Correlation of Elemental Transfer, Bioactive Compounds and Antioxidant Activity on Lactuca sativa L. Grown in Soil with Functionalized CNT and HMs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Plant and Soil Analysis after Harvesting
2.2.1. Determination of Chlorophylls and Total Carotenoids Concentration
2.2.2. Total Polyphenols Evaluation
2.2.3. Antioxidant Capacity Determination via DPPH Method
2.2.4. Multi-Elemental Investigation of Lettuce and Soil Substrate via NAA
2.3. Data Analysis
2.4. Evaluation of the Elemental Translocation
3. Results and Discussion
3.1. Elemental Translocation
3.1.1. Uptake of Minerals from Soil
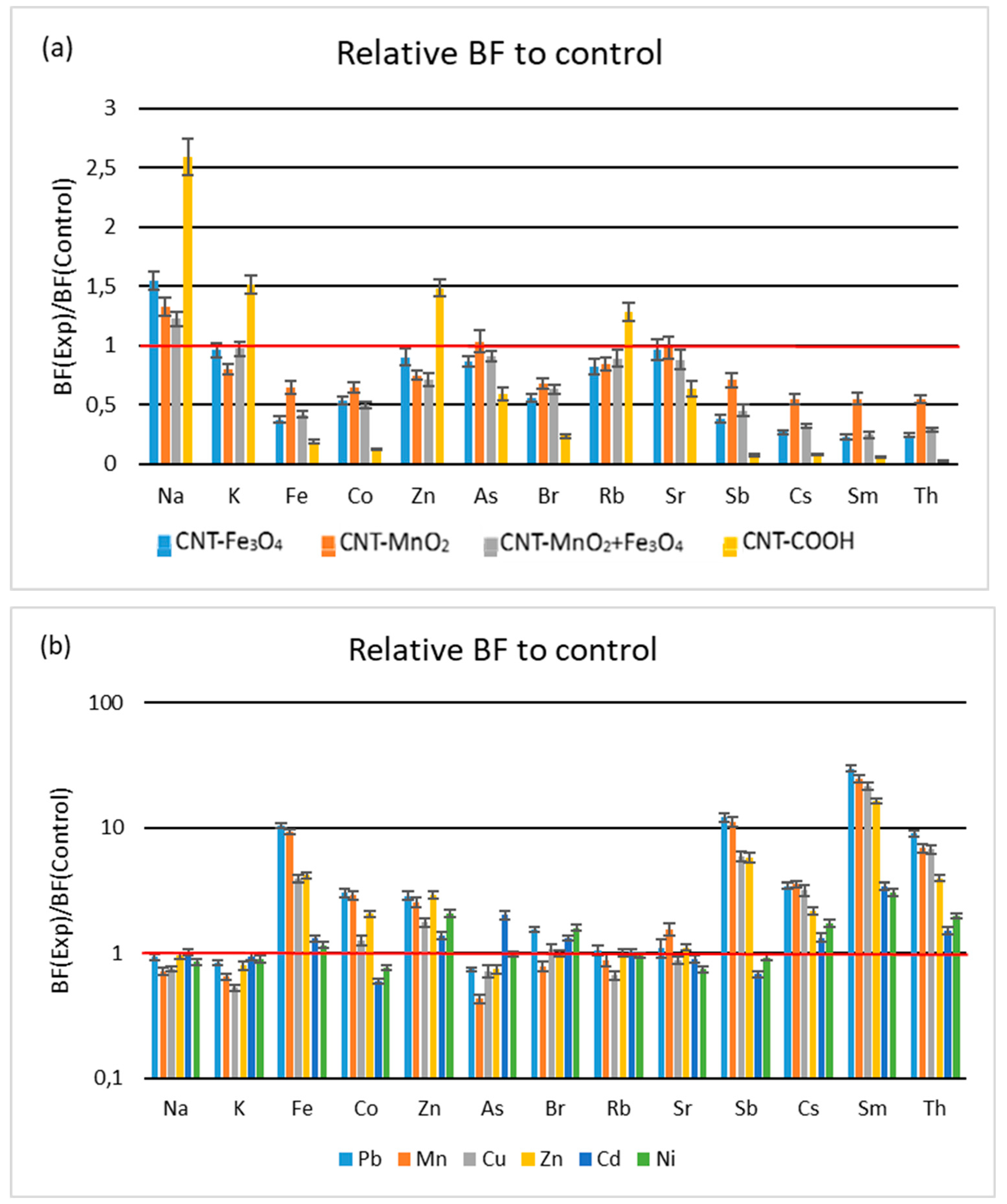
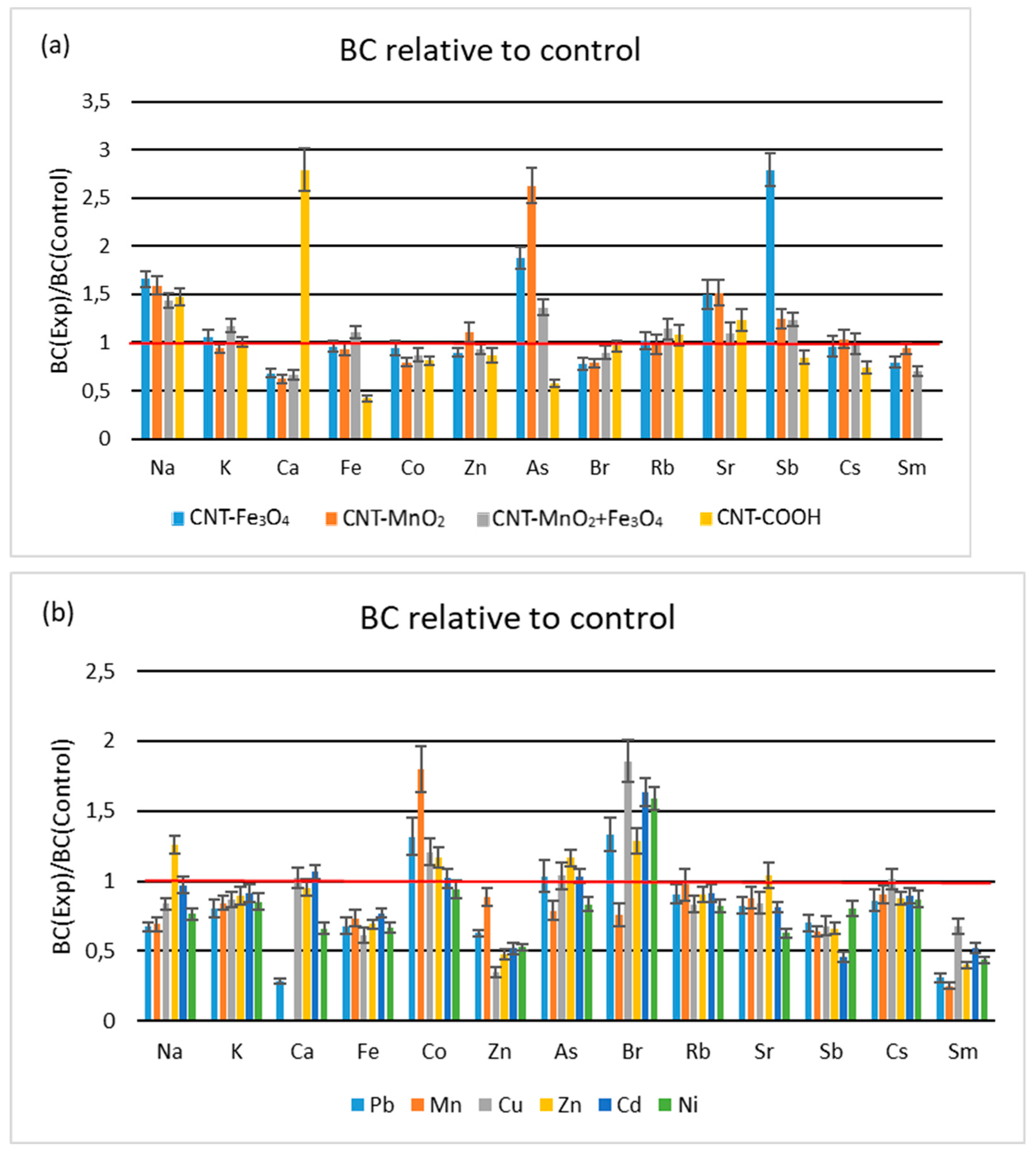
3.1.2. Root-to-Leaves Transfer
3.1.3. Soil-to-Leaves Transfer
3.2. Variation of Bioactive Compounds and Antioxidant Activity
3.3. Correlation of the Elemental Transfer with Bioactive Compounds and Antioxidant Activity
3.3.1. Influence of Functionalized CNTs
3.3.2. Influence of HM Salts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yavuz, N.; Seymen, M.; Kal, U. Impacts of water stress and harvest time on physio-biochemical characteristics of lettuce (Lactuca sativa L.). Int. J. Agric. Nat. Sci. 2021, 14, 61–77. [Google Scholar]
