How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. SPME and Chemicals
2.3. GC–MS Analysis of Silylated Primary Metabolites
2.4. SPME–GC–MS Volatiles Analysis
2.5. Metabolites Identification and Multivariate Data Analyses
3. Results and Discussion
3.1. Primary Metabolites Profiling in Allium Species Aerial Parts via GC–MS Analysis (Post-Silylation)
3.1.1. Sugars
3.1.2. Amino Acids/Nitrogenous Compounds
3.1.3. Fatty Acids/Esters/Sterols
3.1.4. Organic Acids/Alcohols
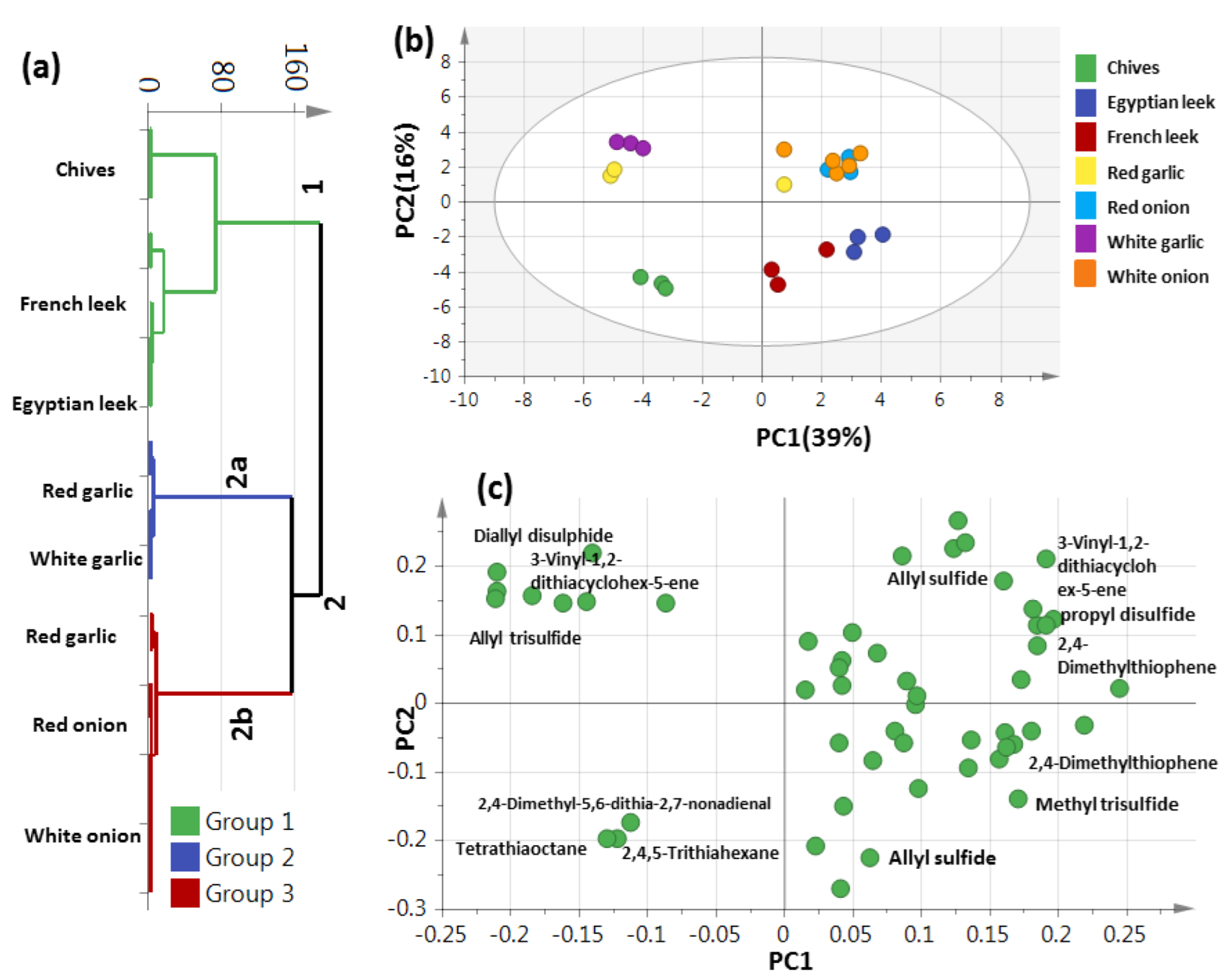
3.2. HCA and PCA Analysis of Post-Silylated Primary Metabolites Allium Species
3.3. OPLS-DA Analysis of Allium Aerial Parts
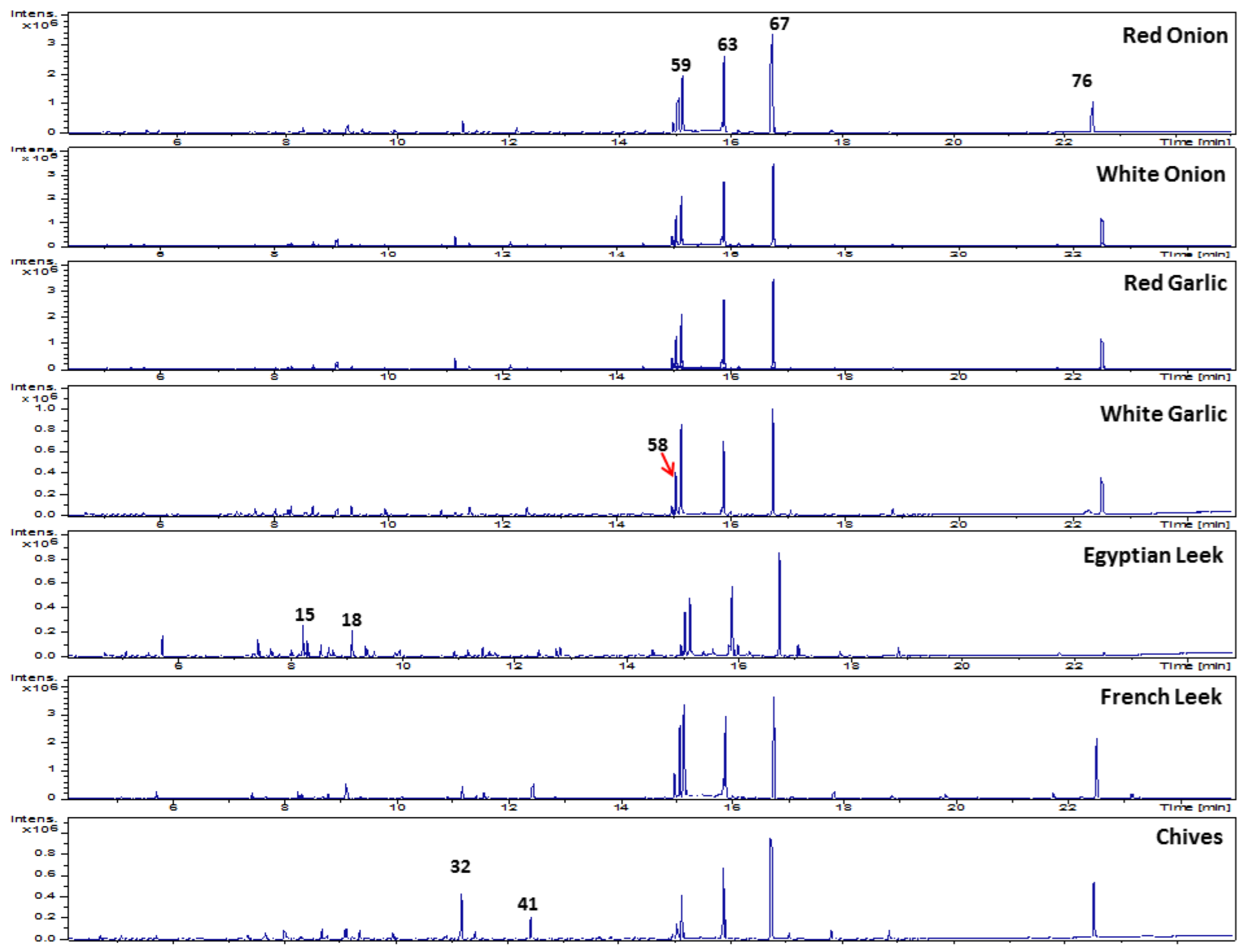
3.4. Headspace-SPME–GC–MS Volatiles Analysis of Allium Species
3.4.1. Sulfur Compounds
3.4.2. Aldehydes/Ketones
3.4.3. Alcohols
3.4.4. Acids/Esters and Furans
3.4.5. Aliphatic and Aromatic Hydrocarbons
3.4.6. Fatty Acids/Esters
3.4.7. Monoterpene Hydrocarbons
3.4.8. Phenols/Ethers and Nitrogenous Compounds
3.5. PCA Analysis of Allium Aroma Profile via HS-GC–MS
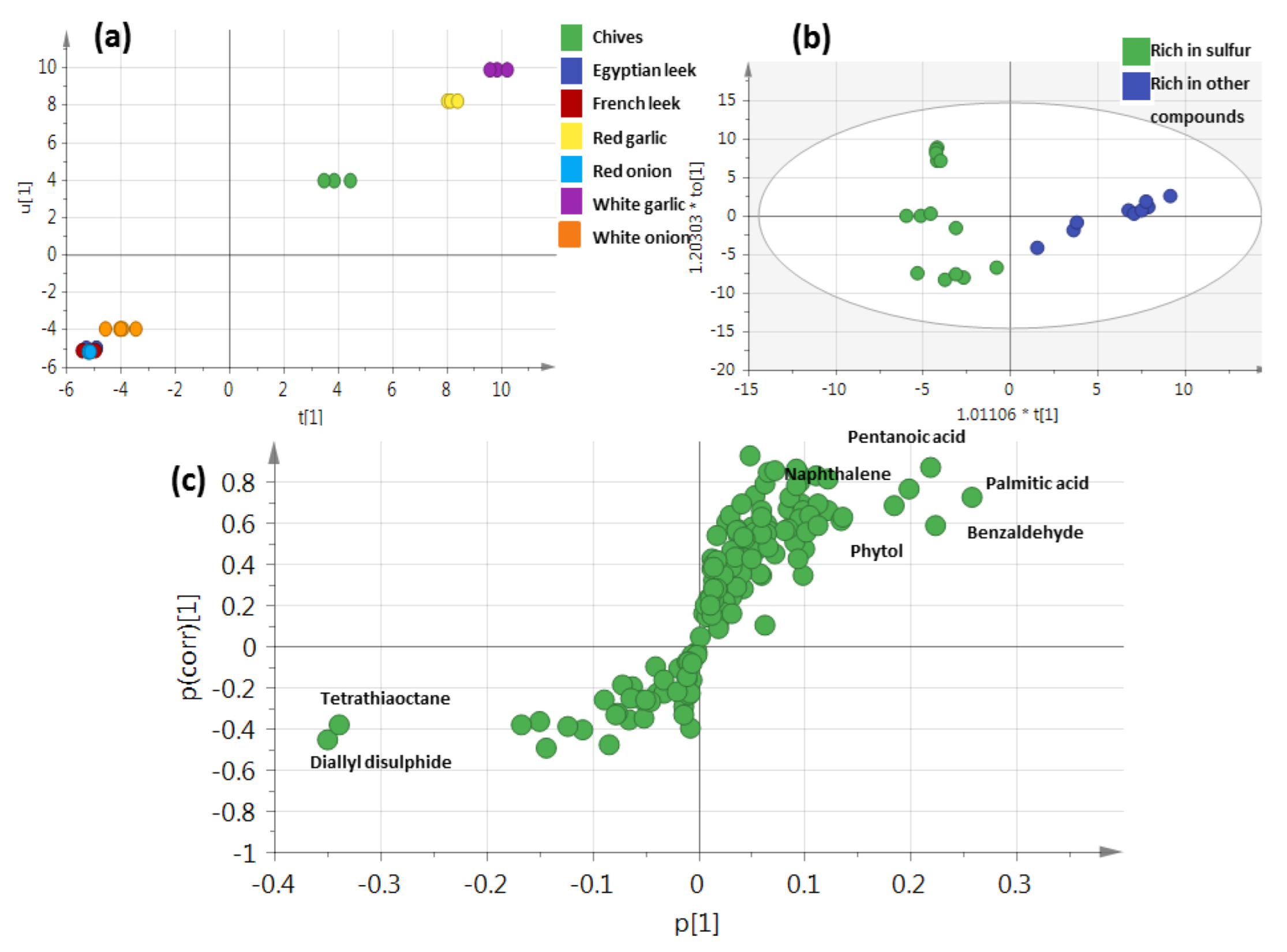
3.6. Unsupervised PCA Model Using Aroma Sulfur Compounds
3.7. Supervised OPLS-DA Analysis of Allium Rich in Sulfur versus Those Rich in Other Volatile Classes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Recommendations
References
- Baky, M.H.; Shamma, S.N.; Xiao, J.; Farag, M.A. Comparative aroma and nutrients profiling in six edible versus nonedible cruciferous vegetables using MS based metabolomics. Food Chem. 2022, 383, 132374. [Google Scholar] [CrossRef]
- Serag, A.; Baky, M.H.; Döll, S.; Farag, M.A. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: A prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020, 10, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; Andreo-Martínez, P.; Mut-Salud, N.; Fonollá, J.; Baños, A. Beneficial Effects of Organosulfur Compounds from Allium cepa on Gut Health: A Systematic Review. Foods 2021, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Garrido-Mesa, J.; Diez-Echave, P.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; García, F.; Sánchez, M.; Toral, M.; Romero, M.; Duarte, J. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Quesada, I.; de Paola, M.; Torres-Palazzolo, C.; Camargo, A.; Ferder, L.; Manucha, W.; Castro, C. Effect of garlic’s active constituents in inflammation, obesity and cardiovascular disease. Curr. Hypertens. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Kim, S.-K. Allium flavonols: Health benefits, molecular targets, and bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Nandagopal MS, G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from Alliums: A potential drug candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef]
