Aldose Reductase (AR) Mediates and Perivascular Adipose Tissue (PVAT) Modulates Endothelial Dysfunction of Short-Term High-Fat Diet Feeding in Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Short-Term High-Fat Diet Feeding Increases Body and PVAT Adiposity
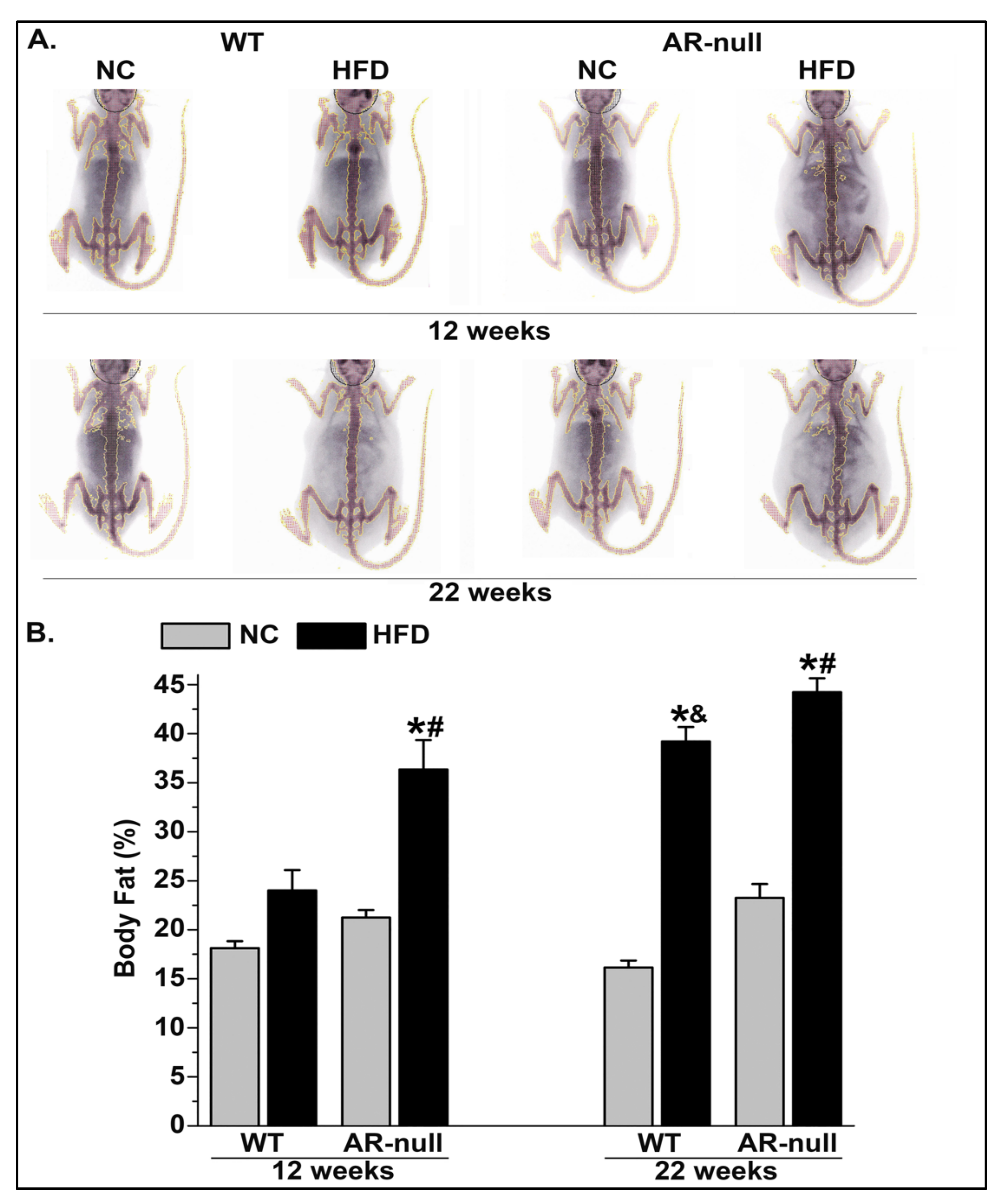
3.1.1. Body Weight and Adiposity
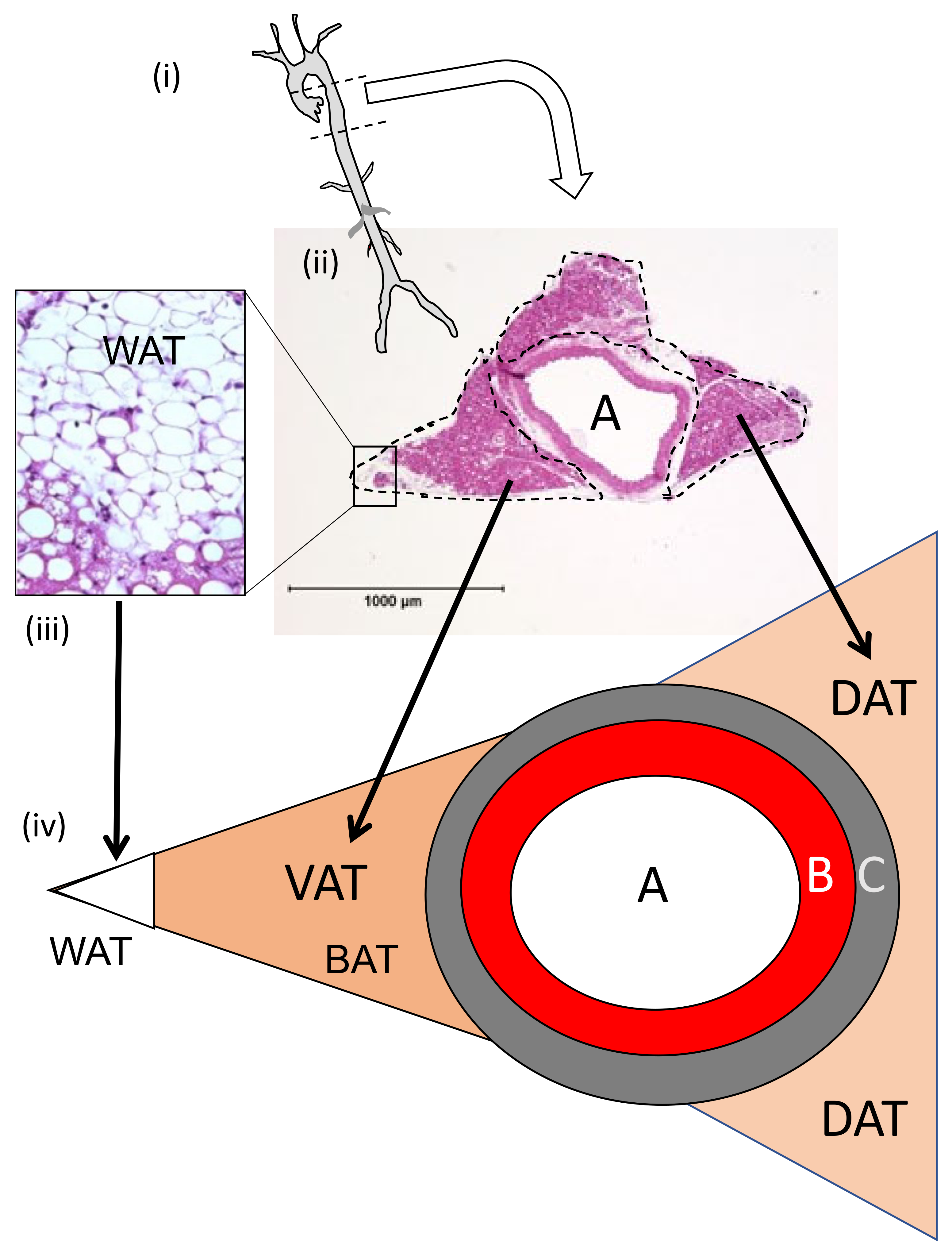
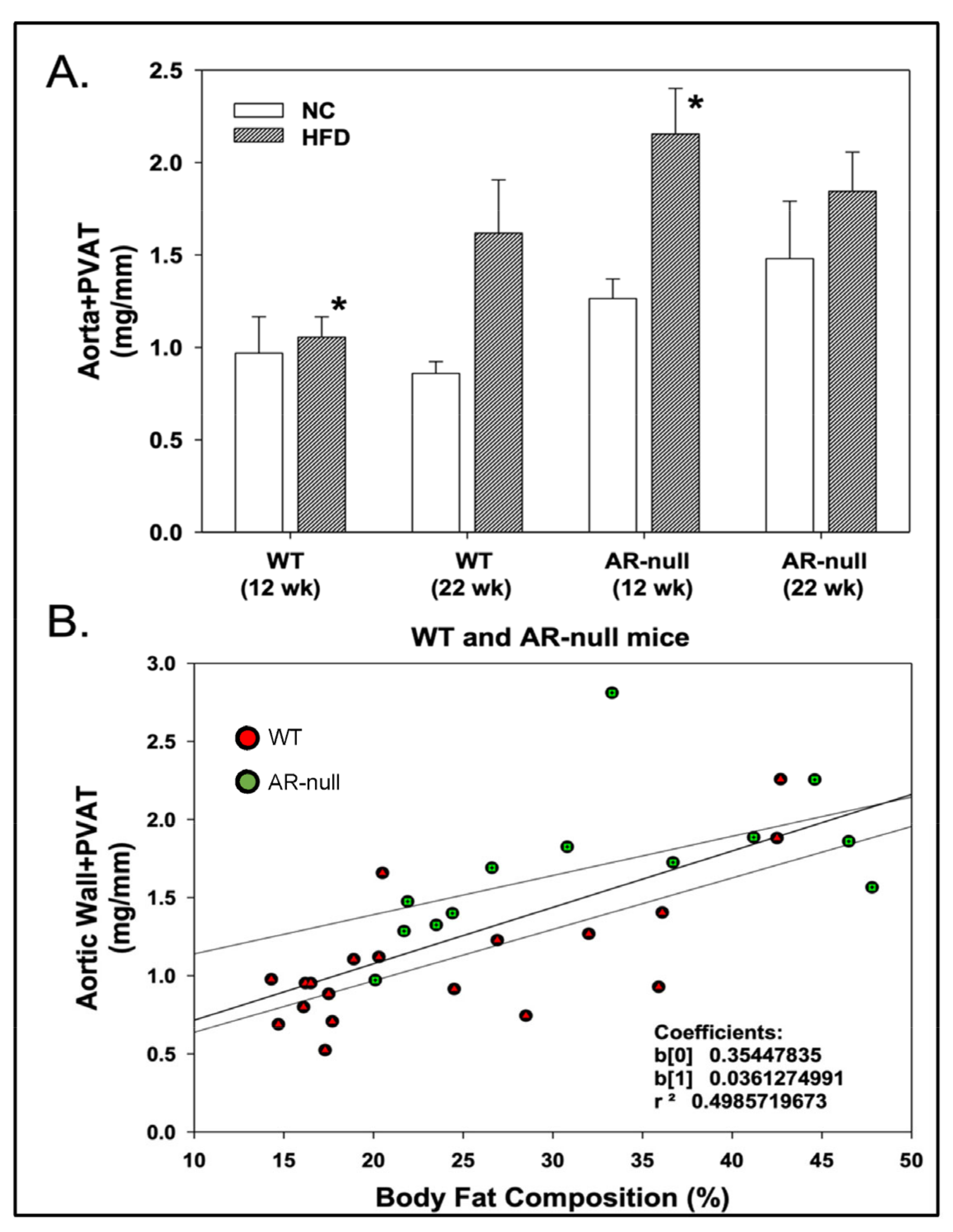
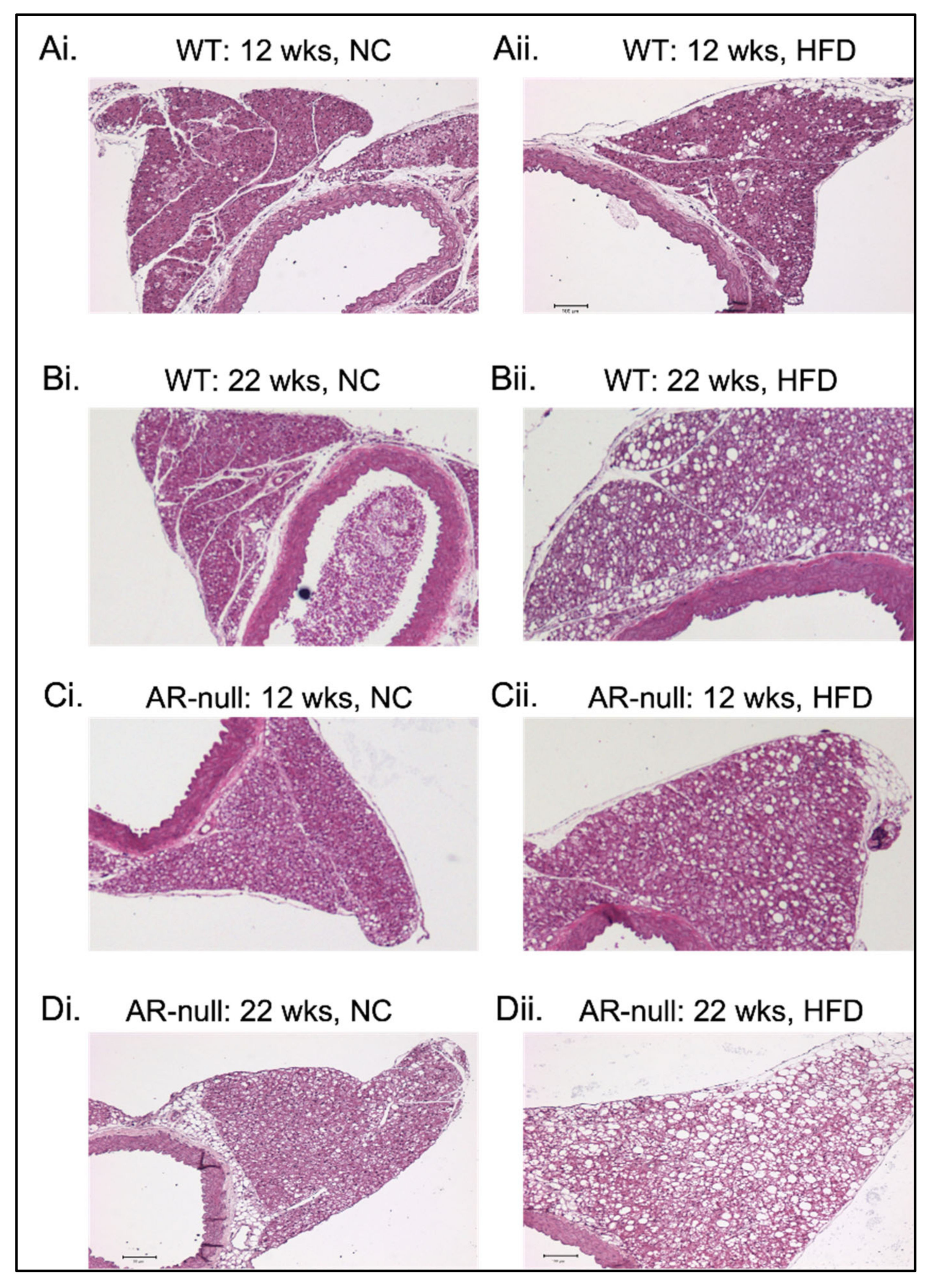
3.1.2. Perivascular Adipose Tissue (PVAT) Morphometry: Effects of HFD
3.2. Aortic Function with and without PVAT: Effects of Age, Genotype, and Diet
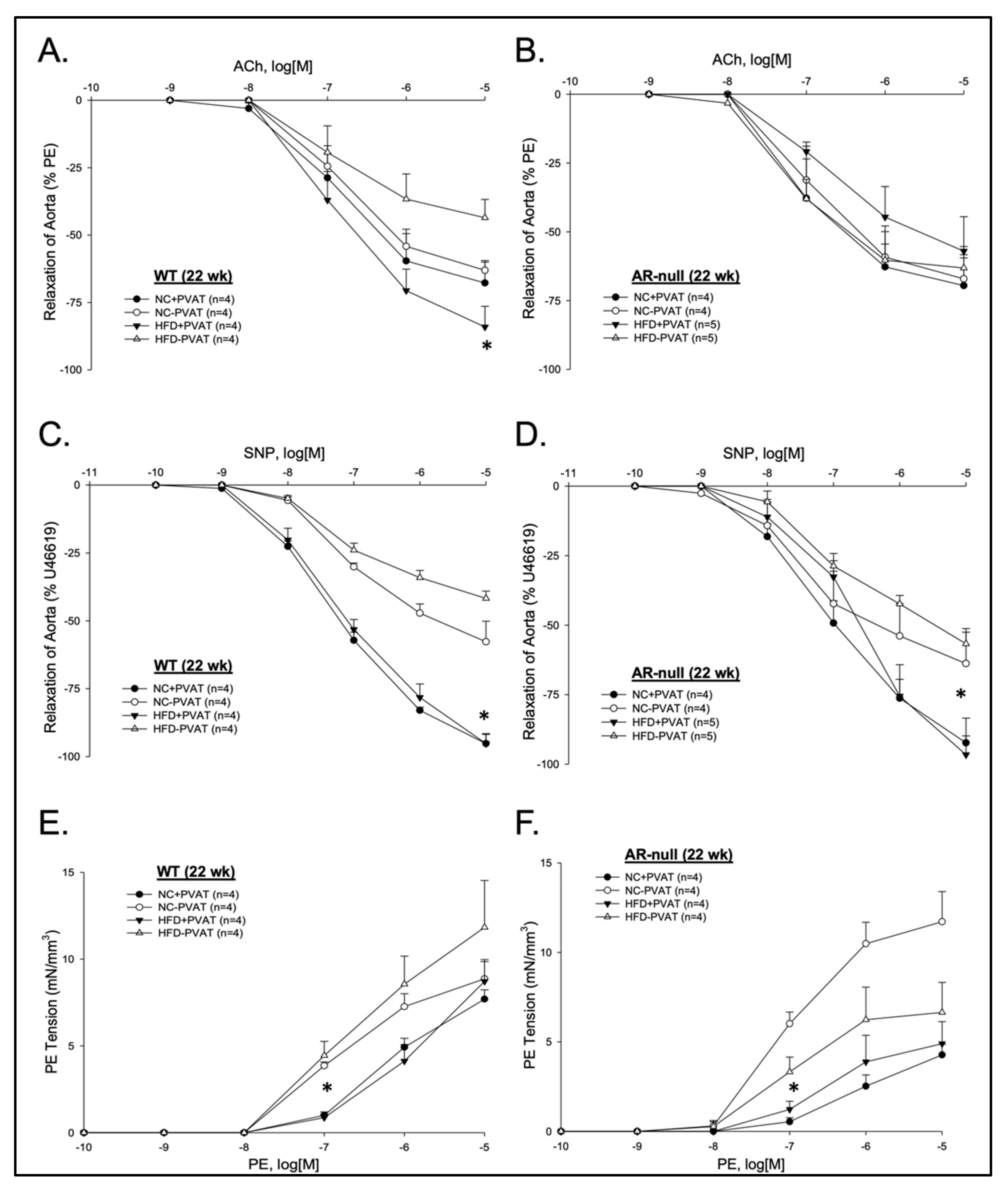
3.2.1. Relaxation Responses
3.2.2. Contractile Responses
3.2.3. Immunofluorescence Localization of AR and Leptin in Aorta and PVAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conklin, D.J.; Schick, S.; Blaha, M.J.; Carll, A.; DeFilippis, A.; Ganz, P.; Hall, M.E.; Hamburg, N.; O’Toole, T.; Reynolds, L.; et al. Cardiovascular injury induced by tobacco products: Assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H801–H827. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P.C. Diabetes is a vascular disease: The role of endothelial dysfunction in pathophysiology of cardiovascular disease in diabetes. Cardiol. Clin. 2004, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Schlett, C.L.; Massaro, J.M.; Lehman, S.J.; Bamberg, F.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int. J. Obes. 2009, 33, 226–232. [Google Scholar] [CrossRef]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Kohr, M.C.; Tune, J.D. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br. J. Pharmacol. 