimicrobial lipopeptide, from molecular dynamics. J. Phys. Chem. B 2018, 122, 2219–2226. [Google Scholar] [CrossRef]
- Deleu, M.; Bouffioux, O.; Razafindralambo, H.; Paquot, M.; Hbid, C.; Thonart, P.; Jacques, P.; Brasseur, R. Interaction of surfactin with membranes: A computational approach. Langmuir 2003, 19, 3377–3385. [Google Scholar] [CrossRef]
- Karamanou, D.A.; Aliferis, K.A. The yeast (Saccharomyces cerevisiae) YCF1 vacuole transporter: Evidence on its implication into the yeast resistance to flusilazole as revealed by GC/EI/MS metabolomics. Pest. Biochem. Physiol. 2020, 165, 104475. [Google Scholar] [CrossRef]
- Sevastos, A.; Kalampokis, I.; Panagiotopoulou, A.; Pelecanou, M.; Aliferis, K. Implication of Fusarium graminearum primary metabolism in its resistance to benzimidazole fungicides as revealed by 1H NMR metabolomics. Pest. Biochem. Physiol. 2018, 148, 50–61. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Haslam, E. Shikimic Acid: Metabolism and Metabolites, 1st ed.; Wiley: Chichester, UK, 1993. [Google Scholar]
- Martínez, J.A.; Bolívar, F.; Escalante, A. Shikimic acid production in Escherichia coli: From classical metabolic engineering strategies to omics applied to improve its production. Front. Bioeng. Biotechnol. 2015, 3, 145. [Google Scholar] [CrossRef]
- Guo, Y.-W.; Gong, B.-Q.; Yuan, J.; Li, H.-J.; Mahmud, T.; Huang, Y.; Li, J.-F.; Yang, D.-P.; Lan, W.-J. L-phenylalanine alters the privileged secondary metabolite production in the marine-derived fungus Trichoderma erinaceum F1-1. J. Nat. Prod. 2019, 83, 79–87. [Google Scholar] [CrossRef]
- Guo, Y.-W.; Liu, X.-J.; Yuan, J.; Li, H.-J.; Mahmud, T.; Hong, M.-J.; Yu, J.-C.; Lan, W.-J. L-tryptophan induces a marine-derived Fusarium sp. to produce indole alkaloids with activity against the Zika virus. J. Nat. Prod. 2020, 83, 3372–3380. [Google Scholar] [CrossRef]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and plant phenylalanine ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef]
- Lomascolo, A.; Lesage-Meessen, L.; Haon, M.; Navarro, D.; Antona, C.; Faulds, C.; Marcel, A. Evaluation of the potential of Aspergillus niger species for the bioconversion of L-phenylalanine into 2-phenylethanol. World J. Microbiol. Biotechnol. 2001, 17, 99–102. [Google Scholar] [CrossRef]
- Liu, S.; Xu, J.-Z.; Zhang, W.-G. Advances and prospects in metabolic engineering of Escherichia coli for l-tryptophan production. World J. Microbiol. Biotechnol. 2022, 38, 22. [Google Scholar] [CrossRef]
- Zelante, T.; Choera, T.; Beauvais, A.; Fallarino, F.; Paolicelli, G.; Pieraccini, G.; Pieroni, M.; Galosi, C.; Beato, C.; De Luca, A. Aspergillus fumigatus tryptophan metabolic route differently affects host immunity. Cell Rep. 2021, 34, 108673. [Google Scholar] [CrossRef]
- Cohen, B.A.; Amsellem, Z.; Maor, R.; Sharon, A.; Gressel, J. Transgenically enhanced expression of indole-3-acetic acid confers hypervirulence to plant pathogens. Phytopathology 2002, 92, 590–596. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ito, M. Valuation of Phytotoxic Activity of Naturally Occurring Phenolic Compounds. J. Weed Sci. Technol. 1998, 43, 341–348. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Cubeta, M.A.; Jabaji, S. Chemotaxonomy of fungi in the Rhizoctonia solani species complex performing GC/MS metabolite profiling. Metabolomics 2013, 9, 159–169. [Google Scholar] [CrossRef]
