Using Machine Vision of Glycolytic Elements to Predict Breast Cancer Recurrences: Design and Implementation
Abstract
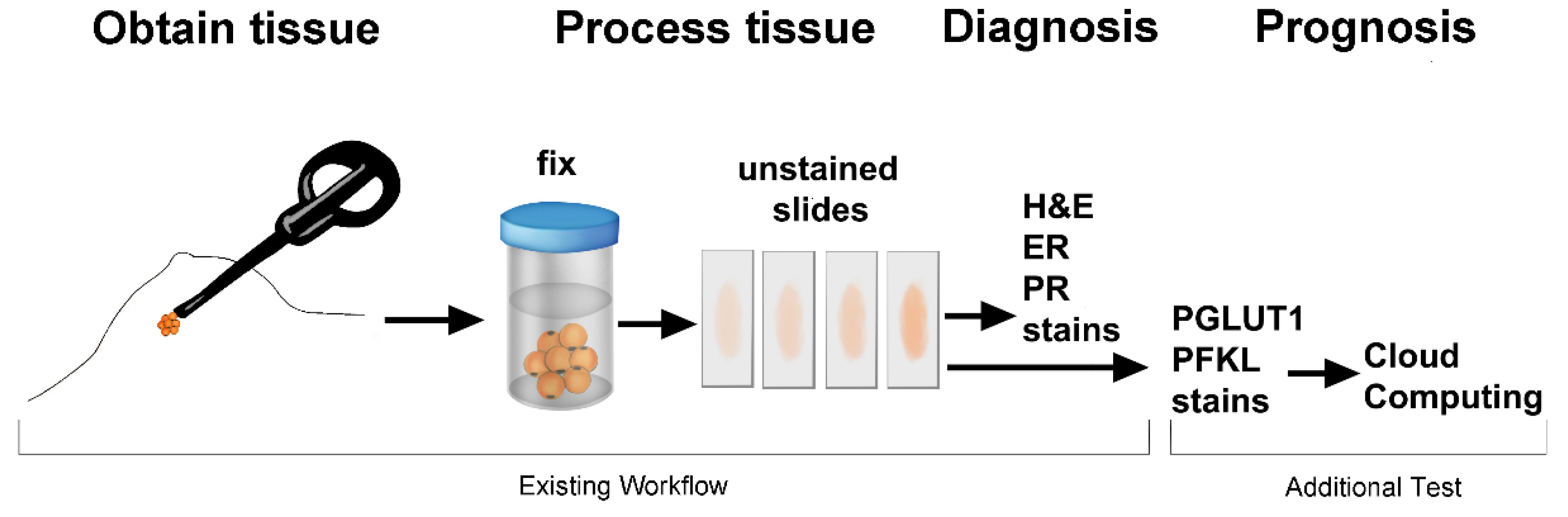
1. Introduction
2. Considerations
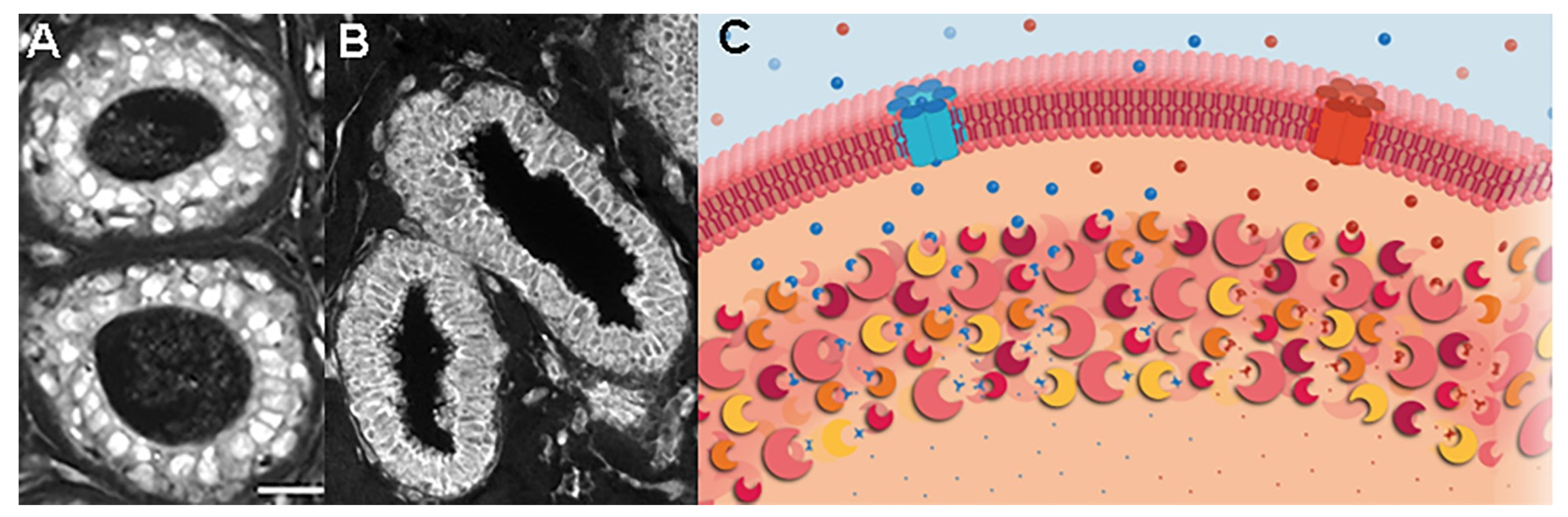
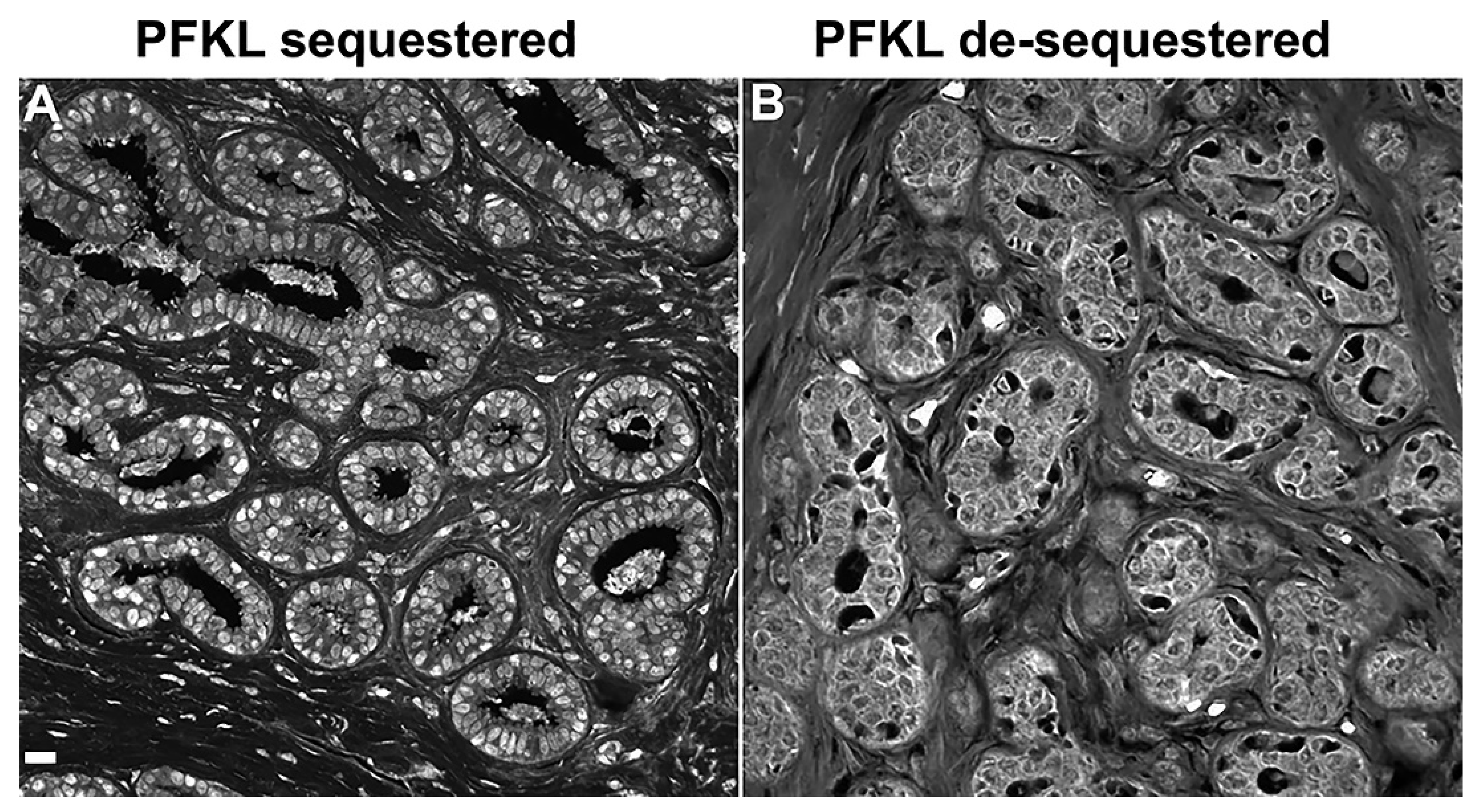
3. Unique Enzyme Patterns Are Found in Pre-Invasive Lesions Prior to Cancer Recurrences
4. Putting Cancer Recurrences into a Biological Perspective
5. Barriers to Progress
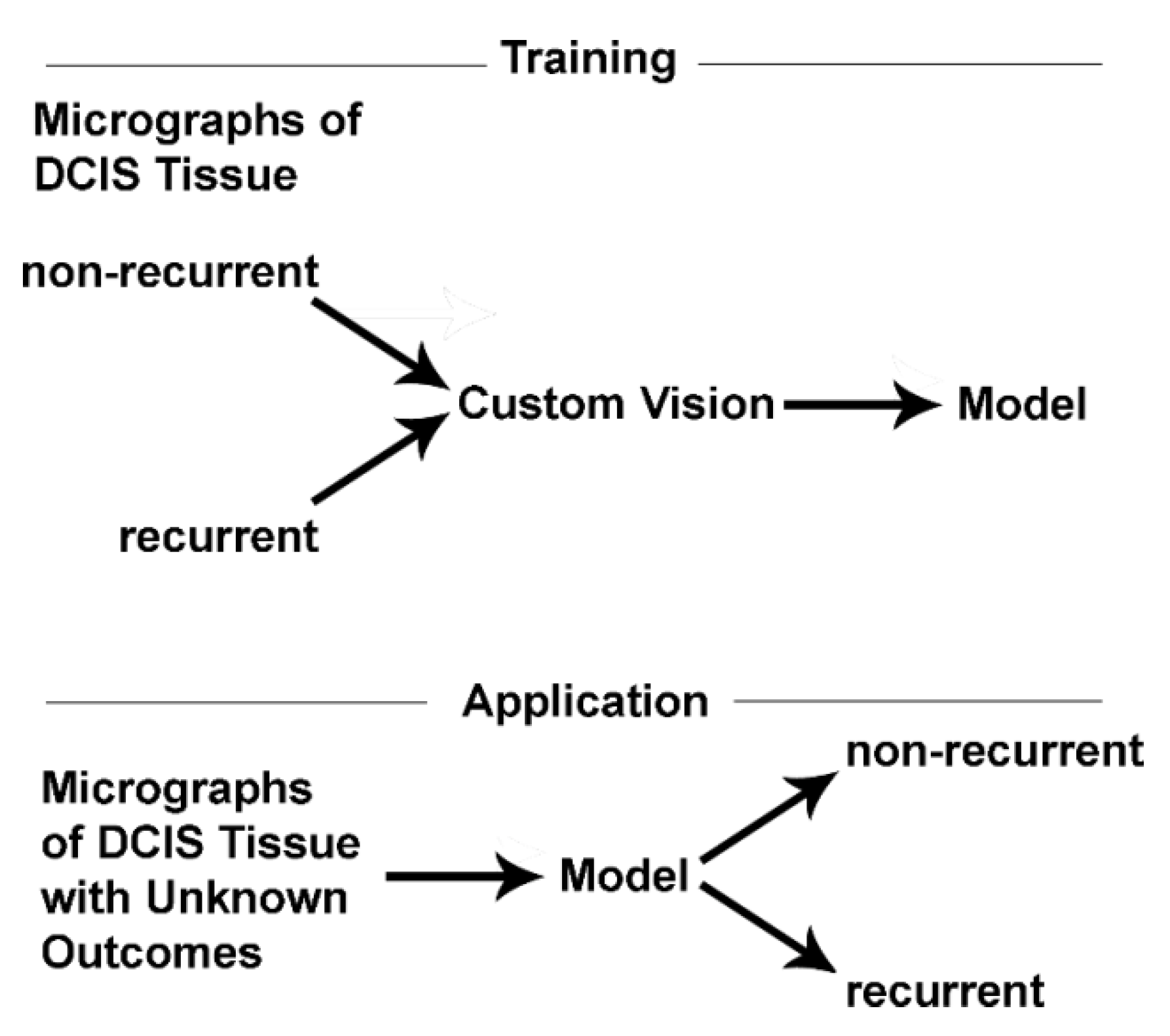
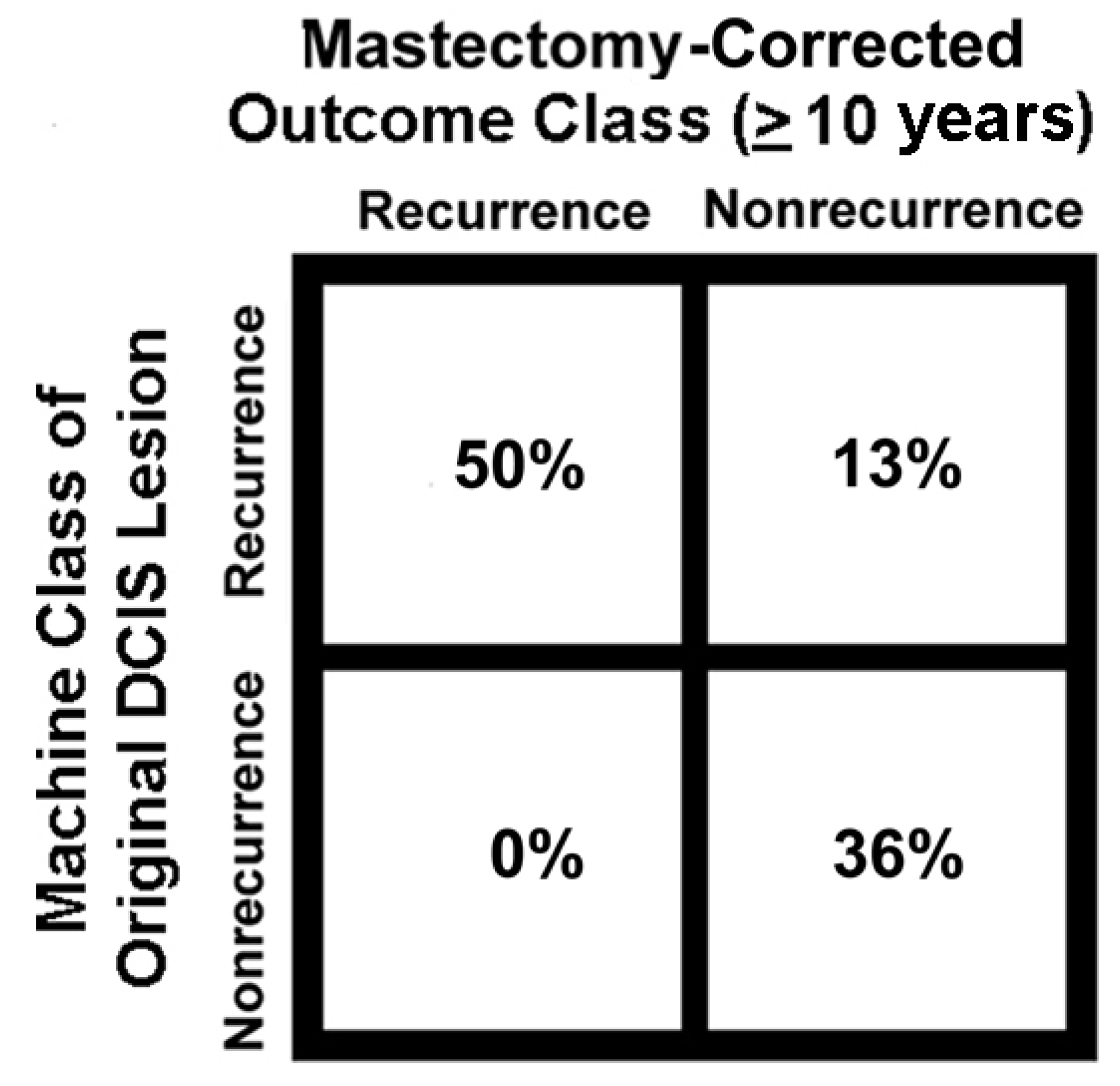
6. Potential Clinical Applications
7. Potential Research Applications
8. Conclusions
8.1. Machine Vision: An Ideal Diagnostic Tool
8.2. From Computer Bench to Beside
8.3. Applications Related to Drug Development
Funding
Conflicts of Interest
References
- Tatapudy, S.; Aloisio, F.; Barber, D.; Nystul, T. Cell Fate Decisions: Emerging Roles for Metabolic Signals and Cell Morphology. EMBO Rep. 2017, 18, 2105–2118. [Google Scholar] [CrossRef]
- Miyazawa, H.; Aulehla, A. Revisiting the Role of Metabolism During Development. Development 2018, 145, dev131110. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Adekola, K.; Rosen, S.T.; Shanmugam, M. Glucose Transporters in Cancer Metabolism. Curr. Opin. Oncol. 2012, 24, 650–654. [Google Scholar] [CrossRef]
- Zhu, A.; Lee, D.; Sim, H. Metabolic Positron Emission Tomography Imaging in Cancer Detection and Therapy Response. Semin. Oncol. 2011, 38, 55–69. [Google Scholar] [CrossRef]