2012, 165, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.K.; Witzmann, F.A.; McKenney, M.L.; Lai, X.; Berwick, Z.C.; Moberly, S.P.; Alloosh, M.; Sturek, M.; Tune, J.D. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation 2013, 128, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Shi, H.; Winkler, M.A.; Lee, R.; Weintraub, N.L. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 2569–2576. [Google Scholar] [CrossRef]
- Payne, G.A.; Tune, J.D.; Knudson, J.D. Leptin-induced endothelial dysfunction: A target for therapeutic interventions. Curr Pharm. Des. 2014, 20, 603–608. [Google Scholar] [CrossRef]
- Ketonen, J.; Pilvi, T.; Mervaala, E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessel. 2010, 25, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ramana, K.V.; Bhatnagar, A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr. Rev. 2005, 26, 380–392. [Google Scholar] [CrossRef]
- Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Complic. 2010, 24, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Rev. Med. Chem. 2016, 16, 120–162. [Google Scholar] [CrossRef]
- Phillips, S.A.; Mirrlees, D.; Thornalley, P.J. Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor Statil. Biochem. Pharmacol. 1993, 46, 805–811. [Google Scholar] [CrossRef]
- Rittner, H.L.; Hafner, V.; Klimiuk, P.A.; Szweda, L.I.; Goronzy, J.J.; Weyand, C.M. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J. Clin. Investig. 1999, 103, 1007–1013. [Google Scholar] [CrossRef]
- Jannapureddy, S.; Sharma, M.; Yepuri, G.; Schmidt, A.M.; Ramasamy, R. Aldose Reductase: An Emerging Target for Development of Interventions for Diabetic Cardiovascular Complications. Front. Endocrinol. 2021, 12, 636267. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.G.; Cote, C.; Couto, J.; Daskiran, M.; Gunnarsson, C.; Haas, K.; Haas, S.; Nigam, S.C.; Schuette, R. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: Findings from the GE Centricity Electronic Medical Record database. Popul. Health Manag. 2010, 13, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Conklin, D.J.; Liu, S.Q.; Prakash, N.; Boor, P.J.; Srivastava, S.K.; Bhatnagar, A. Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells. Atherosclerosis 2001, 158, 339–350. [Google Scholar] [CrossRef]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O’Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 2009, 58, 2486–2497. [Google Scholar] [CrossRef]
- Srivastava, S.; Watowich, S.J.; Petrash, J.M.; Srivastava, S.K.; Bhatnagar, A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry 1999, 38, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Zhang, D.; Singh, M.; Dassanayaka, S.; Xie, Z.; Jagatheesan, G.; Zhao, J.; Schmidtke, V.K.; Brittian, K.R.; Merchant, M.L.; et al. Deficiency of aldose reductase exacerbates early pressure overload-induced cardiac dysfunction and autophagy in mice. J. Mol. Cell Cardiol. 2018, 118, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.T.; Chung, S.K.; Law, J.W.; Ko, B.C.; Tam, S.C.; Brooks, H.L.; Knepper, M.A.; Chung, S.S. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol. Cell Biol. 2000, 20, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, S.; Jagatheesan, G.; Haberzettl, P.; Shah, J.; Hill, B.G.; Bhatnagar, A.; Conklin, D.J. Glutathione S-transferase P deficiency induces glucose intolerance via JNK-dependent enhancement of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1005–E1018. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J.; Haberzettl, P.; Prough, R.A.; Bhatnagar, A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1586–H1597. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lipinski, A.; Conklin, D.J. A simple method for normalization of aortic contractility. J. Vasc. Res. 2018, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Haberzettl, P.; Jin, L.; Riggs, D.W.; Zhao, J.; O’Toole, T.E.; Conklin, D.J. Fine particulate matter air pollution and aortic perivascular adipose tissue: Oxidative stress, leptin, and vascular dysfunction. Physiol. Rep. 2021, 9, e14980. [Google Scholar] [CrossRef] [PubMed]
- Blomkalns, A.L.; Chatterjee, T.; Weintraub, N.L. Turning ACS outside in: Linking perivascular adipose tissue to acute coronary syndromes. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H734–H735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajsheker, S.; Manka, D.; Blomkalns, A.L.; Chatterjee, T.K.; Stoll, L.L.; Weintraub, N.L. Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 2010, 10, 191–196. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Stucchi, P.; Guzman-Ruiz, R.; Cano, V.; Arribas, S.; Gonzalez, M.C.; Ruiz-Gayo, M.; Fernandez-Alfonso, M.S.; Somoza, B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology 2010, 151, 3299–3306. [Google Scholar] [CrossRef]
- Van den Enden, M.K.; Nyengaard, J.R.; Ostrow, E.; Burgan, J.H.; Williamson, J.R. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism. Implications for diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1675–1685. [Google Scholar]
- Shinmura, K.; Bolli, R.; Liu, S.Q.; Tang, X.L.; Kodani, E.; Xuan, Y.T.; Srivastava, S.; Bhatnagar, A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ. Res. 2002, 91, 240–246. [Google Scholar] [CrossRef]
