Neonatal Orally Administered Zingerone Attenuates Alcohol-Induced Fatty Liver Disease in Experimental Rat Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Animal Use Ethical Clearance
2.2. Animals and Animal Management
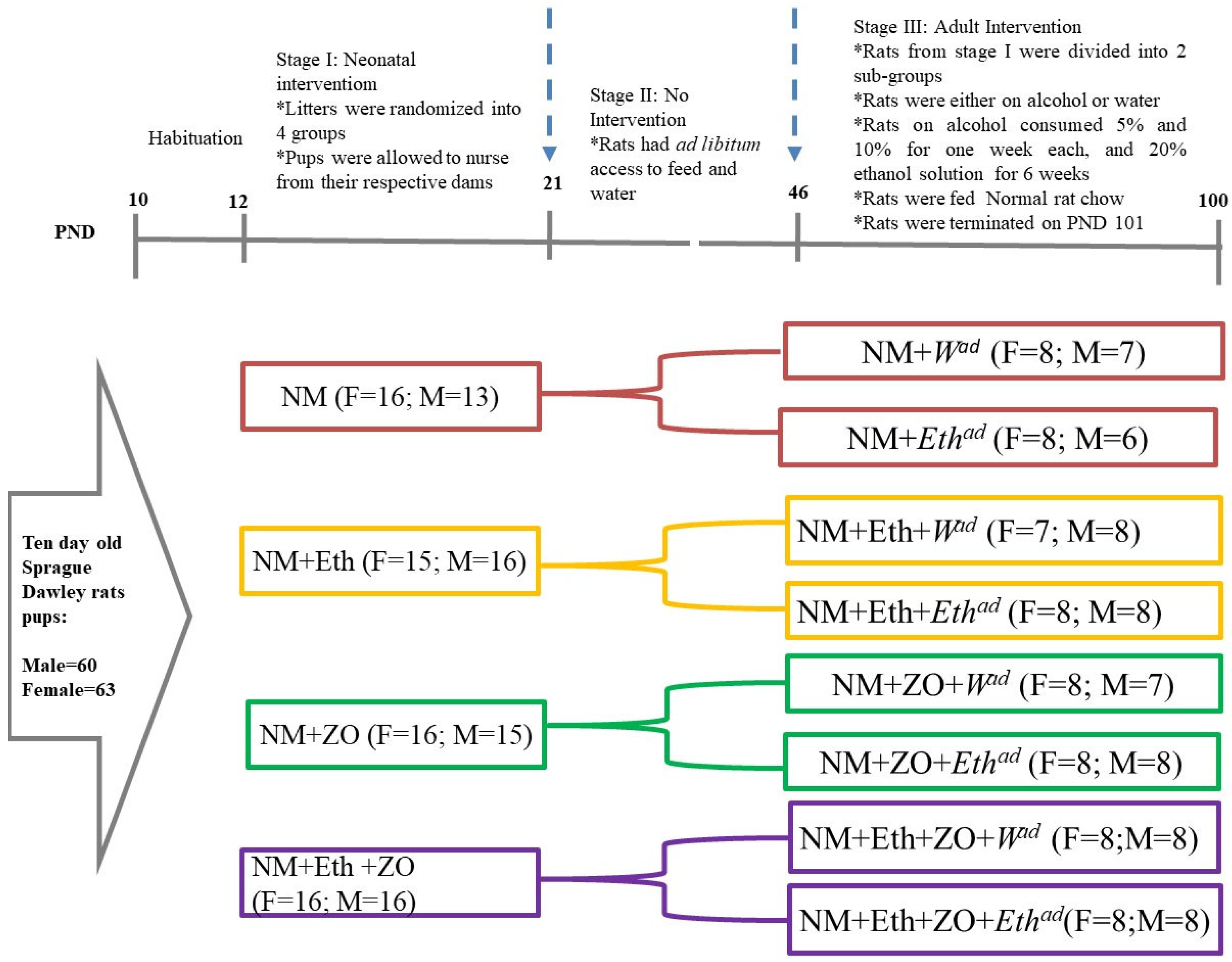
2.3. Experimental Design
2.4. Terminal Procedure
2.5. Computation of the Hepatosomatic Index
2.6. Determination of Hepatic Lipid Peroxidation
2.6.1. Liver Tissue Homogenisation
2.6.2. Determination of Peroxidation in the Liver
2.7. Determination of Liver Lipid Content
2.8. Determination of Liver Histomorphometry
2.9. Determination of Surrogate Plasma Biomarkers of Liver Function
2.10. Determination of Plasma CYP2E1, TNF-α and IL-6 Concentration
2.11. Determination of Hepatic Gene Expression
2.11.1. RNA Extraction and cDNA Synthesis
2.11.2. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Analysis
2.11.3. Statistical Analysis
3. Results
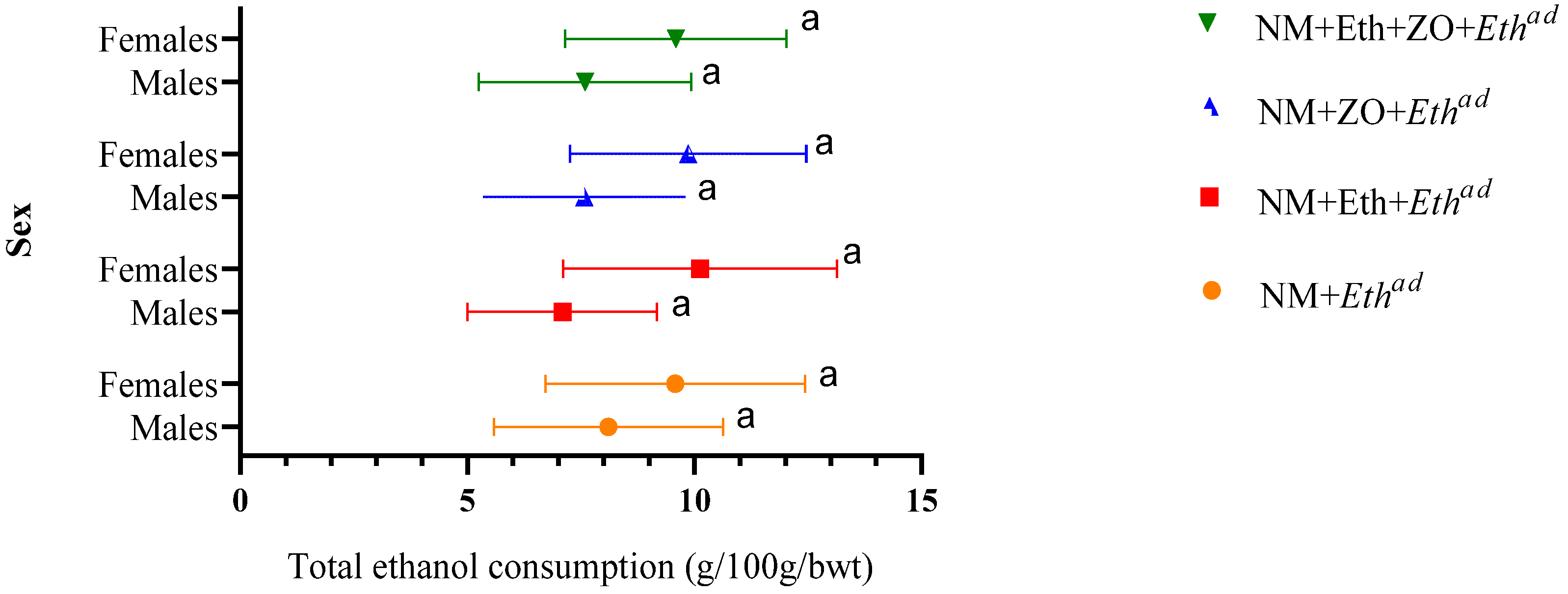
3.1. Effect of Neonatal Orally Administered Zingerone on Ethanol Consumption in Adult Rats
3.2. Effect of Neonatal Orally Administered Zingerone on Hepatosomatic Index
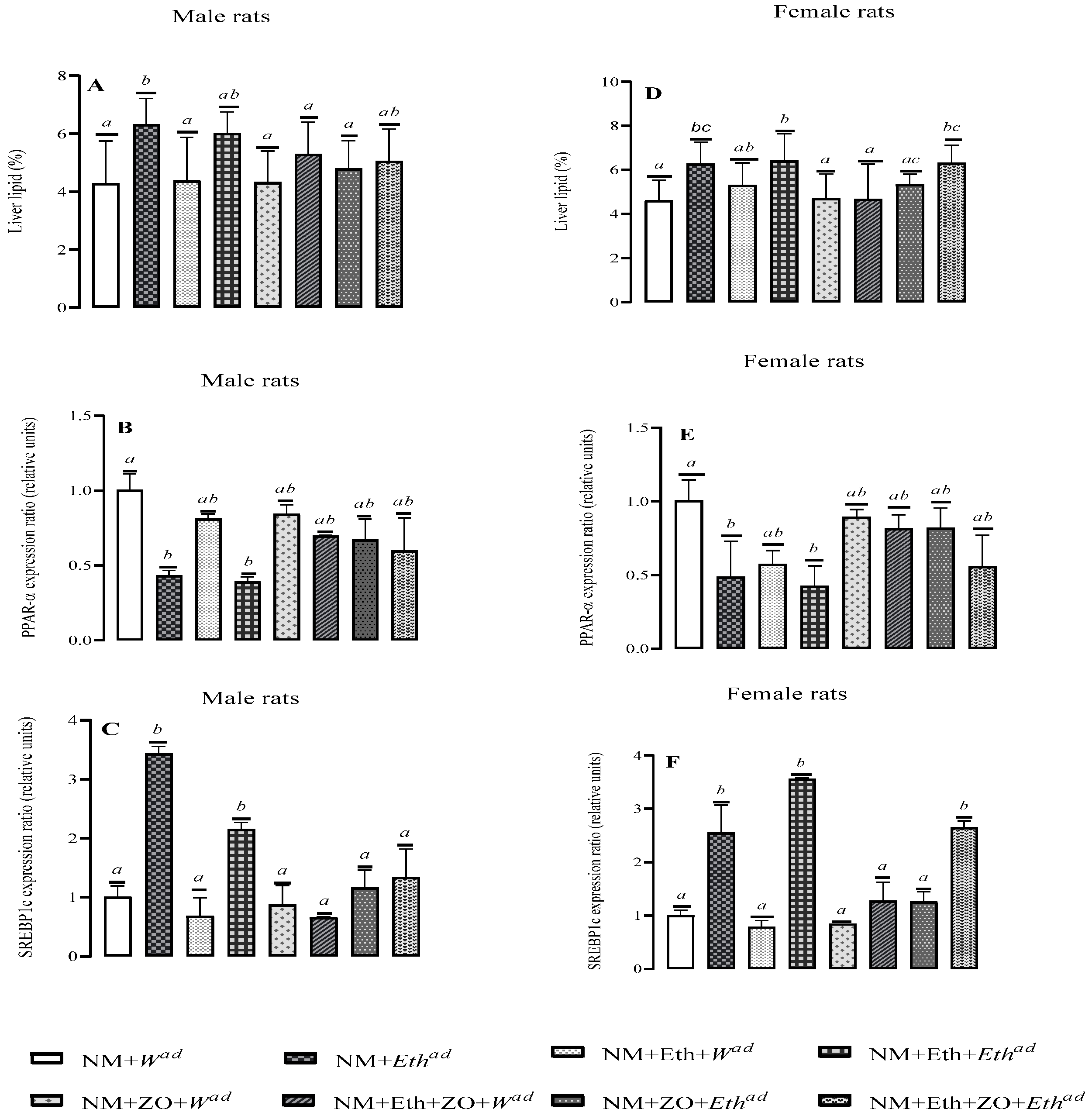
3.3. Effect of Neonatal Orally Administered Zingerone on Liver Fat Content
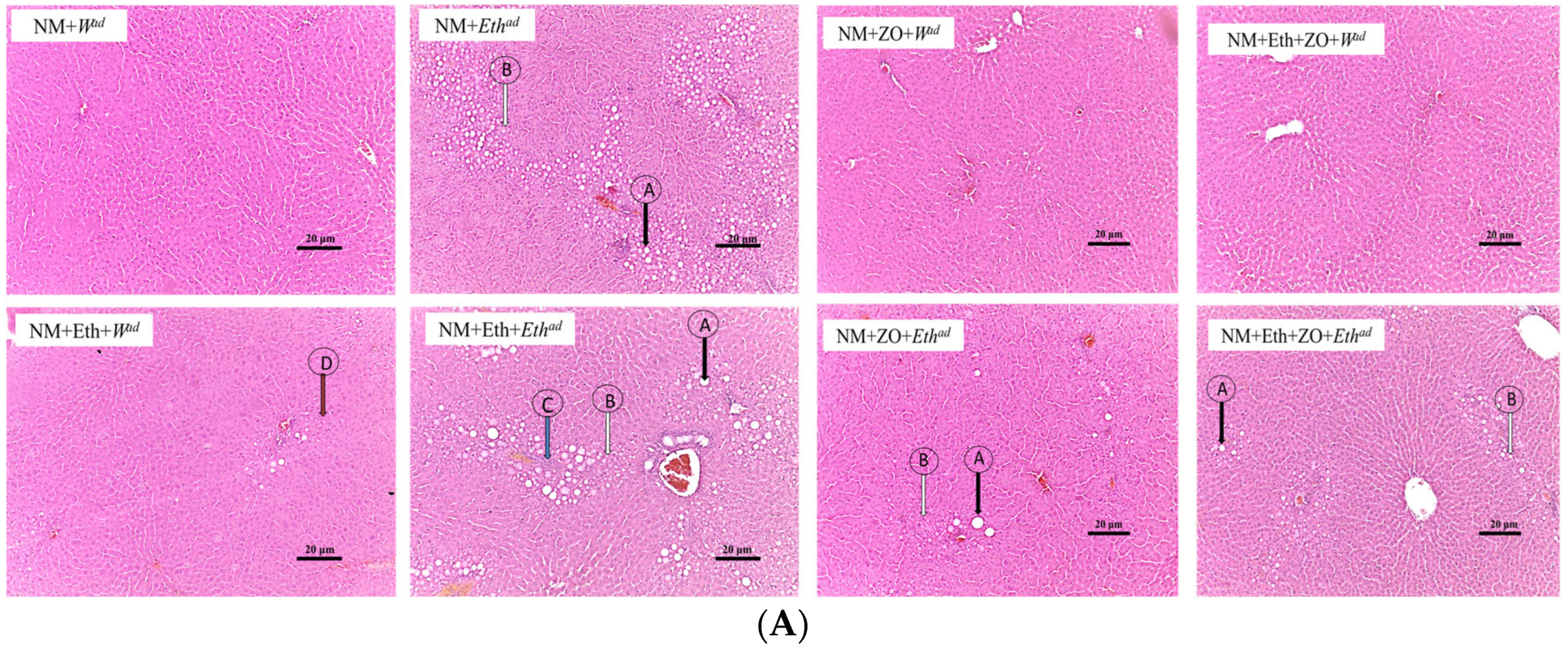
3.4. Effect of Neonatal Orally Administered Zingerone on Hepatic Histomorphometric Changes
3.5. Effect of Neonatal Orally Administered Zingerone on Lipid Regulatory Genes
3.6. Effect of Neonatal Orally Administered Zingerone on Plasma Liver Enzyme Activities
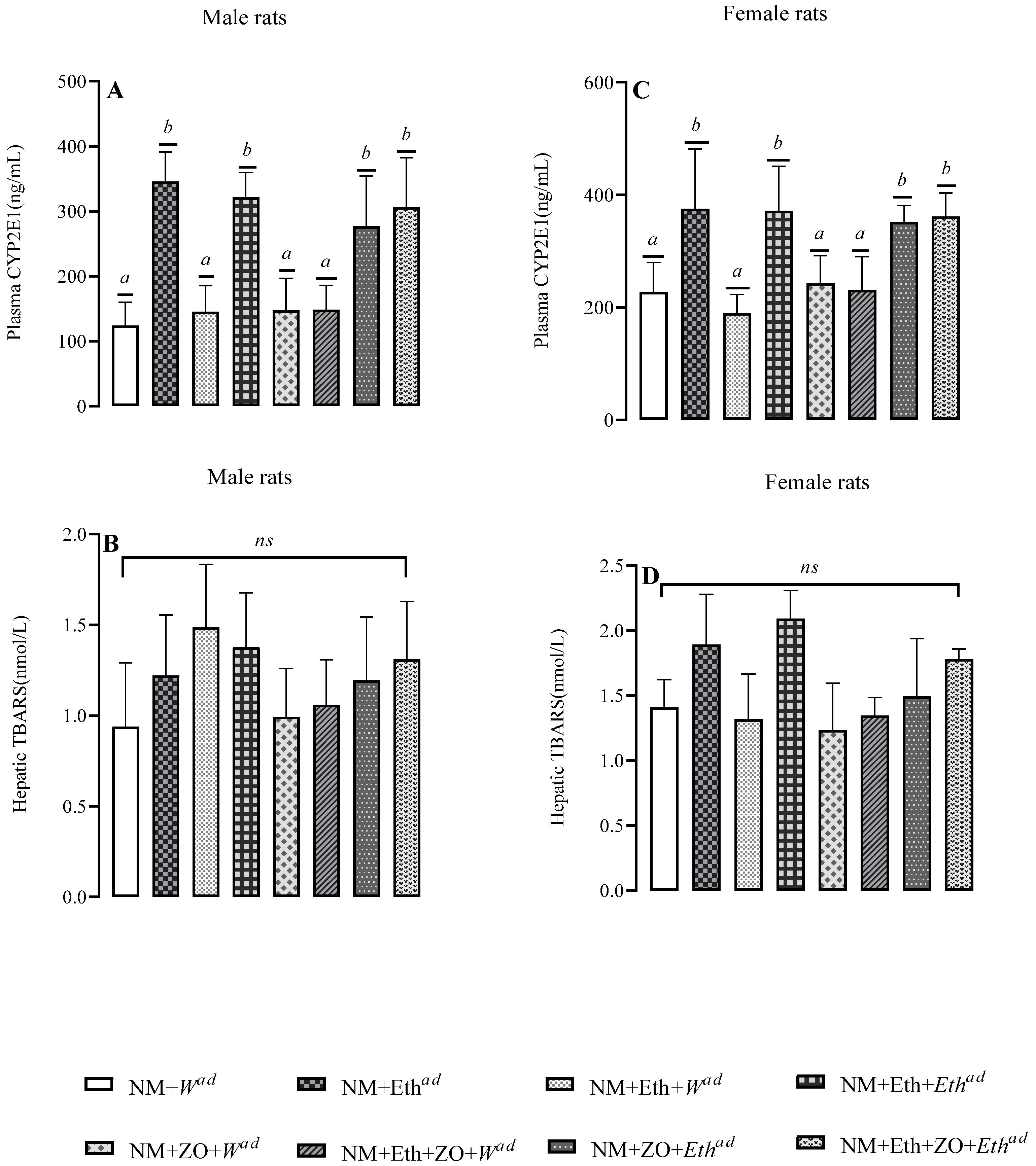
3.7. Effect of Neonatal Orally Administered Zingerone on Plasma CYP2E1 and Hepatic TBARS
3.8. Effect of Neonatal Orally Administered Zingerone on Biomarkers of Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tawa, E.A.; Hall, S.D.; Lohoff, F.W. Overview of the Genetics of Alcohol Use Disorder. Alcohol. Alcohol. 2016, 51, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Siomek-Gorecka, A.; Dlugosz, A.; Czarnecki, D. The Molecular Basis of Alcohol Use Disorder (AUD). Genetics, Epigenetics, and Nutrition in AUD: An Amazing Triangle. Int. J. Mol. Sci. 2021, 22, 4262. [Google Scholar] [CrossRef] [PubMed]
