Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Data Extraction
3. Results
3.1. The Literature Search and Characteristics of Eligible Studies
3.2. Clinical Studies That Evaluated the Effects of Blueberry or Bilberry Consumption on Obesity-, MetS- or T2DM-Related Outcomes
3.3. Studies with Rodent Models That Evaluated the Effects of Blueberry or Bilberry Consumption on Obesity-, MetS- or T2DM-Related Outcomes
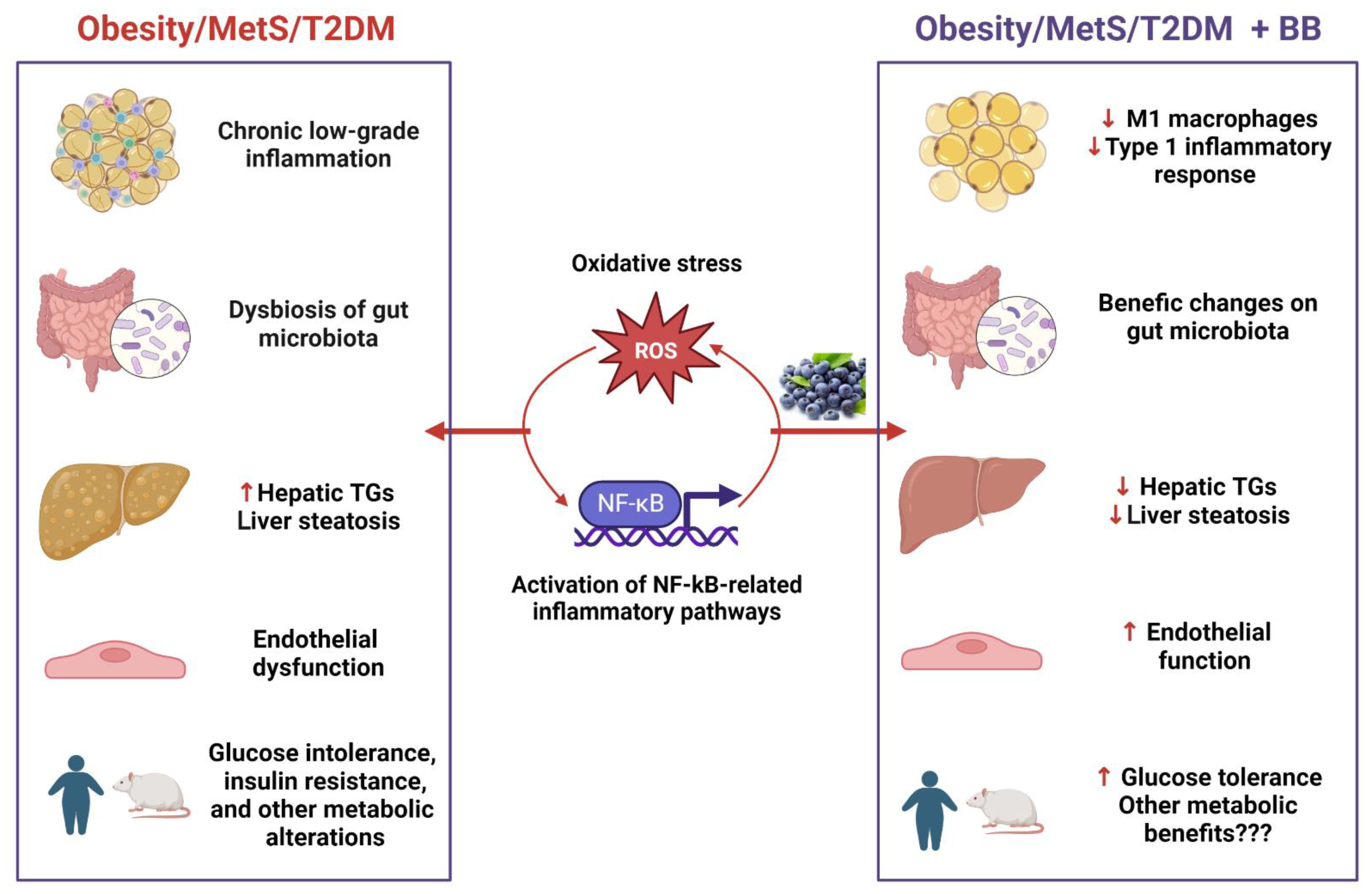
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| BB | Blueberry |
| BiB | Bilberry |
| BW | Body weight |
| BMI | Body mass index |
| BP | Blood pressure |
| CCL2 | C-C motif chemokine ligand 2 |
| MCP1 | Monocyte chemoattractant protein-1 |
| Δ-BW | Delta body weight |
| FFM | Fat-free mass |
| FM | Fat mass |
| FPG | Fasting plasma glucose |
| GLUT | Glucose transporter |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GTT | Glucose tolerance test |
| HbA1c | Glycated hemoglobin |
| HFD | High-fat diet |
| HNE | Hydroxynonenal |
| hs-CRP | High sensitivity C-reactive protein |
| IL | Interleukin |
| IR | Insulin resistance |
| IRS1 | Insulin receptor substrate 1 |
| MDA | Malondialdehyde |
| MetS | Metabolic syndrome |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor-kappa B |
| PPARγ | Peroxisome proliferator-activated receptor-γ |
| RBP4 | Retinol-binding protein 4 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| Th | T helper |
| TG | Triglycerides |
| T2DM | Type 2 diabetes mellitus |
| TNF | Tumor necrosis factor |
| TBARS | Thiobarbituric acid reactive substances |
| V. | Vaccinium |
References
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 26 January 2021).
