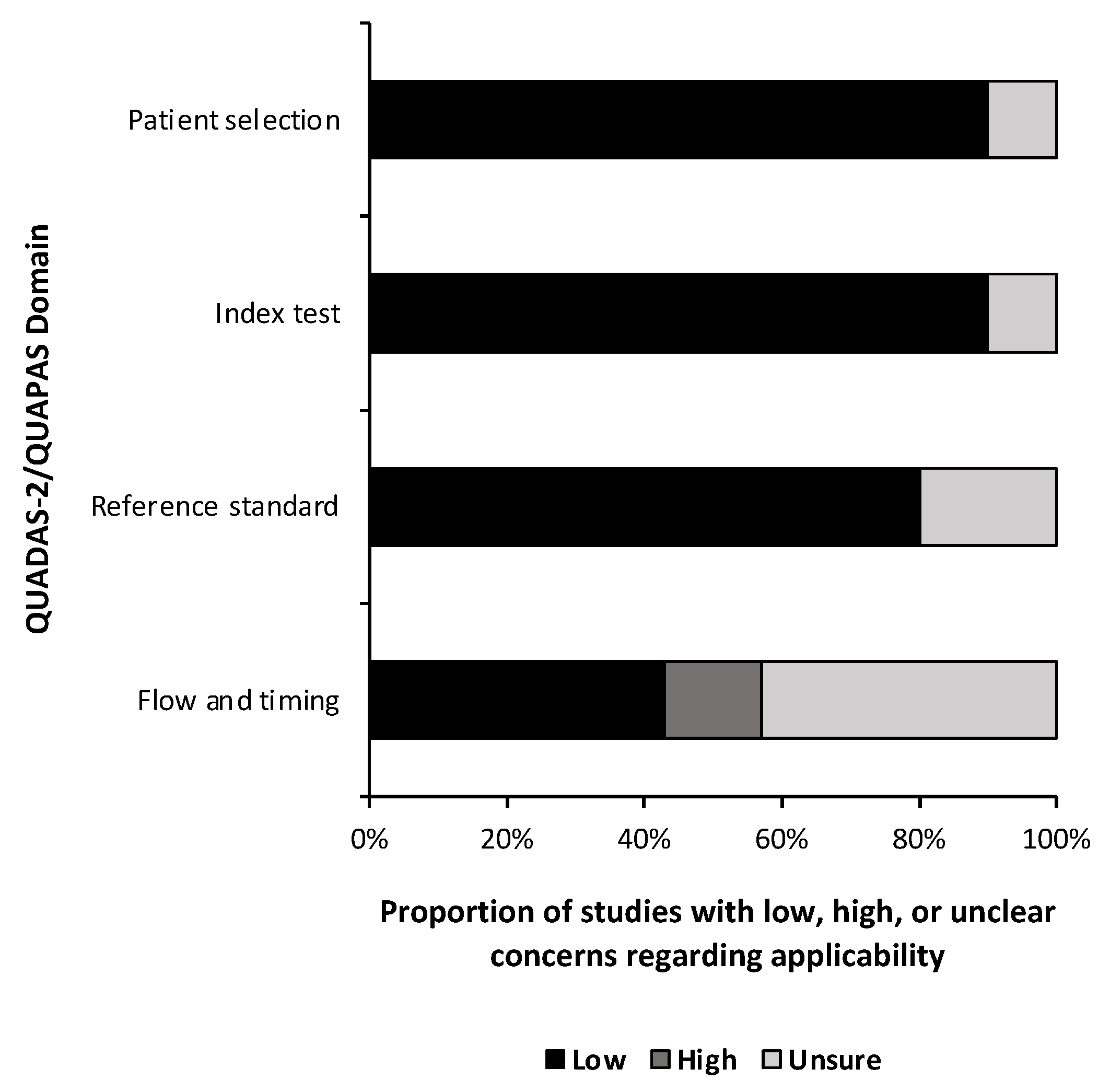Abstract
Glioblastoma (GBM) is a common and deadly brain tumor with late diagnoses and poor prognoses. Machine learning (ML) is an emerging tool that can create highly accurate diagnostic and prognostic prediction models. This paper aimed to systematically search the literature on ML for GBM metabolism and assess recent advancements. A literature search was performed using predetermined search terms. Articles describing the use of an ML algorithm for GBM metabolism were included. Ten studies met the inclusion criteria for analysis: diagnostic (n = 3, 30%), prognostic (n = 6, 60%), or both (n = 1, 10%). Most studies analyzed data from multiple databases, while 50% (n = 5) included additional original samples. At least 2536 data samples were run through an ML algorithm. Twenty-seven ML algorithms were recorded with a mean of 2.8 algorithms per study. Algorithms were supervised (n = 24, 89%), unsupervised (n = 3, 11%), continuous (n = 19, 70%), or categorical (n = 8, 30%). The mean reported accuracy and AUC of ROC were 95.63% and 0.779, respectively. One hundred six metabolic markers were identified, but only EMP3 was reported in multiple studies. Many studies have identified potential biomarkers for GBM diagnosis and prognostication. These algorithms show promise; however, a consensus on even a handful of biomarkers has not yet been made.
1. Introduction
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in the United States, accounting for approximately 56.6% of all gliomas and 47.7% of all primary malignant central nervous system tumors [1]. GBM is 1.58 times more common in males than females, and the annual incidence of GBM is 2.53 per 100,000 population. The highest rate of diagnosis falls with the group aged 75 to 84 years; however, the median age of diagnosis is 65 years. Globally, the incidence is highest in North America, Northern and Western Europe, and Australia [2]. When accounting for race and ethnicity, incidence rates are highest among non-Hispanic whites and lowest among American Indians and Alaskan Natives. Furthermore, 1- and 5-year survival rates are lowest among non-Hispanic whites and highest among American Indians and Alaskan Natives [3].
The prognosis of GBM is notably grim, with a 1-year relative survival rate of 41.4% and a 5-year survival rate of 5.8% following diagnosis [1,3,4]. Negative prognostic factors include advanced age, incomplete resection, and poor mental performance status while the inverse of these factors each indicates a slightly better prognosis [4]. Furthermore, biomarkers indicating isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations indicate a longer survivability [5].
Currently, no screening method for GBM prior to clinical presentation exists. Only once clinical symptoms are present does magnetic resonance imaging (MRI) become the gold standard for diagnosis [6]. This lack of reliable screening leads to diagnoses late into the progression of cancer. The development of techniques leading to early detection of GBM may play an integral role in improving patient outcomes following diagnosis. Current research suggests that early interventions with surgical resection, radiation therapy, and pharmacological targeting of the neoplasm may improve patient outcomes [7]. Additional research suggests that early resection of the tumor may play a role in preventing disease progression as GBM tumors display rapid early progression (REP) indicating that early phases of tumor growth are crucial to the growth of the neoplasm [8]. The identification of metabolic biomarkers such as IDH, platelet-derived growth factor (PDGF), and epidermal growth factor receptor (EGFR) provides an opportunity for early detection of risk factors and prognostic factors relating to GBM [6].
Traditional cancer diagnoses are determined by a physician via clinical, imaging, and population-based data, with confirmation via histology upon biopsy or autopsy [9]. Recently, machine learning (ML, a subset of artificial intelligence [AI]) is improving the diagnostic and prognostic processes for various cancers [10,11,12,13]. Machine learning is a method of teaching computers to learn from data without explicit programming. Instead, algorithms are fed massive amounts of training data to identify patterns and make classifications and predictions about new, untested data. This is accomplished by creating mathematical models that can learn from existing data and then use these models to predict new data.
