Abstract
Many patients in intensive care units, especially the elderly, suffer from chronic critical illness and exhibit a new pathophysiological phenotype: persistent inflammation, immunosuppression, and catabolism syndrome (PICS). Most patients with PICS have a constellation of digestive-system symptoms and gut failure. Akkermansia muciniphila (Akk) is a commensal gut bacterium that reduces inflammation, balances immune responses, modulates energy metabolism, and supports gut health. This study investigated the protective effects and underlying mechanisms of live and pasteurized Akk in treating PICS in a mouse model. PICS was induced on day 14 after performing cecal ligation and puncture (CLP) on day 1 and administrating lipopolysaccharide on day 11. Pasteurized or live Akk, or phosphate-buffered saline was administered twice daily by oral gavage for 7 days. Both live and pasteurized Akk attenuated PICS, as evidenced by reduced weight loss, and a reduction in symptoms and serum cytokine/chemokine levels. Liver and intestinal injuries were mitigated, and intestinal barrier integrity improved with Akk administration. Analysis of 16S rRNA amplicon sequences showed that Akk induced significant intestinal microbiota alterations, including increased abundance of Akk, Muribaculaceae, Parabacterbides goldsteinii, and decreased abundance of Escherichia_Shigella and Enterobacteriaceae. Collectively, Akk alleviates PICS by enhancing gut barrier function and reshaped the microbial community.
1. Introduction
With advances in life support systems, an increasing number of patients can survive lethal multiple organ failure (MOF) in the initial episode of a critical illness; however, long-term survival has not improved significantly [1]. Many survivors, especially patients who are elderly, suffer from severe and prolonged functional disabilities and experience persistent low-grade inflammation and dysregulated immune responses. The devastating pathophysiology is known as “persistent inflammation, immunosuppression, and catabolism syndrome” (PICS) [2]. PICS contributes to poor long-term prognosis in critically ill patients and imposes large financial burdens on families and the healthcare system. Effective treatment protocols are urgently needed. Although the clinical presentation of PICS is prominent, it is difficult to investigate the mechanisms involved and validate relevant treatments in the clinical setting. To elucidate the mechanisms of PICS and explore treatment options, a mouse model was established by conducting a modified sublethal CLP on day 1 and administering low doses of lipopolysaccharide (LPS) on day 11, resulting in critical PICS features on day 14 [3]. The CLP + LPS model involves a composite strike to mimic the main characteristics of PICS in humans. CLP causes severe abdominal infection, and endotoxemia simulated by LPS could further aggravate immune suppression and catabolism. Mice underwent CLP + LPS experienced debilitating damage and repeated inflammation that required rescue treatments, consistent with the physiopathology and clinical characteristics of PICS.
PICS is characterized by a dramatic shift in the composition of bacterial communities in the gut, leading to complications and elevated long-term mortality rates [4]. In addition, the loss of commensal gut microbiota and overgrowth of opportunistic pathogenic flora have been implicated in PICS mice [5]. Over the past decades, there has been increasing interest in modifying the gut microbiome by fecal microbiota transplantation (FMT), probiotics, or prebiotics to improve outcomes in critically ill patients. Probiotics are by far the most widely studied microbiome-based therapeutics to prevent sepsis, the early survivors of which can progress to PICS. Probiotics have been found helpful in reducing ventilator-associated pneumonia, antibiotic-associated diarrhea, and infections in critically ill patients requiring mechanical ventilation [6]. Probiotic intervention in previous studies mostly focused on Lactobacillus and Bifidobacterium species [7,8]; however, several other next-generation probiotics appear promising in animal studies and preclinical research.
Akkermansia muciniphila (Akk), a mucin-degrading bacterium belonging to the phylum Verrucomicrobia, is an intestinal symbiont that colonizes the intestinal mucosal layer of humans and rodents [9]. It is located at the interface between the host and gastrointestinal microbiota, indicating its specific metabolic properties. This symbiont plays a key role in maintaining intestinal health by inducing intestinal adaptive immune responses [10] and promoting epithelial development [11]. Akk is also associated with disease states. Several interventional studies on Akk have revealed its value in improving host metabolic functions [12,13,14] and immune responses [15]. Previous studies have shown an inverse correlation between the abundance of Akk and inflammatory conditions, such as acute appendicitis [16] and inflammatory bowel disease [17,18]. In addition, Akk has been associated with increased sepsis survival in animal models [19], probably because it affects T cell differentiation [10]. These findings indicate that Akk exerts pleiotropic effects on inflammation, modulating energy metabolism and the immune system, which may indicate its capacity to alleviate PICS. Moreover, pasteurized Akk achieves similar or even stronger beneficial effects than live Akk, and both show high safety and tolerability in humans [13,14].
16S rRNA sequencing data from 17 PICS patients revealed a decreased abundance of Akk (0.364 ± 1.11%, not published). Although the role of this bacterium in anti-inflammation, immunomodulation, and energy regulation strongly implies its protective effects against PICS, there have been no published reports to date. Thus, we investigated the protective effects of live and pasteurized Akk against PICS and the potential mechanisms of Akk activity in a mouse model.
2. Materials and Methods
2.1. Materials and Reagents
Fluorescein isothiocyanate (FITC)-labeled dextran 4 kDa (FD-4) and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA). Luminex Mouse Magnetic Assay kits were obtained from R&D Systems (Minneapolis, MN, USA). The antibodies of muscle RING finger-1 (MuRF-1), claudin-1, mucin 2 (Muc2), F4/80, GAPDH, β-Actin and myeloperoxidase (MPO) were obtained from Servicebio (Wuhan, China).
2.2. Animal Experiments
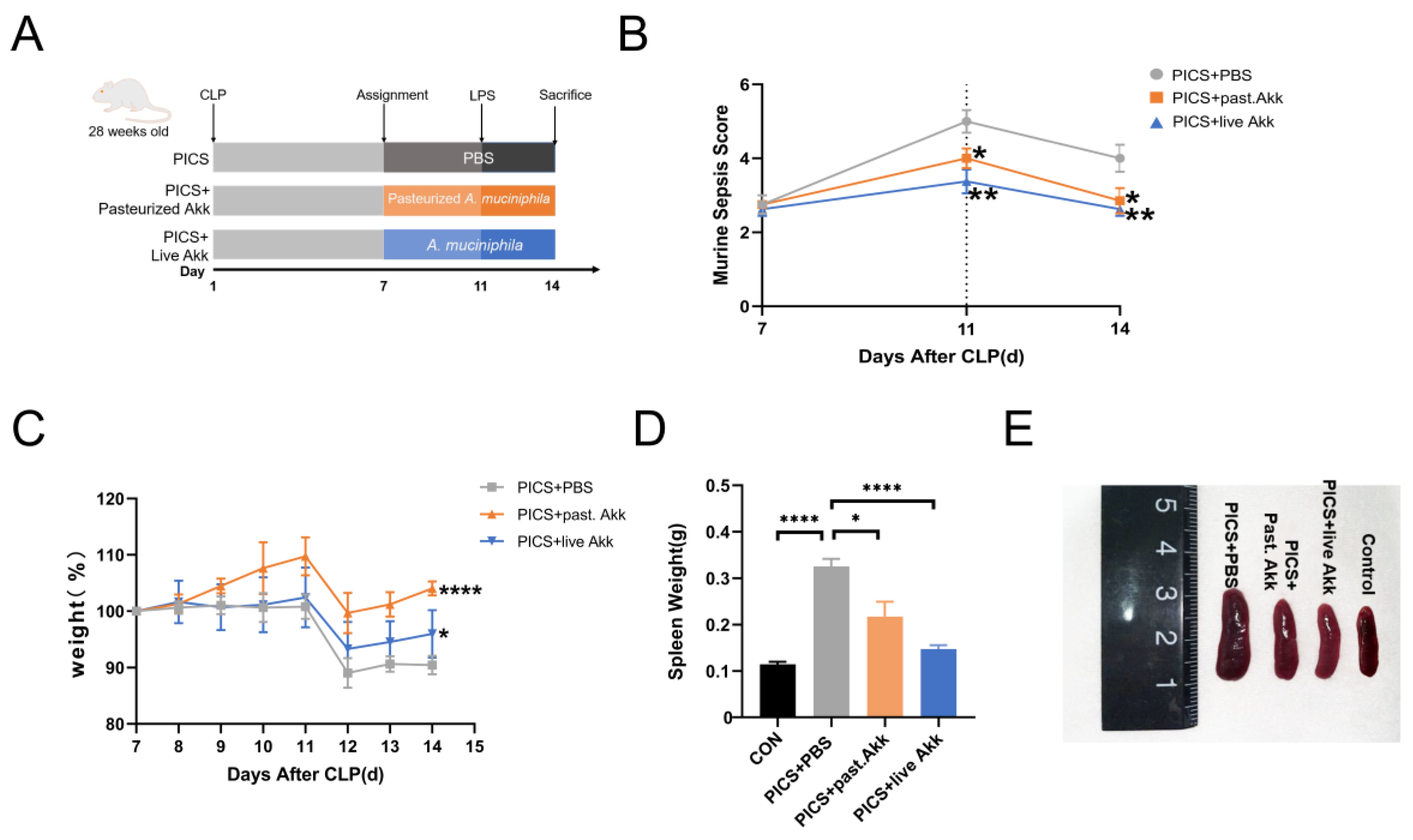