- Abd, F.A.E.-R.A.; Ali, R.F.M. Proximate compositions, phytochemical constituents, antioxidant activities and phenolic contents of seed and leaves extracts of Egyptian leek (Allium ampeloprasum var. kurrat). Eur. J. Chem. 2013, 4, 185–190. [Google Scholar]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Di Rosa, M.; Ianaro, A. Cytotoxic saponins from bulbs of Allium porrum L. J. Agric. Food Chem. 2000, 48, 3455–3462. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum). Biosci. Biotechnol. Biochem. 2008, 72, 2987–2991. [Google Scholar] [CrossRef]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-based profiling of African baobab fruit (Adansonia digitata L.) using a multiplex approach of MS and NMR techniques in relation to its biological activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.M.; El-Mahis, A.; Heiss, A.G.; Farag, M.A. Gas Chromatography–Mass Spectrometry-Based Classification of 12 Fennel (Foeniculum vulgare Miller) Varieties Based on Their Aroma Profiles and Estragole Levels as Analyzed Using Chemometric Tools. ACS Omega 2021, 6, 5775–5785. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical methods for bioactive sulfur compounds in Allium: An integrated review and future directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Gîtin, L.; Dinică, R.; Neagu, C.; Dumitrascu, L. Sulfur compounds identification and quantification from Allium spp. fresh leaves. J. Food Drug Anal. 2014, 22, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kusterer, J.; Keusgen, M. Cysteine sulfoxides and volatile sulfur compounds from Allium tripedale. J. Agric. Food Chem. 2010, 58, 1129–1137. [Google Scholar] [CrossRef]
- Farag, M.A.; Ramadan, N.S.; Shorbagi, M.; Farag, N.; Gad, H.A. Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods 2022, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Khattab, A.R.; Shamma, S.; Afifi, S.M. Profiling of Primary Metabolites and Volatile Determinants in Mahlab Cherry (Prunus mahaleb L.) Seeds in the Context of Its Different Varieties and Roasting as Analyzed Using Chemometric Tools. Foods 2021, 10, 728. [Google Scholar] [CrossRef]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health; Springer: Berlin/Heidelberg, Germany, 2013; Volume 45, pp. 407–411. [Google Scholar]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food products as sources of protein and amino acids—The case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Lalam, C.; Petla, N.; Tantravahi, S. Antiproliferative and Antibacterial Effects of Pyroglutamic Acid Isolated from Enterococcus Faecium (Mcc-2729). Ann. Rom. Soc. Cell Biol. 2021, 25, 7624–7628. [Google Scholar]
- Kubec, R.; Drhová, V.; Velíšek, J. Thermal degradation of S-methylcysteine and its sulfoxide important flavor precursors of Brassica and Allium vegetables. J. Agric. Food Chem. 1998, 46, 4334–4340. [Google Scholar] [CrossRef]
- Farag, M.A.; Mohsen, E.; Abd El Nasser, G. Sensory metabolites profiling in Myristica fragrans (Nutmeg) organs and in response to roasting as analyzed via chemometric tools. LWT 2018, 97, 684–692. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- Kubec, R.; Krejcčovaá, P.; Mansur, L.; García, N. Flavor precursors and sensory-active sulfur compounds in alliaceae species native to South Africa and South America. J. Agric. Food Chem. 2013, 61, 1335–1342. [Google Scholar] [CrossRef]