- Nizhnikov, M.E.; Popoola, D.O.; Cameron, N. Transgenerational Transmission of the Effect of Gestational Ethanol Exposure on Ethanol Use-Related Behavior. Alcohol. Clin. Exp. Res. 2016, 40, 497–506. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Rametta, R.; Dongiovanni, P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Mitchell, M.D. Developmental origins of health and disease: Reducing the burden of chronic disease in the next generation. Genome Med. 2010, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.J.; Morris, T.J.; Skinner, B.M.; Sargent, C.A.; Vickers, M.H.; Gluckman, P.D.; Gilmour, S.; Affara, N.A. Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: Implications for models of developmental programming. BMC Genom. 2014, 15, 49. [Google Scholar] [CrossRef]
- Asiedu, B.; Nyakudya, T.T.; Lembede, B.W.; Chivandi, E. Early-life exposure to alcohol and the risk of alcohol-induced liver disease in adulthood. Birth Defects Res. 2021, 113, 451–468. [Google Scholar] [CrossRef]
- Hannigan, J.H.; Chiodo, L.M.; Sokol, R.J.; Janisse, J.; Delaney-Black, V. Prenatal alcohol exposure selectively enhances young adult perceived pleasantness of alcohol odors. Physiol. Behav. 2015, 148, 71–77. [Google Scholar] [CrossRef]
- Gaztañaga, M.; Angulo-Alcalde, A.; Chotro, M.G. Prenatal Alcohol Exposure as a Case of Involuntary Early Onset of Alcohol Use: Consequences and Proposed Mechanisms from Animal Studies. Front. Behav. Neurosci. 2020, 14, 26. [Google Scholar] [CrossRef]
- Rangmar, J.; Dahlgren-Sandberg, A.; Aronson, M.; Fahlke, C. Cognitive and executive functions, social cognition and sense of coherence in adults with fetal alcohol syndrome. Nord. J. Psychiatry 2015, 69, 1754–1760. [Google Scholar] [CrossRef]
- Hagström, H.; Hemmingsson, T.; Discacciati, A.; Andreasson, A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J. Hepatol. 2018, 68, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, L.; Harman, E.; Padmanabhan, V.; Gregg, B. Lactational programming of glucose homeostasis: A window of opportunity. Reproduction 2018, 156, R23–R42. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P. The Liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–22. ISBN 978-0-12-804274-8. [Google Scholar]
- Singal, A.K.; Anand, B.S. Recent trends in the epidemiology of alcoholic liver disease. Clin. Liver Dis. 2013, 2, 53–56. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO|Global Status Report on Alcohol and Health 2018. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 19 March 2020).