Animal experiments were carried out in accordance with protocols approved by the Animal Care and Use Committee of Nanjing Drum Tower Hospital (2022AE01001). Male C57BL/6 mice (28-week-old) were housed in the Animal Research Center of Nanjing Drum Tower Hospital under constant temperature (22 ± 2 °C) and humidity (55 ± 5%) with free access to food and water. A 12-hr day/night cycle was maintained before the formal experiment. Murine models of PICS were established by CLP + LPS as previously described [3]. Briefly, mice in the PICS group were subjected to CLP surgeries on day 1 and injected intraperitoneally with LPS diluted in PBS at a dose of 1 mg/kg on day 11. The PICS states were generated on day 14. Mice in the control group (n = 5) underwent the same CLP protocol without ligation and puncture and were intraperitoneally administrated equal amounts of PBS on day 11. As shown in Figure 1A, 24 mice in the PICS group were randomly divided into three groups (PICS + PBS, PICS + pasteurized Akk, PICS + live Akk) on day 7 (n = 8 each group). Mice in the PICS + pasteurized Akk and PICS + live Akk groups were gavage-fed pasteurized Akk (1 × 108 colony-forming units (CFU)) or live Akk (1 × 108 CFU) in 0.2 mL PBS solution, respectively, twice a day for 7 days (day 7 to 14). Mice in the PICS + PBS group were administered the same volume of anaerobic sterile PBS via oral gavage. The survival rates, septic severity and body weights were recorded daily throughout the experiment for all mice. Fecal samples were collected on day 7 (before administration of live Akk or pasteurized Akk) and day 14 (the end of the experiments) then stored at −80 °C. On day 14, serum, ileum tissues with fecal materials, liver tissues, spleen tissues, and muscle tissues were collected and stored at −80 °C after the mice were humanely euthanized. A portion of liver and ileum tissues was fixed in Carnoy’s solution for further procedures.
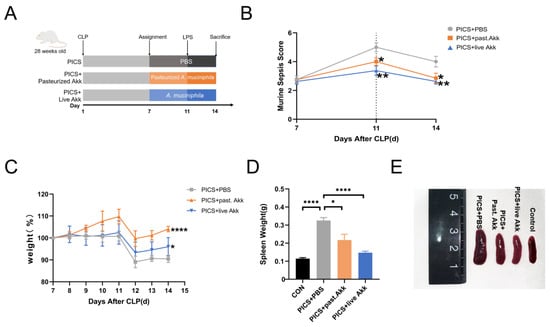
Figure 1.
Oral gavage administration of Akk alleviates phenotype in CLP + LPS-induced PICS. (A) Establishment of the PICS mouse model and schematic diagram of pasteurized Akk and live Akk treatments. (B) MSS calculated with time. (C) Body weight changes are expressed as the mean change from the weight on day 7. (D,E) Representative spleens of four groups (E) and the spleen weight (D) were analyzed at day 14. CON = control. past. = pasteurized. MSS = murine sepsis score. Data are shown as the mean ± SEM. * p < 0.05, ** p < 0.01, **** p < 0.0001.
2.3. Bacterial Strains and Culture Conditions
Akk strain (ATCC BAA-835) was grown in brain-heart-infusion (BHI) media supplemented with 0.4% porcine mucin (Sigma-Aldrich, St. Louis, MO, USA) and 0.05% cysteine (Sigma-Aldrich, St. Louis, MO, USA) under strict anaerobic conditions (99%N2 at 37 °C). The concentration of bacteria was estimated by measuring the absorbance at the wavelength of 600 nm as previously described [20]. In one group of the experiment, Akk was inactivated by pasteurization at 70 °C for 30 min.
2.4. Assessment of PICS Severity
According to the Murine Sepsis Score (MSS) system [21], severity of the inflammatory disease for all PICS mice were evaluated on day 7, 11, and 14 after CLP.
2.5. Pathological Assessment
Ileum and liver specimens were fixed, embedded in paraffin and cut into sections for hematoxylin and eosin (H&E) staining. After that, an experienced pathologist assessed and scored the pathological changes in the ileum and liver tissue at random locations. A 0–4 grading scale for the histological assessment of ileum injuries was used as previously described [22]. The evaluation of liver injuries used a 0–5 grading scale according to our previous study [3].
2.6. Alcian Blue-Periodic Acid-Schif (AB-PAS) Staining
To assess the mucus production and goblet cells, paraffin-embedded ileum sections with fecal materials were prepared and stained with AB-PAS following the manufacturer’s protocol. The tissue slides were then imaged at 20× magnification under a bright field microscope. AB-PAS stains goblet cells within the intestinal villi structure deep purple. The number of goblet cells per intestinal villi were quantified at 4–5 different locations of view, and n = 3–5 per group were used for analysis.
2.7. Immunohistochemistry (IHC) Analysis
IHC was performed as follows: briefly, 4-µm paraffin-embedded liver and ileum sections were dewaxed and processed for antigen retrieval in sodium citrate for 20 min followed by blocking for endogenous peroxidase in 3% H2O2 for 15 min. Subsequently, liver sections were incubated with an anti-mouse F4/80 antibody and an anti-MPO antibody overnight at 4 °C to detect the infiltration into the liver of macrophages and neutrophils, respectively. The ileum sections were incubated with an anti-Muc2 antibody. Afterward, the target proteins were visualized by 3, 3-diaminobenzidine (DBA). The MPO positive cells in the liver were counted under high power field (HPF) as previously described. The intensity of Muc2 and F4/80 staining was analyzed using Image J software (1.8.0, National Institutes of Health, Bethesda, MD, USA).
2.8. Western Bloting
The extensor muscle and ileum tissues were dissolved in lysis buffer. Proteins were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes after being extracted and quantified. The membranes were then incubated with primary antibodies against MURF-1 and claudin-1 overnight at 4 °C and followed secondary antibody incubation. Protein quantification was performed by optical density methods using Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA) and was normalized against GAPDH or β-Actin.
2.9. Real-time Quantitative Polymerase Chain Reaction (qRCR)
Total RNA from the ileum tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s procedures. Real-time quantitative PCR was performed using the One Step SYBR PrimeScript plus RT-PCR Kit (TaKaRa Biotechnology Company Limited, Dalian, China) in the 7500 real-time PCR system (Applied Biosystems, Waltham, MA, USA). The expression of Gapdh mRNA served as an internal control, and the results were analyzed using the ΔΔCT method. The qPCR primers were synthesized and purified by GENERAY Biotechnology (Shanghai, China), and the following primers were used: for MuRF-1, 5′-GATGGAAACGCTATGGAGAACC-3′ and 5′-ACGGAAACGACCTCCAGACAT-3′; for krüppel-like factor 4 (klf4), 5′-AGGAACTCTCTCACATGAAGCG-3′ and 5′-GGTCGTTGAACTCCTCGGTC-3′; for resistin-like molecule-beta (Relm-β), 5′-CCATTTCCTGAGCTTTCTGG-3′ and 5′-AGCACATCCAGTGACAACCA-3′; and for trefoil factor 3 (Tff3), 5′-CAGATTACGTTGGCCTGTCTCC-3′ and 5′-ATGCTTGCTACCCTTGGACCAC-3′.