The first mention of AI was in 1956 during a seminar at Dartmouth College [14,15]. Decades later, ML was born in the mid-1980s after Valiant’s theory of the learnable (1984) and Hopfield’s neural network model of associative memory (1982) first connected statistical mechanics to learning theory, thereby replacing the previous AI approach centered on logic and rules [16]. The first applications of ML in neurosurgery began in the 1990s [17]. An early proof-of-concept by Floyd et al. in 1992 demonstrated that artificial neural networks (ANN) could outperform human efforts in detecting circular lesions in stimulated single-photon emission CT imagery [17,18]. In 1995, ML was utilized in a study by Christy et al. for grading and distinguishing between high and low-grade supratentorial astrocytomas [17,19]. The results of this study, though nonsignificant, further demonstrated the diagnostic capabilities of ML with an accuracy of 61% when compared to 57% for neuroradiologists [17,19].
Cancer gene expression has been a prominent focus of ML in addition to MRI/CT imaging analysis, cancer susceptibility testing, radiation resistance, mortality risk percentages, and tumor grade [10,20,21,22,23]. The ML techniques commonly utilized within cancer research include ANN, K-nearest neighbors (KNN), Bayesian network (BN), Naïve Bayes (NB), support vector machine (SVM), and decision trees (DT). Of these approaches, ANNs, KNN, and SVMs exhibit popular use among researchers working to ascertain a cancer diagnosis [9,20]. Traditional ML methods including DTs, KNNs, NB, and SVMs are more simplistic, which results in greater computational speed, efficiency, and cost savings [20]. Past attempts to diagnose GMB include a notable two-stage ML-based study that created a multimodel, multichannel predictive model consisting of a convolution neural network (CNN) (a subset of ANN) connected to an SVM. The two systems analyzed MRI images (T1, diffusion tensor imaging, and resting state MRI) along with tumor histology and patient age. This ML model projected preoperative high-grade glioma survival rates that were 90.66% accurate (N = 68) [24].
The exponential advancement of AI has led to the creation of ML models that utilize electronic health record data to achieve a 60% positive predictive value (PPV) for a 3-month mortality rate in an advanced cancer population (N = 2041) prognostic algorithm [25]. The algorithm’s PPV markedly surpassed the 34.8% PPV attained by oncologists and advanced practice clinicians [25]. For diagnostic applications, a study from Zhou et al. incorporated liquid chromatography and mass spectrometry into an SVM-based ML algorithm to diagnose malignant brain gliomas (MBGs) by means of plasma lipid biomarker analysis. This method was shown to be a reliable noninvasive screening method for the diagnosis of MBGs [26]. The enhancement of diagnostic/prognostic methods integrated with ML algorithms allows physicians to assign patients more accurate prognoses for the expeditious implementation of treatment plans and conceivably better patient care as the technology continues to improve [4,10].
The heterogeneous nature of GBM along with high rates of re-incidence and therapeutic resistance necessitate the timely identification of novel therapeutic targets in the metabolism of GBM to remain ahead of this rapidly evolving disease [27]. Recent efforts to identify such targets have utilized tumor omics data integrated with clinical information by use of ML techniques [27]. However, there is still a paucity of literature concerning GBM metabolism and ML. To our knowledge, there has been no review on ML and GBM metabolism. Therefore, in this review, the authors systematically search the literature on ML and GBM metabolism and assess recent advancements with commentary on future developments in this novel and the emerging field of study.
2. Methods
2.1. Strategy and Registration
This study was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines. This systematic review was registered on PROSPERO, with details of our initial protocol, and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022367758 (accessed on 25 December 2022) [28].
2.2. Search and Data Sources
A literature search was performed using PubMed (Medline), Embase, Cochrane, OVID, and Web of Science databases from 1975 to October 2022. The following predetermined search terms were used: “metabolism” or “biomarkers” and “glioblastoma” and “artificial intelligence” or “machine learning” or “deep learning” or “predictive model” as title and abstract keywords (Supplementary Materials S1).
2.3. Selection Criteria
Articles obtained from searching the specified databases were imported into the Covidence platform (Veritas Health Innovation) for screening. The screening was independently performed by two investigators (Z.D.N. and C.B.) by title and abstract, and later by full-text review. Conflicts were resolved by consensus. When consensus could not be obtained, a third reviewer (N.P.) broke the tie. Articles describing the use of an ML algorithm for GBM metabolism were included. Additional inclusion criteria included research on human GBM metabolism and a confirmatory diagnosis of GBM for validity. Review articles, case reports, commentary, conference abstracts, unpublished articles, editorials, and purely technical descriptions were excluded. The language was restricted to English.
2.4. Data Extraction
Using a data extraction form, a quality and bias assessment was performed based on the quality assessment of diagnostic accuracy studies 2 (QUADAS-2) [29] for diagnostic studies and the quality assessment of prognostic accuracy studies (QUAPAS) [30] for prognostic studies. QUAPAS is adapted from QUADAS-2 and thus, the two can be compared together. Studies that featured both diagnostic and prognostic models were assessed with both assessments and combined. All studies were also assigned a level of evidence rating based on the American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) joint guidelines for diagnostic and prognostic studies organized into classes (I, II, and III) [31].
The following variables were extracted from the included studies: publication year, lead author, country of origin, study population, the origin of patient data (original or database), type of ML algorithm used, and source of biologic sample (plasma, tissue, etc). Our primary outcome was the accuracy of the ML algorithm. The secondary outcome, if reported, included the top metabolic markers identified by each study.
3. Results
3.1. Search Results
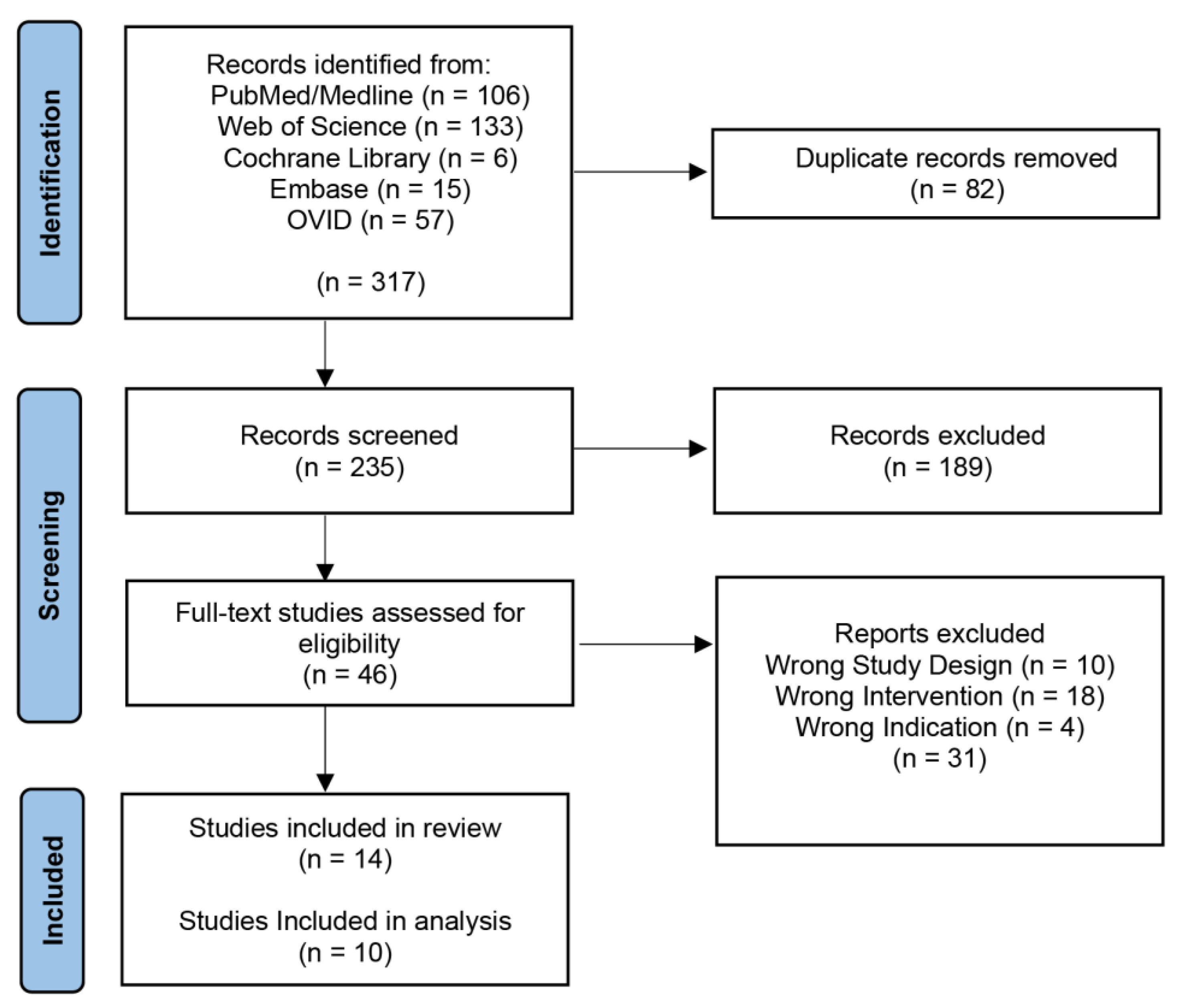
Our initial search yielded 317 records with 235 articles remaining after the removal of duplicates (Figure 1). These articles were screened by title and abstract, which returned 46 articles for a full-text review. Thirty-one articles were excluded, leaving 14 final studies included in this review, ten of which were included in the analysis [32,33,34,35,36,37,38,39,40,41]. Four studies were not included in the analysis because they did not perform an ML algorithm on GBM; however, they did discuss the topic [21,42,43,44,45,46].