- Gao, B.; Bataller, R. Alcoholic Liver Disease: Pathogenesis and New Therapeutic Targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Yeh, M.M.; Brunt, E.M. Pathological Features of Fatty Liver Disease. Gastroenterology 2014, 147, 754–764. [Google Scholar] [CrossRef]
- Mostafa, M.; Abdelkader, A.; Evans, J.J.; Hagen, C.E.; Hartley, C.P. Fatty Liver Disease: A Practical Approach. Arch. Pathol. Lab. Med. 2020, 144, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D. Histopathology of alcoholic liver disease. Clin. Liver Dis. 2013, 2, 64–67. [Google Scholar] [CrossRef]
- Sakhuja, P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J. Gastroenterol. 2014, 20, 16474–16479. [Google Scholar] [CrossRef] [PubMed]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Ünalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 55, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, Y.; Xu, D.; Zhao, Z.; Zhang, Y.; Pan, Y.; Cao, P.; Wang, Z.; Chen, Y. Ethanol-induced hepatic steatosis is modulated by glycogen level in the liver. J. Lipid Res. 2015, 56, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. Curr. Rev. 2017, 38, 147–161. [Google Scholar]
- Stickel, F.; Datz, C.; Hampe, J.; Bataller, R. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver 2017, 11, 173–188. [Google Scholar] [CrossRef]
- Deaciuc, I.V.; D’Souza, N.B.; Spitzer, J.J. Tumor Necrosis Factor-alpha Cell-Surface Receptors of Liver Parenchymal and Nonparenchymal Cells during Acute and Chronic Alcohol Administration to Rats. Alcohol. Clin. Exp. Res. 1995, 19, 332–338. [Google Scholar] [CrossRef]
- Petrasek, J.; Csak, T.; Szabo, G. Toll-Like Receptors in Liver Disease. Adv. Clin. Chem. 2012, 59, 155–201. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Mukwevho, E.; Nkomozepi, P.; Erlwanger, K.H. Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J. Dev. Orig. Health Dis. 2018, 9, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.M. Zingerone ameliorates non-alcoholic fatty liver disease in rats by activating AMPK. J. Food Biochem. 2022, 46, e14149. [Google Scholar] [CrossRef] [PubMed]
