Study the Effect of Relative Energy Deficiency on Physiological and Physical Variables in Professional Women Athletes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
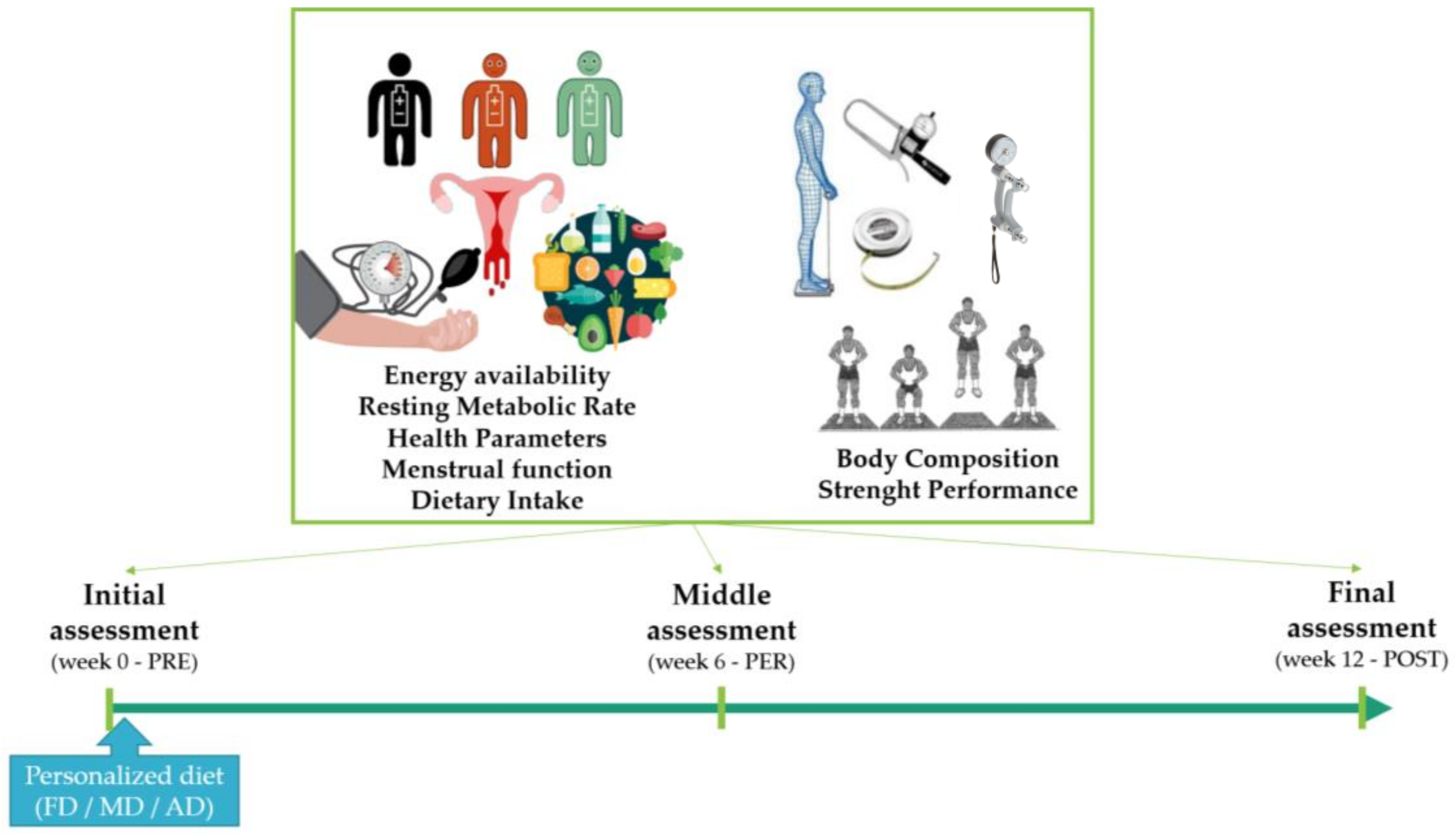
2.1. Study Design
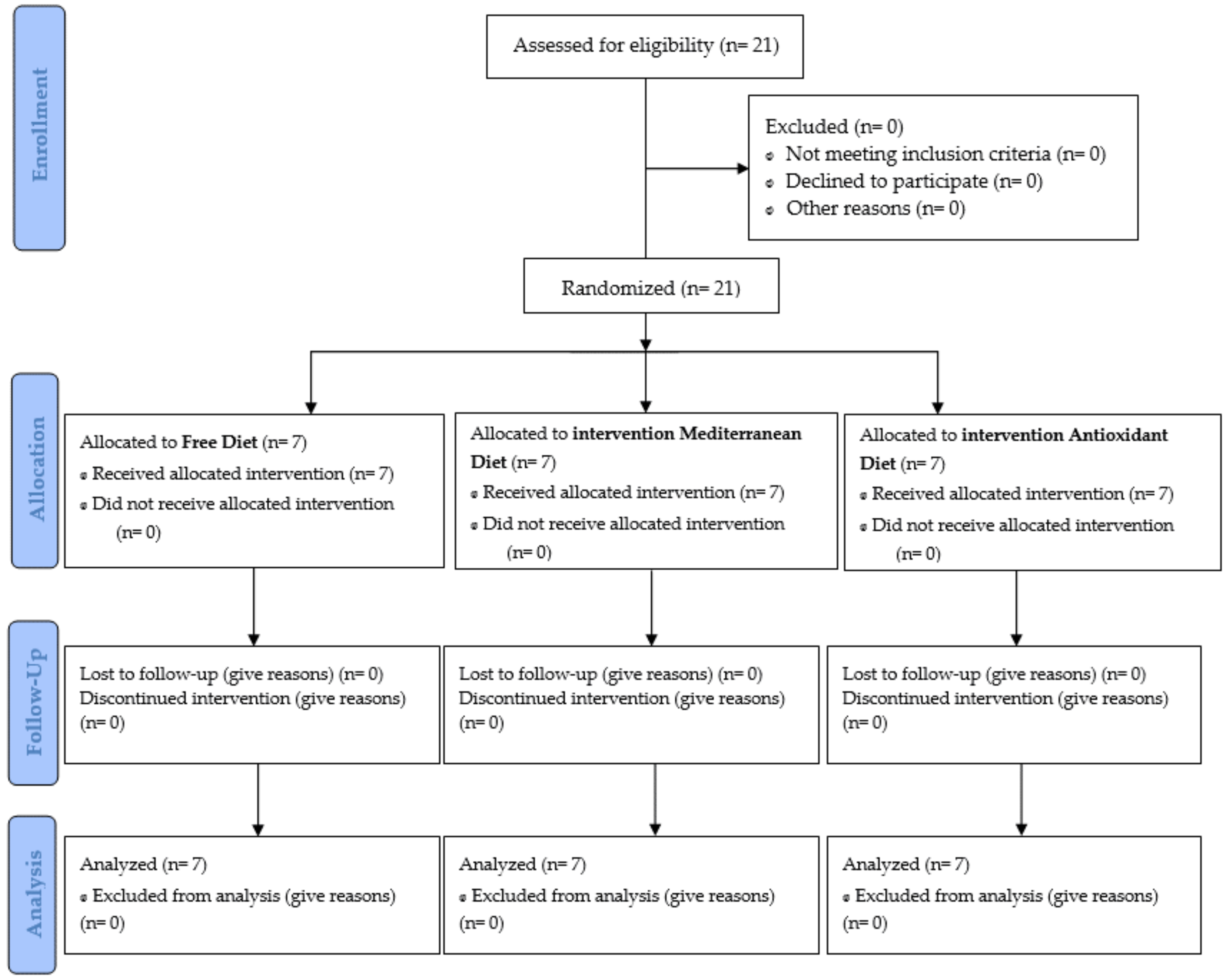
2.2. Subjects
2.3. Data Collection
2.3.1. Physiological Variables
Energy Availability and Resting Metabolic Rate
Health Parameters
Dietary Intake
2.3.2. Physical Variables
Body Composition
Strength Performance
2.4. Intervention
2.5. Statistical Analysis
3. Results
3.1. Physiological Variables
3.1.1. Energy Availability and Resting Metabolic Rate
3.1.2. Health Parameters
3.1.3. Dietary Intake
3.2. Physical Variables
3.2.1. Body Composition
3.2.2. Strength Performance
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loucks, A.B. Exercise training in the normal female: Effects of low energy availability on reproductive function. In Endocrinology of Physical Activity and Sport; Springer: Berlin/Heidelberg, Germany, 2020; pp. 171–191. [Google Scholar]
- Areta, J.L.; Taylor, H.L.; Koehler, K. Low energy availability: History, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur. J. Appl. Physiol. 2021, 121, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. Relative Energy Deficiency in Sport (RED-S). Br. J. Sport. Med. 2015, 49, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Torstveit, M.K.; et al. International Olympic Committee (IOC) Consensus statement on relative energy deficiency in sport (red-s): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G. Female Athlete Triad Coalition Consensus Statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, M. Br. J. Sport. Med. 2014, 48, 289. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. What is the evidence for a triad-like syndrome in exercising men? Curr. Opin. Physiol. 2019, 10, 27–34. [Google Scholar] [CrossRef]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. The Role of Energy Availability in Reproductive Function in the Female Athlete Triad and Extension of its Effects to Men: An Initial Working Model of a Similar Syndrome in Male Athletes. Sport. Med. 2019, 49, 125–137. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Barrack, M.T.; Nattiv, A.; Fredericson, M. Parallels with the Female Athlete Triad in Male Athletes. Sport. Med. 2016, 46, 171–182. [Google Scholar] [CrossRef]
- Sim, A.; Burns, S.F. Review: Questionnaires as measures for low energy availability (LEA) and relative energy deficiency in sport (RED-S) in athletes. J. Eat. Disord. 2021, 9, 41. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R. The IOC consensus statement: Beyond the female athlete triad—Relative energy deficiency in sport (RED-S). Br. J. Sport. Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Møller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sjödin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sport. 2015, 25, 610–622. [Google Scholar] [CrossRef]
- Woods, A.L.; Garvican-Lewis, L.A.; Lundy, B.; Rice, A.J.; Thompson, K.G. New approaches to determine fatigue in elite athletes during intensified training: Resting metabolic rate and pacing profile. PLoS ONE 2017, 12, e0173807. [Google Scholar] [CrossRef]