2.10. Serum Parameter Analysis
The levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, interferon (IFN)-γ, chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-X-C motif) ligand 2 (CXCL2), and IL-33 in the serum were measured according to the standard procedure of kits (Luminex Mouse Magnetic Assay kits, R&D Systems) and then determined by MILLIPLEX Analyst (V5.1) software (Millipore, Billerica, MA, USA).
2.11. In Vivo Intestinal Permeability Measurement
Intestinal permeability was evaluated using the FITC-dextran method, as described previously [23]. Mice were starved for 6 h and then FD-4 (Sigma-Aldrich, 500 mg/kg body weight) was orally gavaged 3 h before sacrifice. The intensity of FITC in the serum was determined by fluorometry (excitation, 485 nm; emission, 520 nm).
2.12. 16S rRNA Analysis
Total genomic DNA was extracted from the fecal aliquots (approximately 0.2 g) by a DNA extraction kit (MN NucleoSpin 96 Soi, Macherey-Nagel, Germany). The NanoDrop-2000C spectrophotometer and gel electrophoresis were used to evaluate the quantity and quality of the DNA. Further, the primers 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) were used to amplify the V3-V4 hypervariable regions of the 16S rRNA gene, as described previously [24]. The purified amplicons were paired-end sequenced using Illumina MiSeq (Illumina, San Diego, CA, USA). After merging the raw data using FLASH software, the reads were filtered using Quantitative Insights into the Microbial Ecology (QIIME, v1.8.0; http://qiime.org/ (accessed on 11 April 2022)) pipeline. The sequences were clustered into operational taxonomic units (OTUs) with ≥97% identity. A representative sequence for each OUT was screened and aligned.
The Silva database was used as the reference database. A linear discriminant analysis (LDA) of effect size (LEfSe) analysis (https://www.omicstudio.cn/tool/ (accessed on 20 April 2022) was performed to identify the differentially abundant taxon among the experimental groups. Reconstruction of Unobserved States (PICRUSt V1.0.0) was applied to predict the metabolic functions of the microbiota based on the 16S rRNA gene library composition.
2.13. Statistical Analysis
Statistical analyses were performed by using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). All data are presented as mean ± standard error of the mean (SEM). One-way ANOVA was applied for comparisons between multiple experimental groups, followed by Tukey’s post hoc test. The distribution normalities were estimated by Kolmogorov–Smirnov test. Data with small sample sizes were analyzed using the Krustal–Wallis test, a non-parametric one-way ANOVA. An unpaired Student’s t test was applied for comparison of the two groups with normal distributions. Values of p < 0.05 were considered statistically significant differences.
3. Results
3.1. Administration of Akkermansia muciniphila Alleviates the Phenotype in CLP + LPS-induced PICS
On day 7, 24 mice were randomly selected and divided into three groups: PICS + PBS, PICS + pasteurized Akk, and PICS + live Akk (n = 8 for each group). The mice were injected intraperitoneally with LPS at a dose of 1 mg/kg on day 11. On day 14, two mice died in the PICS + PBS group; one mouse died in the PICS + pasteurized Akk group; and no mouse died in the PICS + live Akk group. The murine sepsis score (MSS) decreased with time after CLP and increased after the LPS injection on day 11. The score was much higher in the PICS + PBS group than in the PICS + pasteurized Akk and PICS + live Akk groups on days 11 and 14 (Figure 1B). The mice were weighed daily and the percentage weight change relative to their body weight on day 7 was assessed from day 7 to 14. After intraperitoneal injection of low doses of LPS on day 11 (serving as the second challenge), the mice showed a more significant body weight improvement in the PICS + pasteurized Akk (p < 0.0001) and PICS + live Akk groups (p < 0.05) than in the PICS + PBS group (Figure 1C). In the PICS mouse model, splenomegaly occurs as a physiological response to systemic inflammation [25]. Owing to CLP and LPS stimulation, the spleens of mice were more enlarged in the PICS + PBS group than in the control group (p < 0.0001). After 7 days of pasteurized and live Akk administration, much smaller spleens were observed in the PICS + pasteurized Akk (p < 0.05) and PICS + live Akk groups (p < 0.0001) (Figure 1D,E).
3.2. Degrees of Inflammation and Consumptive Catabolism Are Milder in Akk-treated Mice
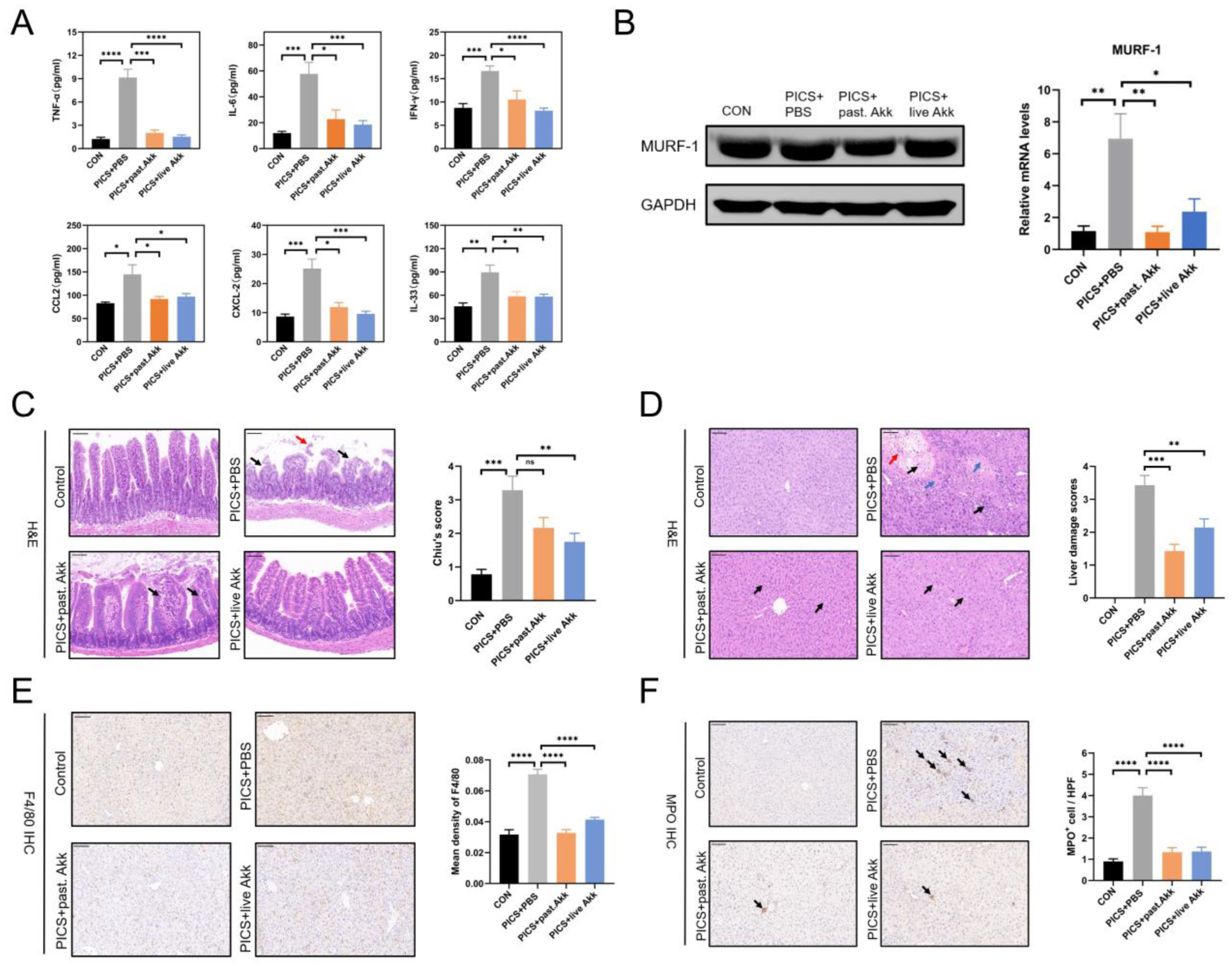
The effects of pasteurized and live Akk on the inflammatory response were investigated in PICS mice at day 14. The blood levels of TNF-α, IL-6, IFN-γ, CCL2, and CXCL2 were measured. TNF-α, IL-6, IFN-γ, CCL2, and CXCL2 levels were increased in the PICS + PBS group compared with those in the control group. Pasteurized and live Akk treatment significantly reduced the release of TNF-α, IL-6, IFN-γ, CCL2, and CXCL2 (Figure 2A). IL-33 is considered an important indicator of sepsis-induced immunosuppression [26]. The levels of IL-33 were higher in the PICS + PBS group than in the control, PICS + pasteurized Akk, and PICS + live Akk groups (Figure 2A). Severe consumptive catabolism with profound muscle wastage has been observed in patients with PICS [27] and in mouse models [3], leading to poor functional outcomes. MuRF-1 contributes to muscle atrophy [28] and showed higher mRNA and protein expression in PICS mice than in the control group, whereas alterations were partially and significantly ameliorated by live and pasteurized Akk treatments (Figure 2B).