- Ajah, A.A.; Lembede, B.W.; Nkomozepi, P.; Erlwanger, K.H.; Nyakudya, T.T. Neonatal Oral Administration of Chrysin Prevents Long-Term Development of Non-Alcoholic Fatty Liver Disease in a Sexually Dimorphic Manner in Fructose Nurtured Sprague Dawley Rats. Life 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Lembede, B.W.; Erlwanger, K.H. Zingerone Administered Neonatally Prevents the Subsequent Development of High Dietary Fructose-Induced Fatty Liver in Sprague Dawley Rats. J. Med. Food 2021, 24, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Arivalagan, S.; Siddique, A.I.; Namasivayam, N. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Mol. Cell. Biochem. 2016, 421, 169–181. [Google Scholar] [CrossRef]
- Cheslock, S.J.; Varlinskaya, E.I.; Silveri, M.M.; Petrov, E.S.; Spear, L.P.; Spear, N.E. Acute Effects of Ethanol and the First Suckling Episode in the Newborn Rat. Alcohol. Clin. Exp. Res. 2000, 24, 996–1002. [Google Scholar] [CrossRef]
- Asiedu, B.; Lembede, B.W.; Nyakudya, T.T.; Chivandi, E. Orally administered zingerone does not mitigate alcohol-induced hepatic oxidative stress in growing Sprague Dawley rat pups. Drug Chem. Toxicol. 2022, 0, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Delgado-Villa, M.J.; Llopis, R.; Murillo, M.L.; Carreras, O. Lipid Metabolism in Ethanol-Treated Rat Pups and Adults: Effects of Folic Acid. Alcohol Alcohol. 2008, 43, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.; Da-Silva, V.; Boaventura, G. Can calories from ethanol contribute to body weight preservation by malnourished rats? Braz. J. Med. Biol. Res. 2004, 37, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Veale, W.L.; Myers, R.D. Tables for Determining Grams and Caloric Values of Ethanol Solutions. Purdue Neuropscycological Ser. 1968, 1, 1–10. [Google Scholar]
- Niehaus, W.G.; Samuelsson, B. Formation of Malonaldehyde from Phospholipid Arachidonate during Microsomal Lipid Peroxidation. JBIC J. Biol. Inorg. Chem. 1968, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi, L. Effects of Prenatal Alcohol Exposure on Neonatal Sleep-wake Behaviour and Adult Alcohol Consumption in Rats. Acta Pharmacol. Toxicol. 2009, 59, 36–42. [Google Scholar] [CrossRef]
