Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target
Abstract
1. Introduction
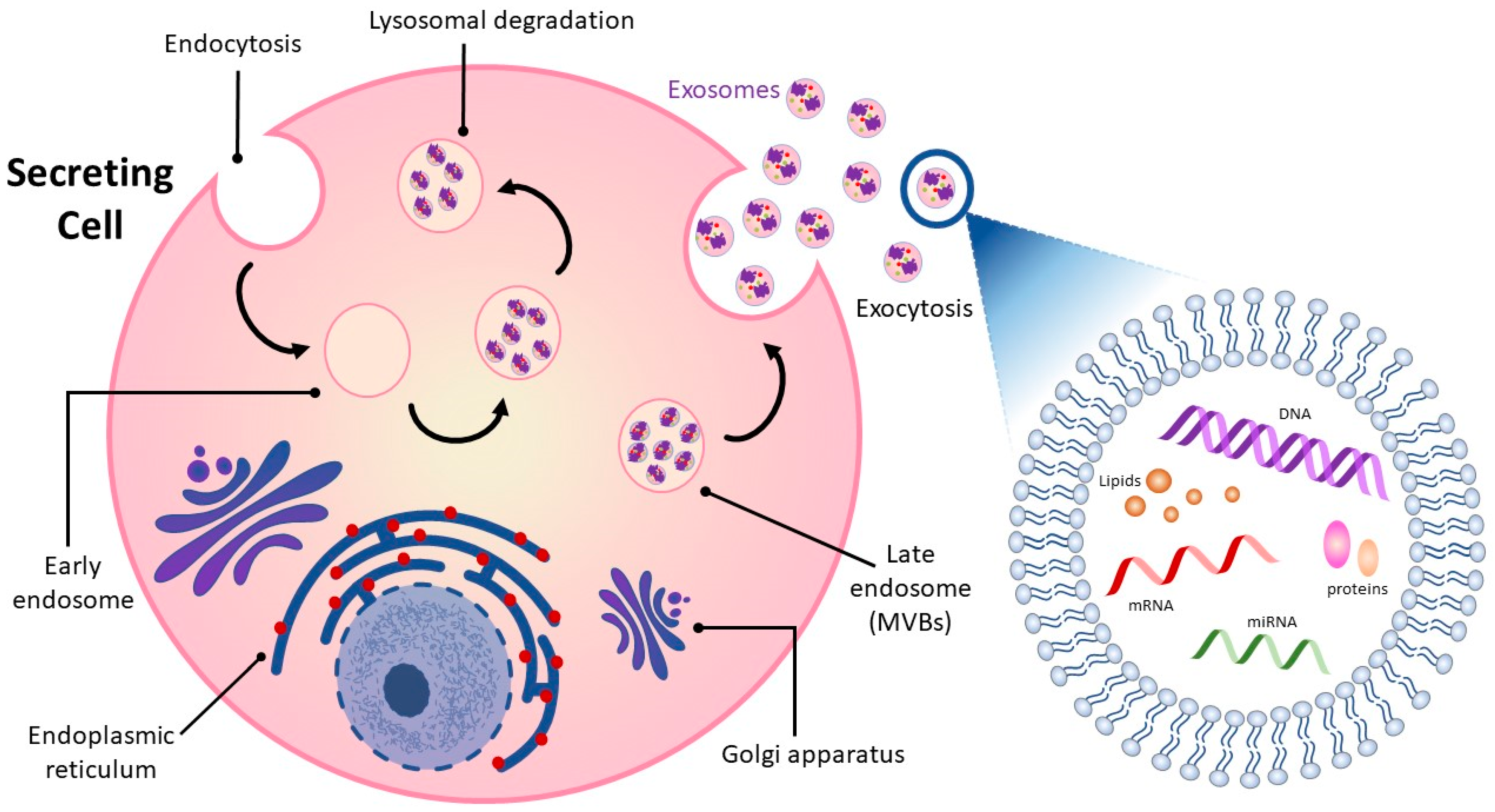
2. Overview of Exosomes
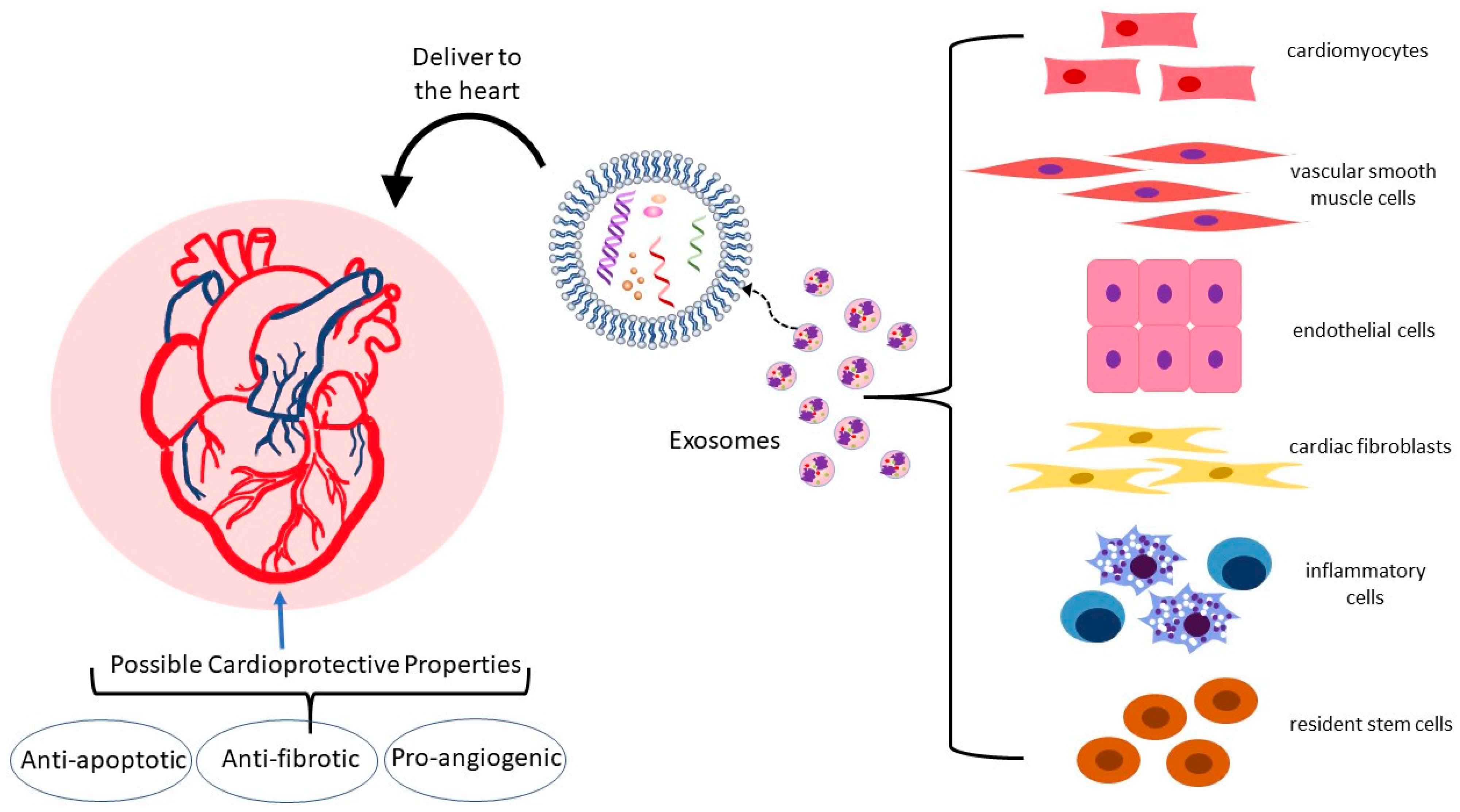
3. The Link between Cardiovascular Disease and Exosomes
3.1. Exosome Cargo and the Cardiovascular System
3.2. Exosomes in Atherosclerosis: Endothelial Dysfunction, Macrophage Recruitment, and Vascular Smooth Muscle Behavior
3.3. Exosomes in Apoptosis
3.4. Exosomes in Hypertrophy
3.5. Exosomes in Angiogenesis
3.6. Exosomes in Cardiac Fibrosis
3.7. Exosomes in Myocardial Infarction
3.8. Adipose Tissue Exosomes
| Cell/Tissue Source of Exosomes | Reference | CVD Impact | Key Cargo Involved |
|---|---|---|---|
| Carotid atherosclerotic tissue | [36] | Endothelial inflammation in rats | Whole exosomes |
| Endothelial progenitor cells (EPC) | [44] | ↓ oxidative stress and inflammation and ↓ plaque area in hyperglycemic mouse model | Whole exosomes |
| Cardiomyocyte | [49] | Protects against apoptosis, remodeling and cardiac hypertrophy in diabetic mice | Heat shock protein (HSP)-20, |
| Cardiomyocyte | [50,51] | Atherogenic via induction of inflammatory mediators | HSP-60 |
| Blood | [56,57] | Anti-inflammatory, atheroprotective effects on macrophages via IL-10 release, facilitation of cholesterol efflux | HSP-27 |
| Blood | [59] | Delivery of free fatty acids to cardiac endothelium and myocytes for energy | CD36 |
| Arterial endothelium | [64,65] | Monocyte activation and adhesion | HSP-70 |
| Macrophage foam cells | [71,72] | Enhanced adhesion and suppressed apoptosis of vascular smooth muscle cells | MiR-186-5p |
| Cardiac fibroblasts | [77,78,79] | ↓ infarct size in rat ischemia-reperfusion injury, limited apoptosis | MiR-423-3p |
| Human mesenchymal stem cells | [88] | ↓ cardiac markers for cardiomyocyte hypertrophy and ↓ inflammatory cytokine levels | Whole exosomes |
| Human cardiospheres | [89] | ↓ left ventricular fibrosis and hypertrophy in a mouse cardiac hypertrophy model | MiRNA-148a |
| Human adipose tissue mesenchymal stem cells | [91] | Promote angiogenesis | MiR-125a |
| Cardiomyocyte after hypoxic preconditioning | [92,93] | Promote angiogenesis and protect microvascular endothelium from oxidative damage | MiR-222 and miR-143 |
| Primary neonatal cardiomyocytes | [94,95] | Anti-angiogenic | MiR-19a-3p |
| Blood | [97,98,99] | ↑ proliferation, migration and tube-formation in endothelial cells | MiR-126 and miR-199a |
| Inflammatory M1 macrophages | [101,102] | Anti-angiogenic | MiR-155 |
| Heart after exercise | [109] | ↓ fibrosis | MiR-29b and miR-455 |
| Cardiomyocyte | [111] | ↑ fibrosis and hypertrophy | MiR-217 |
| Cardiomyocytes after mechanical stress | [112] | ↓ fibrosis | MiR-378 |
| Cardiomyocytes after mechanical stress | [113] | ↑ fibrosis | MiR-494-3p |
| Perivascular adipose tissue | [126] | Atheroprotective, ↑ macrophage cholesterol efflux, ABCA1, and ABCG1 | MiR-382-5p |
| Obese mouse adipose tissue macrophages | [131] | Confer insulin resistance on lean mice | Whole exosomes |
4. Exosomal MiRNAs of Specific Interest in CVD
4.1. MiR-19b
4.2. MiR-130a
4.3. MiR-10b
4.4. MiR-33
4.5. MiR-186-5p
| MiRNA | Reference | Model Where Studied | Effect on the Cardiovascular System |
|---|---|---|---|
| MiR-19b | [137,138] | Elevated in persons with angina, further investigated in mice | ↑atherosclerosis with ↑ lipids, ↑vascular smooth muscle, ↑ apoptosis |
| MiR-130a | [142,143] | Inverse association with coronary disease in humans, further investigated in mice and cultured human endothelial cells | Stimulates ↑ in serum levels of inflammatory cytokines |
| MiR-10b | [146,147,148,149,150] | Elevated level in plasma and in arteries in humans with atherosclerosis, further investigated in mice and cultured human endothelial cells and both mouse and human macrophages | Associated with pro-stenotic endothelial cell phenotype. Downregulates cholesterol efflux proteins ABCA1 and ABCG1 in macrophages. |
| MiR-33 | [161,162,163,164,165,166] | Mice, rats and primates. | Potent inhibitor of ABCA1. Knockdown of miR33 in animal models increases HDL and enhances cholesterol efflux. |
| MiR-186-5p | [167] | Elevated in persons following acute myocardial infarction, further investigated in cell culture and mice | ↓ levels in exosomes after myocardial infarction. Cultured macrophages exposed to these exosomes show ↑ scavenger receptor expression and ↑ cholesterol uptake |
5. Leveraging Exosomes for CVD Treatment
6. Exosome Delivery for Therapeutic Applications
7. Exosomes and miRNAs as Biomarkers of CVD
8. Challenges in the Application of Exosomes in Diagnosis and Treatment
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751. [Google Scholar] [CrossRef]
- Campbell, N.R.C.; Ordunez, P.; Giraldo, G.; Rodriguez Morales, Y.A.; Lombardi, C.; Khan, T.; Padwal, R.; Tsuyuki, R.T.; Varghese, C. WHO HEARTS: A Global Program to Reduce Cardiovascular Disease Burden: Experience Implementing in the Americas and Opportunities in Canada. Can. J. Cardiol. 2021, 37, 744–755. [Google Scholar] [CrossRef]
- Salvatore, F.P.; Spada, A.; Fortunato, F.; Vrontis, D.; Fiore, M. Identification of Health Expenditures Determinants: A Model to Manage the Economic Burden of Cardiovascular Disease. Int. J. Environ. Res. Public Health 2021, 18, 4652. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, J.M.; Eufrosino de Alencar Rodrigues, R.; da Costa Pereira de Arruda Neta, A.; Leite Lima Ferreira, F.E.; Lira Formiga Cavalcanti de Lima, R.; Pinheiro de Toledo Vianna, R.; Vasconcelos Leitão Moreira, L.; Moreira da Silva Neto, J.; Moreira, P.V.L. The direct and indirect costs of cardiovascular diseases in Brazil. PLoS ONE 2022, 17, e0278891. [Google Scholar] [CrossRef]
- Braunwald, E. Control of residual dyslipidaemic risk. Eur. Heart J. 2022, 43, 3824–3825. [Google Scholar] [CrossRef]