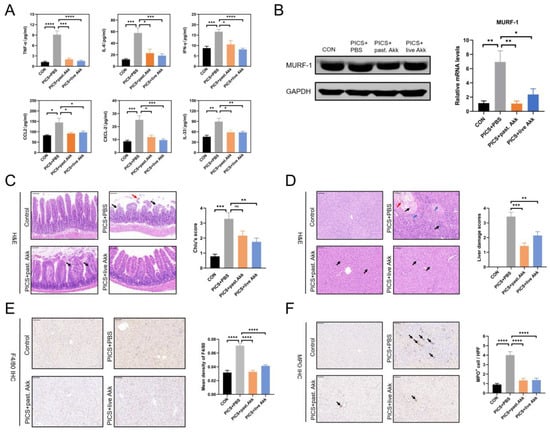
Figure 2.
Akk exhibited systemic, hepatic and intestinal anti-inflammatory effects and alleviated hypercatabolism in PICS mice. (A) Levels of serum inflammatory indices including TNF-α, IL-6, IFN-γ, CCL2, CXCL2 and a novel biomarker in immunosuppression induction, IL-33, in the four groups. (B) The protein (left) and mRNA levels (right) of MuRF-1 were detected by immunoblotting and qRCR. (C) Representative H&E staining of ileum tissue. Black arrows indicate separation of intestinal epithelial cells from the lamina propria. The red arrow indicates shedding of intestinal epithelial cells at the villi tip. (D) Representative H&E staining of liver tissue. Blue arrows indicate focal necrosis. Black arrows indicate inflammatory cell infiltration. The red arrow indicates hemorrhage. (E,F) Representative images and quantification of IHC staining of F4/80 (E) and MPO (F) in liver tissue (indicated by black arrows). Black arrows indicate inflammatory cell infiltration. Scale bars, 100 µm. ns = not significant; Past. = pasteurized. Data are shown as the mean ± SEM (n = 5 mice per group). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
The PICS mice exhibited chronic organ damage due to the combined effects of CLP and LPS. Consistent with the beneficial inflammatory profile, H&E staining of the intestine and liver tissues revealed that the damage in mice treated with pasteurized or live Akk was milder, based on tissue morphology and estimation of immune cell infiltration (Figure 2C,D). In detail, CLP and LPS significantly induced the loss of ileum villi as intestinal epithelial cells were separated from the lamina propria (upper right panel of Figure 2C, black arrows) and shed at the villi tip (upper right panel of Figure 2C, red arrow). In contrast, live Akk treatment significantly improved intestinal damage of PICS mice (lower right panel of Figure 2C), while mild separation of the apical mucosal epithelium from the lamina propria was observed (lower left panel of Figure 2C, black arrows), there was no significant detachment observed in the ileum of the PICS + pasteurized Akk group. In the control group, the hepatocytes were regularly arranged with clear hepatic lobules. The liver tissue in the PICS + PBS group displayed cell swelling, focal necrosis (upper right panel of Figure 2D, blue arrows), apparent inflammatory cell infiltration (upper right panel of Figure 2D, black arrows), and hemorrhage (upper right panel of Figure 2D, red arrow). In contrast, only slight liver cell swelling was observed in the PICS + pasteurized Akk and PICS + live Akk groups (lower panels of Figure 2D, black arrows). Macrophages and neutrophils are the major immune cells in MOF and play an indispensable role in promoting the viscous cycle in PICS [28]. Immunohistochemical analysis of the liver tissue revealed that the levels of F4/80+ macrophages and MPO+ neutrophils were increased in the PICS mice compared with those in the control group. Mice administered Akk (either pasteurized or live) protected macrophage and neutrophil infiltration in the liver of PICS mice (Figure 2E,F). Collectively, these results indicate that Akk attenuated PICS-associated systemic inflammation and immunosuppression, as well as liver injury and small intestinal damage.
3.3. Akk Preserves the Intestinal Mucosal Barrier Function in PICS Mice
Next, we explored the mechanism by which Akk prevents PICS. The intestinal mucosal barrier with its chemical and physical barriers plays an important role in maintaining intestinal homeostasis [29]. Intestinal barrier dysfunction contributes to the transition of luminal contents into circulation, thereby inducing inflammation and activating the immune response. Akk supplementation stimulates mucin production [30,31] and improves the integrity of the epithelial cell layer ex vivo [32]. Given the properties described above, we hypothesized that preservation of the intestinal mucosal barrier contributes to the benefits of Akk in PICS.