- Biggio, F.; Talani, G.; Locci, V.; Pisu, M.; Boero, G.; Ciarlo, B.; Grayson, D.; Serra, M. Low doses of prenatal ethanol exposure and maternal separation alter HPA axis function and ethanol consumption in adult male rats. Neuropharmacology 2018, 131, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bordner, K.; Deak, T. Endogenous opioids as substrates for ethanol intake in the neonatal rat: The impact of prenatal ethanol exposure on the opioid family in the early postnatal period. Physiol. Behav. 2015, 148, 100–110. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, J.A.G.; Duijf, C.M.P.; Pertijs, J.C.L.M.; Peereboom-Stegeman, J.H.J.C.; Bos, R.P. Growth and liver morphology after long-term ethanol consumption of rats. Lab. Anim. 1990, 24, 265–272. [Google Scholar] [CrossRef]
- Al-Humadi, H.; Al-Saigh, R.; Sahib, A. The impact of low alcohol consumption on the liver and inflammatory cytokines in diabetic rats. Adv. Clin. Exp. Med. 2018, 28, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rasineni, K.; Thomes, P.G.; Kubik, J.L.; Harris, E.N.; Kharbanda, K.K.; Casey, C.A. Chronic alcohol exposure alters circulating insulin and ghrelin levels: Role of ghrelin in hepatic steatosis. Am. J. Physiol. Liver Physiol. 2019, 316, G453–G461. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol Induces Fatty Acid Synthesis Pathways by Activation of Sterol Regulatory Element-binding Protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef]
- Ji, C.; Shinohara, M.; Vance, D.; Than, T.A.; Ookhtens, M.; Chan, C.; Kaplowitz, N. Effect of Transgenic Extrahepatic Expression of Betaine-Homocysteine Methyltransferase on Alcohol or Homocysteine-Induced Fatty Liver. Alcohol. Clin. Exp. Res. 2008, 32, 1049–1058. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.-J.; Song, Y.; Jang, S.-H.; Ko, Y.-G.; Kang, S.N.; Chung, B.Y.; Kim, H.-D.; Kim, G.-S.; Cho, J.-H. Schisandra chinensis Prevents Alcohol-Induced Fatty Liver Disease in Rats. J. Med. Food 2014, 17, 103–110. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, J.; Zhou, D.; Zhu, Z.; Sun, L.; Nanji, A.A. The Adiponectin-SIRT1-AMPK Pathway in Alcoholic Fatty Liver Disease in the Rat. Alcohol. Clin. Exp. Res. 2015, 39, 424–433. [Google Scholar] [CrossRef]
- Straub, B.K. Lipid droplet-associated proteins. Importance in steatosis, steatohepatitis and hepatocarcinogenesis. Der Pathol. 2015, 36, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Sundrani, D.P.; Roy, S.S.; Jadhav, A.T.; Joshi, S.R. Sex-specific differences and developmental programming for diseases in later life. Reprod. Fertil. Dev. 2017, 29, 2085–2099. [Google Scholar] [CrossRef] [PubMed]
