Metabolic Engineering of Nicotiana benthamiana to Produce Cannabinoid Precursors and Their Analogues
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Transformation
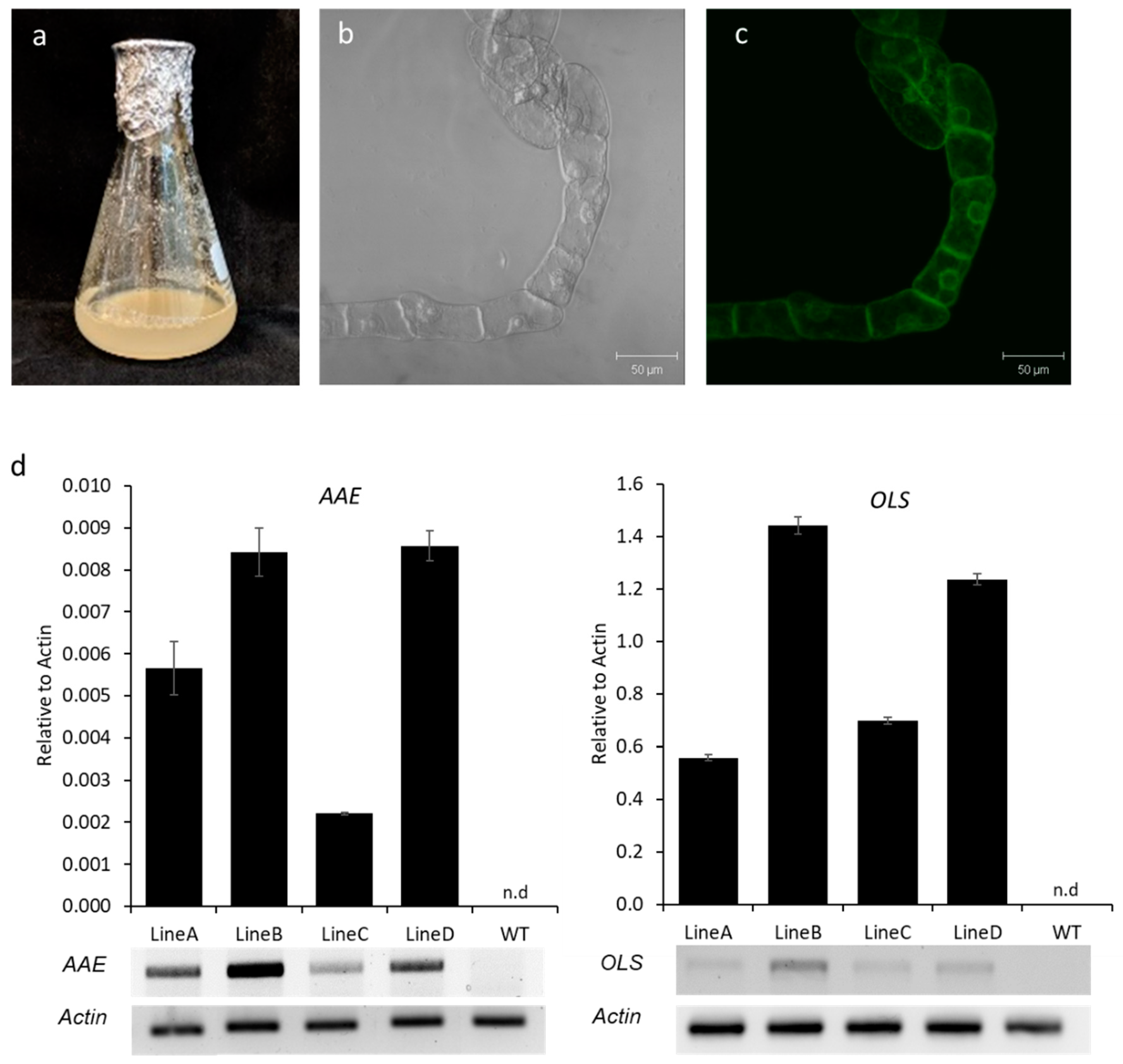
2.2. Generation of Transgenic N. benthamiana Calli and Cell Suspension Cultures
2.3. Vector Construction
2.4. Southern Blot Analysis
2.5. In Vivo Assay and Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis of Transgenic N. benthamiana Leaves
2.6. LC-MS of N. benthamiana Cell Lines
2.7. RNA Isolation, Semi-Quantitative PCR and Quantitative Real-Time PCR (qRT-PCR)
2.8. Statistical Analysis
3. Results
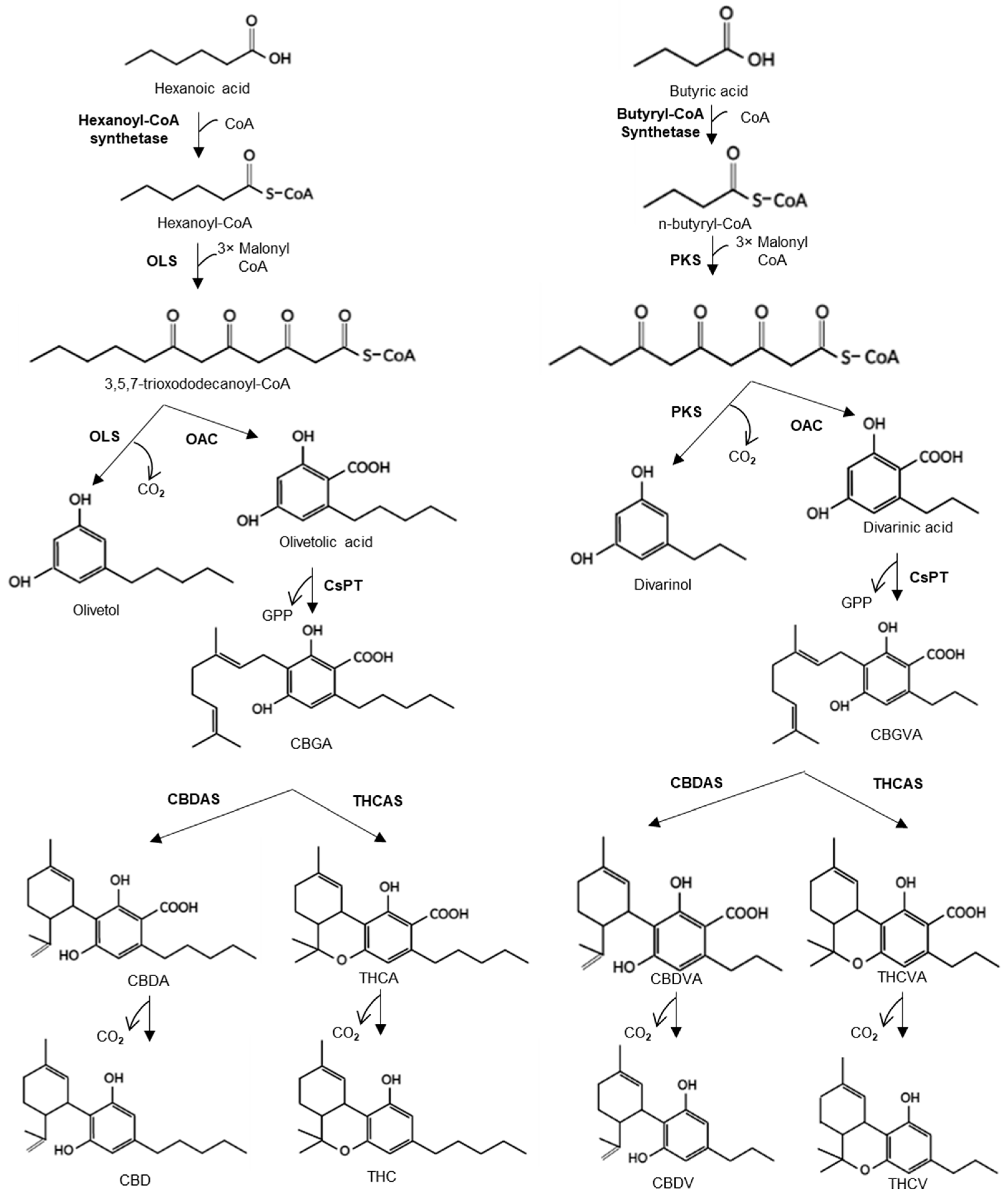
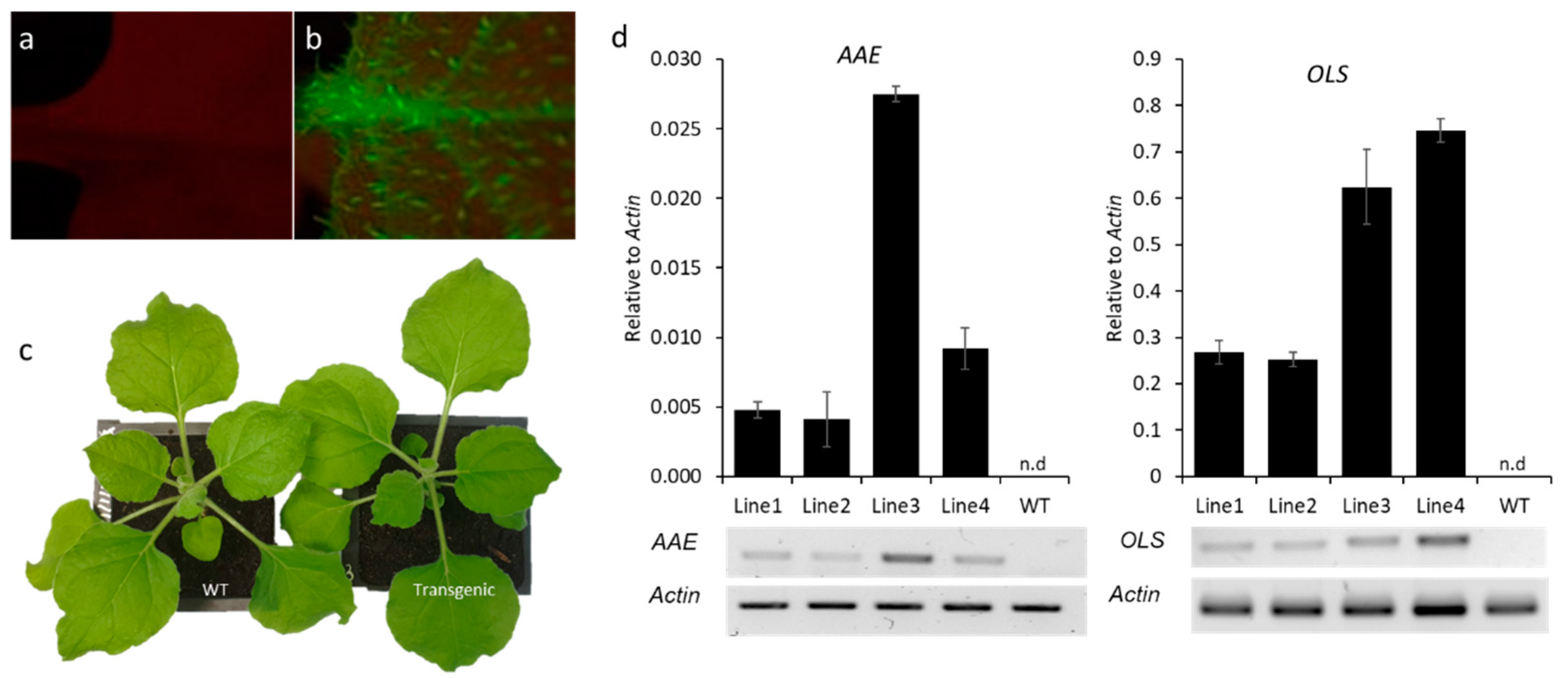
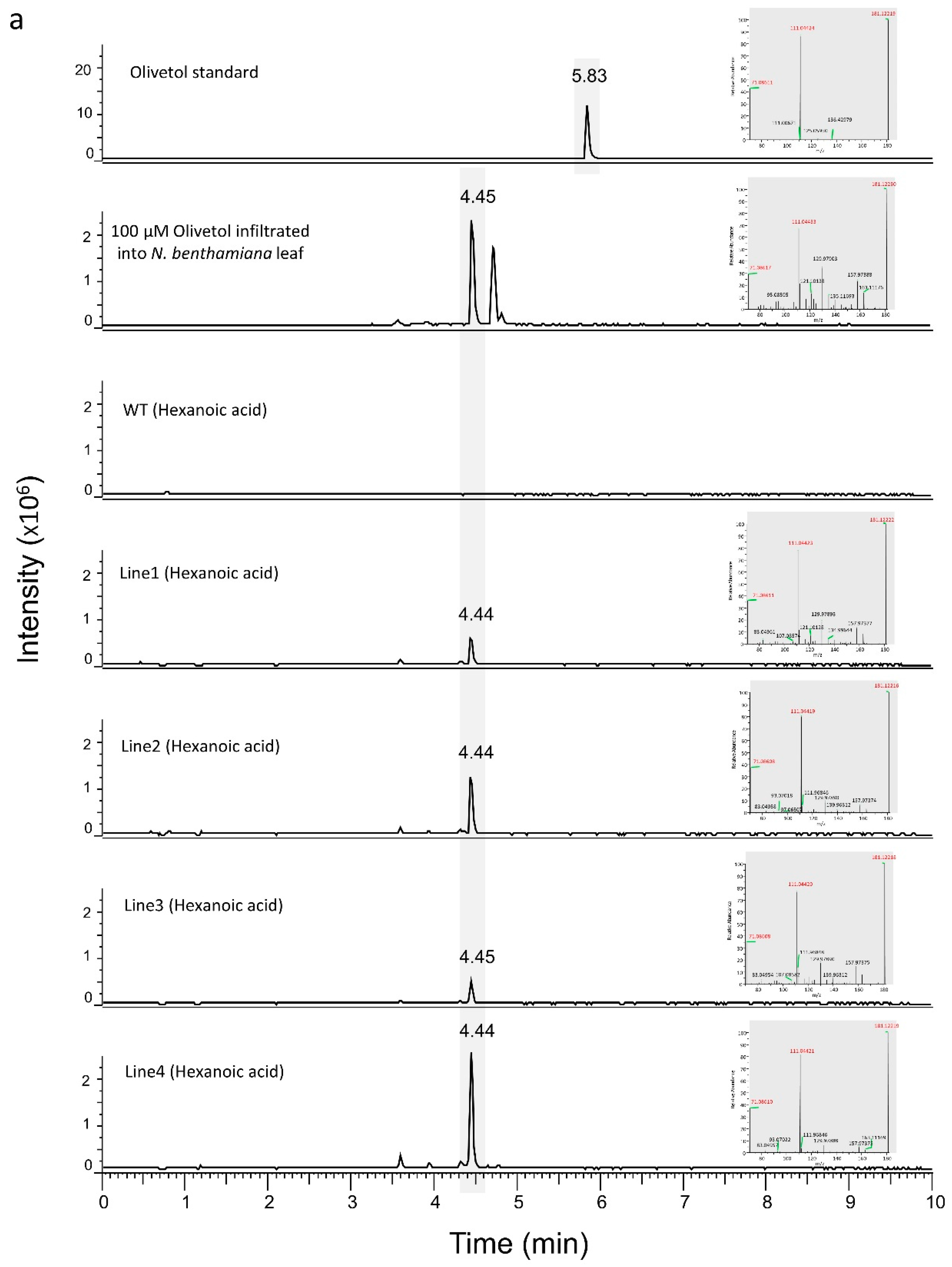
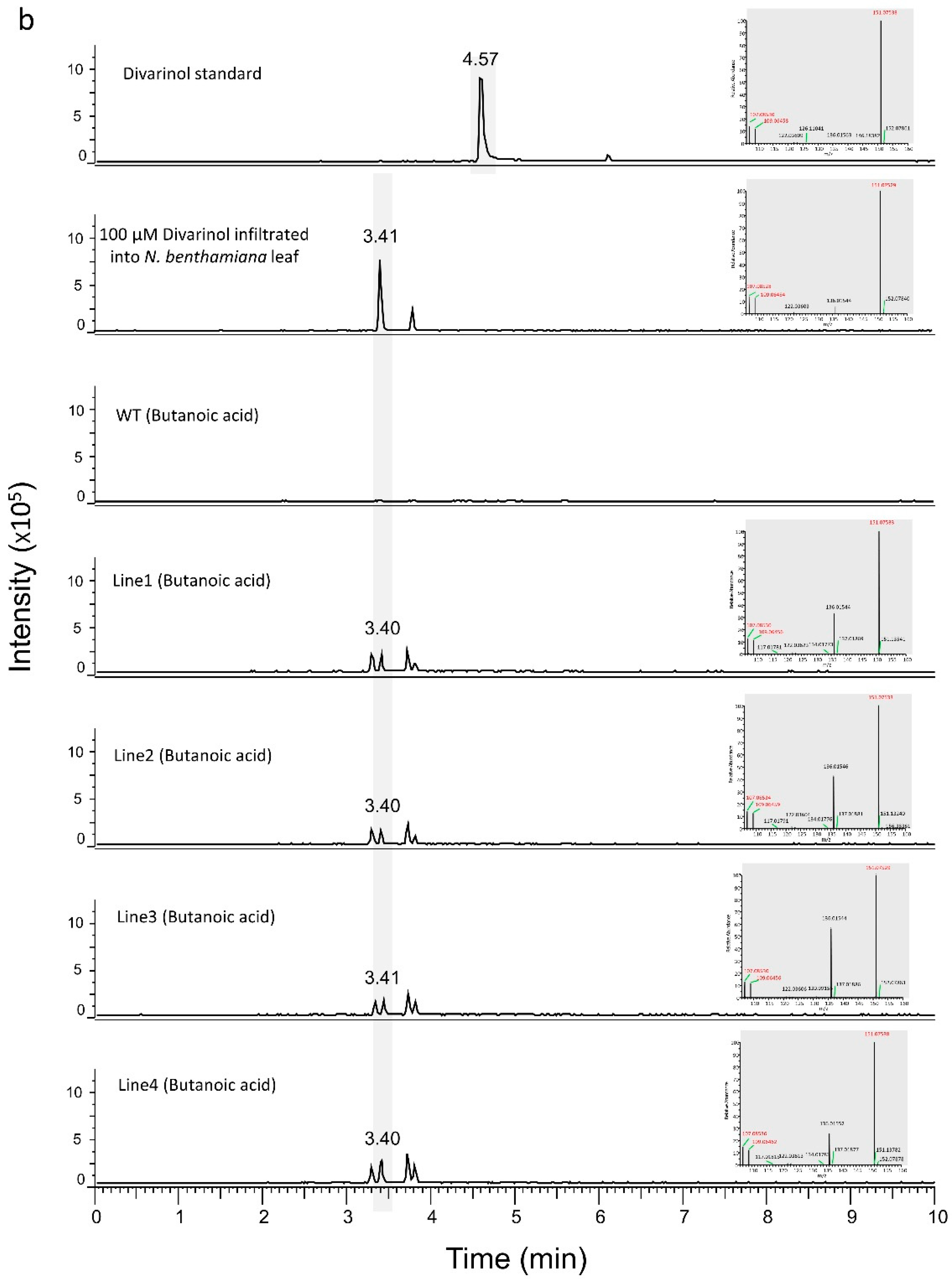
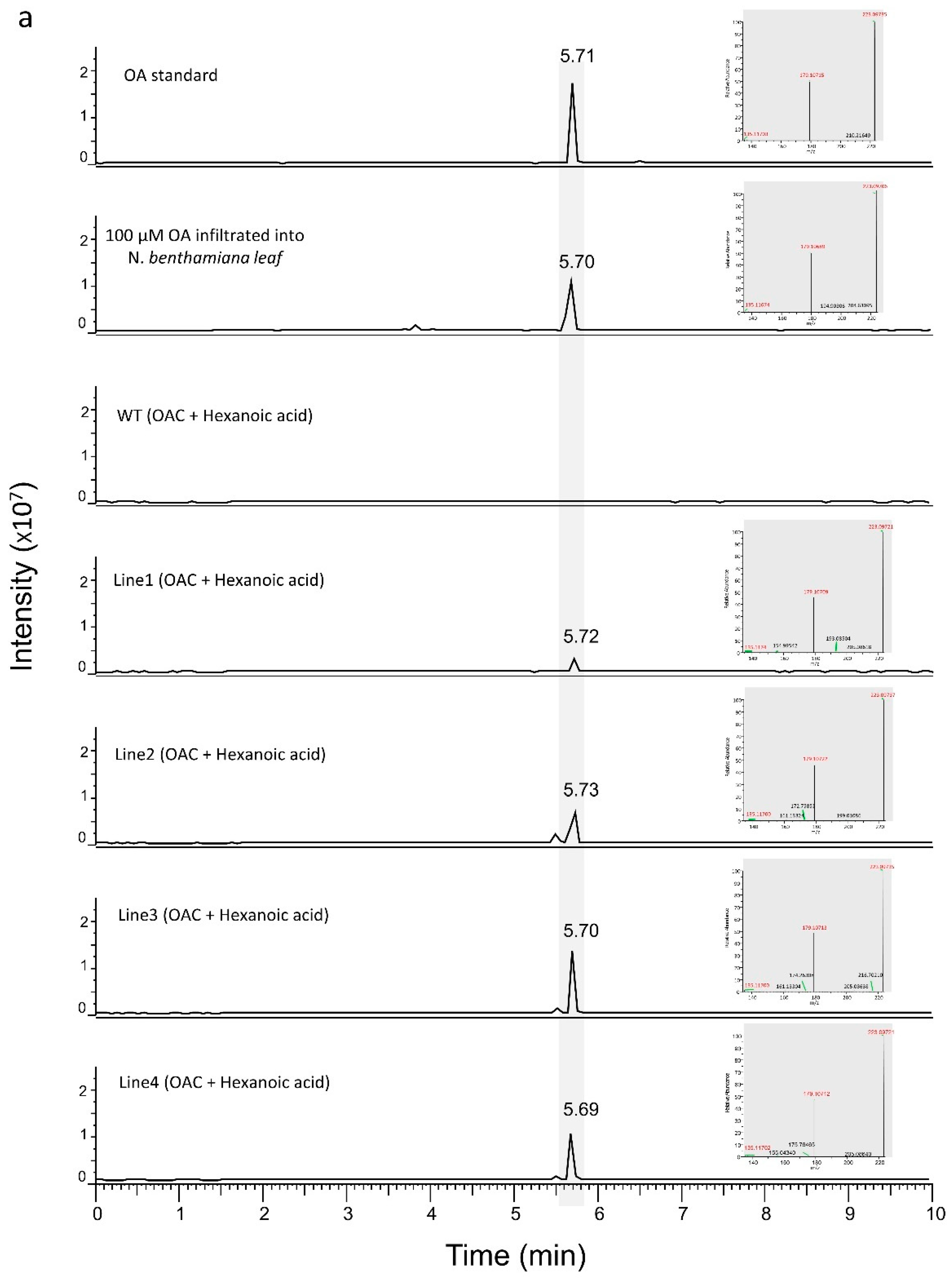
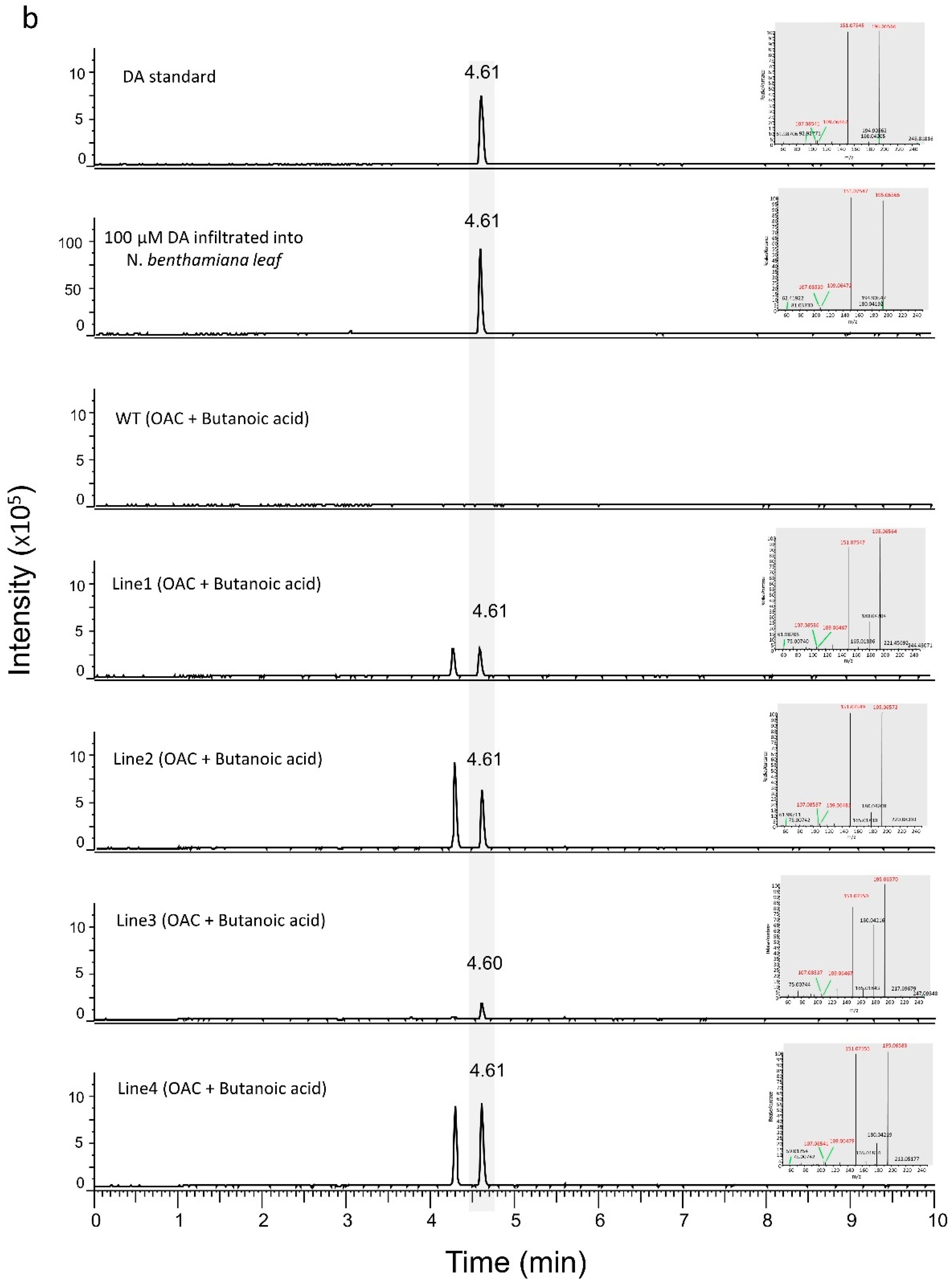
3.1. Engineering the Cannabinoid Precursor Pathway to Produce OA and DA in Transgenic N. benthamiana Plants
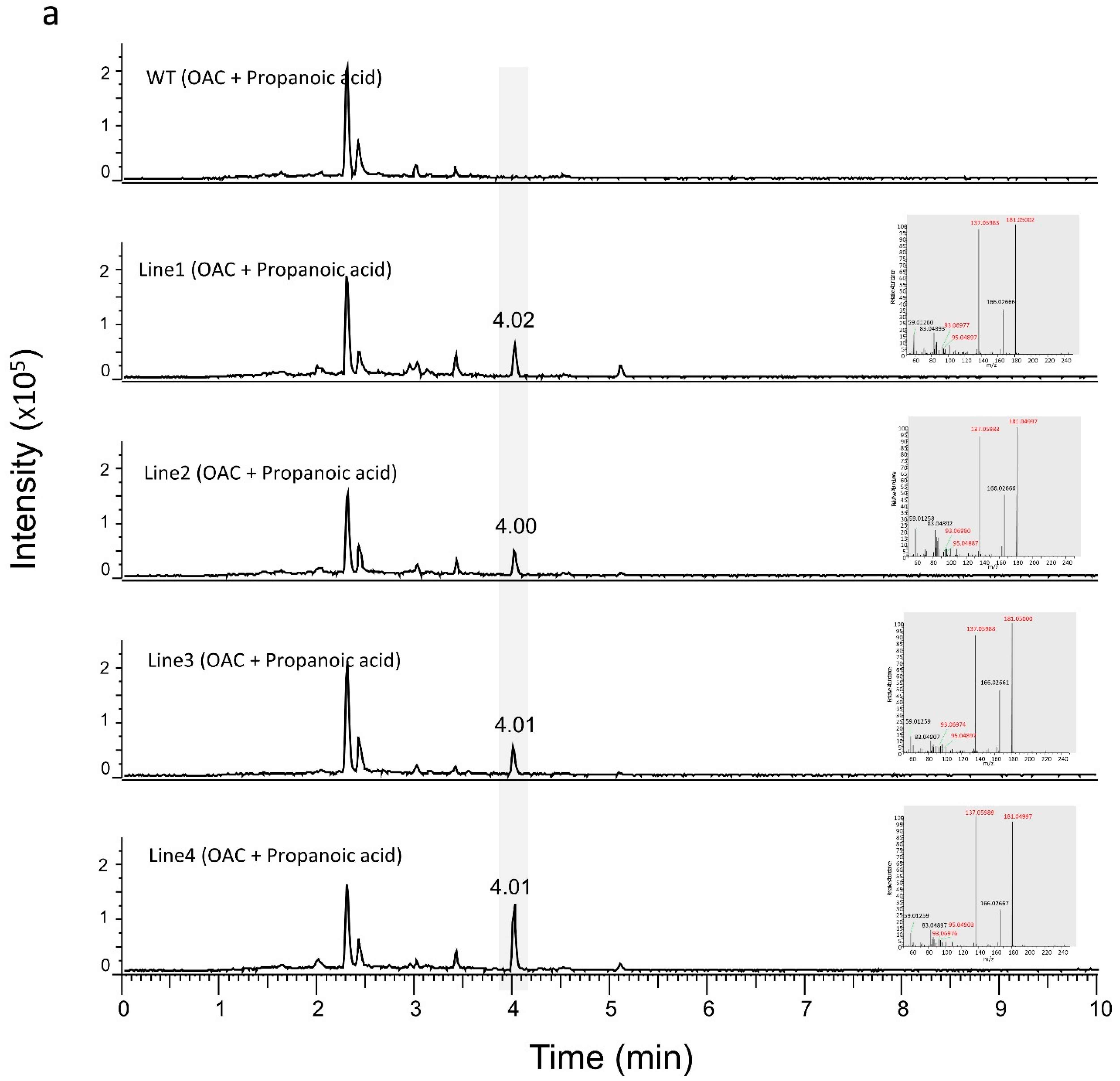
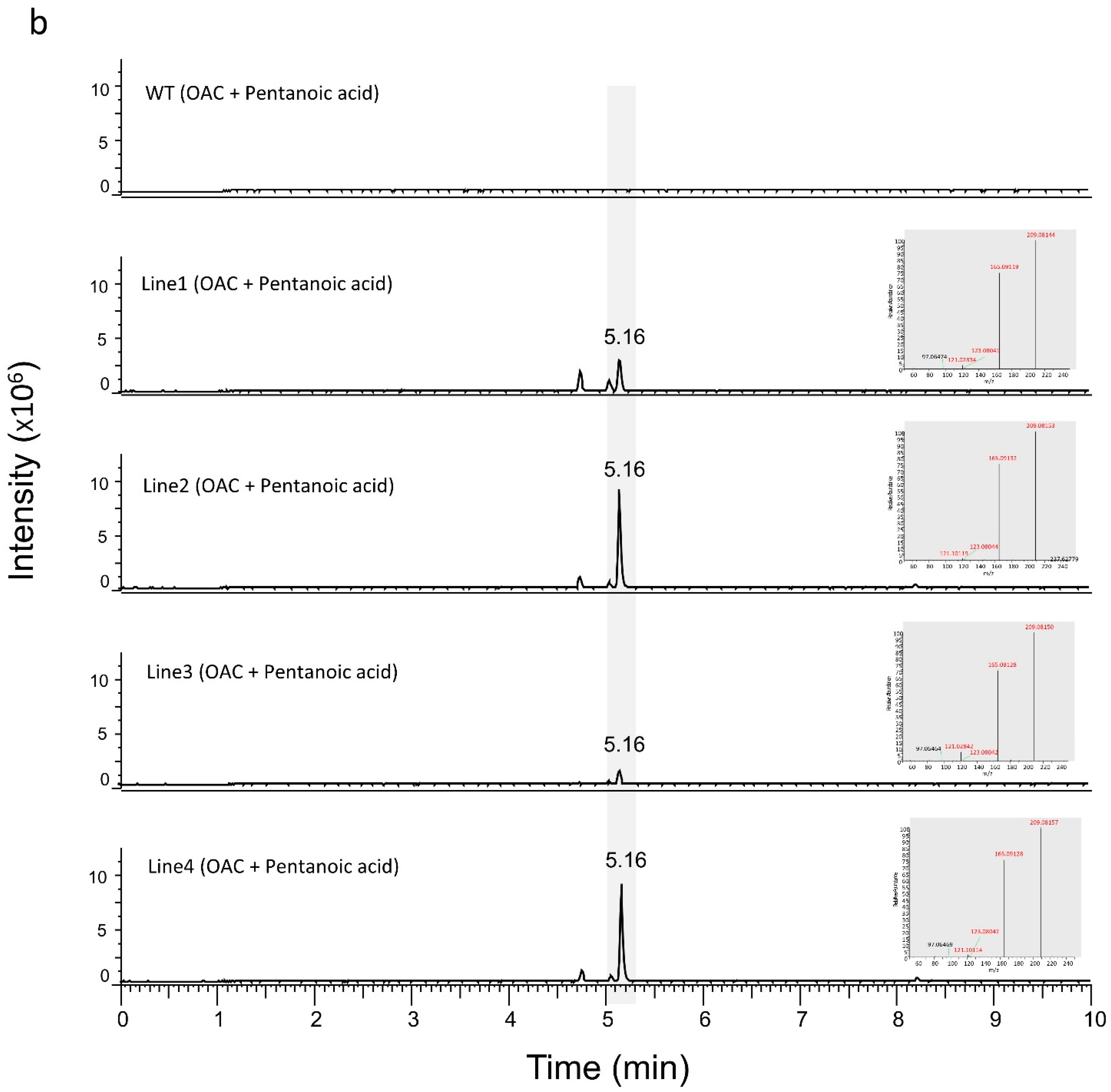
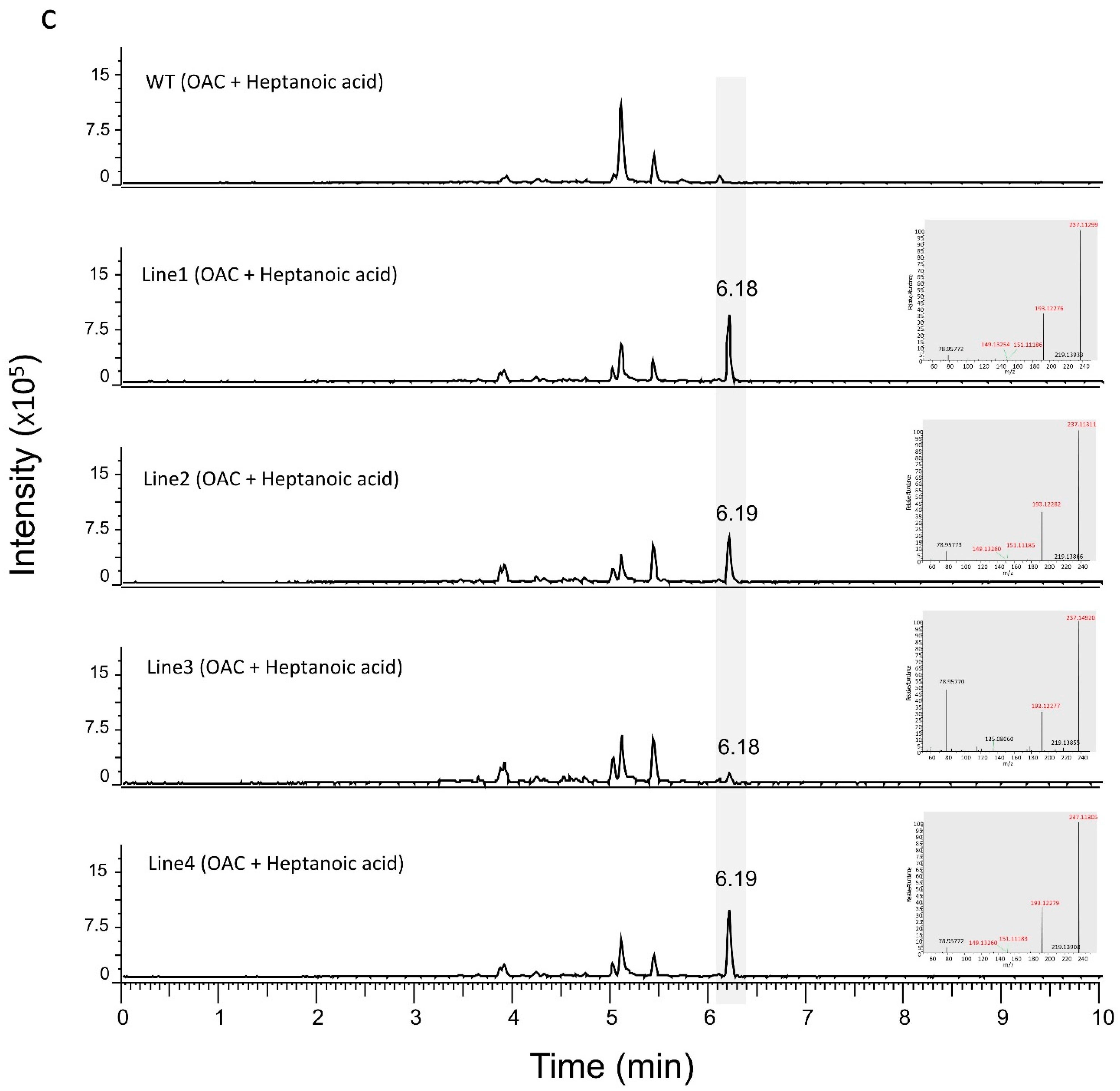
3.2. Production of Analogues of Cannabinoid Precursors in Transgenic N. benthamiana Plants
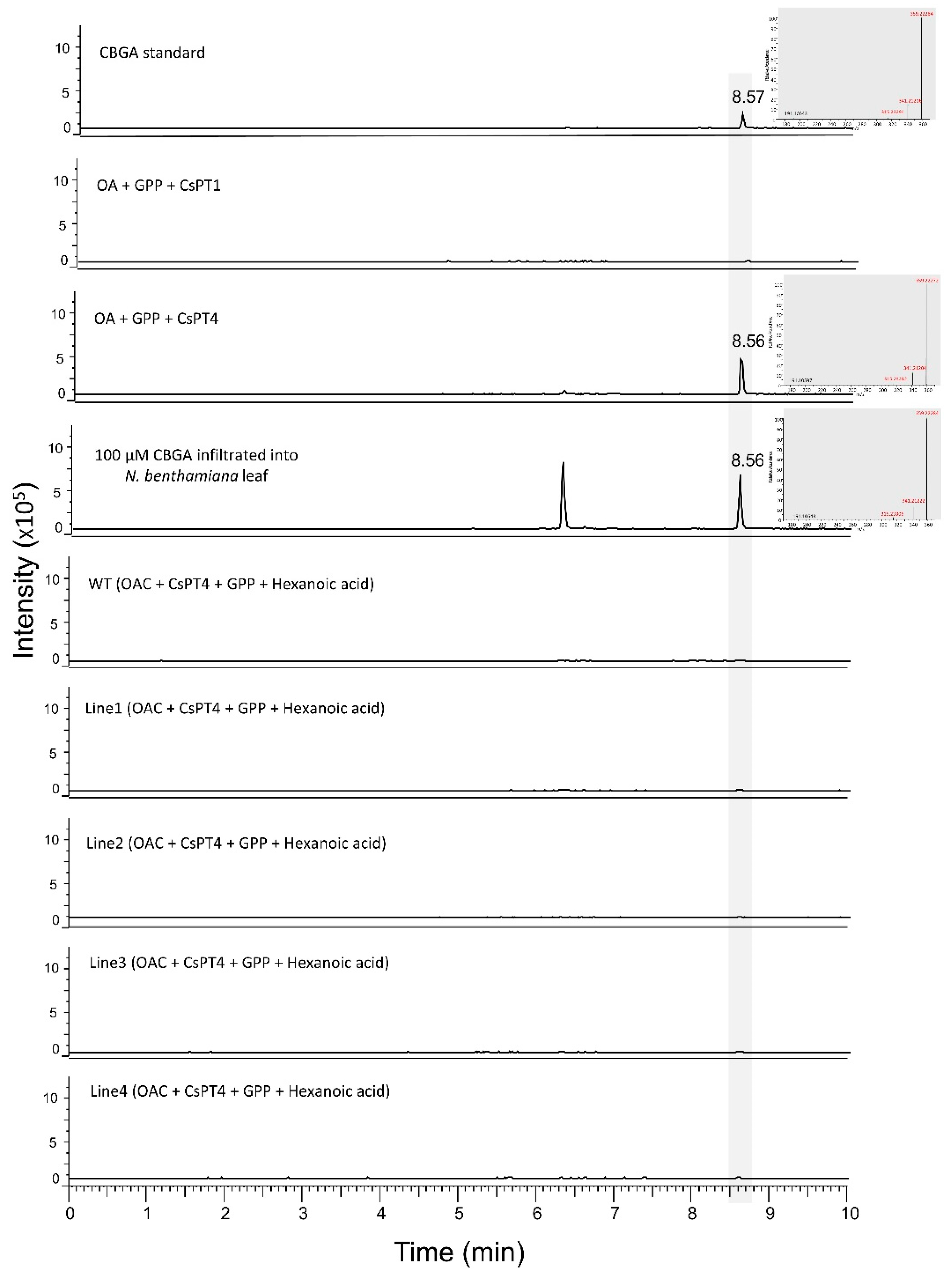
3.3. Production of Cannabigerolic Acid in N. benthamiana Plants
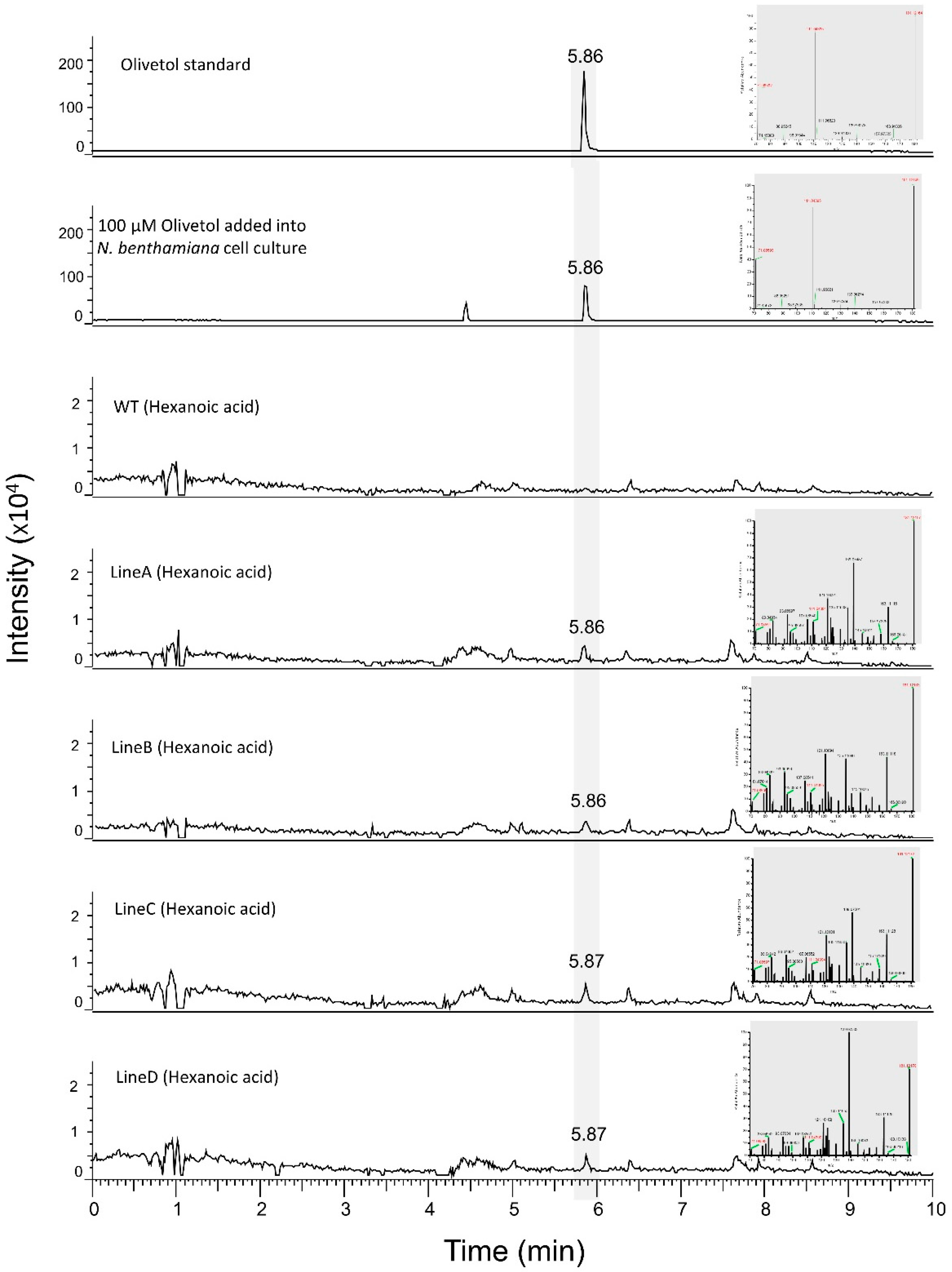