- Yan, J.-K.; Zhu, J.; Liu, Y.; Chen, X.; Wang, W.; Zhang, H.; Li, L. Recent advances in research on Allium plants: Functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Rahimi, H.; Kazemi, A.; Scognamiglio, M.; Naderian, M.; Iraji, A.; Bordbar, F. Allium hooshidaryae (Alliaceae); Chemical compositions, biological and ethnomedicine uses. J. Ethnopharmacol. 2021, 274, 113918. [Google Scholar] [CrossRef]
- Fogang, H.P.; Maggi, F.; Tapondjou, L.A.; Womeni, H.M.; Papa, F.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G. In vitro biological activities of seed essential oils from the cameroonian spices Afrostyrax lepidophyllus Mildbr. and Scorodophloeus zenkeri Harms rich in sulfur-containing compounds. Chem. Biodivers. 2014, 11, 161–169. [Google Scholar] [CrossRef]
- Rapior, S.; Breheret, S.; Talou, T.; Bessière, J.-M. Volatile flavor constituents of fresh Marasmius alliaceus (garlic Marasmius). J. Agric. Food Chem. 1997, 45, 820–825. [Google Scholar] [CrossRef]
- Lim, H.; Kubota, K.; Kobayashi, A.; Seki, T.; Ariga, T. Inhibitory effect of sulfur-containing compounds in Scorodocarpus borneensis Becc. on the aggregation of rabbit platelets. Biosci. Biotechnol. Biochem. 1999, 63, 298–301. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chagnaadorj, A.; Bayarsengee, U.; Leung, T.-K.; Cheng, C.-W. The compound, diallyl disulfide, enriched in garlic, prevents the progression of doxorubicin-induced nephropathy. Food Sci. Technol. 2019, 39, 1040–1046. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Yao, C.-H.; Way, C.-L.; Lee, K.-W.; Tsai, C.-Y.; Ou, H.-C.; Kuo, W.-W. Diallyl trisulfide and diallyl disulfide ameliorate cardiac dysfunction by suppressing apoptotic and enhancing survival pathways in experimental diabetic rats. J. Appl. Physiol. 2013, 114, 402–410. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Habib, T.N.; Andrews, S.C. Diallyl disulfide-induced apoptosis in a breast-cancer cell line (MCF-7) may be caused by inhibition of histone deacetylation. Nutr. Cancer 2012, 64, 1251–1260. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Uddin, T.M.; Zidan, M.; Redwan, B.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M. Allium cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid. Based Complement. Altern. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of volatile and flavonoid composition of different cuts of dried onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC × GC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Altamirano, J.C.; Luco, J.M.; Norlin, R.; Camargo, A.B. Solid phase microextraction coupled to liquid chromatography. Analysis of organosulphur compounds avoiding artifacts formation. Food Chem. 2014, 157, 199–204. [Google Scholar] [CrossRef]