- van Rosendael, S.E.; van den Hoogen, I.J.; Lin, F.Y.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Conte, E.; et al. Clinical and Coronary Plaque Predictors of Atherosclerotic Nonresponse to Statin Therapy. JACC Cardiovasc. Imaging 2022, S1936-878X(22)00655-6. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.W.; Zheng, L.; Wang, Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell. Biol. 2017, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 8, 616161. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Wang, J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, J.; Kim, I.G.; You, G.; Kim, H.; Ahn, J.H.; Mok, H. Exosome-Mediated Delivery of Transforming Growth Factor-β Receptor 1 Kinase Inhibitors and Toll-Like Receptor 7/8 Agonists for Combination Therapy of Tumors. Acta Biomater. 2022, 141, 354–363. [Google Scholar] [CrossRef]
- Gao, P.; Li, X.; Du, X.; Liu, S.; Xu, Y. Diagnostic and Therapeutic Potential of Exosomes in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 790863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Xiong, F.; Mao, R.; Zhao, R.; Zhang, L.; Tan, K.; Liu, C.; Wang, S.; Xu, M.; Li, Y.; Zhang, T. Plasma Exosomal S1PR5 and CARNS1 as Potential Non-invasive Screening Biomarkers of Coronary Heart Disease. Front. Cardiovasc. Med. 2022, 9, 845673. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Saad, M.G.; Beyenal, H.; Dong, W.J. Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques. Biosensors 2021, 11, 518. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of extracellular vesicles: State-of-the-art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Fu, L.; Wu, S.S. Advances in studies on exosomes and microvesicles as markers of cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. Biomed. Res. Int. 2020, 2020, 5424281. [Google Scholar] [CrossRef]
- Barile, L.; Moccetti, T.; Marbán, E.; Vassalli, G. Roles of exosomes in cardioprotection. Eur. Heart J. 2017, 38, 1372–1379. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Peng, M.; Sun, R.; Hong, Y.; Wang, J.; Xie, Y.; Zhang, X.; Li, J.; Guo, H.; Xu, P.; Li, Y.; et al. Extracellular vesicles carrying proinflammatory factors may spread atherosclerosis to remote locations. Cell. Mol. Life Sci. 2022, 79, 430. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J. Cardiovasc. Transl. Res. 2021, 14, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.; Verhage, V.; Deddens, J.; van den Akker, F.; Doevendans, P. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef]
- Pironti, G.; Strachan, R.T.; Abraham, D.; Mon-Wei, Y.U.S.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015, 24, 2120–2130. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Takov, K.; Vicencio, J.M.; Boi-Doku, C.; Khoo, V.; Doreth, C.; Radenkovic, D.; Lavandero, S.; Yellon., D.M. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J. Cell. Mol. Med. 2018, 22, 141–151. [Google Scholar] [CrossRef]
- Xu, M.Y.; Ye, Z.S.; Song, X.T.; Huang, R.C. Differences in the cargos and functions of exosomes derived from six cardiac cell types: A systematic review. Stem Cell Res. Ther. 2019, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Kott, K.; Poe, A.; Kuo, T.; Chen, L.; Ferrara, K.; Knowlton, A. Cardiac myocyte exosomes: Stability, HSP60, and proteomics. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H954–H965. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; Giani, J.F.; Liu, W.; Ijichi, T.; Echavez, A.K.; Valle, J.; Marbán, E. Angiotensin II-induced end-organ damage in mice is attenuated by human exosomes and by an exosomal Y RNA fragment. Hypertension 2018, 72, 370–380. [Google Scholar] [CrossRef]
- Bai, S.; Yin, Q.; Dong, T.; Dai, F.; Qin, Y.; Ye, L.; Du, J.; Zhang, Q.; Chen, H.; Shen, B. Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed. Pharmacother. 2020, 131, 110756. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: A critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 2014, 32, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.W.; Ge, P.P.; Liu, A.L.; Yu, X.Y.; Liu, T.T. HSP20-mediated cardiomyocyte exosomes improve cardiac function in mice with myocardial infarction by activating Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4873–4881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Huang, W.; Peng, J.; Li, Y.; Yang, L.; Qin, D.; Essandoh, K.; Wang, Y.; Peng, T.; et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes 2016, 65, 3111–3128. [Google Scholar] [CrossRef]
- Kim, S.C.; Stice, J.P.; Chen, L.; Jung, J.S.; Gupta, S.; Wang, Y.; Baumgarten, G.; Trial, J.; Knowlton, A.A. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ. Res. 2009, 105, 1186–1195. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat Shock Protein 60 in Cardiovascular Physiology and Diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Ma, J.; Meng, X.W.; Liu, H.; Wang, H.; Song, S.Y.; Chen, Q.C.; Liu, H.Y.; Zhang, J.; Peng, K.; et al. Heat Shock Protein 70 Protects the Heart from Ischemia/Reperfusion Injury through Inhibition of p38 MAPK Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 3908641. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ulke-Lemée, A.; Deng, J.; Batulan, Z.; O’Brien, E.R. Characterization of heat shock protein 27 in extracellular vesicles: A potential anti-inflammatory therapy. FASEB J. 2019, 33, 1617–1630. [Google Scholar] [CrossRef]
- Pulakazhi Venu, V.K.; Adijiang, A.; Seibert, T.; Chen, Y.-X.; Shi, C.; Batulan, Z.; O’Brien, E.R. Heat shock protein 27-derived atheroprotection involves reverse cholesterol transport that is dependent on GM-CSF to maintain ABCA1 and ABCG1 expression in ApoE−/− mice. FASEB J. 2017, 31, 2364–2379. [Google Scholar] [CrossRef]
- Seibert, T.A.; Hibbert, B.; Chen, Y.X.; Rayner, K.; Simard, T.; Hu, T.; Cuerrier, C.M.; Zhao, X.; de Belleroche, J.; Chow, B.J.; et al. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: Observations from a human cohort and treatment of female mice. J. Am. Coll. Cardiol. 2013, 62, 1446–1454. [Google Scholar] [CrossRef]
- Shi, C.; Alvarez-Olmedo, D.; Zhang, Y.; Pattar, B.S.B.; O’Brien, E.R. The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux. Biomedicines 2020, 8, 290. [Google Scholar] [CrossRef]
- Boilard, E. Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.A.; Gonzalez-King, H.; Grueso, E.; Sanchez, R.; Martinez-Romero, A.; Javega, B.; O’Connor, J.E.; Simons, P.J.; Handberg, A.; Sepulveda, P. Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: Possible role of CD36. PLoS ONE 2019, 14, e0217546. [Google Scholar] [CrossRef]