3.4. Production of Olivetol in N. benthamiana Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, U.; Pacchetti, B.; Anand, P.; Sodergren, M.H. Cannabis-based medicines and pain: A review of potential synergistic and entourage effects. Pain Manag. 2021, 11, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.S. Cannabis-based treatments as an alternative remedy for epilepsy. Integr. Med. Res. 2019, 8, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Anis, O.; Poulin, P.; Koltai, H. Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules 2020, 25, 4821. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, F.; Fründt, O.; Hidding, U.; Buhmann, C. Cannabis in Parkinson’s Disease: The Patients’ View. J. Parkinson’s Dis. 2021, 11, 309–321. [Google Scholar] [CrossRef]
- Grenier, K.; Ponnambalam, F.; Lee, D.; Lauwers, R.; Bhalerao, S. Cannabis in the Treatment of Traumatic Brain Injury: A Primer for Clinicians. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2019, 47, 11–17. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Badiola, I.; Doshi, A.; Narouze, S. Cannabis, cannabinoids, and cannabis-based medicines: Future research directions for analgesia. Reg. Anesth. Pain Med. 2022, 47, 437–444. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Shoyama, Y.; Morimoto, S. Phytocannabinoids in Cannabis sativa: Recent Studies on Biosynthetic Enzymes. Chem. Biodiv. 