- De Souza, M.J.; Mallinson, R.J.; Strock, N.C.A.; Koltun, K.J.; Olmsted, M.P.; Ricker, E.A.; Scheid, J.L.; Allaway, H.C.; Mallinson, D.J.; Kuruppumullage Don, P.; et al. Randomised controlled trial of the effects of increased energy intake on menstrual recovery in exercising women with menstrual disturbances: The “REFUEL” study. Hum. Reprod. 2021, 36, 2285–2297. [Google Scholar] [CrossRef]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; Mc Donnell, S.-J.; Corish, C.A. Low Energy Availability in Athletes: A Review of Prevalence, Dietary Patterns, Physiological Health, and Sports Performance. Sport. Med. 2018, 48, 73–96. [Google Scholar] [CrossRef]
- Drew, M.K.; Vlahovich, N.; Hughes, D.; Appaneal, R.; Peterson, K.; Burke, L.; Lundy, B.; Toomey, M.; Watts, D.; Lovell, G. A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J. Sci. Med. Sport 2017, 20, 745–750. [Google Scholar] [CrossRef]
- Drew, M.; Vlahovich, N.; Hughes, D.; Appaneal, R.; Burke, L.M.; Lundy, B.; Rogers, M.; Toomey, M.; Watts, D.; Lovell, G. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br. J. Sport. Med. 2018, 52, 47–53. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- El Ghoch, M.; Soave, F.; Calugi, S.; Dalle Grave, R. Eating disorders, physical fitness and sport performance: A systematic review. Nutrients 2013, 5, 5140–5160. [Google Scholar] [CrossRef]
- Fogelholm, M. Effects of bodyweight reduction on sports performance. Sport. Med. 1994, 18, 249–267. [Google Scholar] [CrossRef]
- Stenqvist, T.B.; Torstveit, M.K.; Faber, J.; Melin, A.K. Impact of a 4-Week Intensified Endurance Training Intervention on Markers of Relative Energy Deficiency in Sport (RED-S) and Performance Among Well-Trained Male Cyclists. Front. Endocrinol. 2020, 11, 512365. [Google Scholar] [CrossRef]
- Baldó Vela, D.; Villarino Marín, A.L.; Bonfanti, N.; Lázaro Martínez, J.L. Prevalence of eating disorders on male team sports players. BMJ Open Sport Exerc. Med. 2021, 7, e001161. [Google Scholar] [CrossRef]
- Baldó Vela, D.; Bonfanti, N. Eating disorders risk assessment on semi-professional male team sports players. Nutr. Hosp. 2019, 36, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sáez, J.A.; Sánchez-Sánchez, J.; Martínez-Rodríguez, A.; Felipe, J.L.; García-Unanue, J.; Lara-Cobos, D. Global positioning system analysis of physical demands in elite women’s beach handball players in an official spanish championship. Sensors 2021, 21, 850. [Google Scholar] [CrossRef] [PubMed]
- Firoozjah, M.H.; Shahrbanian, S.; Homayouni, A.; Hower, H. Comparison of eating disorders symptoms and body image between individual and team sport adolescent athletes during the COVID-19 pandemic. J. Eat. Disord. 2022, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Gastrich, M.D.; Quick, V.; Bachmann, G.; Moriarty, A.M. Nutritional Risks Among Female Athletes. J. Women’s Health 2020, 29, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.; McLay-Cooke, R.; Brown, R.; Black, K. Female Recreational Exercisers at Risk for Low Energy Availability. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 421–427. [Google Scholar] [CrossRef]
- Koehler, K.; Achtzehn, S.; Braun, H.; Mester, J.; Schaenzer, W. Comparison of self-reported energy availability and metabolic hormones to assess adequacy of dietary energy intake in young elite athletes. Appl. Physiol. Nutr. Metab. 2013, 38, 725–733. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef]
- Vanheest, J.L.; Rodgers, C.D.; Mahoney, C.E.; Souza, M.J. De Ovarian suppression impairs sport performance in junior elite female swimmers. Med. Sci. Sport. Exerc. 2014, 46, 156–166. [Google Scholar] [CrossRef]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef]
