Abstract
Giant cell arteritis (GCA) is a potential sight-threatening disease. Although it is associated with polymyalgia rheumatica (PMR), visual loss is not common in PMR. A retinal oximeter can be used to conduct a direct, non-invasive, in vivo assessment of the vascular system. In a cross-sectional study, we measured the retinal oxygen saturation and retinal vessel calibers in GCA patients, PMR patients, and control participants. Twenty GCA patients (38 eyes), 19 PMR patients (33 eyes), and 12 controls (20 eyes) were investigated. Images were analyzed using Oxymap Analyzer software 2.5.0 (Oxymap ehf., Reykjavik, Iceland). Groups were compared using an age- and sex-adjusted linear mixed model regression. The median (IQR) age for GCA patients was 69.0 (66.5–76.5) years, for PMR 69.0 (67.0–72.0) years, and for the controls 75.5 (71.5–81.0) years, respectively. As compared to the controls (115.3 µm), the retinal arterioles were significantly wider in patients with GCA (124.4 µm; p = 0.023) and PMR (124.8 µm; p = 0.049). No difference was found in the retinal venular caliber or vascular oxygen saturation. These results indicate that GCA and PMR patients differ similarly in the retinal arteriolar diameter compared to controls. Further studies are needed in order to clarify the underlying inflammatory mechanisms in retinal arteriolar vessels and if these parameters can be used to predict clinical outcomes.
1. Introduction
Giant cell arteritis (GCA) is a type of medium and large blood vessel vasculitis with a high risk of ocular morbidities, such as visual impairment [1]. In contrast, polymyalgia rheumatica (PMR) is not considered a type of vasculitis, but a syndrome associated with pain and stiffness, usually in the neck, shoulders, upper arms, and hips. Although GCA and PMR are associated, PMR is not known to induce visual loss. The highest incidence and prevalence occur in Northern Europe [2]. It is estimated that, by 2050, approximately 500,000 people will have visual impairment due to GCA worldwide [3].
A direct, non-invasive, in vivo assessment of the retinal metabolism [4,5,6,7,8] and structure [8,9,10,11,12] can be conducted using a retinal oximeter, and the measurement of retinal saturation [13] and retinal vessel diameters [14] has shown acceptable repeatability.
In newly diagnosed GCA patients, retinal oximetry has shown an altered venular oxygen saturation and differences in arterio-venular oxygen saturation, even in GCA patients with no ocular symptoms [15]. However, to our knowledge, retinal oximetry or retinal vascular calibers have never been validated for application in PMR patients and have not been validated for GCA patients at 6–24 months after diagnosis.
Thus, we aimed to investigate the vascular system in terms of the retinal metabolism and retinal vascular calibers in GCA and PMR patients at 6–24 months after diagnosis. Our objectives were to examine if (a) the retinal arteriolar oxygen saturation, (b) retinal venular oxygen saturation, (c) difference in retinal arterio-venular oxygen saturation, (d) retinal arteriolar vessel diameter, and (e) retinal venular vessel diameter differed similarly between GCA and PMR patients, on one hand, and control participants, on the other hand.
2. Materials and Methods
2.1. Study Design
This clinical cross-sectional study was conducted between March 2021 and January 2022. GCA and PMR patients with the International Classification of Diseases (10th revision) and Related Health Problems (ICD-10) codes M315, M315A, M316, M316A (GCA), and M353 (PMR) were identified in medical journal files from the Department of Rheumatology at Odense University Hospital, Odense, Denmark, or Svendborg Sygehus, Svendborg, Denmark, and invited to participate in the study via e-mail.
We divided patients into a group of GCA patients and a group of PMR patients.
GCA patients were included if a positive temporal artery biopsy, positive positron emission tomography/computer tomography scans, or positive ultrasonography were obtained, as described in the 2018 European League Against Rheumatism recommendations [16].