2007, 4, 1649–1663. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. Cell Mol. Biol. 2012, 71, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811. [Google Scholar] [CrossRef]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Page, J.E.; Boubakir, Z. Aromatic Prenyltransferase from Cannabis. Google Patents US9765308B2, 9 October 2014. [Google Scholar]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Gülck, T.; Booth, J.K.; Carvalho, Â.; Khakimov, B.; Crocoll, C.; Motawia, M.S.; Møller, B.L.; Bohlmann, J.; Gallage, N.J. Synthetic Biology of Cannabinoids and Cannabinoid Glucosides in Nicotiana benthamiana and Saccharomyces cerevisiae. J. Nat. Prod. 2020, 83, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.T.; Liu, L.; Raymond, C.A.; Ansari, O.; King, G.J. Developmental Plasticity of the Major Alkyl Cannabinoid Chemotypes in a Diverse Cannabis Genetic Resource Collection. Front. Plant Sci. 2018, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterisation of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2016, 208, 463–475. [Google Scholar] [CrossRef]
- Vree, T.B.; Breimer, D.D.; van Ginneken, C.A.; van Rossum, J.M. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharmacol. 1972, 24, 7–12. [Google Scholar] [CrossRef]
- Smith, R.M. Identification of butyl cannabinoids in marijuana. J. Forensic Sci. 1997, 42, 610–618. [Google Scholar]
