Nutritional Enrichment of Plant Leaves by Combining Genes Promoting Tocopherol Biosynthesis and Storage
Abstract
1. Introduction
2. Results and Discussion
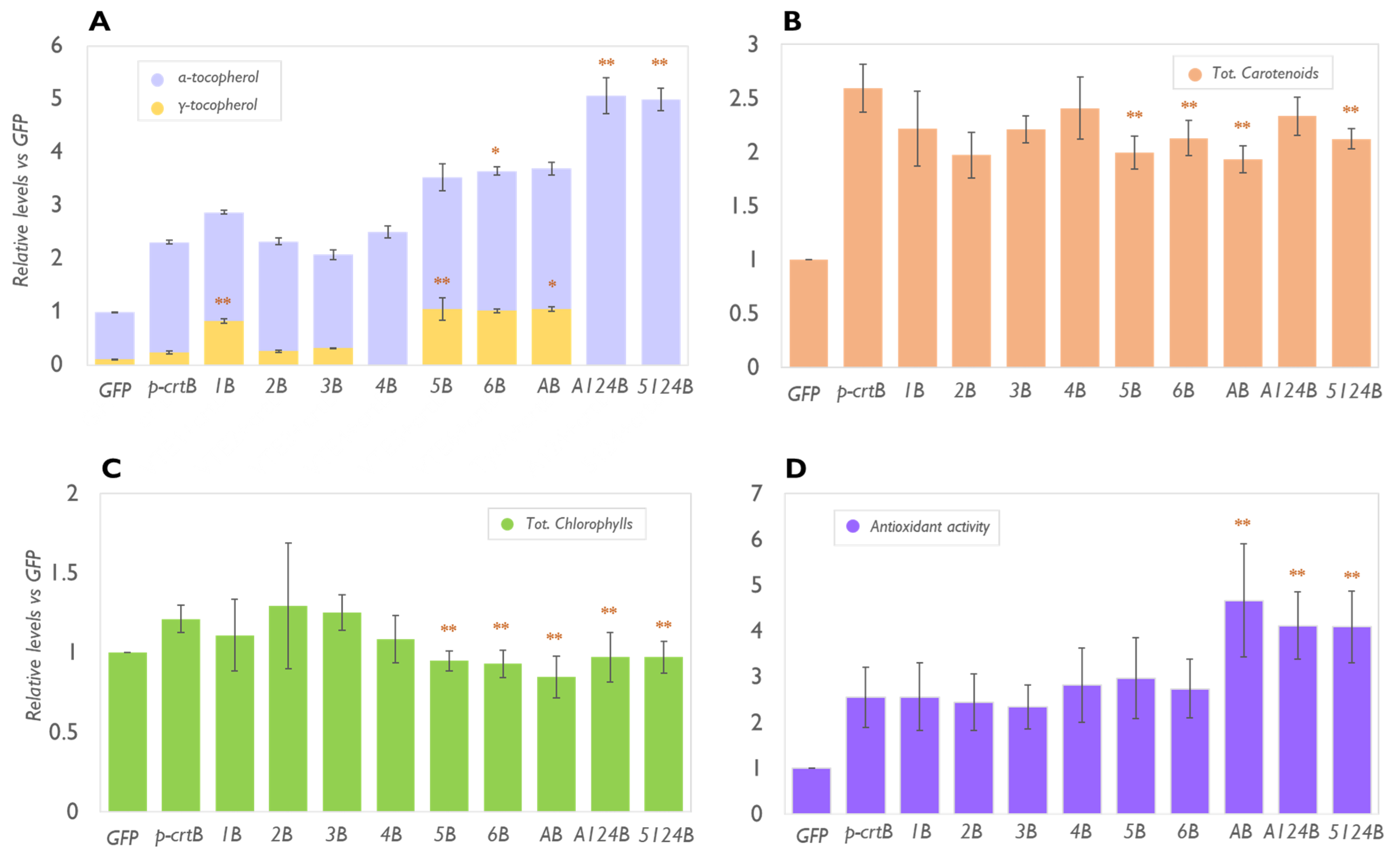
2.1. VitE Content in Leaves Can Be Maximised by Enhancing the Biosynthetic Pathway in Combination with the Formation of Artificial Chromoplasts for Storage
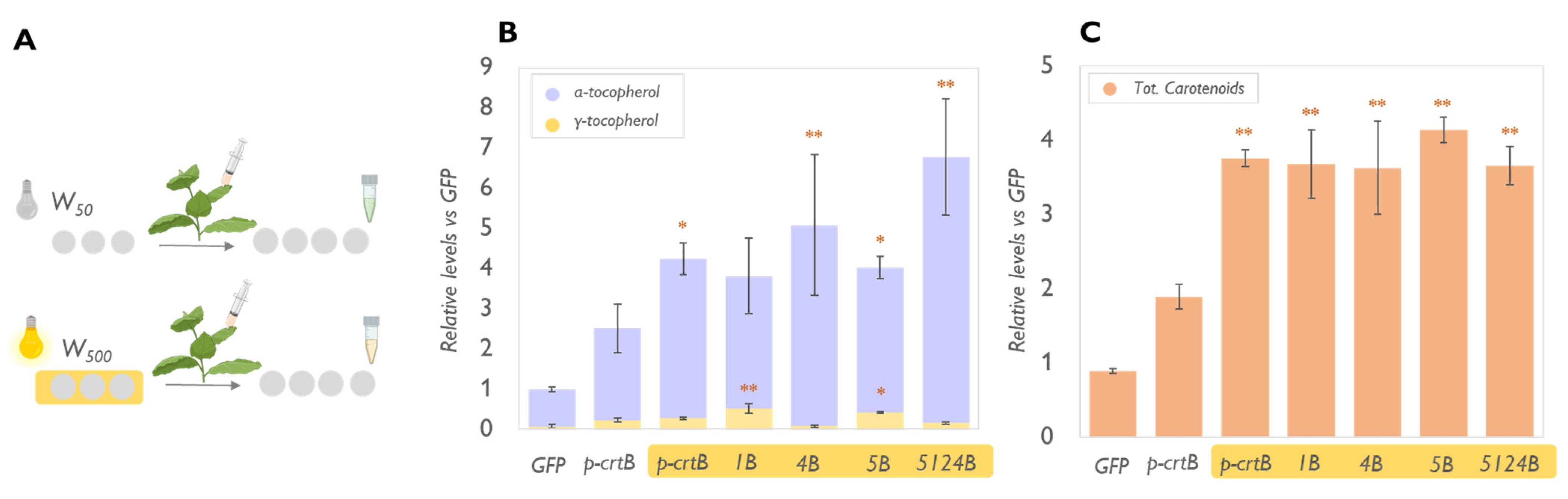
2.2. Physical Treatments Associated with PG Proliferation Can Be Combined with Metabolic Engineering for Additional Tocopherol Enhancement
2.3. The Levels of Plastidial Isoprenoids Other than Tocopherols and Carotenoids Are Also Impacted by Tocopherol Pathway Engineering and Chromoplast Differentiation
3. Conclusions
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Gene Constructs
4.3. Metabolite Analyses
4.4. Antioxidant Capacity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Sequence |
|---|---|
| VTE1-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGGAGATACGGAGCTTGAT-3′ |
| VTE1-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCAGACCCGGTGGCTTGAAGAA-3′ |
| VTE2-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGGAGTCTCTGCTCTCTAG-3′ |
| VTE2-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCTTCAAAAAAGGTAACAGCA-3′ |
| VTE3-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGGCCTCTTTGATGCTCAA-3′ |
| VTE3-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTGATGGGTTGGTCTTTGGGAA-3′ |
| VTE4-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGAAAGCAACTCTAGCAGC-3′ |
| VTE4-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTGAGTGGCTTCTGGCAAGTGATGAT-3′ |
| VTE5-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGGCAGCAACCTTACCTCT-3′ |
| VTE5-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTATATCCGAAACTTAAATAAG-3′ |
| VTE6-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGCTTTCGTCGGGAAGTAG-3′ |
| VTE6-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCTTGACCCAGTTCTGGAGTAT-3′ |
| tyrA-attB1-F | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGGTTGCTGAATTGACCGC-3′ |
| tyrA-attB2-R | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCTGGCGATTGTCATTCGCCT-3′ |
References
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
- Munné-bosch, S.; Alegre, L. Critical Reviews in Plant Sciences The Function of Tocopherols and Tocotrienols in Plants The Function of Tocopherols and Tocotrienols in CRC. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Vitamin E metabolic effects and genetic variants: A challenge for precision nutrition in obesity and associated disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.A. Optimal nutrition: Vitamin E. Proc. Nutr. Soc. 1999, 58, 459–468. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D. A decade of progress in understanding vitamin E synthesis in plants. J. Plant Physiol. 2005, 162, 729–737. [Google Scholar] [CrossRef]