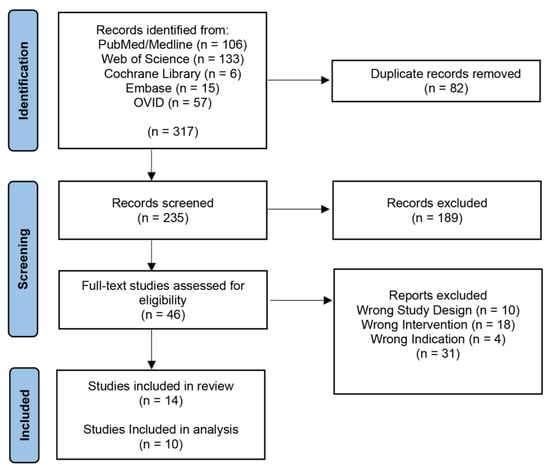
Figure 1.
PRISMA Diagram. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram representing the screening process.
Extraction results from each paper are tabulated below (Table 1). All of the ten analyzed papers were published on or after 2018, except one (2012). The nationalities of the papers were as follows: one Canadian, four Chinese, and one Indian, Pakistani, Polish, Swiss, and American. Three papers (30%) were diagnostic, six were prognostic (60%), and one featured both (10%).

Table 1.
Data extracted from ten studies were included for analysis. ACE (atlas correlation explorer), ANN (artificial neural network), BPNN (backpropagation neural network), AUC of ROC (area under the curve of the receiver operating characteristic curve), CGGA (Chinese glioma genome atlas), CNN (convolutional neural network), GEO (gene expression omnibus), LASSO (least absolute selection and shrinkage operator), NN (neural network), PASNet (pathway-associated sparse deep neural network), PCA (principal component analysis), PLS-DA (partial least-squares discriminant analysis), RSF-SRC (random survival forest–survival regression and classification), SVM (support vector machine), TCGA (The Cancer Genome Atlas), XGBoost (eXtreme gradient boosting).
3.2. Quality and Bias and Level of Evidence
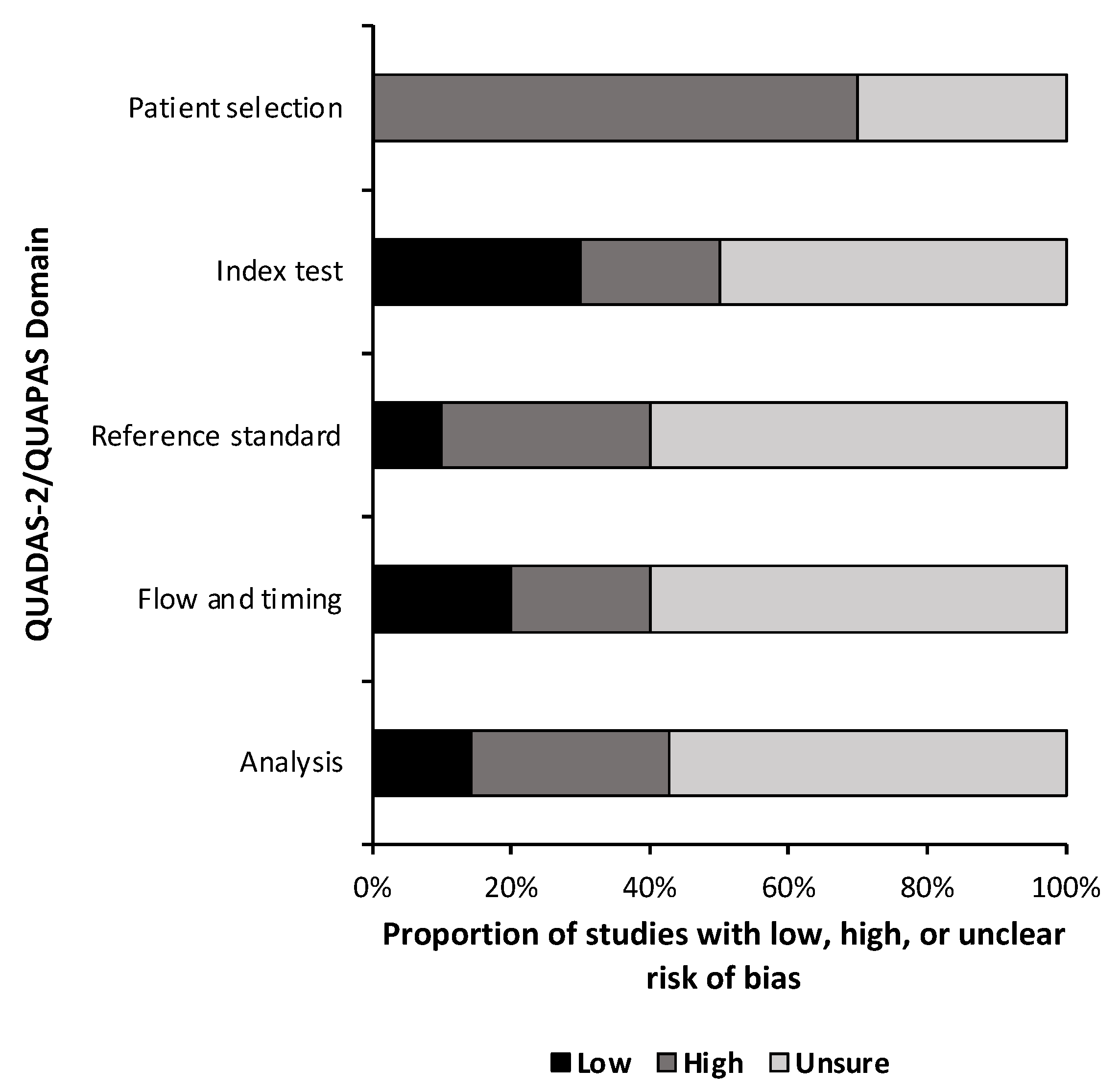
A quality and bias assessment was performed as described above with results tabulated in the Supplementary Materials (Table S1). Weakness in the included studies was most significant for the lack of data in the study design. Fifty-one percent (n = 24) of risk of bias questions were answered as “unsure” due to insufficient information (Figure 2). Patient selection was also a substantial source of bias as most studies used some form of a national database with little information provided on the patient population or control population if used. Conversely, 78% (n = 29) of applicability concern questions were rated as “low” risk of bias (Figure 3). Nine studies (90%) received a level of evidence rating of II, while one study (10%) received a rating of I, based on AANS and CNS joint guidelines for diagnostic and prognostic studies.