- Thiagarajan, D.; Quadri, N.; Jawahar, S.; Zirpoli, H.; Del Pozo, C.H.; Lopez-Diez, R.; Hasan, S.N.; Yepuri, G.; Gugger, P.F.; Finlin, B.S.; et al. Aldose reductase promotes diet-induced obesity via induction of senescence in subcutaneous adipose tissue. Obesity 2022, 30, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Tammali, R.; Reddy, A.B.; Bhatnagar, A.; Srivastava, S.K. Aldose reductase-regulated tumor necrosis factor-alpha production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology 2007, 148, 4371–4384. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Friedrich, B.; Tammali, R.; West, M.B.; Bhatnagar, A.; Srivastava, S.K. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes 2005, 54, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Yadav, U.C.; Reddy, A.B.; Saxena, A.; Tammali, R.; Shoeb, M.; Ansari, N.H.; Bhatnagar, A.; Petrash, M.J.; Srivastava, S.; et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011, 191, 330–338. [Google Scholar] [CrossRef]
- Vladykovskaya, E.; Ozhegov, E.; Hoetker, J.D.; Xie, Z.; Ahmed, Y.; Suttles, J.; Srivastava, S.; Bhatnagar, A.; Barski, O.A. Reductive metabolism increases the proinflammatory activity of aldehyde phospholipids. J. Lipid Res. 2011, 52, 2209–2225. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, S.; Noh, H.; Ananthakrishnan, R.; Son, N.; Hallam, K.; Hu, Y.; Yu, S.; Shen, X.; Rosario, R.; Lu, Y.; et al. Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E−/− mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G.; Ilnytska, O.; Lyzogubov, V.V.; Pavlov, I.A.; Mashtalir, N.; Nadler, J.L.; Drel, V.R. High-fat diet induced neuropathy of pre-diabetes and obesity: Effects of “healthy” diet and aldose reductase inhibition. Diabetes 2007, 56, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wu, X.; Chau, J.F.; Szeto, I.Y.; Tam, W.Y.; Guo, Z.; Chung, S.K.; Oates, P.J.; Chung, S.S.; Yang, J.Y. Aldose reductase regulates hepatic peroxisome proliferator-activated receptor alpha phosphorylation and activity to impact lipid homeostasis. J. Biol. Chem. 2008, 283, 17175–17183. [Google Scholar] [CrossRef]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seica, R.; Sena, C.M. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2020, 146, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Borbouse, L.; Kumar, S.; Neeb, Z.; Alloosh, M.; Sturek, M.; Tune, J.D. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1711–1717. [Google Scholar] [CrossRef]
- Yang, R.; Barouch, L.A. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 2007, 101, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Bohlen, H.G.; Dincer, U.D.; Borbouse, L.; Tune, J.D. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H460–H465. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Borbouse, L.; Bratz, I.N.; Roell, W.C.; Bohlen, H.G.; Dick, G.M.; Tune, J.D. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation 2008, 15, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Pham, M.; Maloney, E.; Rizzo, N.O.; Morton, G.J.; Wisse, B.E.; Kirk, E.A.; Chait, A.; Schwartz, M.W. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Xu, D.; Chen, Y.; Long, Y.; Dai, F.; Gui, L.; Lu, Y. Sitagliptin Reduces Endothelial Dysfunction and Apoptosis Induced by High-Fat Diet and Palmitate in Thoracic Aortas and Endothelial Cells via ROS-ER Stress-CHOP Pathway. Front. Pharmacol. 2021, 12, 670389. [Google Scholar] [CrossRef]