- Kraft, A.M.; Petty, H.R. Spatial Locations of Certain Enzymes and Transporters within Preinvasive Ductal Epithelial Cells Predict Human Breast Cancer Recurrences. Am. J. Physiol. Cell Physiol. 2020, 319, C910–C921. [Google Scholar] [CrossRef]
- Cheung, R.A.; Kraft, A.M.; Petty, H.R. Relocation of Phosphofructokinases Within Epithelial Cells is a Novel Event Preceding Breast Cancer Recurrence that Accurately Predicts Patient Outcomes. Am. J. Physiol. Cell Physiol. 2021, 321, C654–C670. [Google Scholar] [CrossRef]
- Petty, H.R. Enzyme Trafficking and Co-Clustering Precede and Accurately Predict Human Breast Cancer Recurrences: An Interdisciplinary Review. Am. J. Physiol. Cell Physiol. 2022, 322, C991–C1010. [Google Scholar] [CrossRef]
- Petty, H.R. Prognostic Evaluation of Ductal Carcinoma In Situ Lesions Using Monoclonal Antibodies and Machine Learning. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Saatchi, Y.; Schanen, P.; Cheung, R.A.; Petty, H.R. Machine Identification of Recurrent Ductal Carcinoma In Situ Lesions: Special Emphasis on African American Women; Correspondence University of Michigan Medical School: Ann Arbor, MI, USA, 2022; submitted for publication. [Google Scholar]
- Raj, A.; van Oudenaarden, A. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell 2008, 135, 216–226. [Google Scholar] [CrossRef]