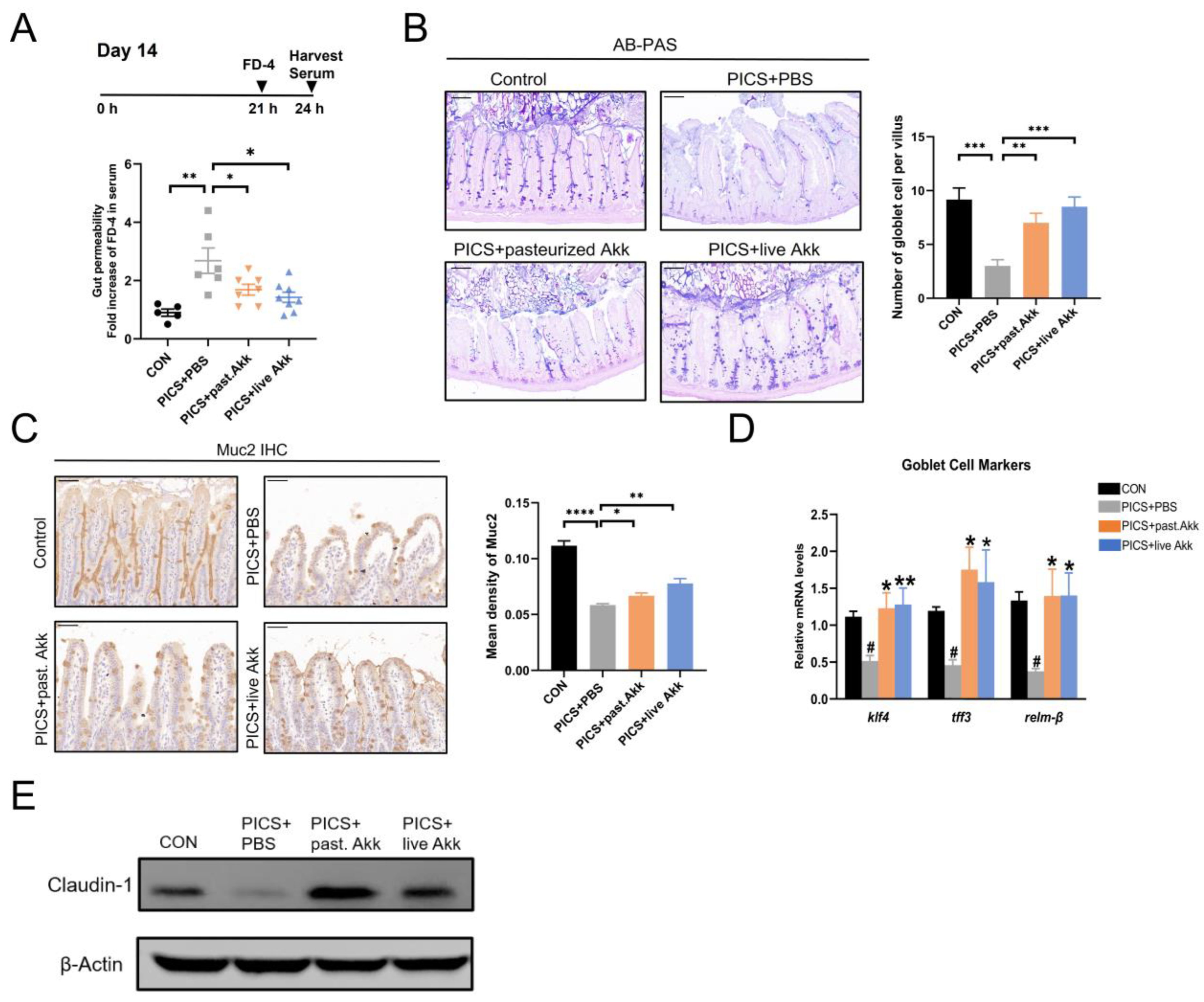
Intestinal permeability was evaluated based on the intensity of FD-4 in the blood at day 14. The PICS mice had higher levels of FD-4 in the bloodstream compared with those of the control group, and they were significantly deceased in the mice treated with pasteurized and live Akk (Figure 3A). This data suggests pasteurized and live Akk could reduce intestinal permeability in PICS mice. Subsequently, mucus thickness and goblet cells in the ileum tissue sections were evaluated using AB-PAS staining. The pasteurized and live Akk treatments significantly ameliorated the production of mucus and the reduction in goblet cells compared with the PICS + PBS group (Figure 3B).
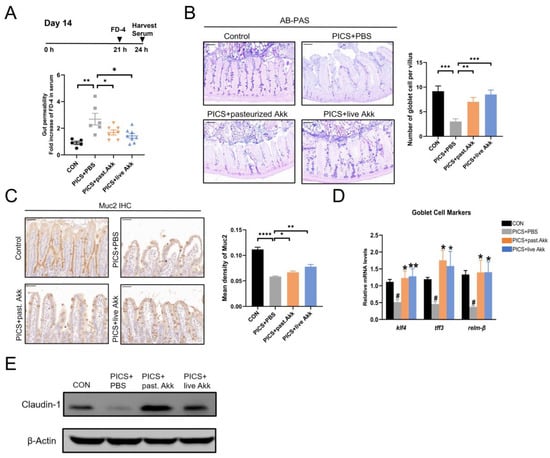
Figure 3.
Akk enhanced intestinal mucosal barrier function. (A) serum concentration of FD-4 at day 14. (B) AB/PAS reaction and the number of acid mucin-producing globlet cells per villus at day 14. Scale bars, 100 µm. (C) IHC staining and quantitation of Muc2 in the ileum at day 14. Scale bars, 50 µm. (D) Gene expression of goblet cell markers in ileum at day 14. (E) Immunoblotting for claudin-1 in ileum at day 14. past. = pasteurized. Data are shown as the mean ± SEM (n = 5 mice per group). # p < 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. * in comparison with the PICS + PBS group, # in comparison with the control group.
Then, the effects of pasteurized and live Akk on goblet cell maturation and function in PICS mice were evaluated. Muc2 secreted by goblet cells is a major component of the ileal mucus barrier. Muc2 expression was examined using IHC. The expression of Muc2 in the ileum was downregulated in the PICS + PBS group compared with that of the control group and moderately but significantly upregulated in the PICS + pasteurized Akk and PICS + live Akk groups (Figure 3C). Compared with the PICS + PBS group, the PICS + pasteurized Akk and PICS + live Akk groups exhibited increased expression of klf4, a zinc-finger transcription factor positively correlated with goblet cell differentiation [33] (Figure 3D). Tff3 and relm-β are important mucosal defense factors secreted by goblet cells [34]. Tff3 cooperates with Muc2 to enhance the protective barrier properties of the intestinal mucosa, whereas Relm-β plays an important role in immunity regulation and host defense against intestinal inflammation. Relm-β and Tff3 expression levels were increased in the PICS + live Akk and PICS + pasteurized Akk groups compared with those in the PICS + PBS group (Figure 3D).
As markers of tight junction structure, claudin-1 was detected in ileum tissue using Western blotting. The protein levels of claudin-1 were lower in the PICS + PBS group than in the control group. However, the levels of the protein increased in the PICS + live Akk group and dramatically increased in the PICS + pasteurized Akk group (Figure 3E).
3.4. Akk Reshapes the Gut Microbiota of PICS Mice
Finally, the composition, abundance, and function of gut microbiota in fecal samples were analyzed using high-throughput sequencing of the V3–V4 regions of the 16S rRNA genes. Paired fecal samples were collected from three groups (PICS + PBS, n = 4; PICS + pasteurized Akk, n = 5; PICS + live Akk, n = 7) on day 7 (before intervention; baseline) and day 14 (after intervention; endpoint). A total of 2,557,182 clean reads were obtained for further analysis, and 2412 identified operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff.
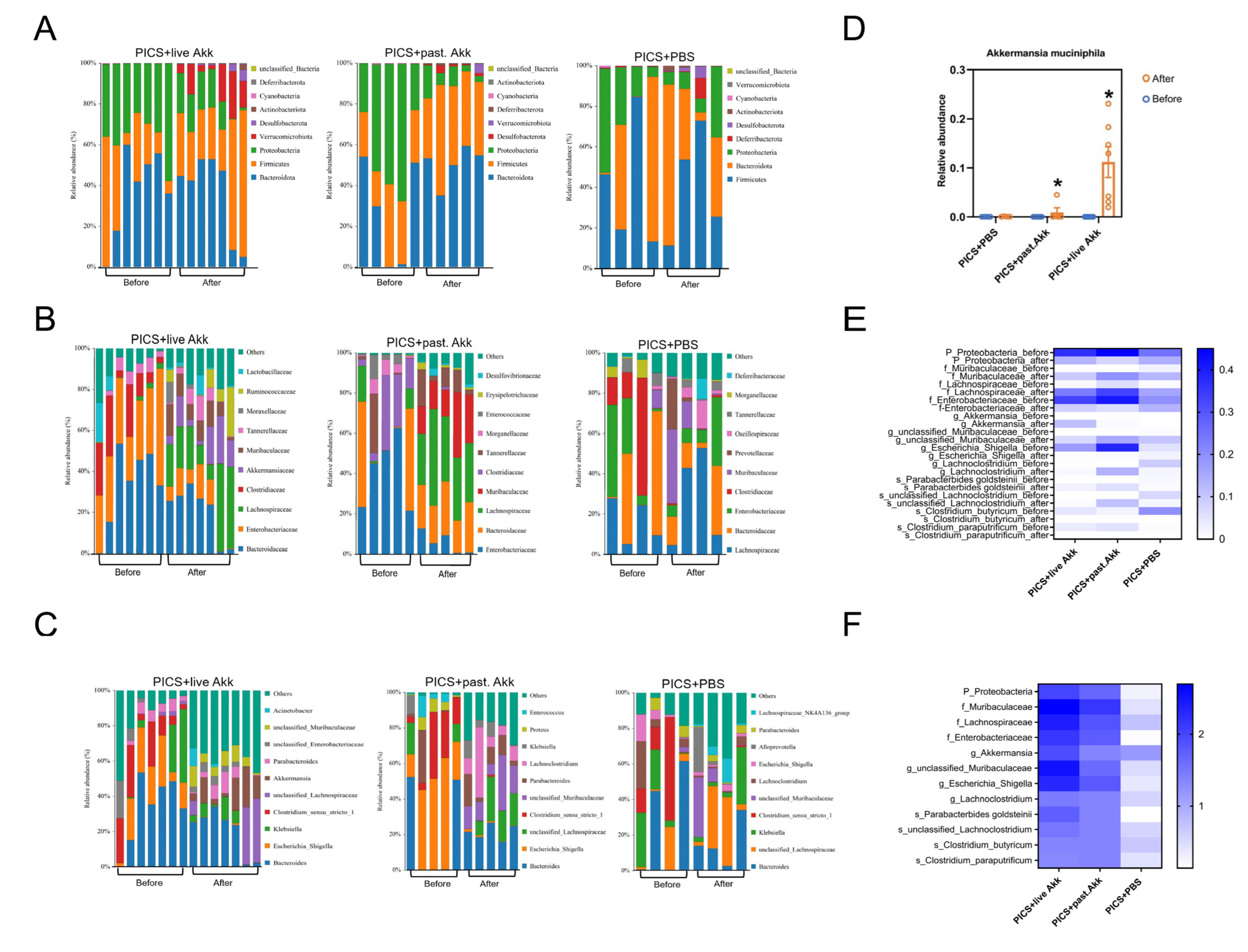