- Welling, M.T.; Liu, L.; Raymond, C.A.; Kretzschmar, T.; Ansari, O.; King, G.J. Complex Patterns of Cannabinoid Alkyl Side-Chain Inheritance in Cannabis. Sci. Rep. 2019, 9, 11421. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front Pharm. 2021, 12, 777804. [Google Scholar] [CrossRef] [PubMed]
- Melzer, R.; McCabe, P.F.; Schilling, S. Evolution, genetics and biochemistry of plant cannabinoid synthesis: A challenge for biotechnology in the years ahead. Curr. Opin. Biotechnol. 2022, 75, 102684. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y.; Chooi, Y.-H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef]
- Sayre, R.T.; Gonçalves, E.C.; Zidenga, T. High Level In Vivo Biosynthesis and Isolation of Water-Soluble Cannabinoids in Plant Systems. Google Patents US10378020B2, 13 August 2019. [Google Scholar]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The Gene Controlling Marijuana Psychoactivity: Molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L.*. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic Acid Synthase, the Enzyme Controlling Marijuana Psychoactivity, is Secreted into the Storage Cavity of the Glandular Trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Geissler, M.; Volk, J.; Stehle, F.; Kayser, O.; Warzecha, H. Subcellular localization defines modification and production of Δ9-tetrahydrocannabinolic acid synthase in transiently transformed Nicotiana benthamiana. Biotechnol. Lett. 2018, 40, 981–987. [Google Scholar] [CrossRef]