- Van Wijk, K.J.; Kessler, F. Plastoglobuli: Plastid Microcompartments with Integrated Functions in Metabolism, Plastid Developmental Transitions, and Environmental Adaptation. Annu. Rev. Plant Biol. 2017, 68, 253–289. [Google Scholar] [CrossRef]
- Morelli, L.; Torres-montilla, S.; Glauser, G. Novel insights on the contribution of plastoglobules and reactive oxygen species to chromoplast differentiation. New Phytol. 2022, 1–36. [Google Scholar] [CrossRef]
- Fritsche, S.; Wang, X.; Jung, C. Recent advances in our understanding of tocopherol biosynthesis in plants: An overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Ischebeck, T.; Zbierzak, A.M.; Kanwischer, M.; Dörmann, P. A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 2006, 281, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Shanmugabalaji, V.; Zita, W.; Collombat, J.; Kessler, F. Plastoglobules: A Hub of Lipid Metabolism in the Chloroplast, 1st ed.; Elsevier Ltd.: Hoboken, NJ, USA, 2022; Volume 101. [Google Scholar]
- Austin, J.R.; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L.A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Photo-oxidative stress during leaf, flower and fruit development. Plant Physiol. 2018, 176, 1004–1014. [Google Scholar] [CrossRef]
- Miret, J.A.; Munné-Bosch, S. Redox signaling and stress tolerance in plants: A focus on vitamin E. Ann. N. Y. Acad. Sci. 2015, 1340, 29–38. [Google Scholar] [CrossRef]
- Hunter, S.C.; Cahoon, E.B. Enhancing vitamin E in oilseeds: Unraveling tocopherol and tocotrienol biosynthesis. Lipids 2007, 42, 97–108. [Google Scholar] [CrossRef]
- Lu, Y.; Rijzaani, H.; Karcher, D.; Ruf, S.; Bock, R. Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proc. Natl. Acad. Sci. USA 2013, 110, 623–632. [Google Scholar] [CrossRef]
- Ajjawi, I.; Shitani, D. Engineered plants with elevated vitamin E: A nutraceutical success story. Trends Biotechnol. 2004, 22, 103–104. [Google Scholar] [CrossRef]