The structure of the intestinal flora can differ significantly even in mice with similar physiological status. In that case, we evaluated the alteration in the intestinal microbiota from baseline to endpoint to minimize the effect of individual differences. To investigate whether Akk administration modulates the structure and composition of intestinal microbiota during PICS, the Wilcoxon rank sum test at three different taxonomic levels was used to compare the distribution of bacteria between day 7 and 14 in the three groups. The histograms illustrate the species and relative abundance of intestinal microbiota at the phylum level (Figure 4A). Consistent with a previous study, Firmicutes and Bacteroidota were the most abundant phyla in all mouse fecal samples [17]. The abundance of Proteobacteria was decreased in the PICS + pasteurized Akk (p < 0.05) and PICS + live Akk group (p < 0.05). At the family level (Figure 4B), the relative abundance of Lachnospiraceae (p < 0.01) and Muribaculaceae (p < 0.01) increased greatly, and Enterobacteriaceae (p < 0.01) decreased after live Akk treatment. Lachnospiraceae (p < 0.05) and Muribaculaceae (p < 0.01) were also increased due to the pasteurized Akk treatment. In addition, Enterobacteriaceae (p < 0.05) was decreased in the PICS + pasteurized Akk group.
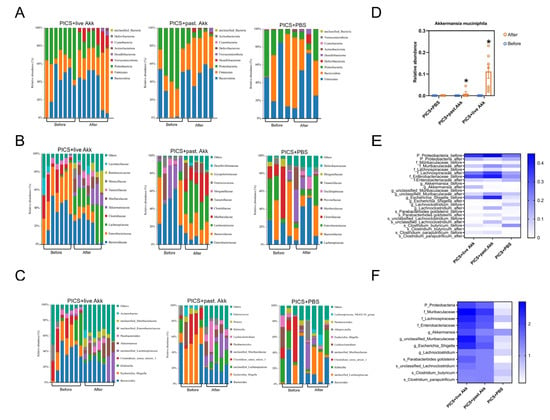
Figure 4.
Pasteurized and live Akk reshapes the gut microbiota community. (A–C) Bar chart of fecal bacterial community composition at the phylum (A), family (B) and genus (C) levels at day 14. (D) Relative abundance of Akk at the genus level before and after treatment at day 14. (E) Heatmap of the statistically different intestinal microbiota after pasteurized and live Akk treatment and PBS in PICS mice at day 14. (F) A heatmap generated by the -log(p-value) was drawn to show the alterative levels of the microbial community in Figure 4E. past. = pasteurized. Data are shown as the mean ± SEM. * p < 0.05, in comparison with the same group before treatment.
The abundances of intestinal microbiota at the genus level were also analyzed. As shown in Figure 4C, both pasteurized and live Akk administration increased the abundance of Akk (p < 0.05), unclassified Muribaculaceae (p < 0.05 and p < 0.01 respectively), and Lachnoclostridium (p < 0.05) and decreased the abundance of Escherichia_Shigella (p < 0.05 and p < 0.01 respectively). There were no significant changes in the PICS + PBS group. The abundances of the genus Akk in the three groups on day 7 (before gavage) and day 14 (after gavage) suggest that Akk could colonize the colon 7 days after twice daily intragastric administration of 108 Akk cells (Figure 4D). These obvious alternations in intestinal microbiota confirmed the regulatory effect of Akk on the gut microbiota. The changed genera might be important regulatory bacteria in the process of both pasteurized and live Akk exerting their effects.
To identify differentially abundant intestinal microbiota, linear discriminant analysis effect size (LEfSe) analysis was used to explore specific bacterial phylotypes associated with the live and pasteurized Akk treatments (Supplementary Figures S1–S3). Higher abundances of potentially beneficial microbes such as Parabacterbides goldsteinii were observed both in the PICS + pasteurized Akk and PICS + live Akk groups. A hierarchically clustered heat map analysis based on LefSe analysis was constructed to identify the intestinal bacterial communities with the same trend of significant changes in PICS + pasteurized Akk and PICS + live Akk groups (Figure 4E). The upregulated potentially beneficial microbes, such as Akk, Parabacterbides goldsteinii and Muribaculaceae, and down-regulated opportunistic pathogenic bacteria, such as Gammaproteobacteria, Enterobacteriaceae and Escherichia_Shigella, were observed after both live and pasteurized Akk treatment but not after PBS treatment (Figure 4F).
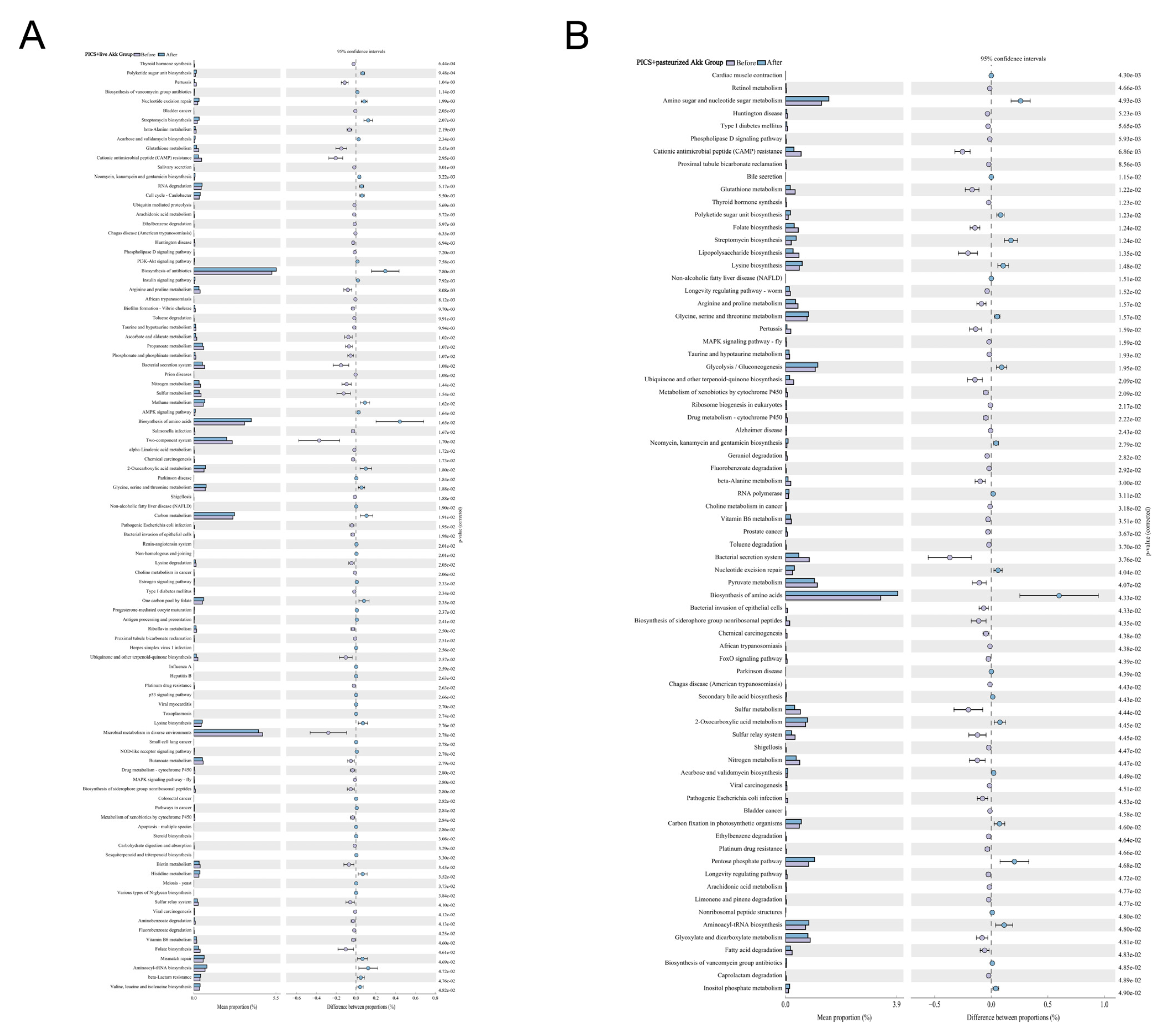
To further explore the biological functions, gut microbiome metabolic functions were predicted at the KEGG pathway Level 3 (Figure 5). The capability for bacterial invasion of epithelial cells, vitamin B6 metabolism, and some inflammatory pathways, for example, the MAPK signaling pathway, were significantly changed after treatment with pasteurized and live Akk. No significant functional differences were observed in the PICS mice treated with PBS.