- Tsuda, K. Plasma homocysteine levels and endothelial dysfunction in cerebro- and cardiovascular diseases in the metabolic syndrome. Am. J. Hypertens. 2015, 28, 1489. [Google Scholar] [CrossRef]
- Liao, J.K. Linking Endothelial Dysfunction with Endothelial Cell Activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F. Inflammation: The new cardiovascular risk factor. Eur. Heart J. 2018, 39, 3483–3487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, B. Circular RNA circCHFR downregulation protects against oxidized low-density lipoprotein-induced endothelial injury via regulation of microRNA-15b-5p/growth arrest and DNA damage inducible gamma. Bioengineered 2022, 13, 4481–4492. [Google Scholar] [CrossRef]
- Zhan, R.; Leng, X.; Liu, X.; Wang, X.; Gong, J.; Yan, L.; Wang, L.; Wang, Y.; Wang, X.; Qian, L.J. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem. Biophys. Res. Commun. 2009, 387, 229–233. [Google Scholar] [CrossRef]
- Xie, F.; Zhan, R.; Yan, L.C.; Gong, J.B.; Zhao, Y.; Ma, J.; Qian, L.J. Diet-induced elevation of circulating HSP70 may trigger cell adhesion and promote the development of atherosclerosis in rats. Cell Stress Chaperones 2016, 21, 907–914. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Tang, N.; Sun, B.; Gupta, A.; Rempel, H.; Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 2016, 30, 3097–3106. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, H.; Zhou, Y.; Song, C. M2 Macrophage-Derived Exosomes Inhibit Apoptosis of HUVEC Cell through Regulating miR-221-3p Expression. Biomed. Res. Int. 2022, 2022, 1609244. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef]
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016, 5, e004099. [Google Scholar] [CrossRef]
- Ren, L.; Chen, S.; Yao, D.; Yan, H. OxLDL-stimulated macrophage exosomes promote proatherogenic vascular smooth muscle cell viability and invasion via delivering miR-186-5p then inactivating SHIP2 mediated PI3K/AKT/mTOR pathway. Mol. Immunol. 2022, 146, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Głuchowska, A.; Cysewski, D.; Baj-Krzyworzeka, M.; Szatanek, R.; Węglarczyk, K.; Podszywałow-Bartnicka, P.; Sunderland, P.; Kozłowska, E.; Śliwińska, M.A.; Dąbrowski, M.; et al. Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions. Geroscience 2022, 44, 2863–2884. [Google Scholar] [CrossRef]
- Wencker, D.; Chandra, M.; Nguyen, K.; Miao, W.; Garantziotis, S.; Factor, S.M.; Shirani, J.; Amstrong, R.C.; Kitsis, R.N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Investig. 2003, 111, 1497–1504. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Neblina, F.; Toledo, A.H.; Toledo-Pereyra, L.H. Molecular biology of apoptosis in ischemia and reperfusion. J. Investig. Surg. 2005, 18, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Abrial, M.; Da Silva, C.C.; Pillot, B.; Augeul, L.; Ivanes, F.; Teixeira, G.; Cartier, R.; Angoulvant, D.; Ovize, M.; Ferrera, R. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2014, 68, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xie, L.; Xiao, P.; Chen, X.; Kong, W.; Lou, Q.; Chen, F.; Lu, X. Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury. Mol. Cell. Biochem. 2022, 477, 1249–1260. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Li, T.; Zhao, L.; He, J.; Zha, L.; Qi, Q.; Yu, Z. microRNA-423-3p exosomes derived from cardiac fibroblasts mediates the cardioprotective effects of ischaemic post-conditioning. Cardiovasc. Res. 2019, 115, 1189–1204. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef]
- Yue, Y.; Garikipati, V.N.S.; Verma, S.K.; Goukassian, D.A.; Kishore, R. Interleukin-10 Deficiency Impairs Reparative Properties of Bone Marrow-Derived Endothelial Progenitor Cell Exosomes. Tissue Eng. Part A 2017, 23, 1241–1250. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.K.; Dhalla, N.S. Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy. Cells 2022, 11, 3336. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279. [Google Scholar] [CrossRef]
- Constantin, A.; Comarița, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vîlcu, A.; Nemecz, M.; Niculescu, L.S.; Păunescu, V.; et al. Stem cell-derived extracellular vesicles reduce the expression of molecules involved in cardiac hypertrophy-In a model of human-induced pluripotent stem cell-derived cardiomyocytes. Front. Pharmacol. 2022, 13, 1003684. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, Y.; He, W.; Tian, Y.; Zhao, X. Exosomes secreted from bone marrow mesenchymal stem cells suppress cardiomyocyte hypertrophy through Hippo-YAP pathway in heart failure. Genet. Mol. Biol. 2023, 46, e20220221. [Google Scholar] [CrossRef]
- Mao, L.; Li, Y.D.; Chen, R.L.; Li, G.; Zhou, X.X.; Song, F.; Wu, C.; Hu, Y.; Hong, Y.X.; Dang, X.; et al. Heart-targeting exosomes from human cardiosphere-derived cells improve the therapeutic effect on cardiac hypertrophy. J. Nanobiotechnol. 2022, 20, 435. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Azam, T.; Wang, X. Cellular signaling cross-talk between different cardiac cell populations: An insight into the role of exosomes in the heart diseases and therapy. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1213–H1234. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7954657. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rodrigues, T.M.; Laundos, T.L.; Pereira-Carvalho, R.; Batista-Almeida, D.; Pereira, R.; Coelho-Santos, V.; Silva, A.P.; Fernandes, R.; Zuzarte, M.; Enguita, F.J.; et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc. Res. 2017, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Xue, C.; Tang, X.; Fang, Z. Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α. Aging 2020, 12, 23609–23618. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-inducible Factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Xue, F.; Liu, W.; Zhang, S. Exosomes derived from cardiac telocytes exert positive effects on endothelial cells. Am. J. Transl. Res. 2017, 9, 5375–5387. [Google Scholar]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Shatseva, T.; Lee, D.Y.; Deng, Z.; Yang, B.B. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell Sci. 2011, 124, 2826–2836. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–267. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 2020, 115, 22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Liu, L.; Axx, X.; Chen, B.; Li, Y.; Du, J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef] [PubMed]