- Tziros, G.T.; Samaras, A.; Karaoglanidis, G.S. Fusarium equiseti as an emerging foliar pathogen of lettuce in Greece: Identification and development of a real-time PCR for quantification of inoculum in soil samples. Pathogens 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L.). Sci. Hortic. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Pérez-Figueroa, C.E.; Salazar-Moreno, R.; Fitz Rodríguez, E.; López Cruz, I.L.; Schmidt, U.; Dannehl, D. Heavy metals accumulation in lettuce and cherry tomatoes cultivated in cities. Pol. J. Environ. Stud. 2023, 32, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- da Silva Freitas, L.; Honscha, L.C.; Volcão, L.M.; de Lima Brum, R.; da Silva Júnior, F.M.R.; Ramos, D.F. Antibiotics in the Environment: Prescribing risks to non-target organisms. Pollutants 2022, 2, 435–443. [Google Scholar] [CrossRef]
- Leitão, I.; Leclercq, C.C.; Ribeiro, D.M.; Renaut, J.; Almeida, A.M.; Martins, L.L.; Mourato, M.P. Stress response of lettuce (Lactuca sativa) to environmental contamination with selected pharmaceuticals: A proteomic study. J. Proteom. 2021, 245, 104291. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.S.; Andrade-Vieira, L.F.; dos Santos, F.E.; Correa, F.F.; das Graças Cardoso, M.; Vilela, L.R. Allelopathic potential and phytochemical screening of ethanolic extracts from five species of Amaranthus spp. in the plant model Lactuca sativa. Sci. Hortic. 2019, 245, 90–98. [Google Scholar] [CrossRef]
- Liné, C.; Manent, F.; Wolinski, A.; Flahaut, E.; Larue, C. Comparative study of response of four crop species exposed to carbon nanotube contamination in soil. Chemosphere 2021, 274, 129854. [Google Scholar] [CrossRef]
- Ramires, P.F.; Dos Santos, M.; Paz-Montelongo, S.; Rubio-Armendáriz, C.; Adamatti, D.; Fiasconaro, M.L.; da Silva Júnior, F.M.R. Multiple exposure pathways and health risk assessment of potentially harmful elements for children and adults living in a coal region in Brazil. Environ. Geochem. Health 2023, 45, 305–318. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; He, X.; Li, W.; Zhang, M.; Cai, Q. Evaluation of heavy metal contamination of soil and the health risks in four potato-producing areas. Front. Environ. Sci. 2023, 11, 1071353. [Google Scholar] [CrossRef]
- Available online: https://www.polarismarketresearch.com/industry-analysis/carbon-nanotubes-market (accessed on 28 August 2023).