- Zhao, Q.; Xi, J.; Xu, X.; Yin, Y.; Xu, D.; Jin, Y.; Tong, Q.; Dong, L.; Wu, F. Volatile fingerprints and biomarkers of Chinese fragrant and non-fragrant japonica rice before and after cooking obtained by untargeted GC/MS-based metabolomics. Food Biosci. 2022, 47, 101764. [Google Scholar] [CrossRef]
- Zito, P.; Tavella, F.; Pacifico, D.; Campanella, V.; Sajeva, M.; Carimi, F.; Ebmer, A.W.; Dötterl, S. Interspecific variation of inflorescence scents and insect visitors in Allium (Amaryllidaceae: Allioideae). Plant Syst. Evol. 2019, 305, 727–741. [Google Scholar] [CrossRef]
- Baky, M.H.; Shawky, E.M.; Elgindi, M.R.; Ibrahim, H.A. Comparative Volatile Profiling of Ludwigia stolonifera Aerial Parts and Roots Using VSE-GC-MS/MS and Screening of Antioxidant and Metal Chelation Activities. ACS Omega 2021, 6, 24788–24794. [Google Scholar] [CrossRef]
- Jing, T.; Zhang, N.; Gao, T.; Zhao, M.; Jin, J.; Chen, Y.; Xu, M.; Wan, X.; Schwab, W.; Song, C. Glucosylation of (Z)-3-hexenol informs intraspecies interactions in plants: A case study in Camellia sinensis. Plant Cell Environ. 2019, 42, 1352–1367. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M. Volatiles profiling in Ceratonia siliqua (Carob bean) from Egypt and in response to roasting as analyzed via solid-phase microextraction coupled to chemometrics. J. Adv. Res. 2017, 8, 379–385. [Google Scholar] [CrossRef]
- Tsiaganis, M.C.; Laskari, K.; Melissari, E. Fatty acid composition of Allium species lipids. J. Food Compos. Anal. 2006, 19, 620–627. [Google Scholar] [CrossRef]
- De Souza, C.O.; Vannice, G.K.; Rosa Neto, J.C.; Calder, P.C. Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol. Nutr. Food Res. 2018, 62, 12. [Google Scholar] [CrossRef]



| Peak Number | Average Rt | Average RI | Metabolite | Class | Chives | Egyptian Leek | French Leek | Red Garlic | Red Onion | White Garlic | White Onion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 5.13 | 1061.59 | Lactic acid, 2TMS | Acid | 0.24 ± 0.12 | 0.23 ± 0.03 | 0.40 ± 0.12 | 0.30 ± 0.11 | 0.26 ± 0.18 | 0.29 ± 0.11 | 0.37 ± 0.12 |
| 4 | 5.34 | 1074.61 | Glycolic acid, 2TMS | Acid | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.17 ± 0.02 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.00 |
| 5 | 5.528 | 1086.41 | Pyruvic acid, 2TMS | Acid | 0.05 ± 0.02 | 0.19 ± 0.07 | 0.34 ± 0.02 | 0.10 ± 0.02 | 0.14 ± 0.04 | 0.03 ± 0.01 | 0.09 ± 0.03 |
| 6 | 5.649 | 1093.85 | Octanoic acid, TMS | Acid | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 8 | 6.365 | 1138.27 | β-Lactic acid, 2TMS | Acid | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.16 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 11 | 7.704 | 1224.87 | γ-Hydroxybutyric acid, 2TMS | Acid | 0.36 ± 0.08 | 0.40 ± 0.05 | 0.48 ± 0.01 | 0.41 ± 0.04 | 0.38 ± 0.07 | 0.40 ± 0.05 | 0.42 ± 0.06 |
| 14 | 8.23 | 1262.65 | Methylmaleic acid, 2TMS | Acid | 0.03 ± 0.01 | 0.08 ± 0.06 | 0.09 ± 0.09 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.01 |
| 17 | 8.808 | 1304.12 | Succinic acid, 2TMS | Acid | 0.17 ± 0.04 | 0.32 ± 0.06 | 1.23 ± 0.10 | 0.17 ± 0.01 | 0.29 ± 0.05 | 0.18 ± 0.03 | 0.24 ± 0.06 |