- Ivey, M.J.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. 2016, 80, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Deems Black, L. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J. Biomech. Eng. 2013, 135, 71001. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Yin, Z.; Wang, F.; Chen, C.; Wang, D.W. Identification of cardiac-related circulating microRNA profile in human chronic heart failure. Oncotarget 2016, 7, 33–45. [Google Scholar] [CrossRef]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids 2018, 12, 254–266. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hou, Y.X.; Shi, P.X.; Zhu, C.H.; Lu, X.; Wang, X.L.; Que, L.L.; Zhu, G.Q.; Liu, L.; Chen, Q.; et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent exosomal communication. FASEB J. 2023, 37, e22699. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Patil, M.; Garikipati, V.; Verma, S.K.; Saheera, S.; Narasimhan, G.; Zhu, W.; Kishore, R.; Zhang, J.; Krishnamurthy, P. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020, 34, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Sakamoto, T.; Fukuta, M.; Kato, H.; Aoki, Y. Stability of exosomes in the postmortem serum and preliminary study on exosomal miRNA expression profiling in serum from myocardial infarction cadavers. Int. J. Legal Med. 2022, 9, 5375–5387. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef]
- Chaulin, A.M. The Metabolic Pathway of Cardiac Troponins Release: Mechanisms and Diagnostic Role. Cardiol Res. 2022, 13, 190–205. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Arroyo-Campuzano, M.; Flores-García, M.; Patlán, M.; Hernández-Díazcouder, A.; Alcántara, D.; Ramírez-Camacho, I.; Arana-Hidalgo, D.; Soria-Castro, E.; Sánchez, F.; et al. Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals 2022, 15, 925. [Google Scholar] [CrossRef]
- Chen, X.; Huang, F.; Liu, Y.; Liu, S.; Tan, G. Exosomal miR-152-5p and miR-3681-5p function as potential biomarkers for ST-segment elevation myocardial infarction. Clinics 2022, 77, 100038. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef]
- Barberio, M.D.; Kasselman, L.J.; Playford, M.P.; Epstein, S.B.; Renna, H.A.; Goldberg, M.; DeLeon, J.; Voloshyna, I.; Barlev, A.; Salama, M.; et al. Cholesterol efflux alterations in adolescent obesity: Role of adipose-derived extracellular vesical microRNAs. J. Transl. Med. 2019, 17, 232. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vascul. Pharmacol. 2022, 143, 106968. [Google Scholar] [CrossRef]
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019, 129, 4041–4049. [Google Scholar] [CrossRef]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.C.; Cheng, P.; Shao, H.G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef]
- Liu, X.; Chu, H.; Ji, Y.; Bosnjak, Z.; Ao, H.; Li, T. Which BMI for Diabetes Patients is Better? From the View of the Adipose Tissue Macrophage-Derived Exosome. Diabetes Metab. Syndr. Obes. 2022, 15, 141–153. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 17, 1372–1784.e12. [Google Scholar] [CrossRef] [PubMed]
- Hubal, M.J.; Nadler, E.P.; Ferrante, S.C.; Barberio, M.D.; Suh, J.H.; Wang, J.; Dohm, G.L.; Pories, W.J.; Mietus-Snyder, M.; Freishtat, R.J. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity 2017, 25, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Iaconetti, C.; Sorrentino, S.; De Rosa, S.; Indolfi, C. Exosomal miRNAs in Heart Disease. Physiology 2016, 31, 16–24. [Google Scholar] [CrossRef]
- Xue, R.; Tan, W.; Wu, Y.; Dong, B.; Xie, Z.; Huang, P.; He, J.; Dong, Y.; Liu, C. Role of Exosomal miRNAs in Heart Failure. Front. Cardiovasc. Med. 2020, 7, 592412. [Google Scholar] [CrossRef]
- Johnston, E.K.; Abbott, R.D. Adipose Tissue Paracrine-, Autocrine-, and Matrix-Dependent Signaling during the Development and Progression of Obesity. Cells 2023, 12, 407. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Li, S.; Geng, Q.; Chen, H.; Zhang, J.; Cao, C.; Zhang, F.; Song, J.; Liu, C.; Liang, W. The potential inhibitory effects of miR-19b on vulnerable plaque formation via the suppression of STAT3 transcriptional activity. Int. J. Mol. Med. 2018, 41, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, S.; Zhang, F.; Wu, M.; Liang, H.; Song, J.; Lee, C.; Chen, H. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE-/- mice. Biochem. Biophys. Res. Commun. 2018, 495, 1922–1929. [Google Scholar] [CrossRef]
- Xue, Y.; Wei, Z.; Ding, H.; Wang, Q.; Zhou, Z.; Zheng, S.; Zhang, Y.; Hou, D.; Liu, Y.; Zen, K.; et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1alpha in the progression of atherosclerosis. Atherosclerosis 2015, 241, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, Y.; Wang, H. microRNA-19b-3p-containing extracellular vesicles derived from macrophages promote the development of atherosclerosis by targeting JAZF1. J. Cell. Mol. Med. 2022, 26, 48–59. [Google Scholar] [CrossRef]
- Liao, B.; Dong, S.; Xu, Z.; Gao, F.; Zhang, S.; Liang, R. MiR-19b-3p regulated by BC002059/ABHD10 axis promotes cell apoptosis in myocardial infarction. Biol. Direct 2022, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.W.; Chen, Z.H.; Ding, X.Q.; Liu, J.Y.; Ge, P.C.; An, F.H.; Li, L.H.; Wang, L.S.; Ma, W.Z.; Yang, Z.J.; et al. Predictive Effects of Circulating miR-221, miR-130a and miR-155 for Coronary Heart Disease: A Multi-Ethnic Study in China. Cell. Physiol. Biochem. 2017, 42, 808–823. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Du, Y.; Li, Y. MiRNA-130a promotes inflammation to accelerate atherosclerosis via the regulation of proliferator-activated receptor γ (PPARγ) expression. Anatol. J. Cardiol. 2021, 25, 630–637. [Google Scholar] [CrossRef]