- Lobstein, T.; Neveux, H.B.; Cavalcanti, O.B.; Barquera, S.; Louise Baur, V.B.; Buse, K.; Dietz, B.; French, A.; Leach, R.J.; Opzeeland, B.; et al. World Obesity Atlas 2022; World Obesity Federation: London, UK, 2022. [Google Scholar]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A.; Myer, P.A.; Singh, G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am. J. Med. 2014, 127, 717–727.e12. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Popkin, B.M. Time use and physical activity: A shift away from movement across the globe. Obes. Rev. 2012, 13, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J.; Balkau, B.; Barengo, N.; Barr, E.; Basit, A.; Bhata, D.; Bommer, C.; Booth, G.; Cariou, B.; et al. IDF Diabetes Atlas 2021, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
- Tian, C.; Hao, L.; Yi, W.; Ding, S.; Xu, F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 7398453. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef]
- Kouki, R.; Schwab, U.; Hassinen, M.; Komulainen, P.; Heikkila, H.; Lakka, T.A.; Rauramaa, R. Food consumption, nutrient intake and the risk of having metabolic syndrome: The DR’s EXTRA Study. Eur. J. Clin. Nutr. 2011, 65, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef]
- Liu, W.; Mao, Y.; Schoenborn, J.; Wang, Z.; Tang, G.; Tang, X. Whole blueberry protects pancreatic beta-cells in diet-induced obese mouse. Nutr. Metab. 2019, 16, 34. [Google Scholar] [CrossRef]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar] [CrossRef]
- Nair, A.R.; Mariappan, N.; Stull, A.J.; Francis, J. Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Food Funct. 2017, 8, 4118–4128. [Google Scholar] [CrossRef]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud.: RDS 2013, 10, 270–282. [Google Scholar] [CrossRef]
- Elks, C.M.; Terrebonne, J.D.; Ingram, D.K.; Stephens, J.M. Blueberries improve glucose tolerance without altering body composition in obese postmenopausal mice. Obesity 2015, 23, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Keirsey, K.I.; Lee, S.; De La Serre, C.B.; Fischer, J.G. Blueberry supplementation alters biomarkers of oxidative stress in high-fat fed rats. FASEB J. 2016, 30, 1174.23. [Google Scholar]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Al-Baghdadi, R.J.T. Effects of refined sugar on some endocrine glands functions in mice. J. Glob. Pharma Technol. 2018, 10, 209–216. [Google Scholar]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield Ii, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Coban, J.; Evran, B.; Ozkan, F.; Cevik, A.; Dogru-Abbasoglu, S.; Uysal, M. Effect of blueberry feeding on lipids and oxidative stress in the serum, liver and aorta of guinea pigs fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem. 2013, 77, 389–391. [Google Scholar] [CrossRef]
- Nair, A.R.; Elks, C.M.; Vila, J.; Del Piero, F.; Paulsen, D.B.; Francis, J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: Potential mechanism of TLR4-MAPK signaling pathway. PLoS ONE 2014, 9, e111976. [Google Scholar] [CrossRef]
- Nunes, S.; Viana, S.D.; Preguiça, I. Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics. Nutrients 2021, 13, 4192. [Google Scholar] [CrossRef]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of Beneficial Metabolic Effects of Berries in High-Fat Fed C57BL/6J Mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Walden, T.B.; Cai, D.; Ahl, D.; Bertilsson, S.; Phillipson, M.; Nyman, M.; Holm, L. Dietary fiber in bilberry ameliorates pre-obesity events in rats by regulating lipid depot, cecal short-chain fatty acid formation and microbiota composition. Nutrients 2019, 11, 1350. [Google Scholar] [CrossRef]