- Loucks, A.B.; Heath, E.M. Induction of low-t3 syndrome in exercising women occurs at a threshold of energy availability. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 266, R817–R823. [Google Scholar] [CrossRef]
- Brattberg, G. Connective tissue massage in the treatment of fibromyalgia. Eur. J. Pain 1999, 3, 235–244. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjödin, A.; Sundgot-Borgen, J. The leaf questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sport. Med. 2014, 48, 540–545. [Google Scholar] [CrossRef]
- Romero-Parra, N.; Cupeiro, R.; Alfaro-Magallanes, V.M.; Rael, B.; Rubio-Arias, J.Á.; Peinado, A.B.; Benito, P.J. Exercise-Induced Muscle Damage During the Menstrual Cycle: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2021, 35, 549–561. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sport. Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Kalampalikis, A.; Chatziioannou, S.S.; Protopapas, A.; Gerakini, A.M.; Michala, L. mHealth and its application in menstrual related issues: A systematic review. Eur. J. Contracept. Reprod. Health Care 2022, 27, 53–60. [Google Scholar] [CrossRef]
- Gabriel García, C.; Sebastià, N.; Blasco, E.; Soriano, J.M. Dietopro. com: Una nueva herramienta de gestión dietoterapéutica basada en la tecnología cloud computing. Nutr. Hosp. 2014, 30, 678–685. [Google Scholar]
- Esparza-Ros, F.; Vaquero-Cristóbal, R.; Marfell-Jones, M. Protocolo internacional para la valoración antropométrica. Perf. Complet. Murcia Int. Soc. Adv. Kinanthropometry-ISAK 2019, 1, 3–82. [Google Scholar]
- Withers, R.T.; Craig, N.P.; Bourdon, P.C.; Norton, K.I. Relative body fat and anthropometric prediction of body density of male athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 191–200. [Google Scholar] [CrossRef]
- Karaba-Jakovljević, D.; Jovanović, G.; Erić, M.; Klašnja, A.; Slavić, D.; Lukač, D. Anthropometric characteristics and functional capacity of elite rowers and handball players. Med. Pregl. 2016, 69, 267–273. [Google Scholar] [CrossRef]
- Neto, C.S.P.; Glaner, M.F. The “faulkner equation” for predicting body fat: The end of a myth. Rev. Bras. Cineantropometría E Desempenho Hum. 2007, 9, 207–213. [Google Scholar]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Ramón, J.; Cruz, A.; Dolores, M.; Porta, J. Protocolo de valoración de la composición corporal para el reconocimiento médico-deportivo. documento de consenso del grupo español de cineantropometría (grec) de la federación española de medicina del deporte (femede). Versión 2010. Arch. Med. Deport. 2009, 26, 166–179. [Google Scholar]
- Heath, B.H.; Carter, J.E.L. A modified somatotype method. Am. J. Phys. Anthropol. 1967, 27, 57–74. [Google Scholar] [CrossRef]
- Carter, J.E.L. Part 1: The Heath-Carter Anthropometric Somatotype; Instruction Manual; TeP and Rosscraft: Surrey, BA, Canada, 2002. [Google Scholar]
- Banquéz Trocha, S.; Fuentes Polo, K.D.; Giraldo Padilla, M.; Ruíz Álvarez, V. Estimación y Comparación de Puntos de Corte de Ángulo de Fase, Resistencia, Reactancia y Coeficiente de Bioimpedancia en una Muestra de Estudiantes de una Institución Universitaria Privada en la Ciudad de Cartagena, 2020-1. Available online: http://repositorio.unisinucartagena.edu.co:8080/jspui/handle/123456789/248 (accessed on 20 November 2022).