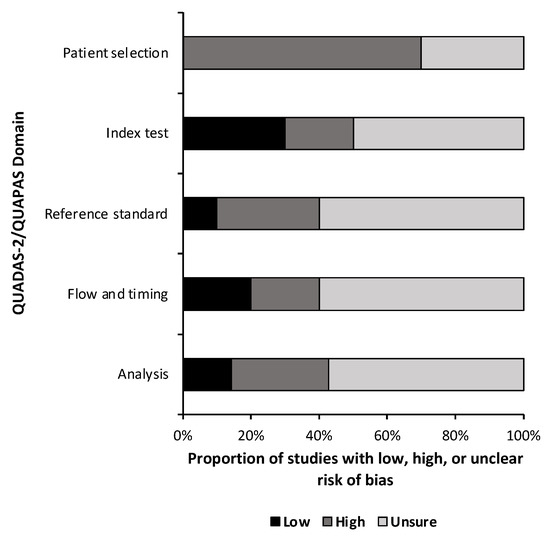
Figure 2.
Risk of Bias. Risk of bias assessment summary based on quality assessment of diagnostic accuracy studies 2 (QUADAS-2) for diagnostic studies and the quality assessment of prognostic accuracy studies (QUAPAS) for prognostic studies.
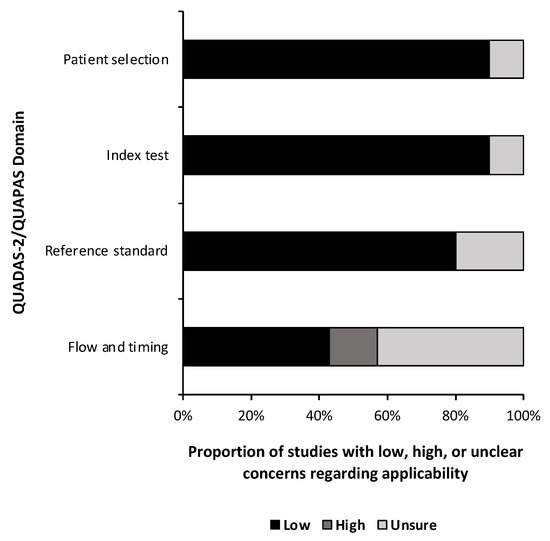
Figure 3.
Risk of bias. Risk of bias applicability assessment summary based on quality assessment of diagnostic accuracy studies 2 (QUADAS-2) for diagnostic studies and the quality assessment of prognostic accuracy studies (QUAPAS) for prognostic studies.
3.3. Patient Samples and Databases
Samples were collected from patients as either tumor/healthy brain tissue (n = 7, 70%) or serum/plasma (n = 3, 30%). Most studies analyzed data from multiple national databases such as The Cancer Genome Atlas (TCGA) (National Institutes of Health) (n = 6, 60%), Chinese Glioma Genome Atlas (CGGA) (Beijing Neurosurgical Institute) (n = 2, 20%), Gene Expression Omnibus (GEO) (National Institutes of Health) (n = 3, 30%), as well as from original samples (n = 5, 50%). However, only three articles (30%) analyzed data from multiple sources. At least 2536 data samples were run through an ML algorithm; however, due to the usage of the same databases by multiple papers at various points of database completeness, an exact number of unique samples could not be determined.
3.4. Machine Learning and Accuracy
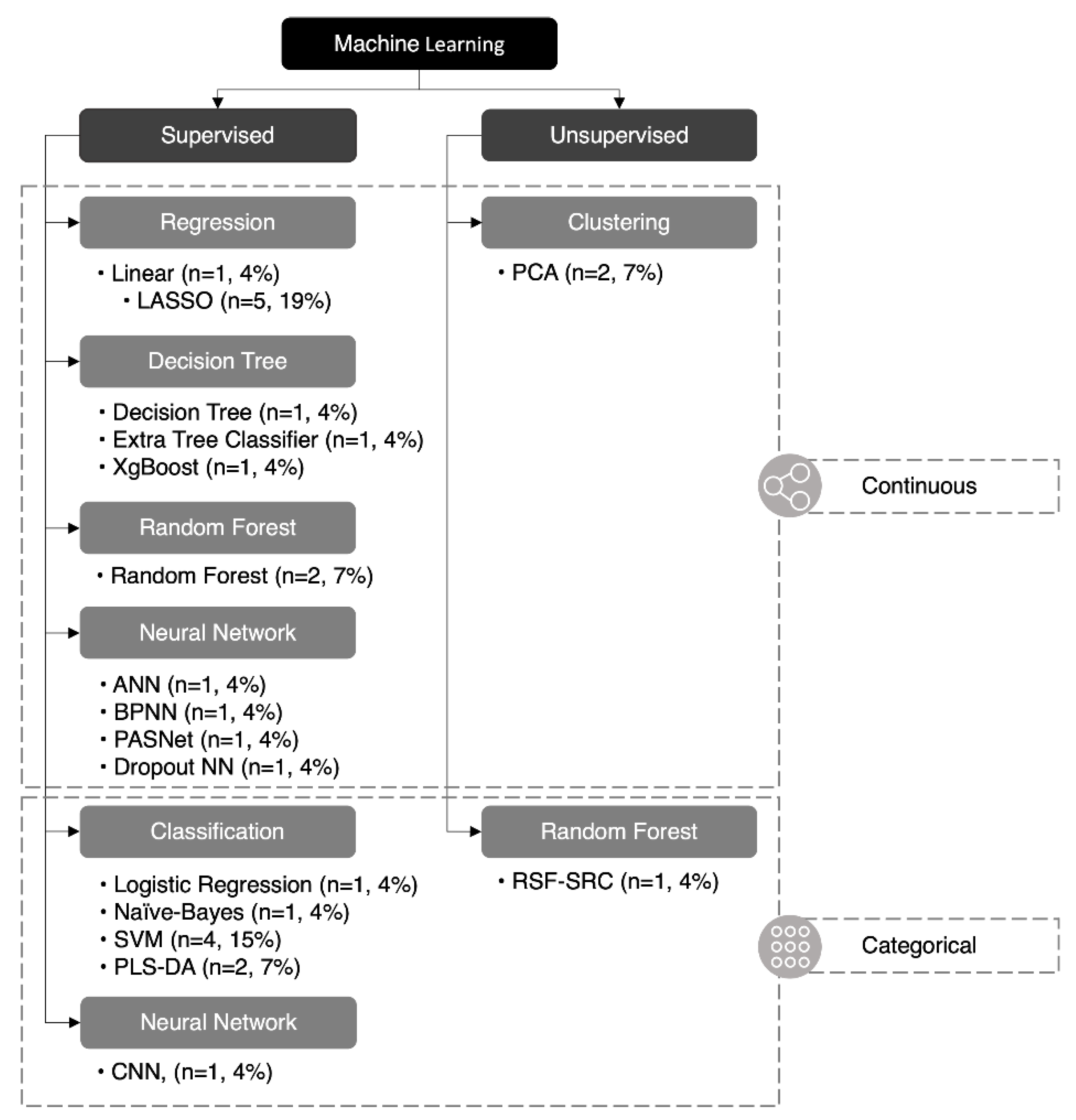
Twenty-seven ML algorithms were found in our analysis, 18 of which were unique (67%) (Figure 4). The least absolute selection and shrinkage operator (LASSO) and support vector machine (SVM) were the two most common methods and were featured in five and four studies, respectively. A mean of 2.7 ML methods was utilized in each study; however, only 50% (n = 5) of papers featured more than two methods of ML. Of the 27 ML methods used, 24 were supervised (89%) and three were unsupervised (11%), while 19 were continuous (70%) and eight were categorical (30%). A summary of each ML method is listed in (Table 2).