- Lembede, B.W.; Joubert, J.; Nkomozepi, P.; Erlwanger, K.H.; Chivandi, E. Insulinotropic Effect of S-Allyl Cysteine in Rat Pups. Prev. Nutr. Food Sci. 2018, 23, 15–21. [Google Scholar] [CrossRef]
- Chung, S.W.; Kim, M.K.; Chung, J.H.; Kim, D.H.; Choi, J.S.; Anton, S.; Seo, A.Y.; Park, K.-Y.; Yokozawa, T.; Rhee, S.H.; et al. Peroxisome Proliferator-Activated Receptor Activation by a Short-Term Feeding of Zingerone in Aged Rats. J. Med. Food 2009, 12, 345–350. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Z.; Gong, J.; Zhang, L.; Wang, L.; Magdalou, J.; Chen, L.; Wang, H. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol. Appl. Pharmacol. 2014, 274, 263–273. [Google Scholar] [CrossRef]
- Larosche, I.; Choumar, A.; Fromenty, B.; Lettéron, P.; Abbey-Toby, A.; Van Remmen, H.; Epstein, C.J.; Richardson, A.; Feldmann, G.; Pessayre, D.; et al. Prolonged ethanol administration depletes mitochondrial DNA in MnSOD-overexpressing transgenic mice, but not in their wild type littermates. Toxicol. Appl. Pharmacol. 2008, 234, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.; Martini, R.; Batey, R. Ethanol-related liver injury in the rat: A model of steatosis, inflammation and pericentral fibrosis. J. Hepatol. 1995, 23, 591–600. [Google Scholar] [CrossRef]
- Greuter, T.; Malhi, H.; Gores, G.J.; Shah, V.H. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: Exploiting similarities and differences in pathogenesis. J. Clin. Investig. 2017, 2, e95354. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, G.; Sowa, J.-P.; Atorrasagasti, C.; Kücükoglu, Ö.; Syn, W.-K.; Canbay, A. Significance of Simple Steatosis: An Update on the Clinical and Molecular Evidence. Cells 2020, 9, 2458. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Morales-González, J.A.; Sernas-Morales, M.D.L.; Morales-Gonzalez, A.; González-López, L.L.; Madrigal-Santillán, E.O.; Vargas-Mendoza, N.; Fregoso-Aguilar, T.A.; Anguiano-Robledo, L.; Madrigal-Bujaidar, E.; Álvarez-González, I.; et al. Morphological and biochemical effects of weekend alcohol consumption in rats: Role of concentration and gender. World J. Hepatol. 