- Baba, H.; Zhang, X.-J.; Wolfe, R.R. Glycerol gluconeogenesis in fasting humans. Nutrition 1995, 11, 149–153. [Google Scholar]
- Beltaifa, L.; Bouguerra, R.; Ben Slama, C.; Jabrane, H.; El-Khadhi, A.; Ben Rayana, M.C.; Doghri, T. Food intake, and anthropometrical and biological parameters in adult Tunisians during fasting at Ramadan. East. Mediterr. Health J. 2002, 8, 603–611. [Google Scholar]
- Sana’a, A.A.; Ismail, M.; Baker, A.; Blair, J.; Adebayo, A.; Kelly, L.; Chandurkar, V.; Cheema, S.; Joanisse, D.R.; Basset, F.A. The effects of diurnal Ramadan fasting on energy expenditure and substrate oxidation in healthy men. Br. J. Nutr. 2017, 118, 1023–1030. [Google Scholar]
- Bosco, C.; Luhtanen, P.; Komi, P.V. A simple method for measurement of mechanical power in jumping. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 273–282. [Google Scholar] [CrossRef]
- García, C.G.; Sebastia, N.; Blasco, E.; Soriano, J.M. Dietopro. com: A new tool for dietotherapeutical management based on cloud computing technology. Nutr. Hosp. 2014, 30, 678–685. [Google Scholar]
- Molina-López, J.; Planells, E. Nutrition and Hydration for Handball. In Handball Sports Medicine; Springer: Berlin/Heidelberg, Germany, 2018; pp. 81–101. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sport. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Watson, T.A.; Callister, R.; Taylor, R.D.; Sibbritt, D.W.; MacDonald-Wicks, L.K.; Garg, M.L. Antioxidant restriction and oxidative stress in short-duration exhaustive exercise. Med. Sci. Sport. Exerc. 2005, 37, 63–71. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Acad. Nutr. Diet. 2000, 100, 637. [Google Scholar]
- de Lima, A.; Chen, M.J.; Abbas, A.; Ramachandran, S.K.; Mitchell, J.D. Evaluating the use of an aerosol box during simulated intubations. Cureus 2021, 13, e16507. [Google Scholar] [CrossRef]
- Dobrowolski, H.; Włodarek, D. Low energy availability in group of Polish female soccer players. Rocz. Panstw. Zakl. Hig. 2020, 71, 89–96. [Google Scholar] [CrossRef]
- Magee, M.K.; Lockard, B.L.; Zabriskie, H.A.; Schaefer, A.Q.; Luedke, J.A.; Erickson, J.L.; Jones, M.T.; Jagim, A.R. Prevalence of Low Energy Availability in Collegiate Women Soccer Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 96. [Google Scholar] [CrossRef]
- Bellissimo, M.P.; Licata, A.D.; Nucci, A.; Thompson, W.; Benardot, D. Relationships between estimated hourly energy balance and body composition in professional cheerleaders. J. Sci. Sport Exerc. 2019, 1, 69–77. [Google Scholar] [CrossRef]
- Hooper, D.R.; Mallard, J.; Wight, J.T.; Conway, K.L.; Pujalte, G.G.A.; Pontius, K.M.; Saenz, C.; Hackney, A.C.; Tenforde, A.S.; Ackerman, K.E. Performance and Health Decrements Associated With Relative Energy Deficiency in Sport for Division I Women Athletes During a Collegiate Cross-Country Season: A Case Series. Front. Endocrinol. 2021, 12, 524762. [Google Scholar] [CrossRef]
- Reed, J.L.; De Souza, M.J.; Mallinson, R.J.; Scheid, J.L.; Williams, N.I. Energy availability discriminates clinical menstrual status in exercising women. J. Int. Soc. Sport. Nutr. 2015, 12, 11. [Google Scholar] [CrossRef]
- Williams, N.I.; Leidy, H.J.; Hill, B.R.; Lieberman, J.L.; Legro, R.S.; Souza, M.J. De Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am. J. Physiol. Metab. 2015, 308, E29–E39. [Google Scholar]
