Potential Role of Copper in Diabetes and Diabetic Kidney Disease
Abstract
1. Introduction
2. Role of Copper on DM and DKD: Animal Studies
3. Copper in T1DM Patients
4. Copper in T2DM Patients
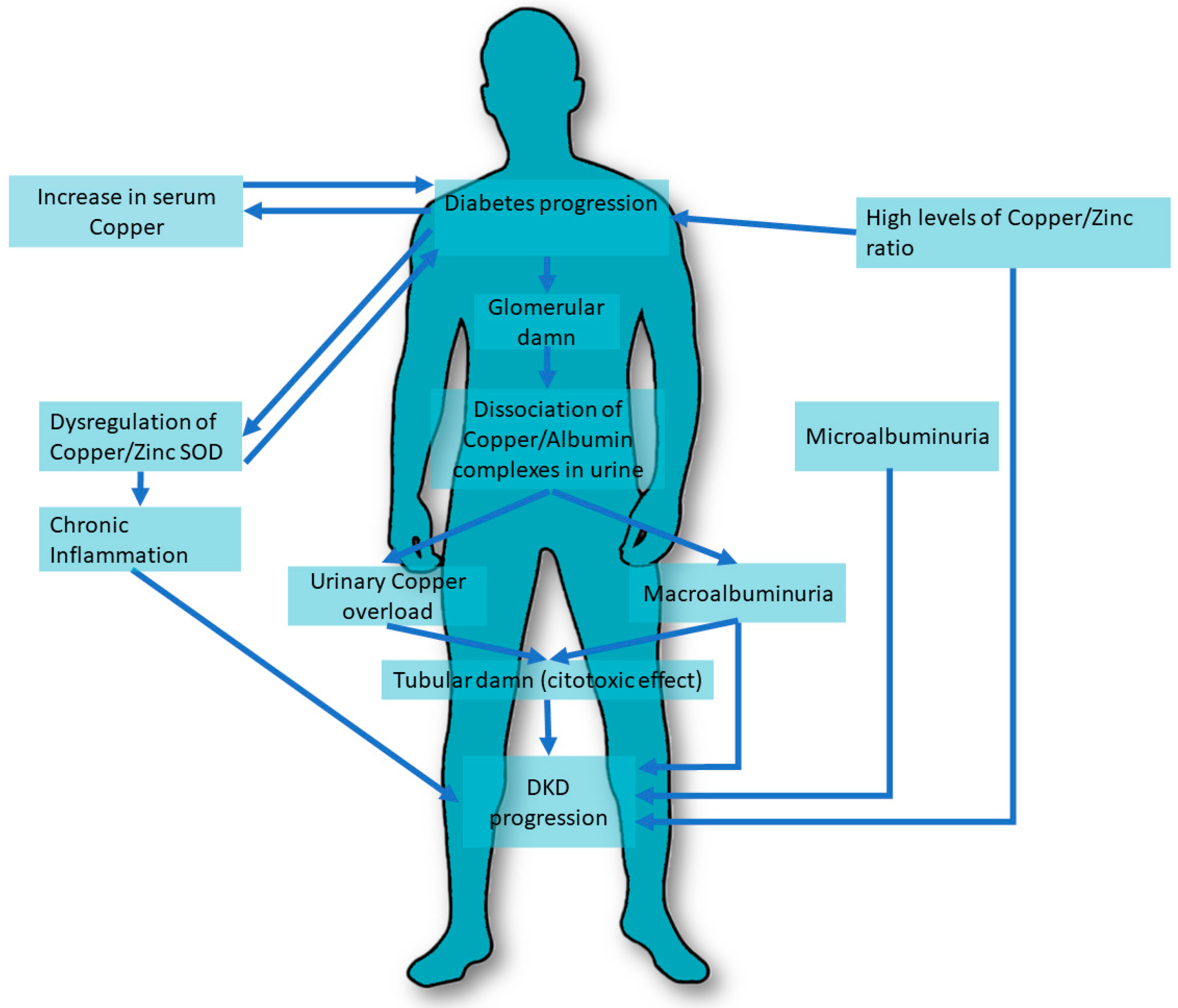
5. Role of Copper in Diabetic Kidney Disease
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CKD | Chronic Kidney Disease |
| Cu | Copper |
| DM | Diabetes Mellitus |
| DKD | Diabetic Kidney Disease |
| GFR | glomerular filtration rate |
| GDM | gestational diabetes mellitus |
| (HbA1c) | Glycosylated hemoglobin (HbA1c) |
| HOMA-β | homeostasis model assessment pancreatic beta cell function |
| HOMA-IR | homeostasis model assessment—insulin resistance |
| HOMA-S | Homeostasis model assessment—insulin sensitivity |
| LDL | low-density lipoprotein |
| Mg | Magnesium |
| Ors | Odds Ratio |
| O2 | oxygen |
| OS | Oxidative stress |
| PAH | sodium p-aminoippurate |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| SMAD | homologous MAD (SMAD) |
| SOD | superoxide dismutase |
| STZ | streptozocin (STZ) |
| TGF-β | Transforming growth factor β |
| (TETA) | thethylenetetramine |
| TINag | antigen of tubulointerstitial nephritis (TINag) |
| Tm | maximal tubular concentration |
| TNF-α | tumor necrosis factor-alpha |
| T1DM | Type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| VDAC | selective voltage-dependent anion channel (VDAC) |
| Zn | Zinc |
References
- Crichton, R.R.; Pierre, J.L. Old iron, young copper: From Mars to Venus. Biometals 2001, 14, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board of the Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2000; Available online: https://www.nap.edu/catalog/10026/dietary-reference-intakes-for-vitamin-a-vitamin-k-arsenic-boron-chromium-copper-iodine-iron-manganese-molybdenum-nickel-silicon-vanadium-and-zinc (accessed on 1 October 2022).
- Johnson, M.A.; Kays, S.E. Copper: Its role in human nutrition. Nutr. Today 1990, 25, 6. [Google Scholar] [CrossRef]
- Wapnir, R.A. Copper absorption and bioavailability. Am. J. Clin. Nutr. 1998, 67 (Suppl. 5), 1054S–1060S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Mason, K.E. A conspectus of research on copper metabolism and requirements of man. J. Nutr. 1979, 109, 1979–2066. [Google Scholar] [CrossRef]
- Linder, M.C.; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. Copper transport. Am. J. Clin. Nutr. 1998, 67 (Suppl. 5), 965S–971S. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper in medicine. Curr. Opin. Chem. Biol. 2003, 7, 207–212. [Google Scholar] [CrossRef]
- Prohaska, J.R. Biochemical functions of copper in animals. In Essential and Toxic Trace Elements in Human Health and Disease; Prasad, A.S., Ed.; Alan R Liss: New York, NY, USA, 1988. [Google Scholar]
- Danks, D.M. Copper deficiency in humans. Annu. Rev. Nutr. 1988, 8, 235–257. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Gembillo, G.; Ingrasciotta, Y.; Crisafulli, S.; Luxi, N.; Siligato, R.; Santoro, D.; Trifirò, G. Kidney Disease in Diabetic Patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int. J. Mol. Sci. 2021, 22, 4824. [Google Scholar] [CrossRef]