2018, 10, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Chang, Y.-Y.; Liu, C.-W.; Kang, W.-Y.; Lin, Y.-L.; Chang, H.-C.; Chen, Y.-C. Fruiting Body of Niuchangchih (Antrodia camphorata) Protects Livers against Chronic Alcohol Consumption Damage. J. Agric. Food Chem. 2010, 58, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Sugamoto, K.; Arakawa, T.; Nishiyama, K.; Yamasaki, M. Chronic intake of high-dose of blueberry leaf extract does not augment the harmful effects of ethanol in rats. PeerJ 2019, 7, e6989. [Google Scholar] [CrossRef] [PubMed]
- Radic, I.; Mijovic, M.; Tatalovic, N.; Mitic, M.; Lukic, V.; Joksimovic, B.; Petrovic, Z.; Ristic, S.; Velickovic, S.; Nestorovic, V.; et al. Protective effects of whey on rat liver damage induced by chronic alcohol intake. Hum. Exp. Toxicol. 2019, 38, 632–645. [Google Scholar] [CrossRef]
- Morales-Gonzalez, J.A.; Gutierrez-Salinas, J.; Yanez, L.; Villagomez-Rico, C.; Badillo-Romero, J.; Hernandez-Munoz, R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: Role of route and timing of administration. Dig. Dis. Sci. 1999, 44, 1963–1974. [Google Scholar] [CrossRef]
- Cheong, K.O.; Shin, D.-S.; Bak, J.; Lee, C.; Kim, K.W.; Je, N.K.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Hepatoprotective effects of zingerone on carbon tetrachloride- and dimethylnitrosamine-induced liver injuries in rats. Arch. Pharmacal Res. 2015, 39, 279–291. [Google Scholar] [CrossRef]
- Narayanan, J.M.; Jesudoss, V.A.S. Hepatoprotective potential of zingerone against nonalcoholic fatty liver disease in rats fed with fructose-enriched diet. Gen. Physiol. Biophys. 2016, 35, 185–194. [Google Scholar] [CrossRef]
- Roberts, B.J.; Song, B.-J.; Soh, Y.; Park, S.S.; Shoaf, S.E. Ethanol Induces CYP2E1 by Protein Stabilization. Role of Ubiquitin Conjugation in the Rapid Degradation of CYP2E1. J. Biol. Chem. 1995, 270, 29632–29635. [Google Scholar] [CrossRef]
- Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Oxidative Stress Parameters in the Liver of Growing Male Rats Receiving Various Alcoholic Beverages. Nutrients 2020, 12, 158. [Google Scholar] [CrossRef]
- Huang, X. Pharmacokinetics Studies Of Zingerone In Rats. Available online: https://www.globethesis.com/?t=1114360275966066 (accessed on 24 September 2022).