| 20 | 9.248 | 1335.82 | Fumaric acid, 2TMS | Acid | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.24 ± 0.07 | 0.01 ± 0.00 | 0.06 ± 0.01 | 0.00 ± 0.00 | 0.07 ± 0.02 |
| 24 | 10.057 | 1393.63 | Methylsuccinic acid, 2TMS | Acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 27 | 10.65 | 1440.33 | Itaconic acid, 2TMS | Acid | 0.05 ± 0.02 | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 |
| 30 | 10.952 | 1465.15 | 2-Ketoisocaproic acid tbo, TMS | Acid | 0.20 ± 0.06 | 0.22 ± 0.05 | 0.27 ± 0.02 | 0.25 ± 0.04 | 0.21 ± 0.05 | 0.25 ± 0.06 | 0.21 ± 0.05 |
| 31 | 11.013 | 1469.98 | Succinic acid, 2TMS | Acid | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.01 |
| 32 | 11.205 | 1485.5 | Malic acid, 3TMS | Acid | 3.13 ± 0.97 | 0.39 ± 0.09 | 3.58 ± 0.32 | 0.32 ± 0.12 | 2.28 ± 0.40 | 0.16 ± 0.09 | 0.92 ± 0.23 |
| 50 | 13.885 | 1712.97 | 2-Ketoglutaric acid, 3TMS | Acid | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 |
| 56 | 14.644 | 1781.21 | Azelaic acid, 2TMS | Acid | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Total Acid | 4.36 ± 1.35 | 2.02 ± 0.45 | 7.08 ± 0.83 | 1.70 ± 0.35 | 3.79 ± 0.83 | 1.46 ± 0.37 | 2.51 ± 0.59 | ||||
| 2 | 5.048 | 1056.5 | 1,3 Propanediol, 2TMS | Alcohol | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| 12 | 7.841 | 1234.73 | Diethylene glycol, 2TMS | Alcohol | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 25 | 10.073 | 1394.89 | 1-Nonanol, TMS | Alcohol | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Total Alcohol | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | ||||
| 7 | 5.758 | 1100.63 | l-Alanine, TMS | Amino acid | 0.22 ± 0.15 | 2.62 ± 0.61 | 1.89 ± 0.49 | 0.07 ± 0.04 | 0.85 ± 0.08 | 0.31 ± 0.10 | 2.36 ± 2.12 |
| 9 | 7.389 | 1202.33 | Glycine, TMS | Amino acid | 0.48 ± 0.12 | 0.50 ± 0.07 | 0.52 ± 0.03 | 0.53 ± 0.05 | 0.51 ± 0.04 | 0.50 ± 0.05 | 0.50 ± 0.03 |
| 10 | 7.464 | 1207.63 | Valine, 2TMS | Amino acid | 0.05 ± 0.02 | 3.51 ± 1.09 | 1.75 ± 0.51 | 0.03 ± 0.01 | 0.22 ± 0.04 | 0.32 ± 0.14 | 1.00 ± 0.49 |
| 15 | 8.276 | 1265.93 | L-Leucine, TMS | Amino acid | 0.02 ± 0.01 | 5.32 ± 1.44 | 3.22 ± 0.92 | 0.02 ± 0.01 | 0.20 ± 0.03 | 0.47 ± 0.22 | 1.41 ± 0.61 |
| 16 | 8.588 | 1288.31 | L-Norleucine, TMS | Amino acid | 0.02 ± 0.01 | 2.34 ± 0.75 | 1.00 ± 0.29 | 0.01 ± 0.01 | 0.08 ± 0.01 | 0.22 ± 0.12 | 0.61 ± 0.30 |
| 21 | 9.54 | 1356.67 | Serine, 3TMS | Amino acid | 0.06 ± 0.02 | 1.34 ± 0.38 | 0.45 ± 0.19 | 0.04 ± 0.02 | 0.40 ± 0.07 | 0.20 ± 0.08 | 0.91 ± 0.28 |
| 22 | 9.911 | 1383.42 | L-Threonine, 3TMS | Amino acid | 0.04 ± 0.01 | 1.10 ± 0.32 | 0.47 ± 0.14 | 0.03 ± 0.01 | 0.11 ± 0.03 | 0.19 ± 0.07 | 0.38 ± 0.11 |
| 26 | 10.376 | 1418.66 | β-Alanine, 2TMS | Amino acid | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.01 |
| 28 | 10.742 | 1448.19 | Leucine, 2TBDMS | Amino acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 29 | 10.918 | 1462.37 | L-Aspartic acid, 3TMS | Amino acid | 0.22 ± 0.11 | 0.07 ± 0.03 | 0.08 ± 0.02 | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.02 |