- Tawfik, A.S. Insights into carbon nanotube-metal oxide composite: Embedding in membranes. Int. J. Sci. Res. Environ. Sci. Toxicol. 2017, 2, 1–4. [Google Scholar]
- Goswami, P.; Yadav, S.; Mathur, J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci. Today 2019, 6, 232–242. [Google Scholar] [CrossRef]
- Abd-Elsalam Kamel, A. Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Oxford, UK, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nano-materials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.H.; Ji, Z.; Bouchard, D.C.; Nisbet, R.M.; Schimel, J.P.; Gardea-Torresdey, J.L.; Holden, P.A. Agglomeration determines effects of carbonaceous nanomaterials on soybean nodulation, dinitrogen fixation potential, and growth in soil. ACS Nano 2017, 11, 5753–5765. [Google Scholar] [CrossRef]
- Baysal, A.; Saygin, H.; Ustabasi, G.S. Risks of graphene nanomaterial contamination in the soil: Evaluation of major ions. Environ. Monit. Assess. 2020, 192, 622. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.; Kolahi, M.; Mohajel, K.E.; Barnaby, G.A. Study of the contamination rate and change in growth features of lettuce (Lactuca sativa Linn.) in response to cadmium and a survey of its phytochelatin synthase gene. Ecotoxicol. Environ. Saf. 2019, 180, 295–308. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Wu, B.; Cheng, H.; Wang, C. Heavy metal pollution improved allelopathy of Canada goldenrod on lettuce germination performance. Plant Biol. 2020, 22, 832–838. [Google Scholar] [CrossRef]
- Paradelo, R.; Villada, A.; Barral, M.T. Heavy metal uptake of lettuce and ryegrass from urban waste composts. Int. J. Environ. Res. Public Health 2020, 17, 2887. [Google Scholar] [CrossRef]
- Kolahi, M.; Mohajel Kazemi, E.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol. Biochem. 2020, 146, 71–89. [Google Scholar] [CrossRef]
- Chen, X.; Chu, S.; Chi, Y.; Wang, J.; Wang, R.; You, Y.; Hayat, K.; Khalid, M.; Zhang, D.; Zhou, P.; et al. Unraveling the role of multi-walled carbon nanotubes in a corn-soil system: Plant growth, oxidative stress and heavy metal(loid)s behavior. Plant Physiol. Biochem. 2023, 200, 107802–107810. [Google Scholar] [CrossRef]
- Soran, M.L.; Opris, O.; Lung, I.; Kacso, I.; Porav, A.S.; Stan, M. The efficiency of the multi-walled carbon nanotubes used for antibiotics removal from wastewaters generated by animal farms. Environ. Sci. Pollut. Res. 2017, 24, 16396–16406. [Google Scholar] [CrossRef]
- Stegarescu, A.; Lung, I.; Leoștean, C.; Kacso, I.; Opris, O.; Lazăr, M.D.; Copolovici, L.; Gutoiu, S.; Stan, M.; Popa, A.; et al. Green synthesis, sharacterization and sest of MnO2 nanoparticles as catalyst in biofuel production from grape residue and seeds oil. Waste Biomass Valorization 2020, 11, 5003–5013. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazăr, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef]
- Podar, D.; Boza, C.-L.; Lung, I.; Soran, M.-L.; Culicov, O.; Stegarescu, A.; Opriș, O.; Ciorîtă, A.; Nekhoroshkov, P. The effect of functionalized multiwall carbon nanotubes with Fe and Mn oxides on Lactuca sativa L. Plants 2023, 12, 1959. [Google Scholar] [CrossRef]
- Soran, M.-L.; Sîrb, A.N.; Lung, I.; Opriș, O.; Culicov, O.; Stegarescu, A.; Nekhoroshkov, P.; Gligor, D.-M. A multi-method approach for impact assessment of some heavy metals on Lactuca sativa L. Molecules 2023, 28, 759. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Current Protocols in Food Analytical Chemistry (Units: F4.3.1–F4.3.8); John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Available online: https://ibr-2.jinr.ru/ (accessed on 1 September 2023).