PMR patients with a score of four or above on the European League Against Rheumatism 2012 classification criteria were included. Age above 50 years, bilateral shoulder pain, and elevated C-reactive protein (CRP) were mandatory, while negative anti-citrullinated protein antibodies (Anti-CCP) and IgM rheumatoid factor (IgM-RF) scored two points and morning stiffness >45 min scored two points. Pelvic pain and no swollen or painful distal joints each scored one point [17]. Furthermore, the GCA and PMR patients had to be diagnosed between 6–24 months before inclusion. A senior specialist in rheumatology (KEB) verified the diagnosis. We excluded images with a quality below 6.0 (range 0–10) as graded by the Oxymap T1 (Oxymap, Reykjavik, ehf.). Moreover, we excluded patients with neurodegenerative disease, including Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and patients with retinal or optic nerve diseases, including glaucoma, larger retinal oedemas, arteritic anterior ischemic optic neuropathy (AAION), non-arteritic anterior ischemic optic neuropathy (NAION), macular degeneration diseases, retinal tears, retinal detachment, macular holes, and retinitis pigmentosa. Furthermore, we excluded patients with diabetic retinopathy, which can affect retinal non-invasive measurements [18]. Yet, minimal small retinal drusen and minor epiretinal fibrosis were not regarded as ophthalmologic diseases. The time of diagnosis of GCA and PMR was defined as the date when the patient received treatment, and the symptom debut was defined as the first time a patient described symptoms of disease.
Control participants were invited the Department of Ophthalmology, Odense University Hospital, Odense, Denmark, prior to cataract surgery and recruited after surgery if no rheumatologic, cancer or relevant retinal or optic nerve diseases were present at the time of inclusion. Minimal small retinal drusen and minor epiretinal fibrosis were not considered ophthalmologic diseases.
2.2. Variables
Our predictors were the study groups (GCA patients and PMR patients, respectively).
The outcomes of the study comprise retinal arteriolar oxygen saturation, a measure of the mean saturation in the largest arteriole of each quadrant of the retina, given as a percentage; retinal venular oxygen saturation, a measure of the mean saturation in the largest venule of each quadrant of the retina, given as a percentage; the difference in retinal arterio-venular oxygen saturation, a measure of the difference between the mean arteriolar oxygen saturation and the mean venular oxygen saturation, given as a percentage; retinal arteriolar vessel diameter, a measure of the mean vessel diameter in the largest arteriole of each quadrant of the retina, given in micrometers; and retinal venular vessel diameter, a measure of the mean vessel diameter in the largest venule of each quadrant of the retina. All means are based on three or four quadrants.
Confounders include age and sex, which were adjusted for in the linear mixed regression model.
We do not believe that any variable had a different effect on the GCA group compared to PMR group, respectively. Thus, we assume that no effect modifiers were present in this study.
2.3. Clinical Examinations
We carried out all clinical examinations at the Department of Ophthalmology at Odense University Hospital, Odense, Denmark. The CRP value closest to inclusion was defined as the latest blood sample drawn before the study visit.
We obtained medical history for diagnoses with giant cell arteritis and polymyalgia rheumatica. Moreover, based on medical journals over a period of five years prior to inclusion, participants were scored according to the Charlson comorbidity index (CCI), using ICD-10 [19]. The CCI score is based on 19 different comorbidities added to a score based on the age of the patient, hence providing one overall score. As the CCI scores of GCA and PMR patients automatically included one point for connective tissue disease, an adjusted CCI was created so as to compensate by subtracting one point from the GCA and PMR patients, respectively.