- Sheedy, P.; Medarova, Z. The fundamental role of miR-10b in metastatic cancer. Am. J. Cancer Res. 2018, 8, 1674–1688. [Google Scholar] [PubMed]
- Hassel, D.; Cheng, P.; White, M.P.; Ivey, K.N.; Kroll, J.; Augustin, H.G.; Katus, H.A.; Stainier, D.Y.; Srivastava, D. MicroRNA-10 regulates the angiogenic behavior of zebrafish and human endothelial cells by promoting vascular endothelial growth factor signaling. Circ. Res. 2012, 111, 1421–1433. [Google Scholar] [CrossRef]
- Bidzhekov, K.; Gan, L.; Denecke, B.; Rostalsky, A.; Hristov, M.; Koeppel, T.A.; Zernecke, A.; Weber, C. microRNA expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thromb. Haemost. 2012, 107, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hakimzadeh, N.; Nossent, A.Y.; van der Laan, A.M.; Schirmer, S.H.; de Ronde, M.W.; Pinto-Sietsma, S.J.; van Royen, N.; Quax, P.H.; Hoefer, I.E.; Piek, J.J. Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS ONE 2015, 10, e0137035. [Google Scholar] [CrossRef]
- Nakahara, M.; Kobayashi, N.; Oka, M.; Nakano, K.; Okamura, T.; Yuo, A.; Saeki, K. miR-10b deficiency affords atherosclerosis resistance. Int. J. Inf. Res. Rev. 2017, 4, 4899–4911. [Google Scholar]
- Kim, J.; Siverly, A.N.; Chen, D.; Wang, M.; Yuan, Y.; Wang, Y.; Lee, H.; Zhang, J.; Muller, W.J.; Liang, H.; et al. Ablation of miR-10b suppresses oncogene-induced mammary tumorigenesis and metastasis and reactivates tumor-suppressive pathways. Cancer Res. 2016, 76, 6424–6435. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Chen, G.; Wu, W.K. MicroRNA-10b induces vascular muscle cell proliferation through Akt pathway by targeting TIP30. Curr. Vasc. Pharmacol. 2015, 13, 679–686. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, W.; Lin, W.; Yang, W.; Zhang, P.; Chen, M.; Ding, D.; Liu, C.; Zheng, J.; Ling, W. Apoptotic cell induction of miR-10b in macrophages contributes to advanced atherosclerosis progression in ApoE-/- mice. Cardiovasc. Res. 2018, 114, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Y.; Chen, Y.; Yang, W.; Han, Y.; Lu, L.; Yang, K.; Cao, J. Effect of HIF-1α/miR-10b-5p/PTEN on Hypoxia-Induced Cardiomyocyte Apoptosis. J. Am. Heart Assoc. 2019, 17, e011948. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Horie, T.; Kuwabara, Y.; Nagao, K.; Baba, O.; Nakao, T.; Nishino, T.; Hakuno, D.; Nakashima, Y.; Nishi, H.; et al. MicroRNA-33 Controls Adaptive Fibrotic Response in the Remodeling Heart by Preserving Lipid Raft Cholesterol. Circ. Res. 2017, 120, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.R.; Wei, L.H.; Chung, A.C.; Yu, C.M.; Lan, H.Y. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol. Ther. 2014, 22, 974–985. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Näär, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef]
- Daemen, S.; Kutmon, M.; Evelo, C.T. A pathway approach to investigate the function and regulation of SREBPs. Genes Nutr. 2013, 8, 289–300. [Google Scholar] [CrossRef]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef]
- Gerin, I.; Clerbaux, L.A.; Haumont, O.; Lanthier, N.; Das, A.K.; Burant, C.F.; Leclercq, I.A.; MacDougald, O.A.; Bommer, G.T. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 2010, 285, 33652–33661. [Google Scholar] [CrossRef]
- Price, N.L.; Rotllan, N.; Canfrán-Duque, A.; Zhang, X.; Pati, P.; Arias, N.; Moen, J.; Mayr, M.; Ford, D.A.; Baldán, Á.; et al. Genetic dissection of the impact of miR-33a and miR-33b during the progression of atherosclerosis. Cell Rep. 2017, 21, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rotllan, N.; Canfrán-Duque, A.; Sun, J.; Toczek, J.; Moshnikova, A.; Malik, S.; Price, N.L.; Araldi, E.; Zhong, W.; et al. Targeted Suppression of miRNA-33 Using pHLIP Improves Atherosclerosis Regression. Circ. Res. 2022, 131, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, H.S.; Guo, X.; Shen, J.J.; Fan, D.; Huang, Y.; Huang, C.X. MiR-33 promotes myocardial fibrosis by inhibiting MMP16 and stimulating p38 MAPK signaling. Oncotarget 2018, 9, 22047–22057. [Google Scholar] [CrossRef]
- Price, N.L.; Rotllan, N.; Zhang, X.; Canfrán-Duque, A.; Nottoli, T.; Suarez, Y.; Fernández-Hernando, C. Specific Disruption of Abca1 Targeting Largely Mimics the Effects of miR-33 Knockout on Macrophage Cholesterol Efflux and Atherosclerotic Plaque Development. Circ. Res. 2019, 124, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; Sharma, M.; Schlegel, M.; van Solingen, C.; Koelwyn, G.J.; Shanley, L.C.; Beckett, L.; Peled, D.; Rahman, K.; Giannarelli, C.; et al. miR-33 Silencing Reprograms the Immune Cell Landscape in Atherosclerotic Plaques. Circ. Res. 2021, 128, 1122–1138. [Google Scholar] [CrossRef]
- Goedeke, L.; Salerno, A.; Ramírez, C.M.; Guo, L.; Allen, R.M.; Yin, X.; Langley, S.R.; Esau, C.; Wanschel, A.; Fisher, E.A.; et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol. Med. 2014, 6, 1133–1141. [Google Scholar] [CrossRef]
- Ding, J.; Li, H.; Liu, W.; Wang, X.; Feng, Y.; Guan, H.; Chen, Z. miR-186-5p Dysregulation in Serum Exosomes from Patients with AMI Aggravates Atherosclerosis via Targeting LOX-1. Int. J. Nanomed. 2022, 17, 6301–6316. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, signaling and its role in atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, F.; Zhang, D.; Qi, S.; Liu, Y. Metabolites as extracellular vesicle cargo in health, cancer, pleural effusion, and cardiovascular diseases: An emerging field of study to diagnostic and therapeutic purposes. Biomed. Pharmacother. 2023, 157, 114046. [Google Scholar] [CrossRef]
- Ning, Y.; Huang, P.; Chen, G.; Xiong, Y.; Gong, Z.; Wu, C.; Xu, J.; Jiang, W.; Li, X.; Tang, R.; et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med. 2023, 21, 96. [Google Scholar] [CrossRef]
- Fuchs, M.; Kreutzer, F.P.; Kapsner, L.A.; Mitzka, S.; Just, A.; Perbellini, F.; Terracciano, C.M.; Xiao, K.; Geffers, R.; Bogdan, C.; et al. Integrative Bioinformatic Analyses of Global Transcriptome Data Decipher Novel Molecular Insights into Cardiac Anti-Fibrotic Therapies. Int. J. Mol. Sci. 2020, 21, 4727. [Google Scholar] [CrossRef]