- Morelli, L.; Rodriguez-Concepcion, M. Open avenues for carotenoid biofortification of plant tissues. Plant Commun. 2022, 4, 100466. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; Torres-Montilla, S.; Morelli, L.; Florez-Sarasa, I.; Matus, J.T.; Ezquerro, M.; D’Andrea, L.; Houhou, F.; Majer, E.; Picó, B.; et al. Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development. Proc. Natl. Acad. Sci. USA 2020, 117, 21796–21803. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.M.; Park, S.-R.; Jung, J.; Moon, J.-K.; Cheong, J.-J.; Kim, M. Overexpression of Arabidopsis homogentisate phytyltransferase or tocopherol cyclase elevates vitamin E content by increasing γ-tocopherol level in lettuce (Lactuca sativa L.). Mol. Cells 2007, 24, 301–306. [Google Scholar] [PubMed]
- Wang, L.; Li, Q.; Zhang, A.; Zhou, W.; Jiang, R.; Yang, Z.; Yang, H.; Qin, X.; Ding, S.; Lu, Q.; et al. The Phytol Phosphorylation Pathway Is Essential for the Biosynthesis of Phylloquinone, which Is Required for Photosystem I Stability in Arabidopsis. Mol. Plant 2017, 10, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Zita, W.; Glauser, G.; Kessler, F.; Shanmugabalaji, V. Chromoplast plastoglobules recruit the carotenoid biosynthetic pathway and contribute to carotenoid accumulation during tomato fruit maturation. PLoS ONE 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Kanwischer, M.; Porfirova, S.; Bergmüller, E.; Dörmann, P. Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol. 2005, 137, 713–723. [Google Scholar] [CrossRef]
- Van Eenennaam, A.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering Vitamin E Content: From Arabidopsis Mutant to Soy Oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar] [CrossRef]
- Vidi, P.-A.; Kanwischer, M.; Baginsky, S.; Austin, J.R.; Csucs, G.; Dörmann, P.; Kessler, F.; Bréhélin, C. Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J. Biol. Chem. 2006, 281, 11225–11234. [Google Scholar] [CrossRef]
- Bréhélin, C.; Kessler, F. The plastoglobule: A bag full of lipid biochemistry tricks. Photochem. Photobiol. 2008, 84, 1388–1394. [Google Scholar] [CrossRef]
- Jin, S.; Daniell, H. Expression of γ-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stress by reducing reactive oxygen species. Bone 2014, 23, 1–7. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2018, 7, 2. [Google Scholar] [CrossRef]
- Bohm, F.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. Carotenoids enhance vitamin E antioxidant efficiency. J. Am. Chem. Soc. 1997, 119, 621–622. [Google Scholar] [CrossRef]
- Espinoza-Corral, R.; Schwenkert, S.; Lundquist, P.K. Molecular changes of Arabidopsis thaliana plastoglobules facilitate thylakoid membrane remodeling under high light stress. Plant J. 2021, 106, 1571–1587. [Google Scholar] [CrossRef]