- Gallois, P.; Marinho, P. Leaf disk transformation using Agrobacterium tumefaciens-expression of heterologous genes in tobacco. Methods Mol. Biol. 1995, 49, 39–48. [Google Scholar] [CrossRef]
- Sukenik, S.C.; Karuppanan, K.; Li, Q.; Lebrilla, C.B.; Nandi, S.; McDonald, K.A. Transient Recombinant Protein Production in Glycoengineered Nicotiana benthamiana Cell Suspension Culture. Int. J. Mol. Sci. 2018, 19, 1205. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stasolla, C.; Brûlé-Babel, A.; Ayele, B.T. Isolation and characterization of rubisco small subunit gene promoter from common wheat (Triticum aestivum L.). Plant Signal Behav. 2015, 10, e989033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, N.J. A Technique Whose Time Has Come. Science 2002, 296, 557. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Ronis, M.J.; Badger, T.M. Matrix effects break the LC behavior rule for analytes in LC-MS/MS analysis of biological samples. Exp. Biol. Med. (MaywoodN.J.) 2015, 240, 488–497. [Google Scholar] [CrossRef]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef]
- Thakur, G.A.; Duclos, R.I.; Makriyannis, A. Natural cannabinoids: Templates for drug discovery. Life Sci. 2005, 78, 454–466. [Google Scholar] [CrossRef]