- Tornberg, Å.B.; Melin, A.; Koivula, F.M.; Johansson, A.; Skouby, S.; Faber, J.; Sjödin, A. Reduced neuromuscular performance in amenorrheic elite endurance athletes. Med. Sci Sport. Exerc 2017, 49, 2478–2485. [Google Scholar] [CrossRef]
- Lane, A.R.; Hackney, A.C.; Smith-Ryan, A.E.; Kucera, K.; Register-Mihalik, J.K.; Ondrak, K. Energy Availability and RED-S Risk Factors in Competitive, Non-elite Male Endurance Athletes. Transl. Med. Exerc. Prescr. 2021, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kyte, K.H.; Stensrud, T.; Berg, T.J.; Seljeflot, I.; Hisdal, J. Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study. Sports 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.D.; Bock, P.M.; Becker, G.F.; Moreira, J.C.F.; Bello-Klein, A.; Oliveira, A.R. Comparison of the effects of two antioxidant diets on oxidative stress markers in triathletes. Biol. Sport 2018, 35, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
| FD (n = 7) | MD (n = 7) | AD (n = 7) | |
|---|---|---|---|
| Age (years) | 22 ± 4 | 21 ± 3 | 22 ± 4 |
| Height (cm) | 171.0 ± 6.0 | 171.0 ± 7.2 | 173.0 ± 2.8 |
| Weight (kg) | 64.4 ± 5.1 | 70.5 ± 7.0 | 70.3 ± 6.7 |
| BMI (kg/m2) | 22.1 ± 1.7 | 24.1 ± 3.2 | 23.5 ± 1.9 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect Time × Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | ||||||||||||||||
| METs per minute | 0.9 | 0.15 | 0.9 | 0.1 | 0.8 | 0.3 | 0.8 | 0.2 | 1.0 | 0.2 | 0.9 | 0.2 | 0.8 | 0.2 | 0.9 | 0.3 | 1.0 | 0.2 | 1.625 | 0.212 | 0.087 | 0.333 | 0.854 | 0.038 |
| RMR 24 h (kcal) | 1399 | 231 | 1497 | 184 | 1369 | 365 | 1349 | 381 | 1691 | 272 | 1532 | 318 | 1436 | 407 | 1619 | 452 | 1595 | 315 | 1.793 | 0.182 | 0.095 | 0.265 | 0.899 | 0.030 |
| EA (kcal/day) | 2.0 | 9.8 | 1.6 | 9.0 | 1.6 | 7.7 | −2.5 | 10.8 | −6.8 | 5.4 | −5.3 | 7.1 | 0.8 | 8.0 | −4.7 | 9.8 | −1.6 | 7.0 | 1.247 | 0.300 | 0.068 | 0.292 | 0.881 | 0.033 |
| LEAF-Q | 8.0 | 1.2 | 9.0 | 2.9 | 9.8 | 3.7 | 9.7 | 3.4 | 9.1 | 2.1 | 8.8 | 2.8 | 9.9 | 3.9 | 10.6 | 3.6 | 10.6 | 3.6 | 1.59 | 0.220 | 0.090 | 1.05 | 0.398 | 0.116 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect time × Group | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2 | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | |||||||||||||||||
| Blood pressure SYS (mmHg) | 98.4 | 8.5 | 105.0 | 5.9 | 105.0 | 8.2 | 106.0 | 4.8 | 107.0 | 10.2 | 105.0 | 0.157 | 109.0 | 12.2 | 110.0 | 7.5 | 112.0 | 8.9 | 3.160 | 0.055 | 0.157 | 1.17 | 0.340 | 0.027 | 0.121 |
| Blood pressure DIA (mmHg) | 56.9 | 3.7 | 61.6 | 5.4 | 63.3 | 2.3 | 62.6 | 5.4 | 63.9 | 5.8 | 64.2 | 0.269 | 68.7 | 5.8 | 70.9 | 3.9 | 70.4 | 6.2 | 6.259 | 0.005 | 0.269 | 0.772 | 0.551 | 0.017 | 0.083 |
| Pulse rate (bpm) | 62.3 | 9.4 | 60.7 | 12.9 | 58.0 | 8.9 | 53.7 | 22.6 | 58.9 | 9.7 | 61.2 | 0.022 | 60.9 | 5.9 | 64.4 | 14.5 | 61.6 | 7.0 | 0.385 | 0.683 | 0.022 | 0.685 | 0.608 | 0.029 | 0.075 |
| Cholesterol (mg/dL) | 165.0 | 24.5 | 154.0 | 10.8 | 159.0 | 18.9 | 153.0 | 7.5 | 155.0 | 10.8 | 158.0 | 0.053 | 172.0 | 28.3 | 163.0 | 22.8 | 154.0 | 9.1 | 0.942 | 0.400 | 0.053 | 0.903 | 0.473 | 0.053 | 0.096 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect time × Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | |||||||||||||||||
| Energy intake (Kcal) | 1784 | 373 | 1850 | 400 | 1740 | 181 | 1484 | 252 | 1606 | 163 | 1496 | 108 | 1820 | 442 | 1673 | 254 | 1854 | 329 | 0.026 | 0.975 | 0.002 | 10.265 | 0.408 | 0.108 |