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef]
- Amatruda, M.; Gembillo, G.; Giuffrida, A.E.; Santoro, D.; Conti, G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina 2021, 57, 868. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Y.; Zhang, Y.Y.; Zhu, Z.; Zhang, X.Q.; Liu, X.; Zhu, S.Y.; Song, Y.; Jin, X.; Lindholm, B.; Yu, C. Elevated intracellular copper contributes a unique role to kidney fibrosis by lysyl oxidase mediated matrix crosslinking. Cell Death Dis. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Iyanda, A.A.; Anetor Adeniyi, F.A. Altered copper level and renal dysfunction in Nigerian women using skin-whitening agents. Biol. Trace Elem. Res. 2011, 143, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Sondheimer, J.H.; Mahajan, S.K.; Rye, D.L.; Abu-Hamdan, D.K.; Migdal, S.D.; Prasad, A.S.; McDonald, F.D. Elevated plasma copper in chronic renal failure. Am. J. Clin. Nutr. 1988, 47, 896–899. [Google Scholar] [CrossRef]
- Ahmad, S.; Ärnlöv, J.; Larsson, S.C. Genetically Predicted Circulating Copper and Risk of Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 509. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lin, Y.; Meng, L.; Peng, L.; Zhang, H.; Zhang, X.; Jin, M.; Wang, J.; Zhang, Y.; Tang, M.; et al. Association of copper exposure with prevalence of chronic kidney disease in older adults. Clin. Nutr. 2022, 41, 2720–2728. [Google Scholar] [CrossRef]
- Smpokou, E.T.; González-Quiroz, M.; Martins, C.; Alvito, P.; Le Blond, J.; Glaser, J.; Aragón, A.; Wesseling, C.; Nitsch, D.; Pearce, N.; et al. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occup. Environ. Med. 2019, 76, 920–926, Erratum in Occup. Environ. Med. 2020, 77, 586. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Rucker, R.B.; Commisso, J.F.; Keen, C.L. Diabetes and dietary copper alter 67Cu metabolism and oxidant defense in the rat. J. Nutr. Biochem. 2005, 16, 312–320. [Google Scholar] [CrossRef]
- Gómez, T.; Bequer, L.; Mollineda, A.; Molina, J.L.; Álvarez, A.; Lavastida, M.; Clapés, S. Concentration of Zinc, Copper, Iron, Calcium, and Magnesium in the Serum, Tissues, and Urine of Streptozotocin-Induced Mild Diabetic Rat Model. Biol. Trace Elem. Res. 2017, 179, 237–246. [Google Scholar] [CrossRef]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.A.; Matsuhisa, M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gong, D.; Choong, S.Y.; Xu, H.; Chan, Y.K.; Chen, X.; Fitzpatrick, S.; Glyn-Jones, S.; Zhang, S.; Nakamura, T.; et al. Copper(II)-selective chelation improves function and antioxidant defences in cardiovascular tissues of rats as a model of diabetes: Comparisons between triethylenetetramine and three less copper-selective transition-metal-targeted treatments. Diabetologia 2010, 53, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Lu, J.; Chen, X.; Reddy, S.; Crossman, D.J.; Glyn-Jones, S.; Choong, Y.S.; Kennedy, J.; Barry, B.; Zhang, S.; et al. A copper(II)-selective chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic TGF-beta activation in the kidneys of rats used as a model of diabetes. Diabetologia 2008, 51, 1741–1751. [Google Scholar] [CrossRef]