- Mykkänen, O.T.; Kalesnykas, G.; Adriaens, M.; Evelo, C.T.; Törrönen, R.; Kaarniranta, K. Bilberries potentially alleviate stress-related retinal gene expression induced by a high-fat diet in mice. Mol. Vis. 2012, 18, 2338–2351. [Google Scholar] [PubMed]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Zhao, A.; Merrow, T.; Klimis-Zacas, D. The effects of wild blueberry consumption on plasma markers and gene expression related to glucose metabolism in the obese Zucker rat. J. Med. Food 2015, 18, 619–624. [Google Scholar] [CrossRef]
- Cochrane, Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V., Eds. Cochrane 2021. Available online: www.training.cochrane.org/handbook (accessed on 2 August 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brondani, L.A.; Assmann, T.S.; de Souza, B.M.; Boucas, A.P.; Canani, L.H.; Crispim, D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1-3 genes with body mass index variability. PLoS ONE 2014, 9, e96411. [Google Scholar] [CrossRef]
- Brondani, L.A.; de Souza, B.M.; Assmann, T.S.; Boucas, A.P.; Bauer, A.C.; Canani, L.H.; Crispim, D. Association of the UCP polymorphisms with susceptibility to obesity: Case-control study and meta-analysis. Mol. Biol. Rep. 2014, 41, 5053–5067. [Google Scholar] [CrossRef]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry anthocyanin intake attenuates the postprandial cardiometabolic effect of an energy-dense food challenge: Results from a double blind, randomized controlled trial in metabolic syndrome participants. Clin. Nutr. 2022, 41, 165–176. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Stote, K.S.; Wilson, M.M.; Hallenbeck, D.; Thomas, K.; Rourke, J.M.; Sweeney, M.I.; Gottschall-Pass, K.T.; Gosmanov, A.R. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: An 8-week, double-blind, randomized, placebo-controlled trial. Curr. Dev. Nutr. 2020, 4, nzaa030. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Morissette, A.; Kropp, C.; Songpadith, J.P.; Junges Moreira, R.; Costa, J.; Mariné Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiology. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Wilkes, S.E.; Rogers, T.J.; Prior, R.L. Effect of dietary blueberry pomace on selected metabolic factors associated with high fructose feeding in growing Sprague-Dawley rats. J. Med. Food 2012, 15, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Ren, Z.; DeFuria, J.; Obin, M.S.; Meydani, S.N.; Wu, D. Dietary supplementation with blueberry partially restores T-cell-mediated function in high-fat-diet-induced obese mice. Br. J. Nutr. 2018, 119, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, O.T.; Huotari, A.; Herzig, K.H.; Dunlop, T.W.; Mykkänen, H.; Kirjavainen, P.V. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE 2014, 9, e114790. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Beyl, R.A. Blueberries improve whole-body insulin action and alter the development of obesity in high-fat fed mice. FASEB J. 2016, 30, 692.7. [Google Scholar]