| 35 | 11.583 | 1516.01 | Pyroglutamic acid, 2TMS | Amino acid | 0.10 ± 0.01 | 0.88 ± 0.32 | 4.10 ± 0.91 | 0.05 ± 0.02 | 0.19 ± 0.03 | 0.07 ± 0.01 | 0.34 ± 0.12 |
| 36 | 11.591 | 1516.99 | Aspartic acid, 3TMS | Amino acid | 0.05 ± 0.02 | 0.35 ± 0.10 | 0.10 ± 0.09 | 0.01 ± 0.01 | 0.16 ± 0.03 | 0.01 ± 0.00 | 0.11 ± 0.03 |
| 43 | 12.767 | 1612.66 | Glutamic acid, 3TMS | Amino acid | 0.17 ± 0.07 | 1.71 ± 0.54 | 0.24 ± 0.05 | 0.07 ± 0.02 | 0.44 ± 0.12 | 0.07 ± 0.02 | 0.38 ± 0.15 |
| 44 | 12.843 | 1619.48 | Phenylalanine, 2TMS | Amino acid | 0.02 ± 0.01 | 2.04 ± 0.66 | 0.85 ± 0.22 | 0.01 ± 0.01 | 0.10 ± 0.03 | 0.18 ± 0.08 | 0.61 ± 0.24 |
| 45 | 12.951 | 1629.1 | Asparagine, 3TMS | Amino acid | 0.02 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.00 ± 0.00 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.03 |
| 48 | 13.368 | 1666.69 | L-Asparagine, 3TMS | Amino acid | 0.07 ± 0.04 | 0.16 ± 0.06 | 0.10 ± 0.05 | 0.00 ± 0.00 | 0.22 ± 0.06 | 0.07 ± 0.04 | 0.15 ± 0.08 |
| 52 | 14.112 | 1733.57 | L-Glutamine, 2TMS | Amino acid | 0.03 ± 0.02 | 0.23 ± 0.08 | 0.11 ± 0.02 | 0.01 ± 0.00 | 0.20 ± 0.02 | 0.03 ± 0.01 | 0.16 ± 0.12 |
| 54 | 14.48 | 1766.68 | L-Glutamine, 2TMS | Amino acid | 0.16 ± 0.07 | 1.48 ± 0.61 | 0.90 ± 0.20 | 0.06 ± 0.02 | 1.18 ± 0.35 | 0.37 ± 0.12 | 0.93 ± 0.51 |
| 65 | 16.209 | 1934.73 | Tyrosine, 3TMS | Amino acid | 0.02 ± 0.00 | 1.33 ± 0.42 | 1.00 ± 0.34 | 0.02 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0.06 | 0.43 ± 0.21 |
| Total Amino Acid | 1.86 ± 0.74 | 25.13 ± 7.51 | 16.94 ± 4.51 | 1.10 ± 0.25 | 5.16 ± 1.01 | 3.27 ± 1.15 | 10.50 ± 5.46 | ||||
| 33 | 11.403 | 1501.26 | Methyl octanoate, TMS | Ester | 0.14 ± 0.03 | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.01 |
| Total Ester | 0.14 ± 0.03 | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.01 | ||||
| 77 | 23.124 | 2759.61 | Monostearin, 2TMS | Fatty acid /Ester | 0.35 ± 0.24 | 0.23 ± 0.24 | 6.71 ± 1.54 | 0.37 ± 0.52 | 0.54 ± 0.25 | 0.75 ± 0.71 | 0.37 ± 0.28 |
| 68 | 17.071 | 2022.13 | Palmitic acid, TMS | Fatty acid/Ester | 1.80 ± 0.46 | 2.45 ± 0.38 | 1.99 ± 0.04 | 1.75 ± 0.77 | 1.69 ± 0.18 | 2.30 ± 0.62 | 1.91 ± 0.61 |
| 71 | 18.593 | 2187.5 | Linoleic acid, TMS | Fatty acid/Ester | 0.09 ± 0.02 | 0.15 ± 0.11 | 0.12 ± 0.05 | 0.05 ± 0.03 | 0.06 ± 0.02 | 0.12 ± 0.03 | 0.07 ± 0.03 |
| 72 | 18.636 | 2192.26 | Oleic acid, TMS | Fatty acid/Ester | 0.09 ± 0.02 | 0.06 ± 0.02 | 0.08 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.01 |
| 73 | 18.656 | 2194.49 | α-Linolenic acid, TMS | Fatty acid/Ester | 0.09 ± 0.02 | 0.42 ± 0.09 | 0.41 ± 0.06 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.12 ± 0.03 | 0.12 ± 0.02 |
| 74 | 18.85 | 2216.87 | Stearic acid, TMS | Fatty acid/Ester | 2.04 ± 0.66 | 2.23 ± 0.26 | 2.53 ± 0.03 | 2.11 ± 0.46 | 1.91 ± 0.13 | 2.11 ± 0.37 | 2.03 ± 0.40 |
| 75 | 21.717 | 2568.34 | 1-Monopalmitin TMS | Fatty acid/Ester | 0.63 ± 0.43 | 0.43 ± 0.45 | 7.32 ± 1.68 | 0.64 ± 0.97 | 0.97 ± 0.51 | 1.40 ± 1.30 | 0.68 ± 0.52 |