Upon the hospital visit, the blood pressure was obtained in mmHg and measured three consecutive times from the left arm using the same monitor. Mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) was calculated. Mean arterial blood pressure (MAP) was defined as DBP + 1/3(SBP − DBP). Height was measured in meters. Weight was measured in kilograms with clothes. Body mass index (BMI) was calculated as height/(meters2). The ophthalmological examination consisted of ophthalmoscopy, with the best-corrected visual acuity tested using the Early Treatment Diabetic Retinopathy Study chart at four meters (Precision Vision, Woodstock, IL, USA). Intraocular pressure was measured using Goldmann applanation tonometry and was provided in mmHg. Pupils were dilated using tropicamide 1% and supplemented with phenylephrine 10% if required for optimal dilation.
2.4. Retinal Oximetry and Image Analysis
We used Oxymap T1 (Oxymap ehf., Reykjavik, Iceland) to detect the retinal arteriolar, venular oxygen saturation, and retinal blood vessel calibers. The Oxymap T1 device comprises two digital cameras and an optical adapter attached to a TRC-50DX fundus camera (Topcon Corporation, Tokyo, Japan). Fundus images were captured using a 50° view at wavelengths of 570 nm and 600 nm of light simultaneously. The fact that light absorbance by the blood affects the brightness in the blood vessels, but not the sides of the blood vessels, is useful for finding the optical density at each wavelength. The ratio between the optical densities of each wavelength is inversely and linearly related to the retinal oxygen saturation [20].
The optic disc was centered in the image, and at least two pictures were captured of each eye, starting with the right eye. The image with the highest overall quality was chosen for further analysis. The image quality was automatically graded on a scale from 0 to 10 by the Oxymap T1 (Oxymap ehf.). The pictures were taken at different light intensities, starting with no light and gradually building up the light intensity for each image. A circle was manually fitted to the optic disc, and two more circles were semi-automatically enlarged to 0.5 times and 3.0 times the size of the circle surrounding the optic disc, respectively. The circle surrounding the optic disc was deleted, and blood vessel measurements were performed manually in the space between the two remaining circles. The required length of the blood vessels for inclusion was between ≥50 pixels and ≤200 pixels. The retinal arteriole and venule with the most considerable length and diameter were chosen from each quadrant, starting with the upper nasal quadrant, and followed by the lower nasal quadrant, the lower temporal quadrant, and the upper temporal quadrant. Mean values for the retinal arteriolar and venular oxygen saturation and blood vessel diameters were calculated.
All images were captured and analyzed by S.J.L. For their conversion from pixels to µm, a conversion factor of 9.3 was used [14]. A retinal oximetry fundus image is graphically depicted in Figure 1 to illustrate the methods. Retinal oximetry is a subclinical measurement, which is not suitable for direct in vivo clinical interpretation. All images were analyzed using Oxymap Analyzer Software 2.5.0 (Oxymap ehf., Reykjavik, Iceland).
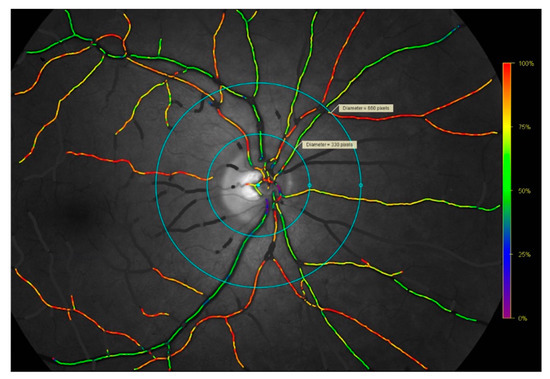
Figure 1.
Retinal oximetry fundus image captured with the TRC-50DX fundus camera and Oxymap T1 oximeter device. Example of a retinal oximetry fundus image depicting the right eye of a patient with giant cell arteritis, included here to illustrate the methods. Images were analyzed using the Oxymap 2.5.0. Oxymap Analyzer software (Oxymap ehf., Reykjavik, Iceland) in the space between the inner and outer circles for the assessment of the arterioles and venules of each quadrant. Purple, blue, and green vessels reflect a lower saturation in the range of approximately 0–65%. Yellow, orange, and red vessels reflect a higher saturation in the range of approximately 66–100%. Retinal oxygen saturation and retinal vessel calibers were manually measured in the space between the inner and outer light-blue circles.