- Vendrame, S.; Kristo, A.S.; Schuschke, D.A.; Klimis-Zacas, D. Wild blueberry consumption affects aortic vascular function in the obese Zucker rat. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Et Metab. 2014, 39, 255–261. [Google Scholar] [CrossRef]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 day oral toxicity study of blueberry polyphenols in ovariectomized sprague-dawley rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fischer, J.; Wicker, L. Intermolecular binding of blueberry pectin-rich fractions and anthocyanin. Food Chem. 2016, 194, 986–993. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Ahmet, I.; Spangler, E.; Shukitt-Hale, B.; Juhaszova, M.; Sollott, S.J.; Joseph, J.A.; Ingram, D.K.; Talan, M. Blueberry-enriched diet protects rat heart from ischemic damage. PLoS ONE 2009, 4, e5954. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Ciampa, A.; Ingallina, C.; Mannina, L.; Capitani, D.; Ernesti, I.; Maggi, E.; Businaro, R.; Del Ben, M.; Engel, P.; et al. Blueberry-based meals for obese patients with metabolic syndrome: A multidisciplinary metabolomic pilot study. Metabolites 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Abdel-Karim, N.E. NF-kappaB cellular and molecular regulatory mechanisms and pathways: Therapeutic pattern or pseudoregulation? Cell. Immunol. 2011, 271, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-gamma gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liu, Y.M.; Wang, J.; Wang, X.N.; Li, C.Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kappaB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Paixao, J.; Dinis, T.C.; Almeida, L.M. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem. Biol. Interact. 2012, 199, 192–200. [Google Scholar] [CrossRef]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstol, L.; Laake, P.; Paur, I.; Bohn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett. 2004, 574, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lazze, M.C.; Pizzala, R.; Perucca, P.; Cazzalini, O.; Savio, M.; Forti, L.; Vannini, V.; Bianchi, L. Anthocyanidins decrease endothelin-1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol. Nutr. Food Res. 2006, 50, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef]
- AlShaalan, R.; Aljiffry, M.; Al-Busafi, S.; Metrakos, P.; Hassanain, M. Nonalcoholic fatty liver disease: Noninvasive methods of diagnosing hepatic steatosis. Saudi J. Gastroenterol. 2015, 21, 64–70. [Google Scholar] [CrossRef]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-beta-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Biophys. Acta 2005, 1733, 137–147. [Google Scholar] [CrossRef]
- Xia, M.; Hou, M.; Zhu, H.; Ma, J.; Tang, Z.; Wang, Q.; Li, Y.; Chi, D.; Yu, X.; Zhao, T.; et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: The role of the peroxisome proliferator-activated receptor gamma-liver X receptor alpha-ABCA1 pathway. J. Biol. Chem. 2005, 280, 36792–36801. [Google Scholar] [CrossRef]
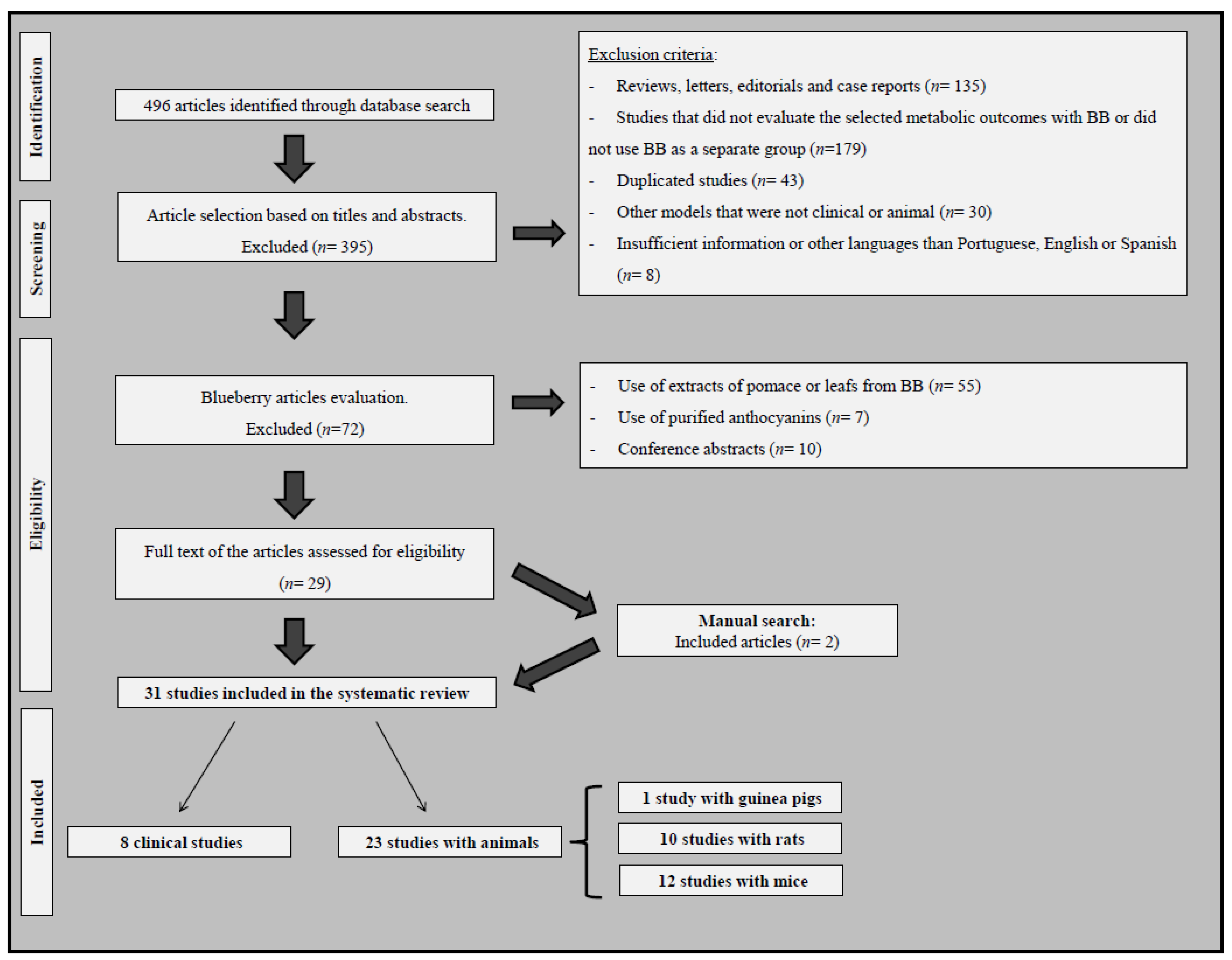
| 1st Author, Year (Ref) | Sample | Blueberry Treatment | Follow-Up | Results (BB vs. Placebo/Control Group) |
|---|---|---|---|---|
| Basu, 2010 [16] | 48 subjects with MetS: BB group (n = 25) vs. controls (n = 23) | 50 g freeze-dried BB (V. angustifolium/V. corymbosum L.) in a beverage, daily | 8 weeks | ↔ No differences in BW, WC, HbA1c, HOMA-IR, FPG, lipid profile, IL6, adiponectin, and hs-CRP; ↓ BP, oxidized LDL, and lipid peroxidation (MDA and HNE); |
| Curtis, 2019 [18] | 115 subjects with MetS: 150 g BB (n = 37), 75 g BB (n = 39) vs. controls (n = 39) | 75 g or 150 g fresh BB | 6 months | ↔ No differences in BP, TC, LDL, FPG, HbA1c, insulin, and IR; ↑ HDL (150 g) and TGs (75 g); improved endothelial function (150 g); |