- Cronin, M.; Pho, M.; Dutta, D.; Stephans, J.C.; Shak, S.; Kiefer, M.C.; Esteban, J.M.; Baker, J.B. Measurement of Gene Expression in Archival Paraffin-Embedded Tissues: Development and Performance of a 92-Gene Reverse Transcriptase-Polymerase Chain Reaction Assay. Am. J. Pathol. 2004, 164, 35–42. [Google Scholar] [CrossRef]
- Elloumi, F.; Hu, Z.; Li, Y.; Parker, J.S.; Gulley, M.L.; Amos, K.D.; Troester, M.A. Systematic Bias in Genomic Classification Due to Contaminating Non-Neoplastic Tissue in Breast Tumor Samples. BMC Med. Genom. 2011, 4, 54. [Google Scholar] [CrossRef]
- Gyanchandani, R.; Lin, Y.; Lin, H.M.; Cooper, K.; Normolle, D.P.; Brufsky, A.; Fastuca, M.; Crosson, W.; Oesterreich, S.; Davidson, N.E.; et al. Intratumor Heterogeneity Affects Gene Expression Profile Test Prognostic Risk Stratification in Early Breast Cancer. Clin. Cancer Res. 2016, 22, 5362–5369. [Google Scholar] [CrossRef]
- Lee, E.E.; Ma, J.; Sacharidou, A.; Mi, W.; Salato, V.K.; Nguyen, N.; Jiang, Y.; Pascual, J.M.; North, P.E.; Shaul, P.W.; et al. A Protein Kinase C Phosphorylation Motif in GLUT1 Affects Glucose Transport and Is Mutated in GLUT1 deficiency syndrome. Mol. Cell 2015, 58, 845–853. [Google Scholar] [CrossRef]
- Castellana, M.; Wilson, M.Z.; Xu, Y.; Joshi, P.; Cristea, I.M.; Rabinowitz, J.D.; Gitai, Z.; Wingreen, N.S. Enzyme Clustering Accelerates Processing of Intermediates Through Metabolic Channeling. Nat. Biotechnol. 2014, 32, 1011–1018. [Google Scholar] [CrossRef]