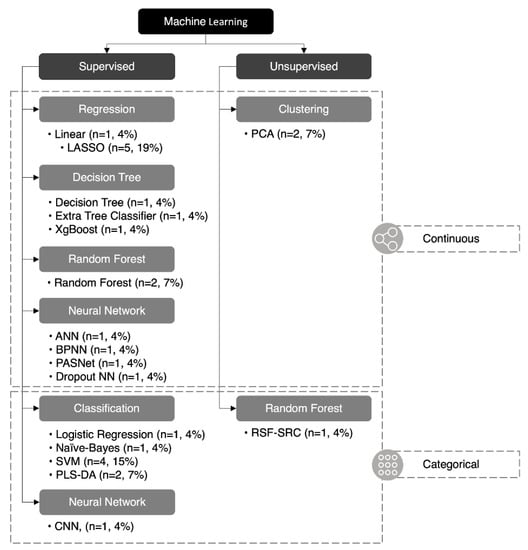
Figure 4.
Classification of machine learning algorithms. Twenty-seven machine learning algorithms reported in this paper were classified: 24 were supervised (89%) and three were unsupervised (11%), 19 were continuous (70%), and eight were categorical (30%). ANN (artificial neural network), BPNN (backpropagation neural network), CNN (convolutional neural network), LASSO (least absolute selection and shrinkage operator), NN (neural network), PASNet (pathway-associated sparse deep neural network), PCA (principal component analysis), PLS-DA (partial least-squares discriminant analysis), RSF-SRC* (random survival forest–survival regression and classification), SVM (support vector machine), XGBoost (eXtreme gradient boosting). *RSF-SRC can be unsupervised or supervised depending on the variation.

Table 2.
A summary of each ML method is listed in these studies. This includes methods that were mentioned; however, some may not have been utilized, but are still included for educational purposes [32,33,34,35,36,37,38,39,40,41]. * Duplicate methods omitted.
Table 2.
A summary of each ML method is listed in these studies. This includes methods that were mentioned; however, some may not have been utilized, but are still included for educational purposes [32,33,34,35,36,37,38,39,40,41]. * Duplicate methods omitted.
| Machine Learning Algorithm | Definition |
|---|---|
| Linear Regression (ACE) | Linear regression is a type of supervised ML algorithm used for predictive modeling. It is used to match observed data with a linear equation to model the correlation between the independent variables and the dependent variable [45]. ACE is a linear regression algorithm specifically designed for use with gene expression data. |
| Logistic Regression | Logistic regression is a statistical method that we use to construct a regression model when the response variable is in binary. It is integrated into a supervised machine learning algorithm to hypothesize an outcome along with a binary response (e.g., Yes/No, True/False) using a set of independent variables [46]. |
| Random Forest * | Random forest is a supervised ML method that creates decision trees and combines them to improve the accuracy of the predictions. It uses a technique called bagging, where each tree is trained on a random grouping of the data [45]. |
| Extra Tree Classifier | Extra tree classifier is a supervised ML algorithm that utilizes a decision tree-based ensemble method. It operates by constructing a set of decision trees and then training them with a random subset of the features. The final class prediction is created by combining all of the trees’ individual class predictions. Extra tree uses more randomization when splitting nodes than is seen in a random forest algorithm [47]. |
| Decision Tree | A decision tree is a type of supervised ML algorithm that is used for classification and regression. It makes predictions based on the feature values of input instances by constructing a tree-like model of decisions and their possible consequences [45]. |
| SVM (Support Vector Machines) * | Support vector machines is a supervised ML algorithm that is used for classification and regression. It works by finding the best boundary (or “hyperplane”) that separates the different classes [45]. |
ANN (Artificial Neural Networks)
| ANN is a class of supervised ML algorithms that are modeled after human neuronal structure and can be applied to a variety of tasks, including the classification of images and the processing of natural language. They are made up of interconnected artificial neurons that can be trained to adjust the weights of connections between nodes. They can use a variety of architectures, including feedforward, convolutional, and recurrent neural networks [45].
|
| XGBoost (eXtreme Gradient Boosting) | XGBoost is a gradient-boosting supervised ML algorithm designed to be efficient and scalable. It is used for supervised ML problems, and it can be used for both classification and regression [51]. |
| K-Means | K-Means is an unsupervised ML clustering algorithm that groups similar n-dimensional observations into k clusters, where k is predefined. The algorithm repetitively assigns points to the closest centroid and updates the centroid based on the mean of assigned points [52]. |
LASSO (Least Absolute Selection and Shrinkage Operator) *
| LASSO is a supervised regularization method for linear regression models. LASSO’s priority is to decrease the absolute values of the independent variable coefficients toward zero. It helps to prevent overfitting by reducing the model’s complexity [53].
|
| PCA (Principal Component Analysis) * | PCA is an unsupervised dimensionality reduction technique. The intention is to convert a group of correlated factors into a group of uncorrelated factors. It does this by switching the data to a new coordinate system. The axis then represents the direction of maximum variance in the data [57]. |
| RSF-SRC (Random Survival Forest–Survival Regression and Classification) | RSF-SRC is a potentially unsupervised ML method for predicting the time-to-event (TTE) outcome in survival analysis (other variations may be supervised). It is an extension of the random forest algorithm, can handle censoring and truncation of time-to-event data, and can be used for both regression and classification [58]. |
| PLS-DA (Partial Least-Squares Discriminant Analysis) | PLS-DA is a supervised ML algorithm that is used for classification. It works by finding a group of latent variables, which are linear combinations of the original variables, and that explain the differences between various different classes [59]. |
| Naïve Bayes | Naïve Bayes is a supervised ML algorithm that is used for classification. It makes predictions based on the probability of certain features appearing in each class. It is called “naïve” because it assumes that all features are independent, which may not always be true [45]. |
Nine algorithms (33%) reported accuracy values and 18 (67%) reported area under the curve of the receiver operating characteristic (AUC of ROC) values, while only 6 (22%) reported neither. Due to the unverifiable nature of unsupervised ML, only accuracy or AUC of ROC values not reported for supervised ML methods were considered “missing.” Only one paper met this criterion [33]. The mean reported accuracy was 95.63% [85.70%, 100.00%], while the mean AUC of ROC was 0.779 [0.590, 1.000].
3.5. Metabolic Markers
One hundred six metabolic markers were identified as the top predictive biomarkers from the analyzed studies. Of these, 23 (22%) were used for diagnosis and 83 (78%) were used for prognostication. Only one metabolic marker, EMP3, was reported in multiple studies; all other biomarkers were reported only once in their respective studies.
4. Discussion
Despite the innovations within the field of GBM research, prognoses remain poor. The studies within this review aim to improve diagnostic and prognostic accuracy by utilizing novel ML algorithms. Although this field exhibits an extensive level of research, there is a paucity of literature pertaining to the ML algorithms used to identify markers underlying GBM metabolism [42].