| Curtis, 2022 [43] | 45 subjects with MetS: BB (n = 23) vs. controls (n = 22) | 26 g of freeze-dried BB (= 150 g of fresh BB) | 24 h | ↔ TG and LDL, BP and Apo-B; ↓ Postprandial glucose (0–24 h), insulin, total cholesterol, and Apo-A1; ↑ HDL; improved endothelial function; |
| Kolehmainen, 2012 [19] | 27 subjects with MetS: BiB diet (n = 15) vs. controls (n = 12) | 400 g fresh BiB [200 g BiB puree + 40 g dried BiB (= 200 g fresh BiB; V. myrtillus), daily | 8 weeks | ↔ No differences in BW, lipid profile, FPG, adiponectin and leptin; ↓ inflammation score, hs-CRP, IL6, and IL12, expression of MMD and CCR2 (monocyte and macrophage function) in PBMCs; |
| Nair, 2017 [22] | 27 subjects with MetS: BB (n = 15) vs. controls (n = 12) | 22.5 g freeze-dried BB, twice a day | 6 weeks | ↓ Superoxide and total ROS (blood and monocytes), TNF, IL6 and TLR4 expressions (monocytes), and GMCSF (serum inflammatory marker); ↑ myeloid dendritic cells; |
| Stote, 2020 [46] | 52 men with T2DM: BB (n = 26) vs. placebo (n = 26) | 22 g freeze dried BB, daily (V. virgatum/V. corymbosum) | ↔ No differences in FPG, insulin, total cholesterol, LDL, HDL, and hs-CRP, BP, and BW; ↓ HbA1c, fructosamine, TG, AST, and ALT; | |
| Stull, 2010 [17] | 32 individuals with obesity and IR: BB (n = 15) vs. controls (n = 17) | 22.5 g freeze-dried BB in a smoothie (V. myrtillus/V. corymbosum, 1:1), twice a day (45 g/day) | 6 weeks | ↔ No differences in BW, BMI, FFM, FM, inflammatory markers (hs-CRP, TNF and MCP-1), lipid profile, insulin, and BP; ↑ insulin sensitivity; |
| Stull, 2015 [47] | 44 individuals with MetS: BB (n = 23) vs. control (n = 21) | 22.5 g freeze-dried BB in a smoothie (V. myrtillus/V. corymbosum, 1:1), twice a day (45 g/day) | 6 weeks | ↔ No differences in BW, BMI, FFM, FM, BP, FPG, insulin, and lipid profile; improved endothelial function. |
| Outcomes | Human | Mouse, Rat, or Guinea Pig | Conclusion |
|---|---|---|---|
| Lipid Profile | |||
| TGs (plasma/serum) | ↔ 5 studies [16,17,19,43,47] ↑ One study [18] ↓ One study [46] | ↔ 5 studies [23,31,34,35,37] ↓ 5 studies [21,24,26,33,49] | ↔ Humans Inconclusive in rodents |
| Hepatic TG content | No study | ↔ Two studies [23,48] ↓ 4 studies [24,31,33,34] | ↓ Rodents |
| Total cholesterol | ↔ 6 studies [16,17,18,19,46,47] ↓ One study [43] | ↔ 5 studies [26,34,37,48,51] ↓ 6 studies [21,23,31,35,49]/↓ liver [34] | ↔ Humans Inconclusive in rodents |
| LDL | ↔ 7 studies [16,17,18,19,43,46,47] | ↔ One study [34] ↓ 2 studies [23,31] | ↔ Humans Inconclusive in rodents |
| HDL | ↔ 5 studies [16,17,19,46,47] ↑ 2 studies [18,43] | ↔ 4 studies [21,26,31,34] ↓ One study [23] | ↔ In general |
| Hepatic steatosis and liver enzymes | ↓ ALT/AST [46] | ↓ Steatosis—3 studies [24,33,35] ↓ ALT/AST [31] ↔ ALT/AST [48] | Inconclusive in humans ↓ Rodents |
| Inflammatory markers | |||
| IL-1β | No study | ↔ 2 studies [33,51] ↓ 2 studies [28,32] | Inconclusive in general |
| IL-6 | ↔ One study [16] ↓ 2 studies [19,22] | ↔ 5 studies [26,28,29,48,51] ↓ One study [30] | Inconclusive in humans ↔ Rodents |
| TNF | ↔ One study [17] ↓ One study [22] | ↔ 4 studies [26,33,48,51] ↓ 3 studies [28,29,30] | Inconclusive in general |
| Nf-kb | No study | ↓ 3 studies [28,30,32] ↔ One study [33] | ↓ Rodents |
| Leptin (pro-inflammatory) | ↔ One study [19] | ↔ 3 studies [29,49,51] ↓ 3 studies [27,37,45] | Inconclusive in general |
| Adiponectin (anti-inflammatory) | ↔ 2 studies [16,19] | ↔ 5 studies [23,24,29,45,51] ↑ One study [30] | ↔ In general |
| BP and endothelial function | |||
| BP | ↔ 5 studies [17,18,43,46,47] ↓ One study [16] | ↔ 2 studies [23,36] ↓ 2 studies [32,51] | ↔ Humans Inconclusive in rodents |