- Available online: https://www.mirion.com/products/technologies/spectroscopy-scientific-analysis/gamma-spectroscopy/gamma-spectroscopy-software/lab-applications/genie-spectroscopy-software-suite (accessed on 27 September 2023).
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of different metal ions with carboxylic acid group: A quantitative study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Zhai, X.; Wu, R.; Liu, W.; Pan, J.; Shohag, M.J.I.; Xin, X.; He, Z.; Yang, X. Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of Cd in wheat (Triticum aestivum L.). J. Hazard Mater. 2022, 440, 129857. [Google Scholar] [CrossRef]
- Wang, J.; Deng, P.; Wei, X.; Zhang, X.; Liu, J.; Huang, Y.; She, J.; Liu, Y.; Wan, Y.; Hu, H.; et al. Hidden risks from potentially toxic metal(loid)s in paddy soils-rice and source apportionment using lead isotopes: A case study from China. Sci. Total Environ. 2023, 856, 158883. [Google Scholar] [CrossRef]
- Abbey, L.; Ijenyo, M.; Spence, B.; Asunni, A.O.; Ofoe, R.; Amo-Larbi, V. Bioaccumulation of chemical elements in vegetables as influenced by application frequency of municipal solidwaste compost. Can. J. Plant Sci. 2021, 101, 967–983. [Google Scholar] [CrossRef]
- Muktar, A.A.; Alhassan, A.J.; Atiku, M.K.; Pedro, S.L.; Wudil, A.M. Assessment of toxicological indices of some heavy metals in soil, cabbage and lettuce samples grown along some rivers in Kano State, Nigeria. Dutse J. Pure Appl. Sci. (DUJOPAS) 2021, 7, 65–76. [Google Scholar] [CrossRef]
- Kumwimba, M.S.; Zeng, X.; Bai, L. Uptake kinetics of arsenic by lettuce cultivars under hydroponics. Afr. J. Environ. Sci. Technol. 2013, 7, 321–328. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soran, M.-L.; Lung, I.; Stegarescu, A.; Culicov, O.; Opriș, O.; Nekhoroshkov, P.; Podar, D. Correlation of Elemental Transfer, Bioactive Compounds and Antioxidant Activity on Lactuca sativa L. Grown in Soil with Functionalized CNT and HMs. Metabolites 2023, 13, 1171. https://doi.org/10.3390/metabo13121171
Soran M-L, Lung I, Stegarescu A, Culicov O, Opriș O, Nekhoroshkov P, Podar D. Correlation of Elemental Transfer, Bioactive Compounds and Antioxidant Activity on Lactuca sativa L. Grown in Soil with Functionalized CNT and HMs. Metabolites. 2023; 13(12):1171. https://doi.org/10.3390/metabo13121171
Chicago/Turabian StyleSoran, Maria-Loredana, Ildiko Lung, Adina Stegarescu, Otilia Culicov, Ocsana Opriș, Pavel Nekhoroshkov, and Dorina Podar. 2023. "Correlation of Elemental Transfer, Bioactive Compounds and Antioxidant Activity on Lactuca sativa L. Grown in Soil with Functionalized CNT and HMs" Metabolites 13, no. 12: 1171. https://doi.org/10.3390/metabo13121171
APA StyleSoran, M.-L., Lung, I., Stegarescu, A., Culicov, O., Opriș, O., Nekhoroshkov, P., & Podar, D. (2023). Correlation of Elemental Transfer, Bioactive Compounds and Antioxidant Activity on Lactuca sativa L. Grown in Soil with Functionalized CNT and HMs. Metabolites, 13(12), 1171. https://doi.org/10.3390/metabo13121171