2.5. Ethics
The tenets of the Declaration of Helsinki were followed, and the study was approved by the Record of Data Processing Activities in the Region of Southern Denmark (20/61779) and by the Research Ethics Committee in the Region of Southern Denmark (20200189). All participants in this study gave informed consent and were allowed to withdraw their consent at any time. Participation in the study was non-invasive and without risk for the participants. However, the dilation of the pupils could burden the participants with blurred vision for 4–6 h afterwards.
2.6. Statistical Methods
For the presentation of the demographic data, categorical variables are presented as counts (n) and proportions (%). Continuous variables are reported as medians with interquartile ranges (IQR). For the comparison of the continuous variables, the Kruskal–Wallis test and Wilcoxon rank sum test were used, as the data were not normally distributed. Pearson’s chi-squared test was used for the categorical data.
As every participant could contribute two eyes, the cluster robust standard error was used in a linear mixed model regression analysis, adjusted for age and sex, to determine the retinal arteriolar and venular oxygen saturation, the arteriolar–venular difference, and the retinal arteriolar and venular vessel diameter. All tests were two-sided, and p-values of <0.05 and 95% confidence intervals that were not null were considered statistically significant. Regarding the regression analysis, there were no missing data among the outcome-variables, including the mean retinal arteriolar and venular oxygen saturation, arterio-venular difference, and mean retinal arteriolar and venular vessel diameters (mean values were based on data of three to four retinal quadrants).
Statistical analyses were carried out in Stata 17 (StataCorp, College Station, TX, USA).
The sample size was estimated from the findings by Türksever et al. [15], according to which the retinal vascular oxygen saturation was determined for the newly diagnosed patients and control participants. On the assumption that PMR patients have the same arteriolar–venular difference as GCA patients, at 32.2% ± 3.8%, and that the control participants have an arteriolar–venular difference of 38.3% ± 2.8%, we deemed that we would require 12 patients in each group (α = 0.05, power 0.90).
3. Results
3.1. Patient Population
We invited 194 patients with either GCA (n = 40) or PMR (n = 154) to participate in the study (Figure 2). The reasons for non-attendance were no response (n = 98) and the fact that the patient was non-contactable by e-mail (n = 36) or did not want to participate (n = 12), and nine participants were excluded for medical reasons, including glaucoma (n = 1), Alzheimer’s disease (n = 1), dry age-related macular degeneration (n = 3), poor image quality under 6.0 (n = 2), bilateral retinal detachment (n = 1), or because they did not fulfill the 2012 European League Against Rheumatism criteria for PMR (n = 1). Two GCA patients participated with a single eye due to former retinal detachment (n = 1) and severe epiretinal fibrosis with lamellar macular holes (n = 1). Among the PMR patients, three participated with a single eye due to poor image quality (n = 2) or retinal tear (n = 1).
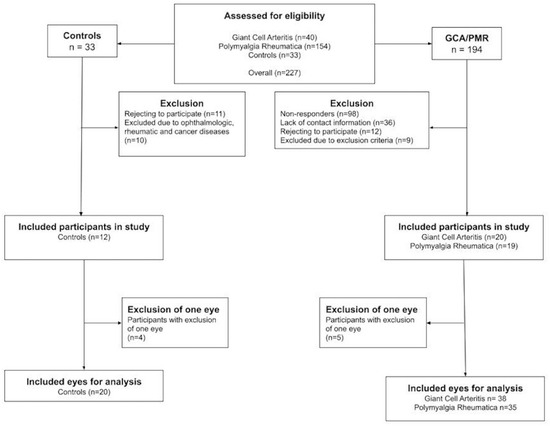
Figure 2.
Flowchart depicting the inclusion process for patients with giant cell arteritis, polymyalgia rheumatica, and control participants.