| Carbohydrates intake (%) | 38.8 | 4.6 | 40.7 | 4.8 | 41.0 | 7.6 | 40.2 | 5.2 | 40.0 | 5.3 | 41.8 | 4.0 | 41.4 | 4.8 | 43.9 | 9.9 | 42.1 | 9.3 | 0.339 | 0.715 | 0.020 | 0.248 | 0.909 | 0.028 |
| Protein intake (%) | 20.8 | 3.4 | 22.2 | 3.4 | 20.1 | 2.8 | 21.6 | 4.3 | 21.6 | 2.4 | 21.1 | 2.3 | 21.3 | 1.5 | 22.0 | 3.3 | 22.7 | 3.9 | 1.123 | 0.337 | 0.062 | 0.896 | 0.477 | 0.095 |
| Lipids intake (%) | 40.3 | 5.2 | 37.2 | 5.5 | 39.2 | 8.1 | 37.8 | 2.5 | 38.0 | 4.5 | 37.2 | 5.0 | 37.4 | 5.2 | 34.2 | 8.0 | 35.0 | 7.9 | 0.995 | 0.380 | 0.055 | 0.197 | 0.938 | 0.023 |
| Animal protein intake (%) | 15.5 | 3.3 | 15.8 | 3.3 | 14.7 | 2.9 | 16.0 | 5.0 | 15.6 | 2.5 | 14.8 | 2.4 | 14.8 | 1.5 | 16.0 | 3.9 | 16.0 | 4.8 | 0.475 | 0.626 | 0.027 | 0.327 | 0.858 | 0.037 |
| Vegetable protein intake (%) | 5.3 | 0.83 | 6.2 | 1.0 | 5.3 | 1.0 | 5.5 | 1.0 | 5.9 | 0.9 | 6.1 | 0.5 | 6.5 | 1.0 | 5.7 | 1.0 | 6.7 | 1.6 | 0.248 | 0.782 | 0.014 | 3.374 | 0.020 | 0.284 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect Time × Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | ||||||||||||||||
| Weight (kg) | 64.4 | 5.1 | 65.9 | 4.7 | 65.6 | 5.3 | 70.5 | 7.0 | 70.8 | 5.7 | 69.4 | 5.6 | 70.3 | 6.7 | 70.3 | 7.3 | 70.5 | 7.1 | 3.914 | 0.030 | 0.187 | 0.912 | 0.468 | 0.097 |
| 6 Skinfolds (mm) | 69.9 | 14.3 | 66.8 | 12.8 | 66.4 | 14.2 | 94.4 | 30.6 | 85.6 | 26.5 | 74.5 | 9.7 | 86.8 | 15.9 | 79.4 | 15.2 | 76.0 | 11.2 | 16.82 | <0.001 | 0.497 | 1.29 | 0.292 | 0.132 |
| 8 Skinfolds (mm) | 90.8 | 17.2 | 85.4 | 15.8 | 85.1 | 16.9 | 116.0 | 36.2 | 106.0 | 33.3 | 91.8 | 11.0 | 110.0 | 23.7 | 98.7 | 20.8 | 94.2 | 15.0 | 16.42 | <0.001 | 0.491 | 1.24 | 0.314 | 0.127 |
| Endomorphy | 2.9 | 0.6 | 2.9 | 0.5 | 2.8 | 0.6 | 3.6 | 1.5 | 3.4 | 1.3 | 2.8 | 0.4 | 3.4 | 0.7 | 3.2 | 0.6 | 3.8 | 0.5 | 6.034 | 0.006 | 0.262 | 0.633 | 0.642 | 0.069 |
| Mesomorphy | 3.1 | 0.8 | 3.3 | 0.8 | 3.6 | 0.7 | 3.3 | 1.3 | 3.4 | 1.2 | 3.1 | 1.1 | 3.4 | 0.8 | 3.6 | 0.8 | 3.5 | 0.7 | 8.561 | <0.001 | 0.335 | 0.521 | 0.721 | 0.058 |
| Ectomorphy | 2.6 | 1.0 | 2.4 | 0.9 | 2.4 | 1.1 | 1.8 | 1.5 | 1.8 | 1.3 | 2.2 | 0.5 | 2.1 | 0.8 | 2.1 | 0.9 | 2.1 | 0.8 | 3.88 | 0.030 | 0.186 | 1.05 | 0.398 | 0.110 |
| Carter FM (%) | 14.4 | 2.2 | 13.9 | 2.0 | 13.9 | 2.2 | 18.2 | 4.7 | 16.8 | 4.1 | 15.1 | 1.5 | 17.0 | 2.5 | 15.9 | 2.4 | 15.3 | 1.7 | 16.84 | <0.001 | 0.498 | 1.29 | 0.293 | 0.132 |
| Faulkner FM (%) | 16.7 | 1.7 | 16.5 | 1.6 | 16.3 | 1.7 | 18.8 | 4.4 | 18.0 | 3.9 | 16.3 | 1.3 | 18.2 | 2.2 | 17.5 | 2.1 | 17.4 | 1.5 | 8.394 | 0.001 | 0.331 | 0.431 | 0.785 | 0.048 |
| Withers FM (%) | 17.6 | 3.3 | 16.3 | 2.1 | 15.6 | 2.7 | 20.9 | 3.1 | 19.3 | 6.2 | 16.9 | 1.1 | 21.1 | 2.7 | 18.4 | 2.8 | 18.5 | 2.1 | 24.13 | <0.001 | 0.587 | 0.857 | 0.499 | 0.092 |
| Carter FM (kg) | 9.3 | 2.0 | 9.2 | 1.8 | 9.0 | 1.8 | 13.0 | 4.7 | 12.0 | 3.6 | 10.4 | 1.4 | 11.9 | 2.0 | 11.1 | 2.0 | 10.8 | 1.7 | 14.21 | <0.001 | 0.455 | 1.34 | 0.274 | 0.136 |
| Faulkner FM (kg) | 10.8 | 1.8 | 10.7 | 1.7 | 10.5 | 1.7 | 13.4 | 4.4 | 12.8 | 3.9 | 11.2 | 1.3 | 12.8 | 2.0 | 12.3 | 2.0 | 12.2 | 1.6 | 8.765 | <0.001 | 0.340 | 0.463 | 0.763 | 0.052 |
| Withers FM (kg) | 11.4 | 2.9 | 10.6 | 2.1 | 10.1 | 2.4 | 14.9 | 3.5 | 13.8 | 5.8 | 11.6 | 1.2 | 14.9 | 2.8 | 13.0 | 2.6 | 13.0 | 2.1 | 25.35 | <0.001 | 0.599 | 1.02 | 0.412 | 0.107 |
| Lee MM (kg) | 25.4 | 2.4 | 22.5 | 2.3 | 22.3 | 2.6 | 25.9 | 2.4 | 22.6 | 2.4 | 22.2 | 2.0 | 26.7 | 3.1 | 23.6 | 3.2 | 23.6 | 3.0 | 198.0 | <0.001 | 0.921 | 0.353 | 0.840 | 0.040 |