| Endothelial function | ↑ 3 studies [18,43,47] | No study | ↑ Humans |
| Oxidative stress and antioxidant status | |||
| Oxidative stress markers | ↓ 2 studies—MDA and HNE [16], total ROS and superoxide [22] | ↔ One study: TBARS [48] ↓ 4 studies: MDA [28,31,33], ROS, superoxide, and peroxynitrite [32] | ↓ In general |
| Antioxidant enzymes | No study | ↔ One study: GSH, GSH-Px, SOD [31] ↑ 4 studies: GST [31], GSH-Px [29], GSH [33], SOD and catalase [32] | ↑ In rodents |
| Glycemic profile and insulin sensitivity | |||
| Glucose (FBG, FPG or postprandial glucose levels) | ↔ 6 studies [16,17,18,19,46,47] ↓ One study [43] | ↔ 12 studies [20,23,26,28,33,34,36,37,38,44,48,49] ↓ 5 studies: fasting [23,24,29,51] * and fed [33] | ↔ In general |
| HbA1c | ↔ 2 studies [16,18] ↓ One study [46] | ↔ One study [23] ↓ One study [38] | Inconclusive in general |
| Glucose tolerance (↓ AUCglucose GTT) | ↑ 2 studies [17,47] | ↔ 4 studies [23,44,48,49] ↑ 10 studies [20,24,26,27,28,29,32,33,44,51] | ↑ In general |
| Insulin resistance (HOMA-IR or ITT) | ↔ 2 studies [16,18] ↓ One study [17] | ↔ 5 studies [23,37,38,48,51] ↓ 7 studies [20,26,28,29,33,34,49] | Inconclusive in general |
| Insulin levels | ↔ 3 studies [17,18,47] ↓ One study [43] | ↔ 7 studies [23,28,29,33,37,38,48] ↓ 4 studies [20,26,34,49] | ↔ Humans Inconclusive in rodents |
| Body measures | |||
| Body weight, BMI, or weight gain | ↔ 5 studies [16,17,19,46,47] | ↔ 17 studies [20,21,23,24,26,28,29,30,32,33,36,37,38,45,48,49,51] ↑ 2 studies [44,50] ↓ 4 studies [27,34,35,51] | ↔ In general |
| Fat mass | ↔ 2 studies [17,47] | ↔ 8 studies [24,26,28,29,37,48,49,51] ↑ 2 studies [44,50] ↓ 2 studies [34,45] | ↔ In general |
| Fat-free mass | ↔ 2 studies [17,47] | ↔ 5 studies [24,26,34,37,48] ↑ 2 studies [44,45] | ↔ In general |
| 1st Author, Year (Ref) | Sample | Treatment | Follow-Up | Results (BB or BiB vs. Diet-Induced Obesity) |
|---|---|---|---|---|
| Mouse | ||||
| Al-Baghdadi, 2018 [27] | Wild type mice: HSD (n = 8) vs. HSD + BB (n = 8) | 4% of total diet BB (V. corymbosum L.) powder | 3 months | Improved glucose tolerance (GTT); ↓ BW and Lep expression in AT; |
| De Furia, 2009 [29] | Male C57Bl/6J mice: HFD (n = 8) vs. HFD + BB (n = 8) | 4% wt:wt freeze-dried BB powder (V. ashei and V. corymbosum 1:1) | 8 weeks | ↔ No differences in BW, FM, insulin, and AdipoQ, Lep, Ccl2 (Mcp1), Il6, and iNos expressions in AT; ↓ blood glucose, AUCglucose GTT, recruitment of pro-inflammatory M1 macrophages (Cd11c+/Mgl-1-) in AT, Tnf and Il10 expressions in AT, adipocyte death, and IR; ↑ Gsh-Px expression in AT; |
| Elks, 2015 [24] | 4-weeks female C57Bl/6J mice with VCB-induced menopause: HFD + VCB (n = 6) vs. HFD + VCB + BB (n = 8) | 4% wt/wt BB powder (V. ashei) | 12 weeks | ↔ No differences in BW, FM, FFM, and adiponectin; ↓ hepatic steatosis and TGs (serum and liver), fasting blood glucose, and AUCglucose GTT; ↑ expression of hepatic fatty-acid oxidation-related genes (Cs, Hadha, and Cd36); |
| Heyman, 2014 [34] | Male C57BL/6J mice: HFD (n = 12) vs. HFD + BiB (n = 12) | 20% wt:wt freeze-dried BiB powder (V. myrtillus) | 13 weeks | ↔ No differences in FFM, FPG, TC, LDL, HDL, TGs, and NEFAs (serum); ↓ BW, FM, IR, insulin, Pai-1, ALT, and hepatic cholesterol and TGs; |
| Lewis, 2018 [50] | Male C57BL/6 mice: HFD (n = 9–11) vs. HFD + BB (n = 9–11) | 4% (wt/wt) freeze-dried BB (V. ashei and V. corymbosum 1:1) | 8 or 12 weeks | ↑ BW, FM, and T cell proliferation; |