- Aoki, H.; Sassa, T.; Tamura, T. Phytotoxic metabolites of Rhizoctonia solani. Nature 1963, 200, 575. [Google Scholar] [CrossRef]
- Kachlicki, P.; Jędryczka, M. Phenylacetic Acid and Methyl p-Hydroxyphenylacetate—Novel Phytotoxins of Fusarium oxysporum. Cereal Res. Commun. 1997, 25, 853–855. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lim, S.W.; Kim, B.S.; Lee, J.Y.; Moon, S.S. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl. Environ. Microbiol. 2001, 67, 3739–3745. [Google Scholar] [CrossRef] [PubMed]
- Bartz, F.E.; Glassbrook, N.J.; Danehower, D.A.; Cubeta, M.A. Elucidating the role of the phenylacetic acid metabolic complex in the pathogenic activity of Rhizoctonia solani anastomosis group 3. Mycologia 2012, 104, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Bartz, F.E.; Glassbrook, N.J.; Danehower, D.A.; Cubeta, M.A. Modulation of the phenylacetic acid metabolic complex by quinic acid alters the disease-causing activity of Rhizoctonia solani on tomato. Phytochemistry 2013, 89, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, Z.; Shi, Y.; Guo, D.; Pang, B.; Chen, X.; Shao, D.; Liu, Y.; Shi, J. Bacillus subtilis inhibits Aspergillus carbonarius by producing iturin A, which disturbs the transport, energy metabolism, and osmotic pressure of fungal cells as revealed by transcriptomics analysis. Int. J. Food Microbiol. 2020, 330, 108783. [Google Scholar] [CrossRef]
- Trotter, P.J. The genetics of fatty acid metabolism in Saccharomyces cerevisiae. Annu. Rev. Nutr. 2001, 21, 97–119. [Google Scholar] [CrossRef]
- Kerwin, J.L. Fatty acids and fungal development: Structure-activity relationships. In Ecology and Metabolism of Plant Lipids; Fuller, G., Nes, W.D., Eds.; ACS Publications: Washington, DC, USA, 1987; Volume 325, pp. 329–342. [Google Scholar]
- Dunlap, C.A.; Schisler, D.A.; Price, N.P.; Vaughn, S.F. Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J. Microbiol. 2011, 49, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wakai, S.; Sasakura, N.; Tsutsumi, H.; Hata, Y.; Ogino, C.; Kondo, A. Pyruvate metabolism redirection for biological production of commodity chemicals in aerobic fungus Aspergillus oryzae. Metab. Eng. 2020, 61, 225–237. [Google Scholar] [CrossRef]
- Zhang, D.; Qiang, R.; Zhou, Z.; Pan, Y.; Yu, S.; Yuan, W.; Cheng, J.; Wang, J.; Zhao, D.; Zhu, J. Biocontrol and action mechanism of Bacillus subtilis lipopeptides’ fengycins Against Alternaria solani in potato as assessed by a transcriptome analysis. Front. Microbiol. 2022, 13, 861113. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Chen, W.; Yu, H.; Jia, W.; Pan, H.; Zhang, X. Multi-Omics techniques for analysis antifungal mechanisms of lipopeptides produced by Bacillus velezensis GS-1 against Magnaporthe oryzae in vitro. Int. J. Mol. Sci. 2022, 23, 3762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; St. Leger, R.J.; Fang, W. Stress-induced pyruvate accumulation contributes to cross protection in a fungus. Environ. Microbiol. 2018, 20, 1158–1169. [Google Scholar] [CrossRef]