| Lee MM (%) | 39.4 | 1.9 | 34.1 | 2.3 | 34.0 | 2.8 | 36.9 | 2.4 | 31.9 | 1.9 | 32.1 | 2.2 | 38.0 | 2.1 | 33.5 | 2.3 | 33.4 | 1.8 | 287.3 | <0.001 | 0.944 | 0.798 | 0.535 | 0.086 |
| Rocha BM (kg) | 10.1 | 0.92 | 10.1 | 0.9 | 10.1 | 0.9 | 10.2 | 1.0 | 10.2 | 1.0 | 10.3 | 1.0 | 10.5 | 0.5 | 10.5 | 0.5 | 10.5 | 0.5 | 0.895 | 0.418 | 0.050 | 0.921 | 0.463 | 0.098 |
| Rocha BM (%) | 15.7 | 1.3 | 15.3 | 1.2 | 15.4 | 1.3 | 14.5 | 1.3 | 14.4 | 1.1 | 14.8 | 0.3 | 15.0 | 1.2 | 15.0 | 1.3 | 14.9 | 1.1 | 3.90 | 0.030 | 0.187 | 1.11 | 0.367 | 0.116 |
| RM (kg) | 18.1 | 1.6 | 22.4 | 2.2 | 22.5 | 2.5 | 20.9 | 1.7 | 25.2 | 2.0 | 25.6 | 2.5 | 20.3 | 2.7 | 24.0 | 3.0 | 24.2 | 2.9 | 290.1 | <0.001 | 0.945 | 0.966 | 0.439 | 0.102 |
| RM (%) | 28.1 | 2.2 | 34.0 | 3.2 | 34.3 | 3.1 | 29.8 | 1.9 | 35.7 | 2.9 | 36.8 | 1.5 | 28.8 | 2.1 | 34.0 | 1.6 | 34.3 | 1.3 | 358.6 | <0.001 | 0.955 | 0.841 | 0.509 | 0.090 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect Time × Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | ||||||||||||||||
| FF mass (kg) | 49.5 | 3.4 | 50.4 | 4.6 | 49.4 | 3.4 | 50.8 | 3.4 | 52.3 | 1.7 | 51.2 | 3.1 | 53.4 | 5.8 | 52.0 | 5.0 | 52.3 | 5.5 | 0.193 | 0.82 | 0.015 | 2.993 | 0.037 | 0.315 |
| FF dry mass (kg) | 14.0 | 1.4 | 14.3 | 1.5 | 14.1 | 0.9 | 14.5 | 1.5 | 15.2 | 0.7 | 14.8 | 1.4 | 14.4 | 1.4 | 14.8 | 1.0 | 14.6 | 1.2 | 4.439 | 0.02 | 0.255 | 0.253 | 0.905 | 0.037 |
| Lean mass (kg) | 47.0 | 3.2 | 47.9 | 4.4 | 46.9 | 3.3 | 48.3 | 3.3 | 49.7 | 1.6 | 48.6 | 3.0 | 50.7 | 5.6 | 49.4 | 4.7 | 49.7 | 5.3 | 0.243 | 0.78 | 0.018 | 2.953 | 0.039 | 0.312 |
| Active cell mass (kg) | 29.7 | 2.2 | 30.6 | 3.1 | 29.8 | 1.9 | 30.4 | 2.3 | 31.6 | 1.2 | 30.9 | 2.1 | 31.6 | 3.3 | 31.4 | 2.7 | 31.3 | 3.1 | 2.21 | 0.13 | 0.145 | 1.67 | 0.188 | 0.204 |
| Skeletal muscle mass (kg) | 26.5 | 2.2 | 27.4 | 3.2 | 26.6 | 2.2 | 27.4 | 2.2 | 28.1 | 1.3 | 27.5 | 2.1 | 29.1 | 4.0 | 28.1 | 3.3 | 28.3 | 3.7 | 0.274 | 0.76 | 0.021 | 2.532 | 0.065 | 0.280 |
| Metabolic protein mass (kg) | 9.0 | 1.6 | 9.3 | 1.2 | 9.1 | 0.6 | 9.3 | 1.3 | 10.0 | 0.6 | 9.7 | 1.2 | 9.1 | 0.9 | 9.7 | 0.7 | 9.4 | 0.8 | 4.774 | 0.01 | 0.269 | 0.185 | 0.944 | 0.028 |
| Protein mass (kg) | 10.9 | 1.2 | 11.2 | 1.3 | 11.0 | 0.7 | 11.3 | 1.4 | 12.0 | 0.6 | 11.6 | 1.3 | 11.1 | 1.1 | 11.6 | 0.8 | 11.4 | 1.0 | 4.714 | 0.01 | 0.266 | 0.180 | 0.947 | 0.027 |
| MM index | 1.2 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 1.3 | 0.1 | 1.2 | 0.1 | 1.2 | 0.1 | 0.275 | 0.76 | 0.021 | 2.493 | 0.068 | 0.277 |
| Constant hydration FM (%) | 23.9 | 2.5 | 23.2 | 3.0 | 24.3 | 3.2 | 27.8 | 3.4 | 27.3 | 3.0 | 25.6 | 2.1 | 24.5 | 3.3 | 25.4 | 2.7 | 25.3 | 3.5 | 0.263 | 0.77 | 0.020 | 1.131 | 0.364 | 0.148 |
| Gross FM (%) | 24.2 | 2.5 | 23.5 | 3.1 | 24.6 | 3.3 | 28.2 | 3.7 | 27.8 | 3.1 | 26.1 | 2.3 | 24.6 | 3.5 | 25.8 | 2.9 | 25.6 | 3.8 | 0.168 | 0.84 | 0.013 | 1.262 | 0.310 | 0.163 |
| FF hydration (%) | 70.4 | 1.6 | 70.3 | 0.9 | 70.0 | 0.6 | 69.8 | 1.8 | 69.1 | 0.6 | 69.5 | 1.4 | 71.6 | 1.2 | 69.8 | 1.4 | 70.5 | 1.6 | 3.07 | 0.06 | 0.191 | 1.47 | 0.241 | 0.184 |
| Total water (L) | 35.5 | 2.4 | 36.2 | 3.2 | 35.3 | 2.6 | 36.4 | 2.1 | 37.1 | 1.1 | 36.4 | 1.8 | 39.0 | 4.6 | 37.2 | 4.1 | 37.8 | 4.5 | 0.228 | 0.79 | 0.017 | 3.359 | 0.024 | 0.341 |