- Teare, J.; Greenfield, S.M.; Watson, D.; Punchard, N.; Miller, N.; Rice-Evans, C.A.; Thompson, R.P. Lipid peroxidation in rats chronically fed ethanol. Gut 1994, 35, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Bradford, B.U.; Yin, M.; Sulik, K.K.; Koop, D.R.; Peters, J.M.; Gonzalez, F.J.; McDonald, T.; Dikalova, A.; Kadiiska, M.B.; et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am. J. Physiol. Liver Physiol. 1999, 277, G1259–G1267. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L. Classifying oxidative stress by F2-Isoprostane levels in human disease: The re-imagining of a biomarker. Redox Biol. 2017, 12, 897–898. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, R.; Vinothkumar, R.; Sudha, M.; Nalini, N. Chemopreventive effect of zingerone against colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Eur. J. Cancer Prev. 2014, 23, 361–371. [Google Scholar] [CrossRef]



| Treatment Group | Males | Females | ||
|---|---|---|---|---|
| Liver Mass (g) | Hepatosomatic Index (%) | Liver Mass (g) | Hepatosomatic Index (%) | |
| NM + Wad | 14.19 ± 1.76 a | 2.99 ± 0.24 a | 8.06 ± 0.49 ab | 2.92 ± 0.10 a |
| NM + Ethad | 13.12 ± 1.21 a | 2.96 ± 0.18 a | 7.73 ± 0.62 ab | 2.78 ± 0.12 a |
| NM + Eth + Wad | 13.85 ± 2.39 a | 2.97 ± 0.25 a | 8.46 ± 0.95 a | 2.90 ± 0.18 a |
| NM + Eth + Ethad | 12.76 ± 1.57 a | 2.84 ± 0.16 a | 7.56 ± 0.61 ab | 2.80 ± 0.21 a |
| NM + ZO + Wad | 14.19 ± 1.76 a | 2.97 ± 0.13 a | 7.81 ± 0.98 ab | 2.82 ± 0.17 a |
| NM + Eth + ZO + Wad | 13.70 ± 1.29 a | 2.94 ± 0.12 a | 8.29 ± 0.48 ab | 2.89 ± 0.18 a |
| NM + ZO + Ethad | 11.73 ± 1.25 a | 2.77 ± 0.21 a | 7.28 ± 0.52 b | 2.75 ± 0.11 a |
| NM + Eth + ZO + Ethad | 12.30 ± 0.95 a | 2.80 ± 0.19 a | 7.38 ± 0.41 b | 2.82 ± 0.10 a |
| Treatment Group | Males | Females | ||
|---|---|---|---|---|
| AST(U/L) | ALT (U/L) | AST (U/L) | ALT (U/L) | |
| NM + Wad | 247.8 ± 155.5 a | 61.8 ± 11.3 a | 147.2 ± 35.9 a | 57.0 ± 19.5 a |
| NM + Ethad | 298.8 ± 130.4 a | 114.0 ± 47.2 a | 168.2 ± 46.6 a | 62.8 ± 22.2 a |
| NM + Eth + Wad | 210.6 ± 81.8 a | 96.2 ± 36.2 a | 161.3 ± 77.0 a | 50.8 ± 19.5 a |
| NM + Eth + Ethad | 156.4 ± 46.2 a | 81.0 ± 30.3 a | 200.7 ± 74.6 a | 69.5 ± 32.6 a |
| NM + ZO + Wad | 183.3 ± 45.7 a | 70.3 ± 21.2 a | 145.0 ± 58.3 a | 51.8 ± 18.9 a |
| NM + Eth + ZO + Wad | 224.6 ± 115.3 a | 79.8 ± 28.1 a | 121.4 ± 28.3 a | 43.0 ± 7.3 a |
| NM + ZO + Ethad | 207.1 ± 102.6 a | 75.8 ± 25.8 a | 117.6 ± 14.1 a | 46.8 ± 9.4 a |
| NM + Eth + ZO + Ethad | 228.7 ± 75.81 a | 95.8 ± 28.7 a | 165.0 ± 69.5 a | 46.83 ± 7.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiedu, B.; Lembede, B.W.; Gomes, M.; Kasonga, A.; Nkomozepi, P.; Nyakudya, T.T.; Chivandi, E. Neonatal Orally Administered Zingerone Attenuates Alcohol-Induced Fatty Liver Disease in Experimental Rat Models. Metabolites 2023, 13, 167. https://doi.org/10.3390/metabo13020167
Asiedu B, Lembede BW, Gomes M, Kasonga A, Nkomozepi P, Nyakudya TT, Chivandi E. Neonatal Orally Administered Zingerone Attenuates Alcohol-Induced Fatty Liver Disease in Experimental Rat Models. Metabolites. 2023; 13(2):167. https://doi.org/10.3390/metabo13020167
Chicago/Turabian StyleAsiedu, Bernice, Busisani Wiseman Lembede, Monica Gomes, Abe Kasonga, Pilani Nkomozepi, Trevor Tapiwa Nyakudya, and Eliton Chivandi. 2023. "Neonatal Orally Administered Zingerone Attenuates Alcohol-Induced Fatty Liver Disease in Experimental Rat Models" Metabolites 13, no. 2: 167. https://doi.org/10.3390/metabo13020167
APA StyleAsiedu, B., Lembede, B. W., Gomes, M., Kasonga, A., Nkomozepi, P., Nyakudya, T. T., & Chivandi, E. (2023). Neonatal Orally Administered Zingerone Attenuates Alcohol-Induced Fatty Liver Disease in Experimental Rat Models. Metabolites, 13(2), 167. https://doi.org/10.3390/metabo13020167