- Razdan, R.K. Structure-activity relationships in cannabinoids. Pharmacol. Rev. 1986, 38, 75–149. [Google Scholar]
- Martin, B.; Jefferson, R.; Winckler, R.; Wiley, J.; Huffman, J.; Crocker, P.; Saha, B.; Razdan, R. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J. Pharmacol. Exp. Ther. 1999, 290, 1065–1079. [Google Scholar]
- Lim, K.J.H.; Lim, Y.P.; Hartono, Y.D.; Go, M.K.; Fan, H.; Yew, W.S. Biosynthesis of Nature-Inspired Unnatural Cannabinoids. Molecules 2021, 26, 2914. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Schachtsiek, J.; Warzecha, H.; Kayser, O.; Stehle, F. Current Perspectives on Biotechnological Cannabinoid Production in Plants. Planta Med 2018, 84, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Panicker, D.; Wang, Q.; Kim, M.J.; Liu, J.; Yin, J.-L.; Wong, L.; Jang, I.-C.; Chua, N.-H.; Sarojam, R. Next generation sequencing unravels the biosynthetic ability of Spearmint (Mentha spicata) peltate glandular trichomes through comparative transcriptomics. Bmc Plant Biol. 2014, 14, 292. [Google Scholar] [CrossRef]
- Sánchez Montero, J.M.; Agis-Torres, A.; Solano, D.; Söllhuber, M.; Fernandez, M.; Villaro, W.; Gómez-Cañas, M.; García-Arencibia, M.; Fernández-Ruiz, J.; Egea, J.; et al. Analogues of cannabinoids as multitarget drugs in the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 895, 173875. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.J.; Bunch, J.E. Determination of Volatile Acids in Tobacco, Tea, and Coffee Using Derivatization-Purge and Trap Gas Chromatography-Selected Ion Monitoring Mass Spectrometry. J. Chromatogr. Sci. 1997, 35, 206–208. [Google Scholar] [CrossRef][Green Version]
- Dolgin, E. The bioengineering of cannabis. Nature 2019, 572, S5-S5. [Google Scholar] [CrossRef]
- Morimoto, S.; Tanaka, Y.; Sasaki, K.; Tanaka, H.; Fukamizu, T.; Shoyama, Y.; Shoyama, Y.; Taura, F. Identification and characterization of cannabinoids that induce cell death through mitochondrial permeability transition in Cannabis leaf cells. J. Biol. Chem. 2007, 282, 20739–20751. [Google Scholar] [CrossRef]
- Wiles, D.; Shanbhag, B.K.; O’Brien, M.; Doblin, M.S.; Bacic, A.; Beddoe, T. Heterologous production of Cannabis sativa-derived specialised metabolites of medicinal significance—Insights into engineering strategies. Phytochemistry 2022, 203, 113380. [Google Scholar] [CrossRef]
- Reimer, C.; Kufs, J.E.; Rautschek, J.; Regestein, L.; Valiante, V.; Hillmann, F. Engineering the amoeba Dictyostelium discoideum for biosynthesis of a cannabinoid precursor and other polyketides. Nat. Biotechnol. 2022, 40, 751–758. [Google Scholar] [CrossRef]