- Gong, D.; Chen, X.; Middleditch, M.; Huang, L.; Vazhoor Amarsingh, G.; Reddy, S.; Lu, J.; Zhang, S.; Ruggiero, K.; Phillips, A.R.; et al. Quantitative proteomic profiling identifies new renal targets of copper(II)-selective chelation in the reversal of diabetic nephropathy in rats. Proteomics 2009, 9, 4309–4320. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.A.; Uriu-Hare, J.Y.; Rucker, R.B.; Keen, C.L. Effect of maternal diabetes and dietary copper on fetal development in rats. Reprod. Toxicol. 1993, 7, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Saunders, G.K. The effects of excess renal copper on kidney function in the diabetic rat. Res. Commun. Chem. Pathol. Pharm. 1986, 52, 45–49. [Google Scholar]
- Squitti, R.; Negrouk, V.; Perera, M.; Llabre, M.M.; Ricordi, C.; Rongioletti, M.C.A.; Mendez, A.J. Serum copper profile in patients with type 1 diabetes in comparison to other metals. J. Trace Elem. Med. Biol. 2019, 56, 156–161. [Google Scholar] [CrossRef]
- Viktorínová, A.; Toserová, E.; Krizko, M.; Duracková, Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism 2009, 58, 1477–1482. [Google Scholar] [CrossRef]
- Zargar, A.H.; Bashir, M.I.; Masoodi, S.R.; Laway, B.A.; Wani, A.I.; Khan, A.R.; Dar, F.A. Copper, zinc and magnesium levels in type-1 diabetes mellitus. Saudi Med. J. 2002, 23, 539–542. [Google Scholar]
- Raudenska, M.; Dvorakova, V.; Pacal, L.; Chalasova, K.; Kratochvilova, M.; Gumulec, J.; Ruttkay-Nedecky, B.; Zitka, O.; Kankova, K.; Adam, V.; et al. Levels of heavy metals and their binding protein metallothionein in type 2 diabetics with kidney disease. J. Biochem. Mol. Toxicol. 2017, 31, e21891. [Google Scholar] [CrossRef]
- Naka, T.; Kaneto, H.; Katakami, N.; Matsuoka, T.A.; Harada, A.; Yamasaki, Y.; Matsuhisa, M.; Shimomura, I. Association of serum copper levels and glycemic control in patients with type 2 diabetes. Endocr. J. 2013, 60, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Zargar, A.H.; Shah, N.A.; Masoodi, S.R.; Laway, B.A.; Dar, F.A.; Khan, A.R.; Sofi, F.A.; Wani, A.I. Copper, zinc, and magnesium levels in non-insulin dependent diabetes mellitus. Postgrad. Med. J. 1998, 74, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, S.K.; Parmar, K.S.; Ahmad, M.K.; Sonkar, G.K.; Gautam, M. An observational study to estimate the level of essential trace elements and its implications in type 2 diabetes mellitus patients. J. Fam. Med. Prim. Care 2021, 10, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.C.; Shih, Y.H.; Bryan, M.S.; Jackson, B.P.; Aguilar, D.; Hanis, C.L.; Argos, M.; Sargis, R.M. Relationships Between Urinary Metals and Diabetes Traits Among Mexican Americans in Starr County, Texas, USA. Biol. Trace Elem. Res. 2022. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Stancic, A.; Rasic-Milutinovic, Z.; Perunicic-Pekovic, G.; Buzadzic, B.; Korac, A.; Otasevic, V.; Jankovic, A.; Vucetic, M.; Korac, B. Relation of CuZnSOD activity with renal insufficiency in hypertensive diabetic patients. Indian J. Biochem. Biophys. 2012, 49, 97–100. [Google Scholar]