Of the control participants, thirty-three responded to the invitation, of whom eleven rejected participation and ten were excluded due to highly suspected glaucoma (n = 1), large retinal oedema (n = 1), dry age-related macular degeneration (n = 2), pseudovitelliform macular degeneration (n = 2), a former PMR diagnosis (n = 1), diabetic retinopathy (n = 1), disseminated colon cancer (n = 1), and small lymphocytic lymphoma (n = 1). Of the remaining 12, four participated with a single eye due to poor image quality (n = 1), highly suspected glaucoma (n = 1), severe epiretinal fibrosis (n = 1), and postoperative cystoid macular edema after cataract surgery (n = 1).
Thus, we included 20 participants (38 eyes) with GCA, 19 participants (35 eyes) with PMR, and 12 control participants (20 eyes).
3.2. Clinical Characteristics
Table 1 demonstrates an overview of the demographic parameters of each group, including the number of individuals per group, stratified by age. Among all participants, the median age (IQR) was 70.0 years (67.0 to 77.0), but the control participants were older compared to the GCA and PMR patients. Twenty participants (39%) were male and twenty participants (39%) were cataract-operated (pseudophakic) in both eyes, including all control participants. No difference was found in the body mass index, systolic and diastolic blood pressure, and likewise in the mean arterial blood pressure between any of the groups.

Table 1.
Clinical characteristics of the controls, giant cell arteritis patients, and polymyalgia rheumatica patients.
While patients with GCA and PMR did not differ in any of the parameters, the controls had a higher age (p = 0.039), higher image quality (p = 0.002), higher best-corrected visual acuity (p = 0.017), and lower intraocular pressure (p = 0.008). Additionally, a difference in the Charlson comorbidity index (p < 0.001) was found between all groups but not in the adjusted Charlson comorbidity index (p = 0.54).
3.3. Retinal Metabolism and Structure
An overview of the linear mixed regression model analysis adjusted for age and sex is found in Table 2. The GCA and PMR patients differed from control participants in the retinal arteriolar diameter (124.4 µm vs. 115.3 µm, p = 0.023 and 124.8 µm vs. 115.3 µm, p = 0.049, respectively).

Table 2.
Differences between patients with giant cell arteritis and polymyalgia rheumatica against the controls.
There were no differences between any of the groups with respect to the retinal arteriolar oxygen saturation, retinal venular oxygen saturation, arterio-venular difference, or retinal venular diameter.
4. Discussion
This cross-sectional study aimed to investigate whether the vascular system in patients with GCA and PMR differed similarly in terms of the retinal metabolism and retinal vascular calibers compared to control participants. The results showed a significantly wider arteriolar diameter in GCA patients compared to control participants and in PMR-patients compared to control participants. This may indicate a microvascular overlap between GCA and PMR.
The retinal vessel diameter can be adjusted by a complex autoregulatory system [21] and has been shown to decrease during breathing with 100% oxygen [9,11]. The increase in the arteriolar diameter among patients with GCA and patients with PMR compared to the control participants in our study may be caused by high metabolic demands or, perhaps, irregularities in the autoregulatory metabolic system, as observed in diabetic retinopathy [22].
Patients with ocular and central nervous system sarcoidosis showed a difference in the retinal arterio-venular oxygen saturation compared to sarcoidosis patients whose ocular and central nervous systems were not affected. However, no difference was found in the retinal venular vessel diameter or arteriolar vessel diameter [8]. Accordingly, newly diagnosed GCA patients were investigated using retinal oximetry by Türksever et al. [15]. In contrast to our results, they found that the retinal arterio-venular difference decreased, and the retinal venular oxygen saturation increased significantly compared to the control participants. However, the retinal vessel calibers were not measured.
The dilation of the retinal venular diameters has previously been linked to inflammation [23]. Although we did not find altered retinal venular calibers in the GCA and PMR patients compared to the control participants, inflammation cannot be ruled out as a potential cause of the retinal arteriolar widening found in our results.