- Taura, F.; Iijima, M.; Kurosaki, F. Daurichromenic acid and grifolic acid: Phytotoxic meroterpenoids that induce cell death in cell culture of their producer Rhododendron dauricum. Plant Signal Behav 2018, 13, e1422463. [Google Scholar] [CrossRef]
- Thomas, F.; Schmidt, C.; Kayser, O. Bioengineering studies and pathway modeling of the heterologous biosynthesis of tetrahydrocannabinolic acid in yeast. Appl. Microbiol. Biotechnol. 2020, 104, 9551–9563. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, V.A.; Leong, S.H.; Jang, I.-C.; Rajani, S. Metabolic Engineering of Nicotiana benthamiana to Produce Cannabinoid Precursors and Their Analogues. Metabolites 2022, 12, 1181. https://doi.org/10.3390/metabo12121181
Reddy VA, Leong SH, Jang I-C, Rajani S. Metabolic Engineering of Nicotiana benthamiana to Produce Cannabinoid Precursors and Their Analogues. Metabolites. 2022; 12(12):1181. https://doi.org/10.3390/metabo12121181
Chicago/Turabian StyleReddy, Vaishnavi Amarr, Sing Hui Leong, In-Cheol Jang, and Sarojam Rajani. 2022. "Metabolic Engineering of Nicotiana benthamiana to Produce Cannabinoid Precursors and Their Analogues" Metabolites 12, no. 12: 1181. https://doi.org/10.3390/metabo12121181
APA StyleReddy, V. A., Leong, S. H., Jang, I.-C., & Rajani, S. (2022). Metabolic Engineering of Nicotiana benthamiana to Produce Cannabinoid Precursors and Their Analogues. Metabolites, 12(12), 1181. https://doi.org/10.3390/metabo12121181