- Ito, S.; Fujita, H.; Narita, T.; Yaginuma, T.; Kawarada, Y.; Kawagoe, M.; Sugiyama, T. Urinary copper excretion in type 2 diabetic patients with nephropathy. Nephron 2001, 88, 307–312. [Google Scholar] [CrossRef]
- Al-Bayati, M.A.; Jamil, D.A.; Al-Aubaidy, H.A. Cardiovascular effects of copper deficiency on activity of superoxide dismutase in diabetic nephropathy. N. Am. J. Med. Sci. 2015, 7, 41–46. [Google Scholar] [CrossRef]
- Talaei, A.; Jabari, S.; Bigdeli, M.H.; Farahani, H.; Siavash, M. Correlation between microalbuminuria and urinary copper in type two diabetic patients. Indian J. Endocrinol. Metab. 2011, 15, 316–319. [Google Scholar] [CrossRef]
- Gembillo, G.; Visconti, L.; Giuffrida, A.E.; Labbozzetta, V.; Peritore, L.; Lipari, A.; Calabrese, V.; Piccoli, G.B.; Torreggiani, M.; Siligato, R.; et al. Role of Zinc in Diabetic Kidney Disease. Nutrients 2022, 14, 1353. [Google Scholar] [CrossRef]
- Hamasaki, H.; Kawashima, Y.; Yanai, H. Serum Zn/Cu Ratio Is Associated with Renal Function, Glycemic Control, and Metabolic Parameters in Japanese Patients with and without Type 2 Diabetes: A Cross-sectional Study. Front. Endocrinol. 2016, 7, 147. [Google Scholar] [CrossRef]
- Takao, T.; Yanagisawa, H.; Suka, M.; Yoshida, Y.; Onishi, Y.; Tahara, T.; Kikuchi, T.; Kushiyama, A.; Anai, M.; Takahashi, K.; et al. Synergistic association of the copper/zinc ratio under inflammatory conditions with diabetic kidney disease in patients with type 2 diabetes: The Asahi Diabetes Complications Study. J. Diabetes Investig. 2022, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Isbir, T.; Tamer, L.; Taylor, A.; Isbir, M. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Res. 1994, 26, 41–45. [Google Scholar] [PubMed]
- Prabodh, S.; Prakash, D.S.; Sudhakar, G.; Chowdary, N.V.; Desai, V.; Shekhar, R. Status of copper and magnesium levels in diabetic nephropathy cases: A case-control study from South India. Biol. Trace Elem. Res. 2011, 142, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Laouali, N.; MacDonald, C.J.; Shah, S.; El Fatouhi, D.; Mancini, F.R.; Fagherazzi, G.; Boutron-Ruault, M.C. Dietary Copper/Zinc Ratio and Type 2 Diabetes Risk in Women: The E3N Cohort Study. Nutrients 2021, 13, 2502. [Google Scholar] [CrossRef]
- Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Rink, L.; Mariani, E.; Fulop, T.; Dedoussis, G.; Herbein, G.; Provinciali, M.; et al. Main biomarkers associated with age-related plasma zinc decrease and copper/zinc ratio in healthy elderly from ZincAge study. Eur. J. Nutr. 2017, 56, 2457–2466. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M.; Lattanzio, F.; Piacenza, F.; Basso, A.; Abbatecola, A.M.; Russo, A.; Giovannini, S.; Capoluongo, E.; Bustacchini, S.; et al. Cu to Zn ratio, physical function, disability, and mortality risk in older elderly (ilSIRENTE study). Age 2012, 34, 539–552. [Google Scholar] [CrossRef]
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| 14–18 years | 890 mcg | 890 mcg | 1000 mcg | 1300 mcg |
| 19+ years | 900 mcg | 900 mcg | 1000 mcg | 1300 mcg |
| ANIMAL STUDIES | Study, Year | Population | Control | Results |