In patients with Voigt–Koyanagi–Harada disease, immunosuppressive treatment decreased the retinal arteriolar and venular diameter at follow-up compared to the baseline [10]. The majority of GCA and PMR-patients in our study had already received corticosteroid treatment. Corticosteroid, in larger doses, is a well-established treatment for GCA and PMR, with good treatment responses [24], which may have influenced the retinal arteriolar- and venular oxygen saturation, the retinal arterio-venular difference in oxygen saturation, and the retinal venular diameter between groups in our study.
Strengths can be found in this study. Firstly, retinal examinations were conducted to eliminate the risk of significant ocular disease. Secondly, this was, to our knowledge, the first study to investigate retinal metabolism and the retinal structure in patients with GCA and PMR at 6–24 months after diagnosis. Finally, the image capturing and analysis were performed by the same investigator (SJL). Limitations can also be found within this study. Firstly, it was a cross-sectional study and, hence, causality cannot be determined. Secondly, we were only able to include a limited number of patients and, thus, the risk of type-2 errors must be considered when interpreting the results. Thirdly, some clinical characteristics differed between the control participants and GCA and PMR patients, potentially affecting the results. Finally, we cannot conclude whether the same patterns would emerge in newly diagnosed treatment-naïve patients.
5. Conclusions
In conclusion, we found a wider arteriolar diameter comparing the GCA and PMR patients to the controls. These results suggest that GCA and PMR patients differ similarly in terms of their retinal structure compared to controls at 6–24 months after diagnosis. Prospective studies on newly diagnosed treatment-naïve patients could help us to determine whether changes in the retinal metabolism and structure can be used predictively or whether they simply reflect the initiated treatment.
Author Contributions
Conceptualization, S.J.L., K.-E.B., J.G., T.J.E. and J.W.; methodology, S.J.L., K.-E.B., J.G., T.J.E. and J.W.; software, S.J.L. and K.-E.B.; validation, S.J.L., T.J.E., J.W., J.K.P., J.G. and K.-E.B.; formal analysis, S.J.L. and K.-E.B.; investigation S.J.L.; data curation, S.J.L.; writing—original draft preparation, S.J.L.; writing—review and editing, J.G., K.-E.B., T.J.E., J.K.P., J.W. and S.J.L.; visualization, S.J.L. and K.-E.B.; supervision, J.G., K.-E.B., T.J.E. and J.W.; project administration, S.J.L., J.G. and K.-E.B.; funding acquisition, S.J.L. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Odense University Hospital Fund for Pre-Graduate Stipends (A4612).
Institutional Review Board Statement
The study was conducted according to the guidelines of the declaration of Helsinki and approved by the Record of Data Processing Activities in the Region of Southern Denmark on the 15 December 2020 (20/61779) and by the Research Ethics Committee in the Region of Southern Denmark on 6 January 2021 (20200189), with the approval of updates on 16 April 2021 and 7 October 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. All participants were allowed to withdraw their consent at any time.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request, with the acceptance of Research Ethics Committee in the Region of Southern Denmark.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ji, J.; Dimitrijevic, I.; Sundquist, J.; Sundquist, K.; Zöller, B. Risk of ocular manifestations in patients with giant cell arteritis: A nationwide study in Sweden. Scand. J. Rheumatol. 2017, 46, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mohammad, A.J.; Turesson, C. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: A systematic literature review. Semin. Arthritis Rheum. 2020, 50, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- De Smit, E.; Palmer, A.J.; Hewitt, A.W. Projected worldwide disease burden from giant cell arteritis by 2050. J. Rheumatol. 2015, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Einarsdottir, A.B.; Hardarson, S.H.; Kristjansdottir, J.V.; Bragason, D.T.; Snaedal, J.; Stefánsson, E. Retinal oximetry imaging in Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 49, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Einarsdottir, A.B.; Olafsdottir, O.B.; Hjaltason, H.; Hardarson, S.H. Retinal oximetry is affected in multiple sclerosis. Acta Ophthalmol. 2018, 96, 528–530. [Google Scholar] [CrossRef]