| Total Fatty Acid/Ester | 5.08 ± 1.85 | 5.98 ± 1.55 | 19.15 ± 3.43 | 5.02 ± 2.78 | 5.30 ± 1.13 | 6.87 ± 3.07 | 5.25 ± 1.86 | ||||
| 13 | 8.181 | 1259.15 | Nicotinic acid-TMS | Nitrogenous | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| 19 | 9.202 | 1332.41 | Uracil, 2TMS | Nitrogenous | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.05 ± 0.02 |
| 23 | 9.981 | 1388.3 | Ethanolamine, 2TMS | Nitrogenous | 0.74 ± 0.17 | 0.71 ± 0.07 | 0.69 ± 0.04 | 0.73 ± 0.04 | 0.76 ± 0.02 | 0.75 ± 0.03 | 0.70 ± 0.05 |
| 37 | 11.677 | 1523.47 | γ-Aminobutyric acid, 3TMS | Nitrogenous | 0.02 ± 0.01 | 1.13 ± 0.48 | 0.88 ± 0.33 | 0.01 ± 0.00 | 0.25 ± 0.10 | 0.17 ± 0.08 | 0.64 ± 0.26 |
| Total Nitrogenous | 0.79 ± 0.20 | 1.90 ± 0.56 | 1.64 ± 0.37 | 0.78 ± 0.04 | 1.06 ± 0.13 | 0.96 ± 0.12 | 1.41 ± 0.33 | ||||
| 1 | 4.842 | 1043.72 | L-Threose, 3TMS (isomer 1) | Sugar | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| 41 | 12.459 | 1586.48 | D-Psicose, 5TMS | Sugar | 0.46 ± 0.21 | 0.28 ± 0.11 | 1.57 ± 0.27 | 0.15 ± 0.09 | 0.04 ± 0.01 | 0.26 ± 0.12 | 0.18 ± 0.04 |
| 42 | 12.58 | 1596.35 | D-Tagatofuranose, 5TMS (isomer 2) | Sugar | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 46 | 12.985 | 1632.41 | L-Rhamnopyranose, 4TMS | Sugar | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 47 | 13.225 | 1654.44 | Arabinose, 4TMS | Sugar | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 49 | 13.418 | 1671.58 | Arabinose, 4TMS | Sugar | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| 51 | 13.899 | 1714.3 | α-D-Lyxopyranose, 4TMS | Sugar | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| 57 | 14.984 | 1813.12 | D-Tagatofuranose, 5TMS (isomer 1) | Sugar | 0.16 ± 0.09 | 0.57 ± 0.17 | 3.77 ± 0.23 | 0.12 ± 0.06 | 0.96 ± 0.17 | 0.56 ± 0.39 | 1.96 ± 0.46 |
| 58 | 15.066 | 1821.23 | D-Tagatofuranose, 5TMS(isomer 2) | Sugar | 0.57 ± 0.24 | 1.68 ± 0.45 | 11.37 ± 0.79 | 0.43 ± 0.16 | 3.29 ± 0.77 | 2.01 ± 1.15 | 5.96 ± 1.08 |
| 59 | 15.145 | 1829.16 | D-Fructopyranose, 5TMS (isomer 1) | Sugar | 1.39 ± 0.47 | 3.86 ± 1.74 | 16.29 ± 1.28 | 0.66 ± 0.32 | 6.46 ± 1.50 | 4.36 ± 2.26 | 10.70 ± 1.54 |
| 60 | 15.383 | 1852.71 | D-Talofuranose, 5TMS (isomer 2) | Sugar | 0.05 ± 0.03 | 0.06 ± 0.03 | 0.26 ± 0.04 | 0.03 ± 0.03 | 0.11 ± 0.01 | 0.05 ± 0.03 | 0.19 ± 0.04 |
| 61 | 15.48 | 1862.34 | D-(-)-Fructopyranose, 5TMS (isomer 1) | Sugar | 0.02 ± 0.01 | 0.09 ± 0.04 | 0.29 ± 0.03 | 0.01 ± 0.01 | 0.11 ± 0.02 | 0.06 ± 0.04 | 0.22 ± 0.04 |
| 62 | 15.854 | 1899.54 | D-Psicose, 5TMS | Sugar | 0.06 ± 0.03 | 0.36 ± 0.16 | 1.47 ± 0.14 | 0.03 ± 0.02 | 0.50 ± 0.11 | 0.24 ± 0.21 | 0.95 ± 0.21 |
| 63 | 15.892 | 1903.29 | β-D-Mannopyranose, 5TMS | Sugar | 2.82 ± 1.02 | 3.34 ± 1.15 | 12.70 ± 1.81 | 0.37 ± 0.15 | 8.68 ± 1.73 | 3.92 ± 2.63 | 13.67 ± 1.91 |
| 64 | 16.006 | 1914.56 | D-Glucose, 5TMS | Sugar | 0.03 ± 0.01 | 0.54 ± 0.11 | 0.51 ± 0.05 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.12 ± 0.05 | 0.31 ± 0.08 |