| Saunders et al. [29] 1986 | 5 diabetic mice treated with penicillamine 5 diabetic mice untreated | 5 non-diabetic mice | GFR and tubular maximum for both p-aminohippurate and glucose were increased in both diabetic groups than in control group p < 0.05 No difference between two diabetic groups | |
| Jankowski M A et al. [28] 1993 Case-Control Study | Diabetic and non-diabetic mice on a diet rich in copper (12 µg/g) | Diabetic and non-diabetic mice on a diet low in copper (1 µg/g) | Diabetic mice had fewer implantation sites, live births, and smaller fetuses with larger placentas than non-diabetic mice Gross external malformation was noted only in diabetic mice Maternal high copper intake did not cause fetal abnormalities more frequently than low copper intake. | |
| Uriu-Adams et al. [22] 2005 Case-Control Study | Diabetic mice, both with adequate and deficient diets in copper | Non-diabetic mice, both with adequate and deficient diet in copper | In diabetic rats, the metabolism of copper was impaired with a low activity of copper-zinc superoxide dismutase (SOD), and this was reduced even more with diets low in copper. The levels of plasma metallothioin and ceruloplasmin in the liver and the kidneys were elevated in diabetic rats compared to control rats. | |
| Gong et al. [26] 2008 | Diabetic mice treated for 8 weeks with thethylene-tetramine | Diabetic mice untreated for diabetes | A marked improvement in the urinary albumin/creatinine ratio after TETA administration | |
| Lu J et al. [25] 2010 Case-Control Study | Diabetic mice treated for 8 weeks with thethylene-tetramine | Diabetic mice treated for 8 weeks with penicillamine, deferiprone, or Zn acetate | TETA treatment increased the resistance of cardiac output to the effects of increasing afterload pressure, while treatment with other chelators did not ameliorate cardiac function. TETA treatment significantly lowered the elevated uACR, while others chelators did not show any significant improvement. | |
| Gomez et al. [23] 2017 Case-Control Study | 10 diabetic mice | 10 non-diabetic mice | Serum (19.87 ± 0.89 vs. 17.08 ± 0.87), liver (40.44 ± 4.09 vs. 33.53 ± 3.98), and kidney tissue (64.15 ± 7.16 vs. 45.71 ± 2.60) concentrations did not differ significantly between diabetic and non-diabetic rats. Urinary concentrations were lower in the diabetic mice group (μmol/L 3.19 ± 0.45) than in the control group (4.71 ± 0.56) p = 0.050 Daily mineral excretion was higher in the diabetic mice group (0.10 ± 0.02) than in the control group (0.03 ± 0.01) p = 0.001 | |
| Tanaka et al. [24] 2017 Case-Control Study | Diabetic mice | Non-diabetic Mice | Serum copper ion levels in diabetic mice tended to be higher compared to those in non-diabetic mice at 10 weeks of age (64.6 ± 15.1 µg/dL vs. 43.0 ± 3.8 µg/dL). After diabetic mice were treated with tetratiomolybdate 0.01 mg/mL or 0.02 mg/mL, serum copper levels were reduced to levels comparable to those of non-diabetic mice, 59.0 ± 17.4 and 46.2 ± 20.3 p < 0.05, respectively. Similarly, serum ROS levels were reduced, 44.8 ± 26.7 and 37.1 ± 11.7 p < 0.05 |
| COPPER IN T1DM PATIENTS | Study, Year | Population | Control | Results |