4.1. Supervised Machine Learning
Supervised ML is broadly used in a predictive scenario where a “ground truth” value can be determined (e.g., a diagnosis of GBM) and the user wishes to identify similar data sets with an unknown “ground truth.” The supervised ML algorithms used by these studies were SVM, random forest, ANN, deep neural networks (e.g., PASnet), DT, NB, partial least-squares discriminant analysis (PLS-DA), logistic regression models, and LASSO-penalized Cox regression analysis [32,34,35,36,37,38,39,40,41,42,43]. A logistic regression model appears to outperform other ML algorithms in classification systems, in this case, the classification of the IDH mutation. The algorithms it outperformed were other supervised ML models such as SVM and random forest models. Specifically, the logistic regression model obtained greater results, which were determined by its performance in determining the AUC of ROC, Bal accuracy, F1 score, precision, recall, and Matthew’s correlation coefficient (MCC) [43].
Supervised ML algorithms are powerful tools in the identification of GBM biomarkers. One study found that by extracting a small amount of peripheral blood (5 µL), a surface-enhanced Raman scattering (SERS) signal-trained supervised ML algorithm was able to distinguish GBM cancer from noncancer without isolating cells. The PLS-DA algorithm exhibited both high sensitivity and specificity. A confirmation test with an ANN validated the previous outcome, and the ANN was crucial in determining the prognosis of the disease [32]. Congruently, Gollapalli et al. used a PLS-DA algorithm to distinguish between GBM patients and healthy controls using predetermined biomarker subsets to discern a high level of classification. Results from this study were confirmed with DT, SVM, and NB algorithms [41].
4.2. Unsupervised Machine Learning
Unsupervised ML techniques are generally used when a user wishes to understand and perhaps categorize their data, without knowing their primary data “ground truth.” The unsupervised ML algorithms used by these studies were K-means and an integrated Kernel PCA. Unsupervised ML methods such as K-means have been used to create continuous clustering models. These models use metabolism-related genes to create stratified clusters with calculated similarity distances between GBM samples [37]. Furthermore, deep neural networks (e.g., PASnet) have been integrated into prediction models, along with kernel principal component analysis (KPCA), as methods to forecast prognostic survival analyses from high-throughput data [39]. The literature on deep learning networks in GBM metabolism is sparse, likely due to the complicated methodology involved in the construction of these algorithms. Sometimes, a combination of both unsupervised and supervised ML is useful. Riviere-Cazaux et al. contrasted the heterogeneity between GBM patients based on IDH status and patient identity. The team utilized PCA for an unbiased evaluation of patient groupings, followed by PLS-DA to identify the predictive variables between the groups. This is a quality example of how several variants of different algorithms can work together harmoniously to achieve results [44].
4.3. Metabolic Markers
A major prognostic metabolic marker researched throughout these studies is isocitrate dehydrogenase-1 (IDH1), which when correlated with various mutations of that marker, as well as when utilized for the characterization of patients, was found to be overexpressed in both high- and low-grade GBM patients [33,37,40]. The type of IDH1 (wild-type vs. mutation) was found to impact the degree of prognosis between different unsupervised clusters of patients, implicating it as a possible prognostic marker, although it should be mentioned that there was a statistically significant difference in age between these clusters [35]. Moreover, IDH status was a risk factor identified as one of the prognostic classifiers with a statistically significant high hazard ratio [40]. Additionally, several studies emphasized the importance of matching metabolic pathway markers with associated genetic alterations in a stepwise fashion to predict the prognosis of GBM patients with greater accuracy [37,39]. The emphasis of ML identification on IDH1 overexpression in the progression of GBM gives credence to an antimetabolic approach, as decreasing the activity of this pathway could impair the growth and development of GBM tumors [42]. However, IDH1 and its role in GBM metabolism has been heavily researched in the literature and is not a new discovery based on these ML papers currently being discussed. Rather, many of these papers used IDH1-positive samples as a starting point for further analysis with ML algorithms.
Various alternate metabolic markers of importance are the levels of dysregulated amino acids. These amino acids have been identified as a product of activated or deactivated metabolic pathways in GBM to increase nutrient availability for tumors [34]. Many of those amino acids were discovered in previous experiments and then analyzed by ML in these studies to differentiate patients’ glioma grading, thereby ascertaining a method for a more precise diagnosis [34,42]. In fact, Firdous et al. found that their diagnostic study utilizing an extra tree classifier, logistic regression integration, and random forest algorithms had greater predictive accuracy than any other previous studies of ML algorithms on the identification of metabolic markers in tissue or liquid-based biopsies [34].
A study conducted by Zeng et al. found that UDP glucose phosphorylase-2 (UGP2) was an upregulated enzyme that exhibited a significant effect on the prognosis of GBM. They identified this marker using a random survival forest algorithm, which is a type of supervised ML method. The overexpression of UGP2 was correlated with a worse prognosis and a higher grade of pathology. As a result, UGP2 may be a useful prognostic marker for GBM patients [38].
In addition, Kałuzińska et al., utilized multiple SVM algorithms to classify the top genes present in multiple types of cancers, including GBM. The team concluded that WWOX-dependent biomarkers PLEK2 and GCSH are possible GBM biomarkers and should serve as a triad along with RRM2. Further investigation is needed pertaining to PLEK2 and GCSH to analyze their prognostic accuracy and ability to differentiate between GBM versus alternative gliomas [36].
Lastly, several independent studies have identified EMP3 as a prognostic gene for high-grade gliomas [35,40]. It has been shown to function as a reliable indicator for prognosis at the mRNA level [40]. In fact, EMP3 was the only gene identified in more than one study. As such, further research involving this genetic marker has the potential to improve the prognostic process for patients diagnosed with glioblastoma.
5. Future Directions
Machine learning has the ability to greatly improve the prognostic and diagnostic capabilities of GBM. However, an integration of ML algorithms for biomarker detection combined with radiomics-based tumor imaging will be necessary to ascertain the greatest level of accuracy and precision [21]. By analyzing the characteristics of the tumor such as shape, size, and texture, radiomics can provide valuable information on the tumor’s current state and progression. Combining the two ML algorithms to analyze the quantitative data from both imaging and biomarkers could improve disease outcomes, once perfected, at a rate higher than any one method alone.
Overall, our findings highlight the importance of further research in this evolving field in order to fully grasp the potential of ML in the diagnosis and prognosis of GBM. Advancements in this area may significantly enhance patient care and treatment outcomes for individuals affected by this devastating disease in the future.
6. Conclusions
Machine learning is a cutting-edge technology that analyzes data and makes predictions or decisions using algorithms and statistical models. It is a formidable research tool and has the potential to completely change how complex diseases such as glioblastoma are studied and understood. The goal of machine learning is to recognize and categorize unknown data samples using training data. The studies we reviewed have found novel insights into the mechanisms of GBM and identified potential biomarkers for diagnosis and prognostication by utilizing this technology in the study of GBM metabolism.
Arguably one of ML’s most significant advantages is its ability to adapt and improve over time as it processes more data, making it ideal for dealing with complex and dynamic tasks. This is particularly useful for assignments that traditional rule-based systems are unable to manage. Additionally, machine learning can automate tasks that would normally require human intervention, increasing efficiency, and decreasing error rates. This can lead to cost savings, increased productivity, and more accurate decision making.
Conversely, machine learning has drawbacks that must be considered despite its benefits. One significant shortcoming is the requirement for large quantities of high-quality training data, which can be expensive and challenging to come by. Furthermore, it can be challenging to understand how ML models make decisions and how to optimize them because the results can be ambiguous and difficult to interpret. It is also important to remember that the effectiveness of ML models depends considerably on the caliber of the data, as well as the specific task it is assigned. Therefore, it is essential to take these factors into account when implementing ML into medical research.