- Maeda, K.; Nakajima, Y.; Tanahashi, Y.; Kitou, Y.; Miwa, A.; Kanamaru, K.; Kobayashi, T.; Nishiuchi, T.; Kimura, M. L-Threonine and its analogue added to autoclaved solid medium suppress trichothecene production by Fusarium graminearum. Arch. Microbiol. 2017, 199, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Kritzman, G.; Okon, Y.; Chet, I.; Henis, Y. Metabolism of L-threonine and its relationship to sclerotium formation in Sclerotium rolfsii. Microbiology 1976, 95, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, J.M.; McCusker, J.H. Threonine biosynthetic genes are essential in Cryptococcus neoformans. Microbiology 2008, 154, 2767. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Arruda, P.; Turner, W.L.; Lea, P.J. The biosynthesis and metabolism of the aspartate derived amino acids in higher plants. Phytochemistry 1997, 46, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, H.; Zhang, X. Metabolic engineering of microorganisms for L-alanine production. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab057. [Google Scholar] [CrossRef]
- Kalampokis, I.F.; Erban, A.; Amillis, S.; Diallinas, G.; Kopka, J.; Aliferis, K.A. Untargeted metabolomics as a hypothesis-generation tool in plant protection product discovery: Highlighting the potential of trehalose and glycerol metabolism of fungal conidiospores as novel targets. Metabolomics 2020, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.; Jabaji, S. 1H NMR and GC-MS metabolic fingerprinting of developmental stages of Rhizoctonia solani sclerotia. Metabolomics 2010, 6, 96–108. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef]
- Boyer, P.D. Energy, life, and ATP (Nobel lecture). Angew. Chem. Int. Ed. 1998, 37, 2296–2307. [Google Scholar] [CrossRef]
- Senior, A.E.; Nadanaciva, S.; Weber, J. The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta Bioenerg. 2002, 1553, 188–211. [Google Scholar] [CrossRef]
- Fernandez, J.; Yang, K.T.; Cornwell, K.M.; Wright, J.D.; Wilson, R.A. Growth in rice cells requires de novo purine biosynthesis by the blast fungus Magnaporthe oryzae. Sci. Rep. 2013, 3, 2398. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.L.; Becker, D.F. Role of proline in pathogen and host interactions. Antioxid. Redox Signal. 2019, 30, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dickman, M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Jenkinson, J.M.; Gibson, R.P.; Littlechild, J.A.; Wang, Z.Y.; Talbot, N.J. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO 2007, 26, 3673–3685. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, E.-A.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. GC/EI/MS and 1H NMR Metabolomics Reveal the Effect of an Olive Tree Endophytic Bacillus sp. Lipopeptide Extract on the Metabolism of Colletotrichum acutatum. Metabolites 2023, 13, 462. https://doi.org/10.3390/metabo13040462
Papadopoulou E-A, Angelis A, Skaltsounis A-L, Aliferis KA. GC/EI/MS and 1H NMR Metabolomics Reveal the Effect of an Olive Tree Endophytic Bacillus sp. Lipopeptide Extract on the Metabolism of Colletotrichum acutatum. Metabolites. 2023; 13(4):462. https://doi.org/10.3390/metabo13040462
Chicago/Turabian StylePapadopoulou, Evgenia-Anna, Apostolis Angelis, Alexios-Leandros Skaltsounis, and Konstantinos A. Aliferis. 2023. "GC/EI/MS and 1H NMR Metabolomics Reveal the Effect of an Olive Tree Endophytic Bacillus sp. Lipopeptide Extract on the Metabolism of Colletotrichum acutatum" Metabolites 13, no. 4: 462. https://doi.org/10.3390/metabo13040462
APA StylePapadopoulou, E.-A., Angelis, A., Skaltsounis, A.-L., & Aliferis, K. A. (2023). GC/EI/MS and 1H NMR Metabolomics Reveal the Effect of an Olive Tree Endophytic Bacillus sp. Lipopeptide Extract on the Metabolism of Colletotrichum acutatum. Metabolites, 13(4), 462. https://doi.org/10.3390/metabo13040462