| Zargar et al [10] (2002) Cross-sectional study | 37 patients with T1DM Age 21.78 ± 1.22 years | 25 healthy subjects | No significant difference in Cu and Mg levels between patients and the control group Plasma Zn levels higher in patients (17.78 ± 0.6 μmol/L) vs. controls (15.80 ± 0.75) p value = 0.022 | |
| Viktorìnovà et al [9] (2009) Cross-sectional study | 11 patients with T1DM Age 49.9 ± 9.4 (37–63) years 25 patients with T2DM Age 49.9 ± 9.4 (37–63) | 34 healthy subjects | Higher copper levels in patients (18.73 ± 2.6) vs. control (17.37 ± 2.4) p value < 0.001 Higher Cu:Zn Ratio in patients (1.42 ± 0.3) vs. control (1.21 ± 0.1) p value < 0.0001 Reduced levels of Zn in patients (13.48 ± 2.2) vs. control (14.41 ± 1.8) p value < 0.01 Reduced levels of Mg in patients (0.77 ± 0.2) vs. control (0.90 ± 0.1) p value < 0.0001 Positive correlation between plasma levels of HbA1c and Cu in patients r = 0.709 p < 0.001 Positive correlation between HbA1c and Cu/Zn ratio in patients r = 0.777 p < 0.001 Negative correlation between plasma levels of HbA1c and Zn in patients r = −0.684 p < 0.001 Negative correlation between plasma levels of HbA1c and Mg in patients r = −0.646 p < 0.001 | |
| Squitti et al [8] (2019) Cross-sectional study | 63 patients With T1DM Age 40.4 ± 15.3 years | 65 healthy subjects | Higher copper levels in patients (17.9 ± 4.8 (μmol/L) vs. control (14.1 ± 3.8 (μmol/L). p value < 0.0001 Higher ceruloplasmin levels in patients (30.1 ± 9.5) vs. control (24.4 ± 6.4) p value < 0.0001 Higher Cu:Zn Ratio in patients (1.3 ± 0.5) vs. control (0.9 ± 0.3) p value < 0.0001 A 15-fold increase of risk of developing T1DM for a standard-deviation increas in copper levels |
| COPPER IN T2DM PATIENTS | Study, Year | Population | Control | Results |
| Zargar et al. [35] 1998 Cross-sectional Study | 83 patients With T2DM Age 50.7 ± 8.47 years | 30 healthy subjects | Higher serum copper levels in patients group (16.87 + 4.69) vs. control group (13.91 ± 3.02) p < 0.01 Higher serum zinc levels in patients group (17.19 + 4.92) vs. control group (15.80 ± 4.12) p < 0.01 | |
| Raudenska et al. [33] 2013 Cross-sectional Study | 70 patients With T2DM Age 69 (54–84)years | 80 healthy subjects Age 52 (45–64) years | Higher copper levels in patients (1.8 [1.4–2.9]) vs. control (1.5 [1.1–2.3]) p = 0.0018 Higher coniugated bilirubin levels in patients (4.8 [3.5–7.2]) vs. control (3.3 [2.7–3.5]) p < 0.0001 Reduced Metallothionins levels in patients (0.8 [0.7–0.9]) vs. control (0.9 [0.8–1.3]) p < 0.0001 Reduced glutathione levels in patients [912 (754–1017)] vs. control [1646 (966–2695)] p < 0.0001 | |
| Naka et al. [34] 2013 Observational Study 3 months of follow up | 132 patients With T2DM Age 58.5 ± 12.5 years | No control group | Positive correlation between serum copper and HbA1c levels r = 0.176, p = 0.044. Serum copper levels were significantly associated with HbA1c (r = 0.18, p < 0.05), Hb (r= −0.170, p = 0.051), and creatinine (r= −0.170, p = 0.051). HbA1c (β = 0.224, p = 0.011) and Hb (β= −0.220, p = 0.013) were independent determinants of serum copper levels. Serum ceruloplasmin or urinal copper levels were not associated with HbA1c levels (n = 53, r= −0.074, p = 0.60 and n = 103, r = 0.031, p = 0.757, respectively). After 3 months glycemic control, As HbA1c levels were decreased (from 8.7% to 6.8%, p < 0.001), copper levels tended to be decreased (from 105.7 µg/dL to 101.8 µg/dL, p = 0.069) | |