GBM is a complicated disease with a limited understanding of the underlying biological mechanisms, making diagnosis and treatment challenging. The use of ML algorithms has demonstrated incredible promise in the enhancement of diagnostic and prognostic capabilities for GBM patients; however, a consensus on even a handful of biomarkers discovered with ML algorithms has not yet been made. Many researchers are still exploring this new field and there is still much to be learned. Despite the challenges and limitations, the potential of ML in the study of GBM metabolism is clear.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13020161/s1, Supplementary Materials S1: Search String; Table S1: Risk of Bias Assessment.
Author Contributions
Conceptualization, J.G. and N.P.; methodology, N.P. and Z.D.N.; formal analysis, N.P.; investigation, Z.D.N.; data curation, N.P. and Z.D.N.; writing—original draft preparation, Z.D.N., N.P., O.L. and C.B.; writing—review and editing, Z.D.N., N.P., N.J.B., C.C.K. and J.G.; visualization, N.P.; supervision, J.G. and N.J.B.; project administration, C.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro-Oncology 2017, 19, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology 2013, 15, ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro-Oncology 2004, 6, 227–235. [Google Scholar] [CrossRef]
- Richardson, T.E.; Kumar, A.; Xing, C.; Hatanpaa, K.J.; Walker, J.M. Overcoming the Odds: Toward a Molecular Profile of Long-Term Survival in Glioblastoma. J. Neuropathol. Exp. Neurol. 2020, 79, 1031–1037. [Google Scholar] [CrossRef]
- Kesari, S. Understanding Glioblastoma Tumor Biology: The Potential to Improve Current Diagnosis and Treatments. Semin. Oncol. 2011, 38, S2–S10. [Google Scholar] [CrossRef]
- Waqar, M.; Trifiletti, D.M.; McBain, C.; O’Connor, J.; Coope, D.J.; Akkari, L.; Quinones-Hinojosa, A.; Borst, G.R. Early Therapeutic Interventions for Newly Diagnosed Glioblastoma: Rationale and Review of the Literature. Curr. Oncol. Rep. 2022, 24, 311–324. [Google Scholar] [CrossRef]
- Alieva, M.; Margarido, A.S.; Wieles, T.; Abels, E.R.; Colak, B.; Boquetale, C.; Noordmans, H.J.; Snijders, T.J.; Broekman, M.L.; van Rheenen, J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 2017, 7, 7529. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.v.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Lewis, J.E.; Kemp, M.L. Integration of machine learning Lewis, J.E.; Kemp, M.L. Integration of machine learning and genome-scale metabolic modeling identifies multi-omics biomarkers for radiation resistance. Nat. Commun. 2021, 12, 2700. [Google Scholar] [CrossRef]
- Bertsimas, D.; Margonis, G.A.; Sujichantararat, S.; Boerner, T.; Ma, Y.; Wang, J.; Kamphues, C.; Sasaki, K.; Tang, S.; Gagniere, J.; et al. Using Artificial Intelligence to Find the Optimal Margin Width in Hepatectomy for Colorectal Cancer Liver Metastases. JAMA Surg. 2022, e221819. [Google Scholar] [CrossRef]
- Farrokhian, N.; Holcomb, A.J.; Dimon, E.; Karadaghy, O.; Ward, C.; Whiteford, E.; Tolan, C.; Hanly, E.K.; Buchakjian, M.R.; Harding, B.; et al. Development and Validation of Machine Learning Models for Predicting Occult Nodal Metastasis in Early-Stage Oral Cavity Squamous Cell Carcinoma. JAMA Netw. Open 2022, 5, e227226. [Google Scholar] [CrossRef]
- Rudie, J.D.; Rauschecker, A.M.; Bryan, R.N.; Davatzikos, C.; Mohan, S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology 2019, 290, 607–618. [Google Scholar] [CrossRef]
- Yousra, M.; Khalid, C. Analysis of The Variables Of Intention Of The Adoption And Acceptance Of Artificial Intelligence And Big Data Tools Among Leaders Of Organizations In Morocco: Attempt Of A Theoretical Study. Eur. Sci. J. ESJ 2021, 17, 106. [Google Scholar] [CrossRef]
- Ball, H.C. Improving Healthcare Cost, Quality, and Access Through Artificial Intelligence and Machine Learning Applications. J. Healthc. Manag. 2021, 66, 271–279. [Google Scholar] [CrossRef]
- Carleo, G.; Cirac, I.; Cranmer, K.; Daudet, L.; Schuld, M.; Tishby, N.; Vogt-Maranto, L.; Zdeborová, L. Machine learning and the physical sciences. Rev. Mod. Phys. 2019, 91, 045002. [Google Scholar] [CrossRef]
- Jin, M.C.; Rodrigues, A.J.; Jensen, M.; Veeravagu, A. A Discussion of Machine Learning Approaches for Clinical Prediction Modeling. Acta Neurochir. 2022, 134, 65–73. [Google Scholar] [CrossRef]
- Floyd, C.E.; Tourassi, G.D. An Artificial Neural Network for Lesion Detection on Single-Photon Emission Computed Tomographic Images. Investig. Radiol. 1992, 27, 667–672. [Google Scholar] [CrossRef]
- Christy, P.S.; Tervonen, O.; Scheithauer, B.W.; Forbes, G.S. Use of a neural network and a multiple regression model to predict histologic grade of astrocytoma from MRI appearances. Neuroradiology 1995, 37, 89–93. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Guo, Y.; Sa, Y.; Li, Q.; Ma, J. Research progress of gliomas in machine learning. Cells 2021, 10, 3169. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Marhold, F.; Oberndorfer, S.; Heinz, G.; Buchfelder, M.; Kinfe, T.M.; Meyer-Bäse, A. Radiophysiomics: Brain Tumors Classification by Machine Learning and Physiological MRI Data. Cancers 2022, 14, 2363. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, H.; Shafaat, O.; Bernstock, J.D.; Brooks, M.D.; Elsayed, G.; Chen, J.A.; Szerip, P.; Chagoya, G.; Gessler, F.; Sotoudeh, E.; et al. Artificial Intelligence in the Management of Glioma: Era of Personalized Medicine. Front. Oncol. 2019, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Farwell, M.D.; Mankoff, D.A. Analysis of Routine Computed Tomographic Scans with Radiomics and Machine Learning: One Step Closer to Clinical Practice. JAMA Oncol. 2022, 8, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Lu, J.; Zhang, H.; Adeli, E.; Wang, J.; Yu, Z.; Liu, L.; Wang, Q.; Wu, J.; Shen, D. Multi-Channel 3D Deep Feature Learning for Survival Time Prediction of Brain Tumor Patients Using Multi-Modal Neuroimages. Sci. Rep. 2019, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, F.J.; Rossi, L.A.; Roberts, L.M.; Bosserman, L.D. Prospective Comparison of Medical Oncologists and a Machine Learning Model to Predict 3-Month Mortality in Patients with Metastatic Solid Tumors. JAMA Netw. Open 2022, 5, e2214514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ji, N.; Wang, G.; Zhang, Y.; Song, H.; Yuan, Y.; Yang, C.; Jin, Y.; Zhang, Z.; Zhang, L.; et al. Metabolic detection of malignant brain gliomas through plasma lipidomic analysis and support vector machine-based machine learning. Ebiomedicine 2022, 81, 97. [Google Scholar] [CrossRef]
- Valdebenito, J.; Medina, F. Machine learning approaches to study glioblastoma: A review of the last decade of applications. Cancer Rep. 2019, 2, 226. [Google Scholar] [CrossRef]
- Neil, Z.; Boyett, C.; Little, O.; Pierzchajlo, N.; Gendreau, J. Integration of Machine Learning Models into the Characterization of Glioblastoma Metabolism to Evaluate Diagnostic and Prognostic Accuracy. PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022367758 (accessed on 4 December 2022).