- Hwang, E.S. The Impact of Surgery on Ductal Carcinoma in Situ Outcomes: The Use of Mastectomy. J. Natl. Cancer Inst. Monogr. 2010, 2010, 197–199. [Google Scholar] [CrossRef]
- Betsill, W.L., Jr.; Rosen, P.P.; Lieberman, P.H.; Robbins, G.F. Intraductal Carcinoma. Long-Term Follow-Up After Treatment by Biopsy Alone. JAMA 1978, 239, 1863–1867. [Google Scholar] [CrossRef]
- Erbas, B.; Provenzano, E.; Armes, J.; Gertig, D. The Natural History of Ductal Carcinoma In Situ of the Breast: A Review. Breast Cancer Res. Treat. 2006, 97, 135–144. [Google Scholar] [CrossRef]
- Page, D.L.; Dupont, W.D.; Rogers, L.W.; Landenberger, M. Intraductal Carcinoma of The Breast: Follow-Up After Biopsy Only. Cancer 1982, 49, 751–758. [Google Scholar] [CrossRef]
- Eusebi, V.; Feudale, E.; Foschini, M.P.; Micheli, A.; Conti, A.; Riva, C.; Di Palma, S.; Rilke, F. Long-Term Follow-Up of in Situ Carcinoma of the Breast. Semin. Diagn. Pathol. 1994, 11, 223–235. [Google Scholar]
- Mokbel, K.; Cutuli, B. Heterogeneity of Ductal Carcinoma in Situ and its Effects on Management. Lancet Oncol. 2006, 7, 756–765. [Google Scholar] [CrossRef]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Metabolic Coupling and the Reverse Warburg Effect in Cancer: Implications for Novel Biomarker and Anticancer Agent Development. Semin. Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef]