- Zhao, Y.; Srivastava, D. A developmental view of microRNA function. Trends Biochem. Sci. 2007, 32, 189–197. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Cordes, K.R.; Srivastava, D. MicroRNA Regulation of Cardiovascular Development. Circ. Res. 2009, 104, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The Human DiGeorge Syndrome Critical Region Gene 8 and Its D. melanogaster Homolog Are Required for miRNA Biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef]
- Shukla, G.C.; Singh, J.; Barik, S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol. Cell. Pharmacol. 2011, 3, 83–92. [Google Scholar]
- Chaudhuri, K.; Chatterjee, R. MicroRNA Detection and Target Prediction: Integration of Computational and Experimental Approaches. DNA Cell Biol. 2007, 26, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, U.; Albayrak, S.; Zafra, R.; Baraa, A.; Vishweswaraiah, S.; Veerappa, A.M.; Mahishi, D.; Saiyed, N.; Mishra, N.K.; Guda, C.; et al. Placental epigenetics for evaluation of fetal congenital heart defects: Ventricular septal defect (VSD). PLoS ONE 2019, 14, e0200229. [Google Scholar] [CrossRef]
- Machado, R.; Sachinidis, A.; Futschik, M.E. Detection of Novel Potential Regulators of Stem Cell Differentiation and Cardiogenesis through Combined Genome-Wide Profiling of Protein-Coding Transcripts and microRNAs. Cells 2021, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- da Costa Martins, P.A.; Bourajjaj, M.; Gladka, M.; Kortland, M.; van Oort, R.J.; Pinto, Y.M.; Molkentin, J.D.; De Windt, L.J. Conditional Dicer Gene Deletion in the Postnatal Myocardium Provokes Spontaneous Cardiac Remodeling. Circulation 2008, 118, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Murchison, E.P.; Tang, R.; Callis, T.E.; Tatsuguchi, M.; Deng, Z.; Rojas, M.; Hammond, S.M.; Schneider, M.D.; Selzman, C.H.; et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2008, 105, 2111–2116. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.W.; Zou, J.; Wang, G.K.; Kremneva, E.; Li, X.Q.; Zhu, N.; Sun, T.; Lappalainen, P.; Yuan, W.J.; et al. Attenuation of microRNA-1 derepresses the cytoskeleton regulatory protein twinfilin-1 to provoke cardiac hypertrophy. J. Cell Sci. 2010, 123 Pt 14, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Heidersbach, A.; Saxby, C.; Carver-Moore, K.; Huang, Y.; Ang, Y.S.; de Jong, P.J.; Ivey, K.N.; Srivastava, D. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife 2013, 2, e01323. [Google Scholar] [CrossRef]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of Cardiogenesis, Cardiac Conduction, and Cell Cycle in Mice Lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef]
- Benzoni, P.; Nava, L.; Giannetti, F.; Guerini, G.; Gualdoni, A.; Bazzini, C.; Milanesi, R.; Bucchi, A.; Baruscotti, M.; Barbuti, A. Dual role of miR-1 in the development and function of sinoatrial cells. J. Mol. Cell. Cardiol. 2021, 157, 104–112. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Loyer, X.; Mallat, Z.; Boulanger, C.M.; Tedgui, A. MicroRNAs as therapeutic targets in atherosclerosis. Expert Opin. Ther. Targets 2015, 19, 489–496. [Google Scholar] [CrossRef]
- Stenvang, J.; Petri, A.; Lindow, M.; Obad, S.; Kauppinen, S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 2012, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhu, L.; Zhu, L.; Wang, P.; Xu, W.; Huang, J. Recent Developments in Delivery of MicroRNAs Utilizing Nanosystems for Metabolic Syndrome Therapy. Int. J. Mol. Sci. 2021, 22, 7855. [Google Scholar] [CrossRef]
- Hammond, S.M. Dicing and slicing the core machinery of the RNA interference pathway. FEBS Lett. 2005, 579, 5822–5829. [Google Scholar] [CrossRef]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Amer. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Exosomes Engineering and Their Roles as Therapy Delivery Tools, Therapeutic Targets, and Biomarkers. Int. J. Mol. Sci. 2021, 22, 9543. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda Redondo, M.; Martínez, G.; Merinero de Los Santos, M.; Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Rezaie, J.; Ajezi, S.; Avci, Ç.B.; Karimipour, M.; Geranmayeh, M.H.; Nourazarian, A.; Sokullu, E.; Rezabakhsh, A.; Rahbarghazi, R. Exosomes and Their Application in Biomedical Field: Difficulties and Advantages. Mol. Neurobiol. 2018, 55, 3372–3393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Gao, J.Q. Exosomes as Novel Bio-Carriers for Gene and Drug Delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mathiyalagan, P.; Sahoo, S. Exosomes-Based Gene Therapy for MicroRNA Delivery. Methods Mol. Biol. 2017, 1521, 139–152. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Zhang, Y.; Wang, J.; Wang, C.; Liu, Y.; Yang, G.; Yuan, L. Exosome-Based Ldlr Gene Therapy for Familial Hypercholesterolemia in a Mouse Model. Theranostics 2021, 11, 2953–2965. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Duong, P.; Phu, T.A.; Alsop, E.; Meechoovet, B.; Reiman, R.; Ng, M.; Yamamoto, R.; Nakauchi, H.; Gasper, W.J.; et al. High glucose macrophage exosomes enhance atherosclerosis by driving cellular proliferation & hematopoiesis. iScience 2021, 24, 102847. [Google Scholar] [CrossRef]
- Schena, G.J.; Murray, E.K.; Hildebrand, A.N.; Headrick, A.L.; Yang, Y.; Koch, K.A.; Kubo, H.; Eaton, D.; Johnson, J.; Berretta, R.; et al. Cortical bone stem cell-derived exosomes’ therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1014–H1029. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Metra, M.; Filippatos, G.S.; Davison, B.A.; Bartunek, J.; Terzic, A.; Gersh, B.J.; Povsic, T.J.; Henry, T.D.; Alexandre, B.; et al. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: Results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur. J. Heart Fail. 2017, 19, 1520–1529. [Google Scholar] [CrossRef]