| Age | 12 Weeks Old | |||
|---|---|---|---|---|
| Groups | WT and NC | WT and HFD | AR-Null and NC | AR-Null and HFD |
| n | 8 | 8 | 5 | 4 |
| Glucose (mg/dL) | 92 ± 11 | 108 ± 15 * | 89 ± 6 | 97 ± 4 * |
| Body weight (g) | 23.9 ± 0.5 | 28.8 ± 1.1 * | 24.3 ± 0.3 | 30.0 ± 1.7 * |
| Gonadal fat pad: BW (%) | 1.3 ± 0.1 | 3.2 ± 0.6 * | 2.1 ± 0.1 | 6.0 ± 1.0 * |
| Age | 22 weeks old | |||
| n | 6 | 8 | 6 | 8 |
| Glucose (mg/dL) | 81 ± 8 | 102 ± 21 * | 81 ± 8 | 113 ± 16 * |
| Body weight (g) | 24.4 ± 0.5 | 34.2 ± 2.0 * | 27.4 ± 1.0 | 40.4 ± 1.1 * |
| Gonadal fat pad: BW (%) | 1.3 ± 0.1 | 6.2 ± 0.7 * | 2.5 ± 0.4 | 7.5 ± 0.3 * |
| Groups | WT and NC | WT and HFD | AR-Null and NC | AR-Null and HFD |
|---|---|---|---|---|
| Measurement | 12 weeks old | |||
| DAT Area | 398,367 ± 76,883 | 748,735 ± 125,439 | 481,555 ± 72,726 | 784,229 ± 161,008 |
| VAT Area | 266,800 ± 26,695 | 386,549 ± 32,881 | 353,759 ± 45,750 | 394,109 ± 65,879 |
| PVAT Area | 665,168 ± 91,534 | 1,135,285 ± 143,463 | 835,314 ± 101,186 | 1,178,337 ± 222,735 |
| WAT Cluster Area | 3459 ± 942 | 12,163 ± 7042 | 19,662 ± 7850 | 21,169 ± 13,925 |
| WAT Cell # | 37 ± 3 | 96 ± 54 | 167 ± 108 | 89 ± 58 |
| WAT Cell Area | 128 ± 22 | 198 ± 12 * | 206 ± 24 | 302 ± 60 * |
| Aorta, X-sec Area | 135,781 ± 14,048 | 156,874 ± 25,033 | 146,826 ± 18,009 | 153,411 ± 20,258 |
| 22 weeks old | ||||
| DAT Area | 461,501 ± 82,989 | 839,290 ± 191,444 | 704417 ± 136492 | 1,084,809 ± 119,937 |
| VAT Area | 290,257 ± 41,676 | 252,562 ± 19,120 | 254685 ± 115211 | 500,949 ± 66,649 |
| PVAT Area | 751,758 ± 119,178 | 1,091,852 ± 185,357 | 959102 ± 21281 | 1,585,758 ± 149,400 |