| Bone Mineral Content (kg) | 2.5 | 0.2 | 2.5 | 0.2 | 2.5 | 0.2 | 2.6 | 0.2 | 2.6 | 0.9 | 2.6 | 0.2 | 2.7 | 0.2 | 2.6 | 0.2 | 2.6 | 0.2 | 0.869 | 0.43 | 0.063 | 2.264 | 0.090 | 0.258 |
| Base metabolism (kcal) | 1502 | 69.4 | 1512 | 75.7 | 1503 | 52.9 | 1518 | 77.2 | 1556 | 43.2 | 1542 | 73.5 | 1516 | 73.6 | 1536 | 57.7 | 1525 | 64.6 | 4.256 | 0.02 | 0.247 | 0.248 | 0.908 | 0.037 |
| Energy expenditure (kcal) | 2063 | 200 | 1921 | 251 | 1909 | 239 | 1903 | 141 | 1952 | 141 | 1973 | 94.1 | 1959 | 201 | 1928 | 71.6 | 1915 | 78.3 | 1.228 | 0.30 | 0.086 | 0.765 | 0.557 | 0.105 |
| Free Diet (n = 7) | Mediterranean Diet (n = 7) | Antioxidant Diet (n = 7) | Effect Time | Effect Time × Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | PER | POST | PRE | PER | POST | PRE | PER | POST | F | p | η2p | F | p | η2p | ||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | ||||||||||||||||
| CMJ (cm) | 33.6 | 4.0 | 31.1 | 3.9 | 30.8 | 5.2 | 28.7 | 5.5 | 25.6 | 6.4 | 32.0 | 4.2 | 29.5 | 4.1 | 29.0 | 4.6 | 29.4 | 2.5 | 2.24 | 0.136 | 0.199 | 2.52 | 0.078 | 0.359 |
| Abalakov (cm) | 36.7 | 4.0 | 32.5 | 3.6 | 34.2 | 3.1 | 32.1 | 6.7 | 27.6 | 8.9 | 34.4 | 5.4 | 31.8 | 4.2 | 31.4 | 3.8 | 33.1 | 2.4 | 7.01 | 0.006 | 0.438 | 1.77 | 0.178 | 0.283 |
| Handgrip dominant (kg) | 36.8 | 3.1 | 35.5 | 3.2 | 37.2 | 4.1 | 37.1 | 5.2 | 39.0 | 7.6 | 39.3 | 6.6 | 41.9 | 5.6 | 36.3 | 4.3 | 40.0 | 2.0 | 2.891 | 0.077 | 0.208 | 0.962 | 0.448 | 0.149 |
| Handgrip non-dominant (kg) | 34.0 | 4.6 | 31.2 | 2.5 | 33.0 | 2.4 | 34.0 | 8.6 | 36.5 | 10.0 | 36.0 | 8.9 | 36.5 | 6.5 | 34.1 | 4.8 | 38.6 | 6.6 | 0.951 | 0.402 | 0.080 | 0.436 | 0.782 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles-Amorós, L.; Asencio-Mas, N.; Martínez-Olcina, M.; Vicente-Martínez, M.; Frutos, J.M.G.-D.; Peñaranda-Moraga, M.; Gonzálvez-Alvarado, L.; Yáñez-Sepúlveda, R.; Cortés-Roco, G.; Martínez-Rodríguez, A. Study the Effect of Relative Energy Deficiency on Physiological and Physical Variables in Professional Women Athletes: A Randomized Controlled Trial. Metabolites 2023, 13, 168. https://doi.org/10.3390/metabo13020168
Miralles-Amorós L, Asencio-Mas N, Martínez-Olcina M, Vicente-Martínez M, Frutos JMG-D, Peñaranda-Moraga M, Gonzálvez-Alvarado L, Yáñez-Sepúlveda R, Cortés-Roco G, Martínez-Rodríguez A. Study the Effect of Relative Energy Deficiency on Physiological and Physical Variables in Professional Women Athletes: A Randomized Controlled Trial. Metabolites. 2023; 13(2):168. https://doi.org/10.3390/metabo13020168
Chicago/Turabian StyleMiralles-Amorós, Laura, Nuria Asencio-Mas, María Martínez-Olcina, Manuel Vicente-Martínez, José Manuel García-De Frutos, Marcelo Peñaranda-Moraga, Lucía Gonzálvez-Alvarado, Rodrigo Yáñez-Sepúlveda, Guillermo Cortés-Roco, and Alejandro Martínez-Rodríguez. 2023. "Study the Effect of Relative Energy Deficiency on Physiological and Physical Variables in Professional Women Athletes: A Randomized Controlled Trial" Metabolites 13, no. 2: 168. https://doi.org/10.3390/metabo13020168
APA StyleMiralles-Amorós, L., Asencio-Mas, N., Martínez-Olcina, M., Vicente-Martínez, M., Frutos, J. M. G.-D., Peñaranda-Moraga, M., Gonzálvez-Alvarado, L., Yáñez-Sepúlveda, R., Cortés-Roco, G., & Martínez-Rodríguez, A. (2023). Study the Effect of Relative Energy Deficiency on Physiological and Physical Variables in Professional Women Athletes: A Randomized Controlled Trial. Metabolites, 13(2), 168. https://doi.org/10.3390/metabo13020168