- Bartunek, J.; Terzic, A.; Behfar, A.; Wijns, W. Clinical Experience With Regenerative Therapy in Heart Failure: Advancing Care With Cardiopoietic Stem Cell Interventions. Circ. Res. 2018, 122, 1344–1346. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Sun, Z.; Chi, B.; Zou, A.; Mao, L.; Xiong, X.; Jiang, J.; Sun, L.; Zhu, W.; et al. HIF-1α overexpression in mesenchymal stem cell-derived exosome-encapsulated arginine-glycine-aspartate (RGD) hydrogels boost therapeutic efficacy of cardiac repair after myocardial infarction. Mater Today Bio. 2021, 12, 100171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, J.; Yan, W.; Li, Y.; Shen, Z.; Asahara, T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J. Am. Heart Assoc. 2016, 5, e002856. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Li, Y.; Peng, N.; Liu, Q.; Qiu, D.; Cho, J.; Borlongan, C.V.; Yu, G. Exosomes Derived From Mesenchymal Stem Cells Pretreated With Ischemic Rat Heart Extracts Promote Angiogenesis via the Delivery of DMBT1. Cell Transplant. 2022, 31, 9636897221102898. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Zhang, O.; Wu, X.; Guan, X.; Xue, Y.; Li, S.; Zhuang, X.; Zhou, B.; Miao, G.; et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. Int. Immunopharmacol. 2020, 80, 106156. [Google Scholar] [CrossRef]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339–353. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Ahmed, L.; Al-Massri, K. New Approaches for Enhancement of the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Diseases. Tissue Eng. Regen. Med. 2022, 19, 1129–1146. [Google Scholar] [CrossRef]
- De Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ousaka, D.; Fukushima, Y.; Kondo, M.; Eitoku, T.; Shigemitsu, Y.; Hara, M.; Baba, K.; Iwasaki, T.; Kasahara, S.; et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci. Transl. Med. 2020, 12, eabb3336. [Google Scholar] [CrossRef]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef]
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; de Couto, G.; Aminzadeh, M.; Middleton, R.; Valle, J.; Weixin, L.; Marbán, E. Fibroblasts rendered anti-fibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol. 2015, 66, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Mentkowski, K.I.; Mursleen, A.; Snitzer, J.D.; Euscher, L.M.; Lang, J.K. CDC-derived extracellular vesicles reprogram inflammatory macrophages to an arginase 1-dependent proangiogenic phenotype. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1447–H1460. [Google Scholar] [CrossRef]
- Namazi, H.; Mohit, E.; Namazi, I.; Rajabi, S.; Samadian, A.; Hajizadeh-Saffar, E.; Aghdami, N.; Baharvand, H. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J. Cell. Biochem. 2018, 119, 4150–4160. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H.; Namazi, I.; Ghiasi, P.; Ansari, H.; Rajabi, S.; Hajizadeh-Saffar, E.; Aghdami, N.; Mohit, E. Exosomes secreted by normoxic and hypoxic cardiosphere-derived cells have anti- apoptotic effect. Iran. J. Pharm. Res. 2018, 17, 377–385. [Google Scholar] [PubMed]
- Mentkowski, K.I.; Lang, J.K. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention in Vivo. Sci. Rep. 2019, 9, 10041. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Saha, P.; Sharma, S.; Korutla, L.; Datla, S.R.; Shoja-Taheri, F.; Mishra, R.; Bigham, G.E.; Sarkar, M.; Morales, D.; Bittle, G.; et al. Circulating Exosomes Derived from Transplanted Progenitor Cells Aid the Functional Recovery of Ischemic Myocardium. Sci. Transl. Med. 2019, 11, eaau1168. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Liao, Z.; Luo, X.; Chen, J.Q.; Deng, M.; Huang, Y.; Wang, Z.; Wei, M. Endothelial colony-forming cell-derived exosomal miR-21-5p regulates autophagic flux to promote vascular endothelial repair by inhibiting SIPL1A2 in atherosclerosis. Cell Commun. Signal. 2022, 20, 30. [Google Scholar] [CrossRef]
- Hu, H.; Wang, B.; Jiang, C.; Li, R.; Zhao, J. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21–5p to modulate Thrombospondin-1 expression. Clin. Sci. 2019, 133, 1629–1644. [Google Scholar] [CrossRef]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic Biomaterials Based on Extracellular Vesicles: Classification of Bio-Engineering and Mimetic Preparation Routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Bheri, S.; Kassouf, B.P.; Park, H.J.; Hoffman, J.R.; Davis, M.E. Engineering Cardiac Small Extracellular Vesicle-Derived Vehicles with Thin-Film Hydration for Customized microRNA Loading. J. Cardiovasc. Dev. Dis. 2021, 8, 135. [Google Scholar] [CrossRef]
- Leggio, L.; Arrabito, G.; Ferrara, V.; Vivarelli, S.; Paternò, G.; Marchetti, B.; Pignataro, B.; Iraci, N. Mastering the Tools: Natural versus Artificial Vesicles in Nanomedicine. Adv. Healthc. Mater. 2020, 9, e2000731. [Google Scholar] [CrossRef]
- Bheri, S.; Hoffman, J.R.; Park, H.J.; Davis, M.E. Biomimetic nanovesicle design for cardiac tissue repair. Nanomedicine 2020, 15, 1873–1896. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H.A.; Lee, M.; Shin, Y.; Song, J.J.; Kang, S.W.; Nam, D.H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef]
- Patel, S.; Schmidt, K.F.; Farhoud, M.; Zi, T.; Jang, S.C.; Dooley, K.; Kentala, D.; Dobson, H.; Economides, K.; Williams, D.E. In vivo tracking of [89Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl. Med. Biol. 2022, 112–113, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Martinez Arroyo, O.; Forner, M.J.; Cortes, R. Exosomes as Drug Delivery Systems: Endogenous Nano vehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics 2020, 13, 3. [Google Scholar] [CrossRef]
- Vandergriff, A.C.; de Andrade, J.B.; Tang, J.; Hensley, M.T.; Piedrahita, J.A.; Caranasos, T.G.; Cheng, K. Intravenous Cardiac Stem Cell-Derived Exosomes Ameliorate Cardiac Dysfunction in Doxorubicin Induced Dilated Cardiomyopathy. Stem Cells Int. 2015, 2015, 960926. [Google Scholar] [CrossRef]
- Charles, C.J.; Li, R.R.; Yeung, T.; Mazlan, S.M.I.; Lai, R.C.; de Kleijn, D.P.V.; Lim, S.K.; Richards, A.M. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization With MRI in a Porcine Model. Front. Cardiovasc. Med. 2020, 7, 601990. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Li, Y.; Chen, L.; Wang, X.; Guo, W.; Zhang, X.; Qin, G.; He, S.H.; Zimmerman, A.; et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 2015, 192, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhu, D.; Huang, K.; Caranasos, T.G. Minimally invasive delivery of a hydrogel-based exosome patch to prevent heart failure. J. Mol. Cell. Cardiol. 2022, 169, 113–121. [Google Scholar] [CrossRef]