| Sonkar et al. [36] 2021 Cross-sectional Study | 150 patients With T2DM Age 52.5 (9.69) years | 50 healthy subjects | Lower serum copper levels in patients group (µg/dL 116.30) vs. control group (µg/dL 150.39) p < 0.001 Lower serum zinc levels in patients group (µg/dL 62.89) vs. control group (µg/dL 74.95) p = 0.0091 Lower serum selenium levels in patients group (µg/dL 8.57) vs. control group (µg/dL 16.16) p < 0.001 Lower serum magnesium levels in patients group (mg/dL 1.92) vs. control group (2.31) p = 0.046 |
| DIABETIC KIDNEY DISEASE | Study, Year | Population | Control | Results |
| Ito et al. [39] 2001 Case-Control Study | Group I: 15 diabetic patients with normoalbuminuria Age 60 ± 7 Group II: 14 diabetic patients with microalbuminuria Age 61 ± 9 Group III: 12 patients with macroalbuminuria Age 66 ± 8 | 10 healthy subjects Age 56 ± 10 | Serum copper levels, serum ceruloplasmin levels, and serum copper/ceruloplasmin ratio were not different among the four groups Serum copper/albumin ratio increased in group III in comparison with group I (p < 0.05) Urinary copper concentration did not differ between groups I, II, and the control group, but its concentration was significantly higher in group III compared to other groups p < 0.001. Urinary cerulopasmin concentration significantly increased in group III in comparison with the other groups (p < 0.001), and it also increased in group II when compared with group I and the control group (p < 0.001). The copper/ceruloplasmin ratio in urine remarkably decreased in group III in comparison with the other groups (p < 0.001), and it also significantly decreased in group II when compared with group I and the control group (p < 0.001). The urinary copper/albumin ratio also decreased in group III compared to the other groups (p < 0.01) and decreased in group II in comparison with group I and control group (p < 0.001). Increase in urinary ceruloplasmin concentration correlated with urinary concentration of NAG (r = 0.846, p < 0.001)) and alfa-1-microglobulin (r = 0.608, p < 0.001) NAG and ·alfa-1-microglobulin concentrations in group II slightly increased in comparison with those in group I and control group (p < 0.05). NAG in group I was slightly higher than that in control group (p < 0.05). NAG and alfa- 1-microglobulin concentrations were clearly higher in group III than in the other groups (p < 0.001 and p < 0.01 | |
| Talaei et al. [41] 2011 Cross-sectional study | 42 TD2M patients with microalbuminuria | 40 T2DM patients without microalbuminuria | Higher 24h urinary copper levels in microalbuminuria group compared to control group 36.14 μcg/L(14.54–57.74) vs. 14.77 μcg/L(10.17–19.37) p = 0.003 No significant difference between different subgroups based on HbA1C% levels | |