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2 Group: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Lee, M.J.; Mulder, F.; Leeflang, D.M.; Wolff, R.; Whiting, P.; Bossuyt, P.M. QUAPAS: An Adaptation of the QUADAS-2 Tool to Assess Prognostic Accuracy Studies. Ann. Intern. Med. 2022, 175, 1010–1018. [Google Scholar] [CrossRef]
- Methodology of guideline development. Neurosurgery 2002, 50, 4. [CrossRef]
- Ishwar, D.; Haldavnekar, R.; Das, S.; Tan, B.; Venkatakrishnan, K. Glioblastoma Associated Natural Killer Cell EVs Generating Tumour-Specific Signatures: Noninvasive GBM Liquid Biopsy with Self-Functionalized Quantum Probes. ACS Nano 2022, 16, 10859–10877. [Google Scholar] [CrossRef]
- McInerney, C.E.; Lynn, J.A.; Gilmore, A.R.; Flannery, T.; Prise, K.M. Using AI-Based Evolutionary Algorithms to Elucidate Adult Brain Tumor (Glioma) Etiology Associated with IDH1 for Therapeutic Target Identification. Curr. Issues Mol. Biol. 2022, 44, 2982–3000. [Google Scholar] [CrossRef]
- Firdous, S.; Abid, R.; Nawaz, Z.; Bukhari, F.; Anwer, A.; Cheng, L.L.; Sadaf, S. Dysregulated Alanine as a Potential Predictive Marker of Glioma—An Insight from Untargeted HRMAS-NMR and Machine Learning Data. Metabolites 2021, 11, 507. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, W.; Tang, B.; Feng, Q.; Dong, Z. Hub gene identification and prognostic model construction for isocitrate dehydrogenase mutation in glioma. Transl. Oncol. 2021, 14, 100979. [Google Scholar] [CrossRef]
- Kałuzińska, Z.; Kołat, D.; Bednarek, A.K.; Zbieta Płuciennik, E.; Pollok, K.E.; Schönthal, A.H. PLEK2, RRM2, GCSH: A Novel WWOX-Dependent Biomarker Triad of Glioblastoma at the Crossroads of Cytoskeleton Reorganization and Metabolism Alterations. Cancers 2021, 13, 2955. [Google Scholar] [CrossRef]
- He, Z.; Wang, C.; Xue, H.; Zhao, R.; Li, G. Identification of a Metabolism-Related Risk Signature Associated with Clinical Prognosis in Glioblastoma Using Integrated Bioinformatic Analysis. Front. Oncol. 2020, 10, 1631. [Google Scholar] [CrossRef]
- Zeng, C.; Xing, W.; Liu, Y. Identification of UGP2 as a progression marker that promotes cell growth and motility in human glioma. J. Cell. Biochem. 2019, 120, 12489–12499. [Google Scholar] [CrossRef]
- Hao, J.; Kim, Y.; Kim, T.-K.; Kang, M. PASNet: Pathway-associated sparse deep neural network for prognosis prediction from high-throughput data. BMC Bioinform. 2018, 19, 510. [Google Scholar] [CrossRef]
- Shu, C.; Wang, Q.; Yan, X.; Wang, J. Whole-Genome Expression Microarray Combined with Machine Learning to Identify Prognostic Biomarkers for High-Grade Glioma. J. Mol. Neurosci. 2018, 64, 491–500. [Google Scholar] [CrossRef]
- Gollapalli, K.; Ray, S.; Srivastava, R.; Renu, D.; Singh, P.; Dhali, S.; Dikshit, J.B.; Srikanth, R.; Moiyadi, A.; Srivastava, S. Investigation of serum proteome alterations in human glioblastoma multiforme. Proteomics 2012, 12, 2378–2390. [Google Scholar] [CrossRef]
- Gilard, V.; Ferey, J.; Marguet, F.; Fontanilles, M.; Ducatez, F.; Pilon, C.; Lesueur, C.; Pereira, T.; Basset, C.; Schmitz-Afonso, I.; et al. Integrative Metabolomics Reveals Deep Tissue and Systemic Metabolic Remodeling in Glioblastoma. Cancers 2021, 13, 5157. [Google Scholar] [CrossRef] [PubMed]
- Nuechterlein, N.; Shapiro, L.G.; Holland, E.C.; Cimino, P.J. Machine learning modeling of genome-wide copy number alteration signatures reliably predicts IDH mutational status in adult diffuse glioma. Acta Neuropathol. Commun. 2021, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Riviere-Cazaux, C.; Carlstrom, L.P.; Rajani, K.; Sarkaria, J.; Rodriguez, M.; Rahman, M.; Brown, D.; White, J.; Ikram, S.; Hirte, R.; et al. Individualized diversity in the extracellular metabolome of live human gliomas. Biol. Med. 2021, 2921, 320. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Hossain, E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform. Decis. Mak. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Connelly, L. Logistic Regression. Med. Surg Nurs. 2020, 29, 353–354. [Google Scholar] [CrossRef]
- Mitchell, J.B.O. Three machine learning models for the 2019 Solubility Challenge. ADMET DMPK 2020, 8, 215. [Google Scholar] [CrossRef]
- Liimatainen, K.; Huttunen, R.; Latonen, L.; Ruusuvuori, P. Convolutional Neural Network-Based Artificial Intelligence for Classification of Protein Localization Patterns. Biomolecules 2021, 11, 264. [Google Scholar] [CrossRef]
- Kriegeskorte, N.; Golan, T. Neural network models and deep learning. Curr. Biol. 2019, 29, R231–R236. [Google Scholar] [CrossRef]
- Poernomo, A.; Kang, D.-K. Biased Dropout and Crossmap Dropout: Learning towards effective Dropout regularization in convolutional neural network. Neural Netw. 2018, 104, 60–67. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Shan, B.; He, M.; Xu, J. XGBoost Machine Learning Algorithm for Prediction of Outcome in Aneurysmal Subarachnoid Hemorrhage. Neuropsychiatr. Dis. Treat. 2022, 18, 659–667. [Google Scholar] [CrossRef]
- Govindarajulu, U.; Bedi, S. K-means for shared frailty models. BMC Med. Res. Methodol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Ranstam, J.; Cook, J.A. LASSO regression. Br. J. Surg. 2018, 105, 1348. [Google Scholar] [CrossRef]
- Jardillier, R.; Koca, D.; Chatelain, F.; Guyon, L. Prognosis of lasso-like penalized Cox models with tumor profiling improves prediction over clinical data alone and benefits from bi-dimensional pre-screening. BMC Cancer 2022, 22, 1045. [Google Scholar] [CrossRef]
- Patel, S.J.; Chamberlain, D.B.; Chamberlain, J.M. A Machine Learning Approach to Predicting Need for Hospitalization for Pediatric Asthma Exacerbation at the Time of Emergency Department Triage. Acad. Emerg. Med. 2018, 25, 1463–1470. [Google Scholar] [CrossRef]
- Wang, S.; Nan, B.; Rosset, S.; Zhu, J. Random lasso. Ann. Appl. Stat. 2011, 5, 468–485. [Google Scholar] [CrossRef]
- Boutsidis, C.; Mahoney, M.W.; Drineas, P. Unsupervised feature selection for principal components analysis. In Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Las Vegas, NV, USA, 24 August 2008. [Google Scholar] [CrossRef]
- Ishwaran, H.; Lu, M. Standard errors and confidence intervals for variable importance in random forest regression, classification, and survival. Stat. Med. 2019, 38, 558–582. [Google Scholar] [CrossRef]
- Chevallier, S.; Bertrand, D.; Kohler, A.; Courcoux, P. Application of PLS-DA in multivariate image analysis. J. Chemom. 2006, 20, 221–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).