- Yang, D.; Wang, M.; Hu, Z.; Ma, Y.; Shi, Y.; Cao, X.; Guo, T.; Cai, H.; Cai, H. Extracorporeal Cardiac Shock Wave-Induced Exosome Derived From Endothelial Colony-Forming Cells Carrying miR-140-3p Alleviate Cardiomyocyte Hypoxia/Reoxygenation Injury via the PTEN/PI3K/AKT Pathway. Front. Cell Devel. Biol. 2022, 9, 779936. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Venkidasamy, B.; Subramanian, U.; Thiruvengadam, M. Exosomes: Current Use and Future Applications. Clin. Chim. Acta 2020, 500, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Soares Martins, T.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; da Cruz E Silva, O.A.B.; Henriques, A.G. Diagnostic and Therapeutic Potential of Exosomes in Alzheimer’s Disease. J. Neurochem. 2021, 156, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.W.; Ronan, G.; Ren, X.; Bahcecioglu, G.; Senapati, S.; Anderson, D.; Handberg, E.; March, K.L.; Chang, H.C.; Zorlutuna, P. Human Heart Anoxia and Reperfusion Tissue (HEART) Model for the Rapid Study of Exosome Bound miRNA Expression As Biomarkers for Myocardial Infarction. Small 2022, 18, e2201330. [Google Scholar] [CrossRef]
- Cheow, E.S.H.; Cheng, W.C.; Lee, C.N.; de Kleijn, D.; Sorokin, V.; Sze, S.K. Plasma-derived Extracellular Vesicles Contain Predictive Biomarkers and Potential Therapeutic Targets for Myocardial Ischemic (MI) Injury. Mol. Cell. Proteomics 2016, 15, 2628–2640. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position paper from the working group on cellular biology of the heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Bye, A.; Røsjø, H.; Nauman, J.; Silva, G.J.; Follestad, T.; Omland, T.; Wisløff, U. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals—The HUNT study. J. Mol. Cell. Cardiol. 2016, 97, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Burtenshaw, D.; Regan, B.; Owen, K.; Collins, D.; McEneaney, D.; Megson, I.L.; Redmond, E.M.; Cahill, P.A. Exosomal Composition, Biogenesis and Profiling Using Point-of-Care Diagnostics-Implications for Cardiovascular Disease. Front. Cell Dev. Biol. 2022, 10, 853451. [Google Scholar] [CrossRef]
- Gholipour, A.; Shakerian, F.; Zahedmehr, A.; Irani, S.; Malakootian, M.; Mowla, S.J. Bioinformatics Analysis to Find Novel Biomarkers for Coronary Heart Disease. Iran J. Public Health 2022, 51, 1152–1160. [Google Scholar] [CrossRef]
- Moreira-Costa, L.; Barros, A.S.; Lourenço, A.P.; Leite-Moreira, A.F.; Nogueira-Ferreira, R.; Thongboonkerd, V.; Vitorino, R. Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach. Proteomes 2021, 9, 8. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Xu, B.; Liu, Y.L.; Liu, Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J. Med. Sci. 2018, 34, 626–633. [Google Scholar] [CrossRef]
- Zampetaki, A.; Mayr, M. Analytical challenges and technical limitations in assessing circulating MiRNAs. Thromb. Haemost. 2012, 108, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Khamina, K.; Diendorfer, A.B.; Skalicky, S.; Weigl, M.; Pultar, M.; Krammer, T.L.; Fournier, C.A.; Schofield, A.L.; Otto, C.; Smith, A.T.; et al. A MicroRNA Next-Generation-Sequencing Discovery Assay (miND) for Genome-Scale Analysis and Absolute Quantitation of Circulating MicroRNA Biomarkers. Int. J. Mol. Sci. 2022, 23, 1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Gu, H.; Wang, J.; Zhang, J.; Zen, K.; Li, D. Comparative Analyses of Human Exosome Proteomes. Protein J. 2023. published online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, E.; Bozbeyoglu, N.; Gursel, I.; Korkusuz, F.; Bakan Misirlioglu, F.; Korkusuz, P. Comparative analysis of magnetically activated cell sorting and ultracentrifugation methods for exosome isolation. PLoS ONE 2023, 18, e0282238. [Google Scholar] [CrossRef]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 2023, 10, 1100892. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Pedersen, K.W.; Kierulf, B.; Neurauter, A. Specific and generic isolation of extracellular vesicles with magnetic beads. Methods Mol. Biol. 2017, 1660, 65–87. [Google Scholar] [CrossRef]
- Jiawei, S.; Zhi, C.; Kewei, T.; Xiaoping, L. Magnetic bead-based adsorption strategy for exosome isolation. Front. Bioeng. Biotechnol. 2022, 10, 942077. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Getting, S.J.; Moschos, S.A. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol. Ther. 2018, 192, 170–187. [Google Scholar] [CrossRef]
- Fu, W.; Li, T.; Chen, H.; Zhu, S.; Zhou, C. Research Progress in Exosome-Based Nanoscale Drug Carriers in Tumor Therapies. Front. Oncol. 2022, 12, 919279. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Frolova, L.; Li, I.T.S. Targeting Capabilities of Native and Bioengineered Extracellular Vesicles for Drug Delivery. Bioengineering 2022, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Lamichhane, T.N.; Wang, S.; Zou, L.; Dahal, E.; Kronstadt, S.M.; Levy, D.; Parajuli, B.; Knudsen, D.R.; Chao, W.; et al. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2020, 28, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, R.; Sun, X.; Duan, Y.; Han, Y.; Zhao, Y.; Qian, H.; Zhu, W.; Xu, W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 2016, 18, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiss, A.B.; Ahmed, S.; Johnson, M.; Saeedullah, U.; De Leon, J. Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target. Metabolites 2023, 13, 479. https://doi.org/10.3390/metabo13040479
Reiss AB, Ahmed S, Johnson M, Saeedullah U, De Leon J. Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target. Metabolites. 2023; 13(4):479. https://doi.org/10.3390/metabo13040479
Chicago/Turabian StyleReiss, Allison B., Saba Ahmed, Maryann Johnson, Usman Saeedullah, and Joshua De Leon. 2023. "Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target" Metabolites 13, no. 4: 479. https://doi.org/10.3390/metabo13040479
APA StyleReiss, A. B., Ahmed, S., Johnson, M., Saeedullah, U., & De Leon, J. (2023). Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target. Metabolites, 13(4), 479. https://doi.org/10.3390/metabo13040479