- Piller, L.E.; Glauser, G.; Kessler, F.; Besagni, C. Role of plastoglobules in metabolite repair in the tocopherol redox cycle. Front. Plant Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Falk, J. New insights into the function of tocopherols in plants. Planta 2004, 218, 323–326. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Mène-Saffrané, L. Vitamin E. Adv. Bot. Res. 2011, 59, 179–227. [Google Scholar] [CrossRef]
- Dorp, K.V.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of phytol from chlorophyll degradation is essential for Tocopherol synthesis and growth of arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, J.; Wang, L.; Pan, Z.; Xu, Q.; Xiao, S.; Deng, X. A comprehensive analysis of chromoplast differentiation reveals complex protein changes associated with plastoglobule biogenesis and remodeling of protein systems in sweet orange flesh. Plant Physiol. 2015, 168, 1648–1665. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Zeng, X.-M.; Lu, Y.-Y.; Lu, W.-J.; Feng, L.-L.; Yang, X.-Q.; Zeng, Q.-P. Senescent leaves of artemisia annua are one of the most active organs for overexpression of artemisinin biosynthesis responsible genes upon burst of singlet oxygen. Planta Med. 2010, 76, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Rottet, S.; Devillers, J.; Glauser, G.; Douet, V.; Besagni, C.; Kessler, F. Identification of plastoglobules as a site of carotenoid cleavage. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. β-Cyclocitral and derivatives: Emerging molecular signals serving multiple biological functions. Plant Physiol. Biochem. 2020, 155, 35–41. [Google Scholar] [CrossRef]
- Shimada, H.; Ohno, R.; Shibata, M.; Ikegami, I.; Onai, K.; Ohto, M.-A.; Takamiya, K.-I. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis. Plant J. 2005, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Shrader, W.D.; Amagata, A.; Barnes, A.; Hinman, A.; Jankowski, O.; Lee, E.; Kheifets, V.; Komatsuzaki, R.; Mollard, P.; Murase, K.; et al. Towards a modern definition of vitamin e—Evidence for a quinone hypothesis. Bioorganic Med. Chem. Lett. 2012, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Szymańska, R.; Nowicka, B.; Dłużewska, J. Function of isoprenoid quinones and chromanols during oxidative stress in plants. New Biotechnol. 2016, 33, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- Martí, M.C.; Camejo, D.; Olmos, E.; Sandalio, L.M.; Fernández-García, N.; Jiménez, A.; Sevilla, F. Characterisation and changes in the antioxidant system of chloroplasts and chromoplasts isolated from green and mature pepper fruits. Plant Biol. 2009, 11, 613–624. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Daròs, J.A. Transient expression systems to rewire plant carotenoid metabolism. Curr. Opin. Plant Biol. 2022, 66, 102190. [Google Scholar] [CrossRef]