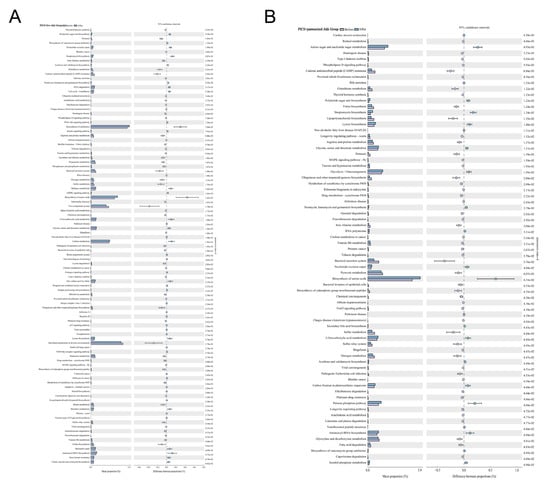
Figure 5.
Pasteurized and live Akk regulates metabolic pathways of microbial communities. Fecal microbial community function differences against the KEGG database on Level 3 before and after (A) live Akk and (B) pasteurized Akk administration at day 14. For p-value, the letter e is used to mean “10 to the power of”.
4. Discussion
Recent studies have demonstrated that the decreased or lack of abundance of Akk is highly linked with increasing inflammation in the context of multiple diseases, such as Crohn’s disease, ulcerative colitis, HIV, diabetes and obesity [12,13,14,15,16,17,18]. Moreover, a similar finding suggests that both live and pasteurized Akk could restore LPS-mediated intestinal barrier damage [35]. However, the therapeutic effects of Akk against PICS have not been demonstrated. Here, we investigated the impacts of Akk (live and pasteurized) and the underlying mechanisms in a CLP + LPS-induced mouse model of PICS. Our study showed that both live and pasteurized Akk administration in CLP + LPS-induced PICS mice reduced aggravated inflammation and increased muscle catabolism; reduced hepatic inflammation, liver injury, and intestinal injury; preserved the mechanical and chemical barrier function of the intestinal mucosa; and reshaped the gut microbiota composition in the mice.
In previous studies, higher levels of inflammatory cytokine/chemokine in serum were observed in patients with PICS [36] and in a mouse model of PICS [3]. Increased levels of cytokines (TNF-α, IL-6, and IFN-γ) and chemokines (CCL2 and CXCL2) are the main characteristics of PICS [3]. Our results demonstrated that Akk treatment alleviated the inflammatory response by inhibiting systemic pro-inflammatory cytokines and chemokines in mice with PICS. In addition, IL-33, which has been reported to play a major role in the induction of long-term immunosuppression [26], showed significant relief in the PICS + live Akk and PICS + pasteurized Akk groups, suggesting that Akk may have an important effect on immune dysfunction in mice with PICS. Profound muscle atrophy and weight loss are common in PICS patients during their hospitalization despite the nutritional support [37]. The expression of MuRF-1 is upregulated in critically ill patients and contributes to muscle wasting [38]. In the present study, CLP + LPS markedly upregulated MuRF-1 expression at both transcriptional and translational levels, which was recovered by treatment with Akk.
Patients with PICS are subject to protracted organ dysfunction and recurrent nosocomial infections, and develop a vicious, self-stimulating cycle. The gastrointestinal tract has long been considered to play a crucial role in the pathophysiology of the acute phase of PICS and functions as a driver of multiple organ dysfunction syndrome [39]. Traditionally, intestinal injury and compromise in the intestinal barrier function facilitate the translocation of bacteria and exogenous pathogen-associated molecular patterns (PAMPs), with subsequent induction of proinflammatory pathways and distant-organ dysfunction [39,40]. PICS patients often suffer from different degrees of gastrointestinal dysfunction leading to reduced absorption of nutrients [41]. In addition, the construction and function of intestinal microbiota is demonstrated to be significantly changed in PICS patients and subsequently affects the organism’s metabolism [42]. The state of malnutrition could impair immune cell metabolism and suppress the host’s immune response [43]. In this regard, gut microenvironment dysfunction may act as a significant initial event that results in the vicious cycle of PICS. Akk emerged as sentinel of the gut and its protective effects on inflammation-related conditions by restoring gut permeability have been extensively described. Western diet-induced inflammation and circulating endotoxin levels have been prevented by Akk treatment through the upregulation of intestinal tight junction proteins [44]. Akk can improve dextran sulfate sodium-induced ulcerative colitis and protect gut barrier function [17]. Moreover, Akk can reduce intestinal permeability and alleviate inflammation, thereby stimulating bone fracture healing [20]. Consistent with these findings, our study showed that the intestinal mucosal barrier function was compromised in the PICS mice. Akk restored the intestinal mechanical and chemical barrier, as evidenced by the lower level of FD-4 in the serum, normalized mucus thickness, increased number of goblet cells, and upregulated expression of tight-junction proteins (claudin-1). The results indicate that Akk may exert protective anti-inflammatory effects on PICS through restoring the intestinal microenvironment. Interestingly, we observed similar protective effects of both pasteurized and live Akk against PICS. This is possibly due to the fact that the outer membrane of Akk could retain biological activities after pasteurization [14], suggesting the critical role of the composition of the outer membrane, such as Amuc_1100 [15], on systemic inflammation and hypercatabolism.