- Olafsdottir, O.B.; Vandewalle, E.; Abegão Pinto, L.; Geirsdottir, A.; De Clerck, E.; Stalmans, P.; Gottfredsdottir, M.S.; Kristjansdottir, J.V.; Van Calster, J.; Zeyen, T.; et al. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br. J. Ophthalmol. 2014, 98, 329–333. [Google Scholar] [CrossRef]
- Osaka, R.; Nakano, Y.; Takasago, Y.; Fujita, T.; Yamashita, A.; Shiragami, C.; Muraoka, Y.; Tsujikawa, A. Retinal oximetry in branch retinal vein occlusion. Acta Ophthalmol. 2019, 97, e896–e901. [Google Scholar] [CrossRef]
- Kindt, A.; Byg, K.E.; Wied, J.; Ellingsen, T.; Davidsen, J.R.; Grauslund, J. Altered retinal oxygen metabolism in patients with combined ocular and central nervous system sarcoidosis. Rheumatology 2021, 60, 3301–3306. [Google Scholar] [CrossRef]
- Olafsdottir, O.B.; Eliasdottir, T.S.; Kristjansdottir, J.V.; Hardarson, S.H.; Stefánsson, E. Retinal Vessel Oxygen Saturation during 100% Oxygen Breathing in Healthy Individuals. PLoS ONE 2015, 10, e0128780. [Google Scholar] [CrossRef][Green Version]
- Abu El-Asrar, A.M.; Alotaibi, M.D.; Gikandi, P.W.; Stefánsson, E. Effect of immunosuppressive therapy on oxygen saturation and diameter of retinal vessels in initial onset acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2021, 99, 75–82. [Google Scholar] [CrossRef]
- Palkovits, S.; Lasta, M.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; Schmetterer, L. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4707–4713. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, L.; Yi, C.; Huang, X.; Fu, Y.; Dong, Y.; Qian, X.; Li, Y.; Gao, Q. Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa at various ages. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.; Geirsdottir, A.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefánsson, E. Retinal oximetry images must be standardized: A methodological analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- Blondal, R.; Sturludottir, M.K.; Hardarson, S.H.; Halldorsson, G.H.; Stefánsson, E. Reliability of vessel diameter measurements with a retinal oximeter. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Türksever, C.; Daikeler, T.; Konieczka, K.; Todorova, M.G. Retinal vessel oxygen saturation in giant cell arteritis patients without ocular symptoms. Klin. Monbl. Augenheilkd 2014, 231, 442–446. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Dasgupta, B.; Cimmino, M.A.; Maradit-Kremers, H.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2012, 71, 484–492. [Google Scholar] [CrossRef]
- Jørgensen, C.M.; Hardarson, S.H.; Bek, T. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 2014, 92, 34–39. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Geirsdottir, A.; Palsson, O.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefánsson, E. Retinal vessel oxygen saturation in healthy individuals. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5433–5442. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef] [PubMed]
- Bek, T. Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.J.; Ikram, M.K.; Witteman, J.C.; Hofman, A.; de Jong, P.T.; Breteler, M.M. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann. Neurol. 2007, 61, 491–495. [Google Scholar] [CrossRef]
- Emamifar, A.; Hess, S.; Ellingsen, T.; Gerke, O.; Ahangarani Farahani, Z.; Syrak Hansen, P.; Hansen, I.M.J.; Thye-Rønn, P. Clinical presentation and treatment response in patients with polymyalgia rheumatica and giant cell arteritis during a 40-week follow-up. Rheumatol. Adv. Pract. 2021, 5, rkab091. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).