- Andersen, T.B.; Llorente, B.; Morelli, L.; Torres-Montilla, S.; Bordanaba-Florit, G.; Espinosa, F.A.; Rodriguez-Goberna, M.R.; Campos, N.; Olmedilla-Alonso, B.; Llansola-Portoles, M.J.; et al. An engineered extraplastidial pathway for carotenoid biofortification of leaves. Plant Biotechnol. J. 2021, 19, 1008–1021. [Google Scholar] [CrossRef]
- Nakagawa, T.; Suzuki, T.; Murata, S.; Nakamura, S.; Hino, T.; Maeo, K.; Tabata, R.; Kawai, T.; Tanaka, K.; Niwa, Y.; et al. Improved gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 2007, 71, 2095–2100. [Google Scholar] [CrossRef]
- Barja, M.V.; Ezquerro, M.; Beretta, S.; Diretto, G.; Florez-Sarasa, I.; Feixes, E.; Fiore, A.; Karlova, R.; Fernie, A.R.; Beekwilder, J.; et al. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol. 2021, 231, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Martinis, J.; Kessler, F.; Glauser, G. A Novel Method For Prenylquinone Profiling in Plant Tissues by Ultra-High Pressure Liquid Chromatography-Mass Spectrometry. 2011. Available online: http://www.plantmethods.com/content/7/1/23 (accessed on 18 January 2020).
- Re, R.; Pellegrini, N.; Proteggente, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, L.; García Romañach, L.; Glauser, G.; Shanmugabalaji, V.; Kessler, F.; Rodriguez-Concepcion, M. Nutritional Enrichment of Plant Leaves by Combining Genes Promoting Tocopherol Biosynthesis and Storage. Metabolites 2023, 13, 193. https://doi.org/10.3390/metabo13020193
Morelli L, García Romañach L, Glauser G, Shanmugabalaji V, Kessler F, Rodriguez-Concepcion M. Nutritional Enrichment of Plant Leaves by Combining Genes Promoting Tocopherol Biosynthesis and Storage. Metabolites. 2023; 13(2):193. https://doi.org/10.3390/metabo13020193
Chicago/Turabian StyleMorelli, Luca, Laura García Romañach, Gaetan Glauser, Venkatasalam Shanmugabalaji, Felix Kessler, and Manuel Rodriguez-Concepcion. 2023. "Nutritional Enrichment of Plant Leaves by Combining Genes Promoting Tocopherol Biosynthesis and Storage" Metabolites 13, no. 2: 193. https://doi.org/10.3390/metabo13020193
APA StyleMorelli, L., García Romañach, L., Glauser, G., Shanmugabalaji, V., Kessler, F., & Rodriguez-Concepcion, M. (2023). Nutritional Enrichment of Plant Leaves by Combining Genes Promoting Tocopherol Biosynthesis and Storage. Metabolites, 13(2), 193. https://doi.org/10.3390/metabo13020193