To date, the mechanisms that are responsible for the improved gut barrier caused by Akk are controversial. Akk protected gut barrier function by reshaping the microbial community in a colitis mouse model [17]. However, a study on overweight and obese humans found that supplementation with either live or pasteurized Akk did not affect the overall structure of the gut microbiome [13]. Consistent with this finding, alteration of the gut microbiota was not observed in mice supplemented with live Akk [45]. This variation in the shift of the microbiota composition could be the result of sample size and the species involved in the experiments. Evaluation strategies such as pairing individuals from baseline to endpoint and group comparisons at the end of interventions may also contribute to the variation. In the present study, we provided evidence that live and pasteurized Akk treatment increased the abundance of Muribaculaceae, Lachnospiraceae, Akk, Lachnoclostridium, and Parabacterbides goldsteinii, whereas it reduced the abundance of Proteobacteria, Enterobacteriaceae, Escherichia_Shigella, Clostridium_butyricum, and Clostridium_paraputrificum. These effects may explain the benefits of live and pasteurized Akk on PICS mice. Muribaculaceae abundance is negatively correlated with inflammation status and could tolerate the immunity stimulation by inhibiting CD8+ T cell activation [46]. Members of Lachnospiraceae and Lachnoclostridium are among the main producers of health-promoting short-chain fatty acids [47,48]. The bacterium Parabacterbides goldsteinii was recently reported as a novel probiotic associated with enhanced gut integrity and reduced inflammation [49]. An abnormal expansion of the bacterial phylum Proteobacteria is a marker for dysbiosis and a diagnostic criterion for disease [50]. In addition, an expansion of the Enterobacteriaceae family is also frequently associated with gut inflammation [50,51]. Escherichia_Shigella is an adherent-invasive bacterium and reported to cause gut microbiota disturbance in the development of colitis [52]. The decreased abundance of Clostridium_butyricum and Clostridium_paraputrificum in the PICS + pasteurized Akk and PICS + live Akk groups seems to conflict with the conclusion. Previous studies have shown that Clostridium_butyricum produces butyrate and is safely used as a probiotic [53,54]. These alternations could be the results of interactions between the intestinal microorganisms.
Taken together, this study demonstrated that pasteurized and live Akk administration alleviated PICS-related symptoms, systemic inflammation, hypercatabolism, and immunosuppression; inhibited hepatic and intestinal damage; and restored intestinal mucosal barrier function. The mechanism of enhanced barrier function could be the regulation of the structure of intestinal microbiota through reductions in the abundance of pathological bacteria (Proteobacteria, Enterobacteriaceae, and Escherichia_Shigella) and increases in the abundance of beneficial bacteria (Muribaculaceae, Lachnospiraceae, Akk, Lachnoclostridium, and Parabacterbides goldsteinii).
5. Conclusions
In summary, this study reveals a protective role for Akk as a critical regulator of chronic systemic inflammation. Live and pasteurized Akk could regulate fecal gut microbiota community and promote intestinal mucosal barrier function to alleviate PICS associated inflammation and multiple organ dysfunction. Thus, Akk as a potential therapeutic agent holds promise for the treatment of systemic inflammation, immunosuppression, and hypercatabolism syndrome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13020194/s1. Figure S1: Discriminative biomarkers with an LDA score >4.0 in the PICS + live Akk group before (blue) and after (orange) intervention (left); the LEfSe cladogram represents the taxa enriched before (blue) and after (orange) intervention (right). Figure S2: Discriminative biomarkers with an LDA score >4.0 in the PICS + pasteurized Akk group before (blue) and after (orange) intervention (left); the LEfSe cladogram represents the taxa enriched before (blue) and after (orange) intervention (right). Figure S3: Discriminative biomarkers with an LDA score >4.0 in the PICS + PBS group before (blue) and after (orange) intervention (left); the LEfSe cladogram represents the taxa enriched before (blue) and after (orange) intervention (right).
Author Contributions
Study conceptualization, W.Y.; methodology/formal analysis/investigation, Y.X., J.D., D.W., J.L., X.C., X.-Y.Q. and W.Y.; writing—original draft preparation, Y.X.; writing—review and editing, X.-Y.Q. and W.Y.; supervision, X.-Y.Q. and W.Y.; funding acquisition, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 81927808 (to Y.W.). This research was also partly funded by the Program for Basic and Clinical Research on Hepatitis from AMED under Grant Number JP22fk0210100h0001, by the e-ASIA Joint Research Program from AMED under Grant Number 22jm0210092s0101, by the Grant-in-Aid for Scientific Research (JP20K07349) from the Japan Society for the Promotion of Science (JSPS) (to X.-Y.Q.). Y.X. has been awarded the International Program Associate (IPA) fellowship from RIKEN, Japan.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Nanjing Drum Tower Hospital (2022AE01001).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets involved in this study are available through the NCBI home page: www.ncbi.nlm.nih.gov/sra/?term=PRJNA916125 (released on 28 February 2023).
Acknowledgments
We thank Qian Wei (Nanjing University) and the Department of Dermatology (General Hospital of Eastern Theater Command, Nanjing) for support in the animal experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nelson, J.E.; Cox, C.E.; Hope, A.A.; Carson, S.S. Chronic critical illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Lu, H.; Xu, Y.; Wei, Y.; Cao, K.; Zhu, Z.; Chen, M.; Yu, W. Mouse Model of Critical Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Shock 2022, 57, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P. The microbiome and critical illness. Lancet Respir. Med. 2016, 4, 59–72. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Thomas, R.M.; Darden, D.B.; Gharaibeh, R.Z.; Hawkins, R.B.; Cox, M.C.; Apple, C.; Nacionales, D.C.; Ungaro, R.F.; Dirain, M.L.; et al. Septic Stability? Gut Microbiota in Young Adult Mice Maintains Overall Stability After Sepsis Compared to Old Adult Mice. Shock 2021, 55, 519–525. [Google Scholar] [CrossRef]
- Manzanares, W.; Lemieux, M.; Langlois, P.L.; Wischmeyer, P.E. Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit. Care 2016, 19, 262. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Probiotics in preterm infants study collaborative G: Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; O, E.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Theissig, F.; Rückert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kühn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Di Caro, V.; Pang, D.; Cummings, J.; Firek, B.; Rogers, M.B.; Ranganathan, S.; Clark, R.S.B.; Aneja, R.K. Dietary Supplementation With Nonfermentable Fiber Alters the Gut Microbiota and Confers Protection in Murine Models of Sepsis. Crit. Care Med. 2017, 45, e516–e523. [Google Scholar] [CrossRef]
- Huck, O.; Mulhall, H.; Rubin, G.; Kizelnik, Z.; Iyer, R.; Perpich, J.D.; Haque, N.; Cani, P.D.; de Vos, W.M.; Amar, S. Akkermansia muciniphila ermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodontol. 2020, 47, 202–212. [Google Scholar] [CrossRef]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ren, J.; Chen, G.; Wu, L.; Song, X.; Li, G.; Deng, Y.; Wang, G.; Gu, G.; Li, J. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci. Rep. 2017, 7, 4364. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Chan, H.; Liang, Y.; Liu, X.; Zhang, L.; Li, Q.; Zhang, Y.; Zeng, J.; Ugwu, F.N.; Ho, I.H.T.; et al. Cathelicidin preserves intestinal barrier function in polymicrobial sepsis. Crit. Care 2020, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.M.; Zhou, W.T.; Chen, D.; Huang, K.Y.; Yu, S.J.; Mi, J.; Lu, L.; Zeng, X.X.; Cao, Y.L. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- McKenzie, C.V.; Colonne, C.K.; Yeo, J.H.; Fraser, S.T. Splenomegaly: Pathophysiological bases and therapeutic options. Int. J. Biochem. Cell Biol. 2018, 94, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.C.; Melo, P.H.; Piñeros, A.R.; Ferreira, R.G.; Colón, D.F.; Donate, P.B.; Castanheira, F.V.; Gozzi, A.; Czaikoski, P.G.; Niedbala, W.; et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat. Commun. 2017, 8, 14919. [Google Scholar] [CrossRef]
- Kress, J.P.; Hall, J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D., 2nd; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia muciniphila ermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila ermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia muciniphila ermansia muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Fay, K.T.; Ford, M.L.; Coopersmith, C.M. The intestinal microenvironment in sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2574–2583. [Google Scholar] [CrossRef]
- Simbrunner, B.; Mandorfer, M.; Trauner, M.; Reiberger, T. Gut-liver axis signaling in portal hypertension. World J. Gastroenterol. 2019, 25, 5897–5917. [Google Scholar] [CrossRef]
- Puleo, F.; Arvanitakis, M.; Van Gossum, A.; Preiser, J.C. Gut failure in the ICU. Semin. Respir. Crit. Care Med. 2011, 32, 626–638. [Google Scholar] [CrossRef]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila ermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila ermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Keskey, R.; Cone, J.T.; DeFazio, J.R.; Alverdy, J.C. The use of fecal microbiota transplant in sepsis. Transl. Res. 2020, 226, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).