| Liu, 2019 [20] | Male C57BL/6 mice: HFD (n = 5) vs. HFD + BB (n = 5) | 4% (wt/wt) non-specified freeze-dried BB | 14 weeks | ↔ No differences in BW and fasting blood glucose; ↓ β-cell expansion, AUCglucose GTT, insulin, and IR; ↑ β-cell survival; |
| Morissette, 2020 [48] | C57BL/6 male mice: HFHS diet (n = 13) vs. HFHS + BB (n = 14) | 4% (wt/wt) freeze-dried BB powder (V. ashei and V. corymbosum 1:1) | 12 weeks | ↔ No differences in BW, FM, FFM, fasting blood glucose, AUCglucose GTT, insulin, IR, hepatic TGs, TC, AST and ALT, TBARS * and Il2, Il6, Ifnγ, Tnf, Mcp1, and Rantes expressions in AT; |
| Mykkänen, 2012 [36] | Male C57BL/6 mice: HFD (n = 6) vs. HFD + BB (n = 6) | 5% (wt/wt) freeze-dried powder (V. myrtillus) | 12 weeks | ↔ No differences in BW, weight gain, fasting blood glucose, FFAs, and systolic BP; |
| Mykkänen, 2014 [51] | Male C57BL mice: HFD (n = 20–27) vs. HFD + 5% BiB (n = 20–27) vs. HFD + 10% BiB (n = 20–27) | 5% and 10% (wt/wt) freeze-dried BiB powder (V. myrtillus) | 12 weeks | ↔ No differences in BW, FM, serum adiponectin, leptin, TC, AUCglucose GTT, IR, Tnf, Il1b, Il12, Il7, Il6, and Gm-Csf; ↓ blood glucose, serum resistin and Mcp1 (5 and 10%), Δ-BW (10%), type-1 pro-inflammatory responsiveness (↓ NKT, Th1, Th1/Th2, and Ifnγ-producing T cells—10% BiB), and systolic BP; |
| Prior, 2008 [44] | Male C57BL/6J mice: HFD (n = 12) vs. HFD + BB (n = 12) | 10% (wt/wt) non-specified freeze-dried whole BB | 92 days | ↔ No differences in fasting blood glucose and AUCglucose GTT; ↑ BW, FM, and FFM; |
| Prior, 2010 [37] | Male C57BL/6J mice: HFD vs. HFD + BB | 10% (wt/wt) non-specified BB juice | 72 days | ↔ No differences in BW, weight gain, FFM, FM, TC, TGs, and glucose, insulin, and IR; ↓ serum leptin levels; |
| Skates, 2018 [45] | Male C57BL/6J mice: HFD (n = 12) vs. HFD + BB (n = 8 per group) | Non specified freeze-dried whole BB powder (normalized to 400 µg/g total anthocyanins) | 12 weeks | ↔ BW, AdipoQ, Fasn, Cpt1a, Pparg, and Ppargc1a expressions (AT); ↓ Lep expression (AT) and FM; ↑ FFM, VO2 and energy expenditure; |
| Rats | ||||
| Brader, 2013 [23] | Male Zucker diabetic fatty rats: Control (n = 12) vs. BiB diet (n = 12) | 15g of standard chow substituted with freeze-dried BiB (V. myrtillus) | 8 weeks | ↔ No differences in BW, BP, TGs (plasma and liver), FFAs, HbA1c, FPG, AUCglucoce GTT, insulin, adiponectin, and IR; ↓ whole blood glucose, TC, HDL, LDL, and Glut2, Irs1, Jnk1, Lxr-α, and Gfat expressions (liver); ↑ Glut4 expression (AT); |
| Khanal, 2012 [49] | Male Sprague Dawley rats: HFrD (n = 6) vs. HFrD + 1.5% BB pomace (n = 6 + 6) vs. HFrD + 3% BB pomace (n = 6 + 6) | Extruded (n = 6) and unextruded (n = 6) pomace from non-specified freeze-dried BB | 8 weeks | ↔ No differences in BW, FM, FPG and leptin, and AUCglucose GTT, ↓ TGs, insulin and TC, and IR (3%); |
| Lee, 2018 [28] | Male Wistar rats: HFD (n = 8) vs. HFD + BB (n = 8) | 10% (wt/wt) freeze-dried BB (V. ashei + V. corymbosum 1:1) | 8 weeks | ↔ No differences in BW, FM, FPG and insulin, AUCglucose GTT, and Il6 expression in AT; ↓ Tnf, Il1B, and Cd11d expressions (AT), phospho to total Nf-kb p65 (AT), and MDA ** (liver); ↑ Pparα and Pparδ expressions (AT); improvement of insulin sensitivity markers; |
| Liu, 2019 [35] | Male Sprague Dawley rats: HFD (n = 8) vs. HFD + BiB (n = 8) | 7% of the dietary fiber (dry weight basis) of V. mirtyllus | 8 weeks | ↔ No differences in TGs, FFAs, and hepatic Pparα, Pparγ, Fasn, Fabp5, and Cpt1α expressions; ↓ BW, hepatic steatosis, and TC; ↑ iBAT mass and Ucp1 expression in iBAT; |