- Mertz, B.G.; Duriaud, H.M.; Kroman, N.; Andersen, K.G. Pain, Sensory Disturbances and Psychological Distress are Common Sequelae After Treatment of Ductal Carcinoma in Situ: A Cross-Sectional Study. Acta Oncol. 2017, 56, 724–729. [Google Scholar] [CrossRef]
- Mertz, B.G.; Duriaud, H.M.; Kroman, N.; Andersen, K.G. Pain, Sensory Disturbances, and Psychological Distress Among Danish Women Treated for Ductal Carcinoma in Situ: An Exploratory Study. Pain Manag. Nurs. 2017, 18, 309–317. [Google Scholar] [CrossRef]
- Aran, D.; Camarda, R.; Odegaard, J.; Paik, H.; Oskotsky, B.; Krings, G.; Goga, A.; Sirota, M.; Butte, A.J. Comprehensive Analysis of Normal Adjacent to Tumor Transcriptomes. Nat. Commun. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Smaglik, P. The Genetic Microscope. Nature 2017, 545, S25–S27. [Google Scholar] [CrossRef]
- Glass-Marmor, L.; Beitner, R. Taxol (paclitaxel) Induces a Detachment of Phosphofructokinase from Cytoskeleton of Melanoma Cells and Decreases the Levels of Glucose 1,6-Bisphosphate, Fructose 1,6-Bisphosphate and ATP. Eur. J. Pharmacol. 1999, 370, 195–199. [Google Scholar] [CrossRef]
- Schwartz, D.; Beitner, R. Detachment of the Glycolytic Enzymes, Phosphofructokinase and Aldolase, from Cytoskeleton of Melanoma Cells, Induced by Local Anesthetics. Mol. Genet. Metab. 2000, 69, 159–164. [Google Scholar] [CrossRef]
- Tejeda-Muñoz, N.; Mei, K.C.; Sheladiya, P.; Monka, J. Targeting Membrane Trafficking as a Strategy for Cancer Treatment. Vaccines 2022, 10, 790. [Google Scholar] [CrossRef]