| Prabodh et al. [46] 2011 Case-control Study | 40 patients with DKD Age 45–70 years | 40 healthy subjects Age matched | No significant difference in serum copper levels between DKD group (165.42 ± 5.71 μg/dL) and control group (166.6 ± 5.48 μg/dL) (p> 0.05). No relation of Cu with microalbumin in DKD patients | |
| Stancic et al. [38] 2012 Case-Control Study | Hypertensive diabetic patients with or without renal insufficiency | Healthy subjects | Copper zinc superoxide dismutase activity was higher only in hypertensive diabetic with renal insufficiency | |
| Al Bayati et al. [40] 2015 Cross-sectional Study | Group I: 31 T2DM patients with microalbuminuria between 30 and 299 μg/mg Age 49.5 ± 7.6 years Group II: 29 T2DM patients with microalbuminuria below 30 μg/mg Age 52.2 ± 8.2 years | 37 healthy subjects Age 48.9 ± 8.9 years | Group I showed a significant increase in urinary Cu/creatinine ratio compared with controls: 53.3 ± 3.2 vs. 44.2 ± 5.3 p < 0.05 No significant difference between Group II and controls in urinary Cu/creatinine ratio SOD was significantly decreased in group I compared to control group 30.6 ± 3.3 vs. 45 ± 6 p < 0.05 No significant difference between Group II and controls in SOD | |
| Hasamaki et al. [43] 2016 Cross-Sectional Study | 149 T2DM patients Age 61.1 ± 17.6 years | 206 non-diabetic patients | A high Zn/Cu ratio was associated with improved renal function levels (β = 0.137, p = 0.014) A high Zn/Cu ratio was associated with reduced risk of poor glycemic control in patients with type 2 diabetes, assessed by multivariate logistic regression analysis. (HbA1c ≥ 7%) (odds ratio = 0.382; 95% confidence interval, 0.165–0.884; p = 0.025) | |
| Takao et al. [44] 2022 Cross-sectional Study | 651 patients with T2DM Age 65.1 ± 9.7 years | No control group | Diabetic kidney disease was identified in 220 patients A Higher Cu/Zn ratios is correlated with more frequent renal involvement Higher Cu levels in DKD patients compared to non DKD patients (100.5–15.5 vs. 97.0–15.6) p = 0.007 Higher Cu/Zn ratio in DKD patients compared to non DKD patients (1.247–0.265 vs. 1.155–0.242) p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2023, 13, 17. https://doi.org/10.3390/metabo13010017
Gembillo G, Labbozzetta V, Giuffrida AE, Peritore L, Calabrese V, Spinella C, Stancanelli MR, Spallino E, Visconti L, Santoro D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites. 2023; 13(1):17. https://doi.org/10.3390/metabo13010017
Chicago/Turabian StyleGembillo, Guido, Vincenzo Labbozzetta, Alfio Edoardo Giuffrida, Luigi Peritore, Vincenzo Calabrese, Claudia Spinella, Maria Rita Stancanelli, Eugenia Spallino, Luca Visconti, and Domenico Santoro. 2023. "Potential Role of Copper in Diabetes and Diabetic Kidney Disease" Metabolites 13, no. 1: 17. https://doi.org/10.3390/metabo13010017
APA StyleGembillo, G., Labbozzetta, V., Giuffrida, A. E., Peritore, L., Calabrese, V., Spinella, C., Stancanelli, M. R., Spallino, E., Visconti, L., & Santoro, D. (2023). Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites, 13(1), 17. https://doi.org/10.3390/metabo13010017