| Nair, 2014 [32] | Obese Zucker rats (OZR): Control diet (n = 7) vs. BB diet (n = 7) | 2% non-specified freeze-dried BB | 15 weeks | ↔ No differences in BW; ↓ mean BP, AUCglucose GTT, kidney expressions of Il1β, Il18, TgfB, Tlr4 and p38/Mapk phosphorylation, Nf-kb activity, total ROS, superoxide and peroxynitrite ***, UAE levels, glomerular sclerosis, and interstitial nephritis; ↑ eGFR, Sod and catalase; |
| Nunes, 2021 [33] | Male Wistar rats: HSuHF + BB juice (n = 10) vs. HSuHF (n = 10) vs. CTRL (n = 8) | 25 g/kg BW/day of BB juice (BB blended with 35% sucrose solution) (V. corymbosum) | ↔ No differences in BW, Δ-BW, fasting insulin and glucose; Il10, Adipor1 e 2, Tnf, Fasn, Ifnγ, Il1β, Nf-kb, Irs1, Insr, and Stat3 expressions; ↓ fed insulin and glucose, AUCglucose GTT, IR, serum and hepatic TGs, MDA and MDA/total antioxidant status ratio ****, hepatic steatosis; ↑ hepatic GSH; | |
| Seymour, 2011 [26] | Male OZR: HFD (n = 12) vs. HFD + BB (n = 12) | 2% (wt/wt) freeze-dried whole BB powder (V. ashei + V. corymbosum) | 90 days | ↔ No differences in BW, FFM, FM, fasting blood glucose, CT, FFAs, HDL, Tnf, and Il6; ↓ TGs, insulin, IR, AUCglucose GTT; ↑ Ppara (AT), Ppargc1a, Glut4, Irs1, and Fasn (AT and skeletal muscle), and Pparγ and Ucp3 (skeletal muscle) expressions; |
| Vendrame, 2013 [30] | Male OZR: Control diet (n = 10) vs. BB diet (n = 10) | 8% wt/wt freeze-dried BB powder (V. angustifolium) | 8 weeks | ↔ No differences in BW and weight gain; ↓ Tnf and Il6 (plasma, liver, and AT), CRP (plasma and liver), and Nf-kb levels (AT and liver); ↑ plasma adiponectin; |
| Vendrame, 2014 [21] | Male OZR: Control diet (n = 10) vs. BB diet (n = 10) | 8% wt/wt freeze-dried BB powder (V. angustifolium) | 8 weeks | ↔ No differences in BW, HDL, Ppara, Pparg, and Abca1 expressions (liver); ↓ TGs, TC, and Srebp1, and Fasn expressions (AT and liver), ↑ Pparγ, Pparα, and Abca1 expressions (AT); |
| Vendrame, 2015 [38] | Male OZR: Control diet (n = 10) vs. BB diet (n = 10) | 8% wt/wt non-specified freeze-dried BB powder | 8 weeks | ↔ No differences in BW, IR, fasting blood glucose and insulin, and Glut4 expression (liver/AT); ↓ HbA1c, Rbp4 and resistin (plasma), Rbp4 (liver and AT) and resistin (liver) expressions; |
| Guinea pig | ||||
| Çoban, 2013 [31] | Male Dankin Hartley guinea pigs: HCD (n = 6) vs. HCD + BB (n = 6) | 8% wt/wt whole BB (V. corymbosum L.) powder | 75 days | ↔ HDL and TGs (serum), and GSH, GSH-Px, and SOD (liver); ↓ TC (serum, liver and aorta), LDL, ALT and AST (serum), and TGs and MDA **** (liver); ↑ GST (liver); |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.S.; Pellenz, F.M.; de Souza, B.M.; Crispim, D. Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review. Metabolites 2023, 13, 19. https://doi.org/10.3390/metabo13010019
de Oliveira MS, Pellenz FM, de Souza BM, Crispim D. Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review. Metabolites. 2023; 13(1):19. https://doi.org/10.3390/metabo13010019
Chicago/Turabian Stylede Oliveira, Mayara Souza, Felipe Mateus Pellenz, Bianca Marmontel de Souza, and Daisy Crispim. 2023. "Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review" Metabolites 13, no. 1: 19. https://doi.org/10.3390/metabo13010019
APA Stylede Oliveira, M. S., Pellenz, F. M., de Souza, B. M., & Crispim, D. (2023). Blueberry Consumption and Changes in Obesity and Diabetes Mellitus Outcomes: A Systematic Review. Metabolites, 13(1), 19. https://doi.org/10.3390/metabo13010019