- Kajiho, H.; Kajiho, Y.; Scita, G. Harnessing Membrane Trafficking to Promote Cancer Spreading and Invasion: The Case of RAB2A. Small GTPases 2018, 9, 304–309. [Google Scholar] [CrossRef]
- Goldenring, J. A Central Role for Vesicle Trafficking in Epithelial Neoplasia: Intracellular Highways to Carcinogenesis. Nat. Rev. Cancer 2013, 13, 813–820. [Google Scholar] [CrossRef]
- Grandhi, R.K.; Perona, B. Mechanisms of Action by which Local Anesthetics Reduce Cancer Recurrence: A Systematic Review. Pain Med. 2020, 21, 401–414. [Google Scholar] [CrossRef]
- Kindzelskii, A.L.; Huang, J.B.; Chaiworapongsa, T.; Fahmy, R.M.; Kim, Y.M.; Romero, R.; Petty, H.R. Pregnancy alters glucose-6-phosphate dehydrogenase trafficking, cell metabolism, and oxidant release of maternal neutrophils. J. Clin. Investig. 2002, 110, 1801–1811. [Google Scholar] [CrossRef]
- Huang, J.B.; Espinoza, J.; Romero, R.; Petty, H.R. Transaldolase is Part of a Supramolecular Complex Containing Glucose-6-Phosphate Dehydrogenase in Human Neutrophils that Undergoes Retrograde Trafficking During Pregnancy. Metabolism 2005, 54, 1027–1033. [Google Scholar] [CrossRef]
| Ductal Epithelial Cells | Additional Tissue Sites |
|---|---|
| Metabolic platforms | Myoepithelial cells |
| Nucleus/nucleoli | Blood vessels |
| Cytoplasmic vesicles/enzyme clumps | Tumor-associated fibroblasts |
| Diagnosis | Prognosis | Possible Action | |
|---|---|---|---|