| WAT Cluster Area | 2983 ± 354 | 14,909 ± 5043 * | 15442 ± 5979 | 24,003 ± 6708 |
| WAT Cell # | 46 ± 5 | 100 ± 33 | 158 ± 70 | 96 ± 1 |
| WAT Cell Area | 103 ± 4 | 402 ± 71 * | 158 ± 16 | 511 ± 93 * |
| Aorta, X-sec Area | 171,875 ± 37,600 | 200,722 ± 65,029 | 160,636 ± 1338 | 152,541 ± 18,914 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conklin, D.J.; Haberzettl, P.; MacKinlay, K.G.; Murphy, D.; Jin, L.; Yuan, F.; Srivastava, S.; Bhatnagar, A. Aldose Reductase (AR) Mediates and Perivascular Adipose Tissue (PVAT) Modulates Endothelial Dysfunction of Short-Term High-Fat Diet Feeding in Mice. Metabolites 2023, 13, 1172. https://doi.org/10.3390/metabo13121172
Conklin DJ, Haberzettl P, MacKinlay KG, Murphy D, Jin L, Yuan F, Srivastava S, Bhatnagar A. Aldose Reductase (AR) Mediates and Perivascular Adipose Tissue (PVAT) Modulates Endothelial Dysfunction of Short-Term High-Fat Diet Feeding in Mice. Metabolites. 2023; 13(12):1172. https://doi.org/10.3390/metabo13121172
Chicago/Turabian StyleConklin, Daniel J., Petra Haberzettl, Kenneth G. MacKinlay, Daniel Murphy, Lexiao Jin, Fangping Yuan, Sanjay Srivastava, and Aruni Bhatnagar. 2023. "Aldose Reductase (AR) Mediates and Perivascular Adipose Tissue (PVAT) Modulates Endothelial Dysfunction of Short-Term High-Fat Diet Feeding in Mice" Metabolites 13, no. 12: 1172. https://doi.org/10.3390/metabo13121172
APA StyleConklin, D. J., Haberzettl, P., MacKinlay, K. G., Murphy, D., Jin, L., Yuan, F., Srivastava, S., & Bhatnagar, A. (2023). Aldose Reductase (AR) Mediates and Perivascular Adipose Tissue (PVAT) Modulates Endothelial Dysfunction of Short-Term High-Fat Diet Feeding in Mice. Metabolites, 13(12), 1172. https://doi.org/10.3390/metabo13121172