| 67 | 16.747 | 1988.06 | β-D-Glucopyranose, 5TMS | Sugar | 3.95 ± 1.31 | 4.68 ± 1.56 | 15.88 ± 2.09 | 0.49 ± 0.21 | 11.22 ± 2.13 | 5.32 ± 3.48 | 17.07 ± 2.24 |
| 76 | 22.495 | 2673.08 | Sucrose, 8TMS | Sugar | 2.29 ± 0.67 | 0.19 ± 0.12 | 10.34 ± 2.54 | 1.45 ± 0.63 | 3.47 ± 0.91 | 3.03 ± 1.90 | 1.93 ± 1.97 |
| Total Sugar | 11.87 ± 4.10 | 15.76 ± 5.70 | 74.55 ± 9.29 | 3.80 ± 1.68 | 34.97 ± 7.40 | 20.04 ± 12.29 | 53.25 ± 9.62 | ||||
| 18 | 9.142 | 1328.08 | Glyceric acid, 3TMS | Sugar acid | 0.24 ± 0.07 | 1.35 ± 0.59 | 1.71 ± 0.26 | 0.84 ± 0.09 | 0.17 ± 0.05 | 0.18 ± 0.06 | 0.24 ± 0.05 |
| 39 | 11.972 | 1547.35 | Erythronic acid, 4TMS | Sugar acid | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| 40 | 12.172 | 1563.47 | L-Threonic acid, 4TMS | Sugar acid | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.08 ± 0.01 | 0.06 ± 0.02 | 0.44 ± 0.11 | 0.05 ± 0.01 | 0.44 ± 0.12 |
| 53 | 14.437 | 1762.66 | Glucaric acid, 6TMS | Sugar acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 55 | 14.583 | 1775.59 | Ribonic acid, 5TMS | Sugar acid | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 69 | 17.156 | 2031.28 | D-Glucuronic acid, 5TMS | Sugar acid | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Total Sugar Acid | 0.26 ± 0.08 | 1.39 ± 0.60 | 1.85 ± 0.27 | 0.91 ± 0.11 | 0.64 ± 0.16 | 0.25 ± 0.08 | 0.71 ± 0.19 | ||||
| 34 | 11.526 | 1511.17 | Erythritol, 4TMS | Sugar alcohol | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| 38 | 11.931 | 1543.65 | Threitol, 4TMS | Sugar alcohol | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.02 |
| 66 | 16.384 | 1952.03 | Mannitol, 6TMS | Sugar alcohol | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.05 | 0.01 ± 0.00 | 0.08 ± 0.02 | 0.01 ± 0.01 | 0.13 ± 0.15 |
| 70 | 17.825 | 2104.04 | Myoinositol, 6TMS | Sugar alcohol | 0.24 ± 0.07 | 0.17 ± 0.04 | 0.68 ± 0.07 | 0.07 ± 0.03 | 0.15 ± 0.04 | 0.06 ± 0.02 | 0.28 ± 0.05 |
| Total Sugar Alcohol | 0.24 ± 0.07 | 0.18 ± 0.05 | 0.77 ± 0.14 | 0.08 ± 0.03 | 0.25 ± 0.06 | 0.08 ± 0.03 | 0.44 ± 0.23 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baky, M.H.; Shamma, S.N.; Khalifa, M.R.; Farag, M.A. How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics. Metabolites 2023, 13, 18. https://doi.org/10.3390/metabo13010018
Baky MH, Shamma SN, Khalifa MR, Farag MA. How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics. Metabolites. 2023; 13(1):18. https://doi.org/10.3390/metabo13010018
Chicago/Turabian StyleBaky, Mostafa H., Samir N. Shamma, Mohamed R. Khalifa, and Mohamed A. Farag. 2023. "How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics" Metabolites 13, no. 1: 18. https://doi.org/10.3390/metabo13010018
APA StyleBaky, M. H., Shamma, S. N., Khalifa, M. R., & Farag, M. A. (2023). How Does Allium Leafy Parts Metabolome Differ in Context to Edible or Inedible Taxa? Case Study in Seven Allium Species as Analyzed Using MS-Based Metabolomics. Metabolites, 13(1), 18. https://doi.org/10.3390/metabo13010018