| Computed Recurrence Prediction | |||
| Lesion | Normal Adjacent | ||
| Tissue | |||
| Atypical Ductal Hyperplasia | - | - | none |
| + | - | partial mastectomy | |
| DCIS | - | - | none |
| + | - | partial mastectomy | |
| + | + | full mastectomy | |
| Agents | Actions |
|---|---|
| Taxol | dissociates PFK from cytokeleton |
| KU55933 | blocks GLUT1 translocation |
| Colchicine | disrupts microtubules, intracellular trafficking |
| Local anesthetics | disrupts intracellular trafficking; dissociates enzymes from cytoskeleton |
| Prenylation inhibitor | disrupts membrane association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petty, H.R. Using Machine Vision of Glycolytic Elements to Predict Breast Cancer Recurrences: Design and Implementation. Metabolites 2023, 13, 41. https://doi.org/10.3390/metabo13010041
Petty HR. Using Machine Vision of Glycolytic Elements to Predict Breast Cancer Recurrences: Design and Implementation. Metabolites. 2023; 13(1):41. https://doi.org/10.3390/metabo13010041
Chicago/Turabian StylePetty, Howard R. 2023. "Using Machine Vision of Glycolytic Elements to Predict Breast Cancer Recurrences: Design and Implementation" Metabolites 13, no. 1: 41. https://doi.org/10.3390/metabo13010041
APA StylePetty, H. R. (2023). Using Machine Vision of Glycolytic Elements to Predict Breast Cancer Recurrences: Design and Implementation. Metabolites, 13(1), 41. https://doi.org/10.3390/metabo13010041








