Skeletal Muscle Mitochondrial and Perilipin Content in a Cohort of Obese Subjects Undergoing Moderate and High Intensity Training
Abstract
:1. Introduction
2. Results
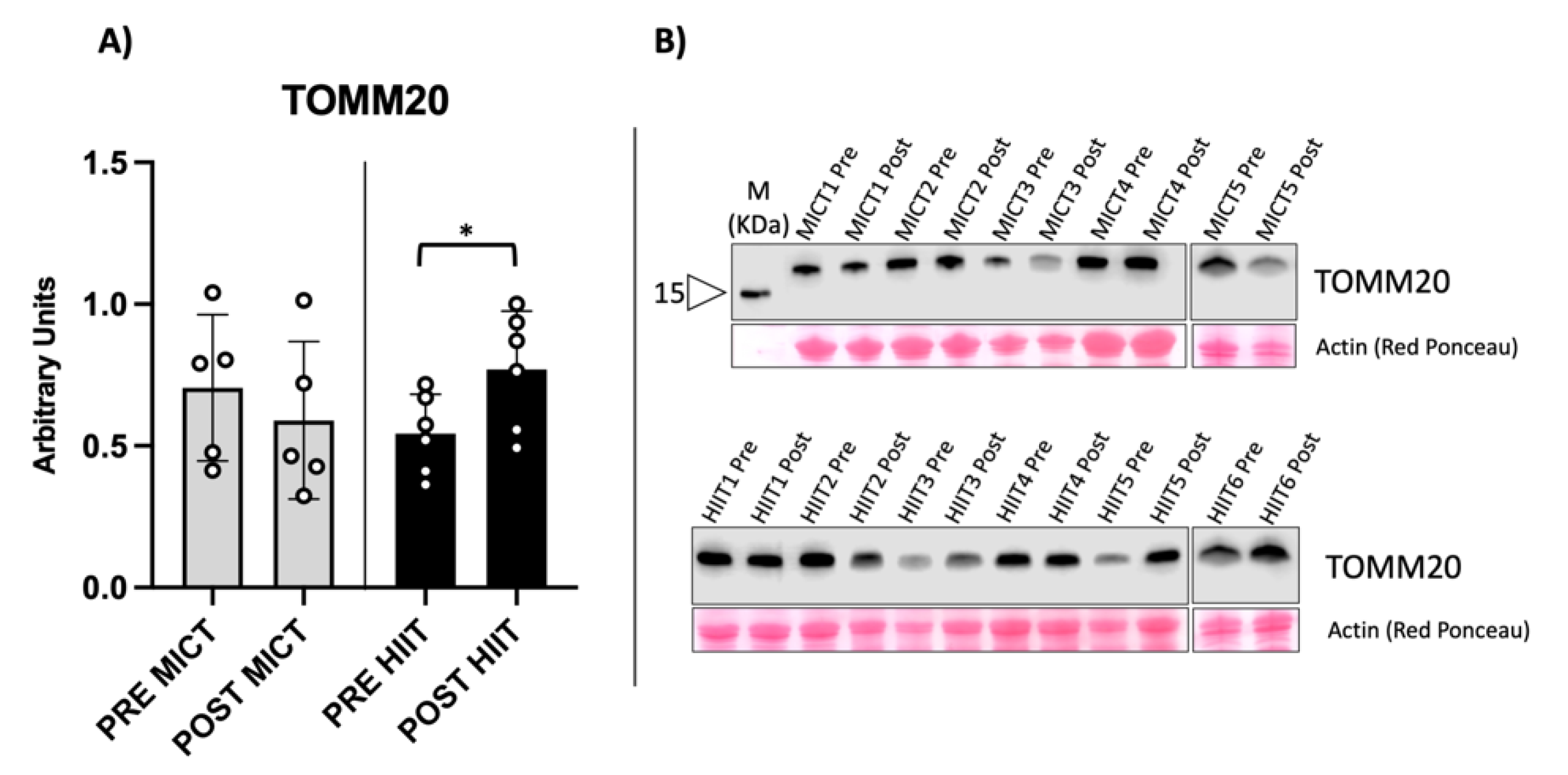
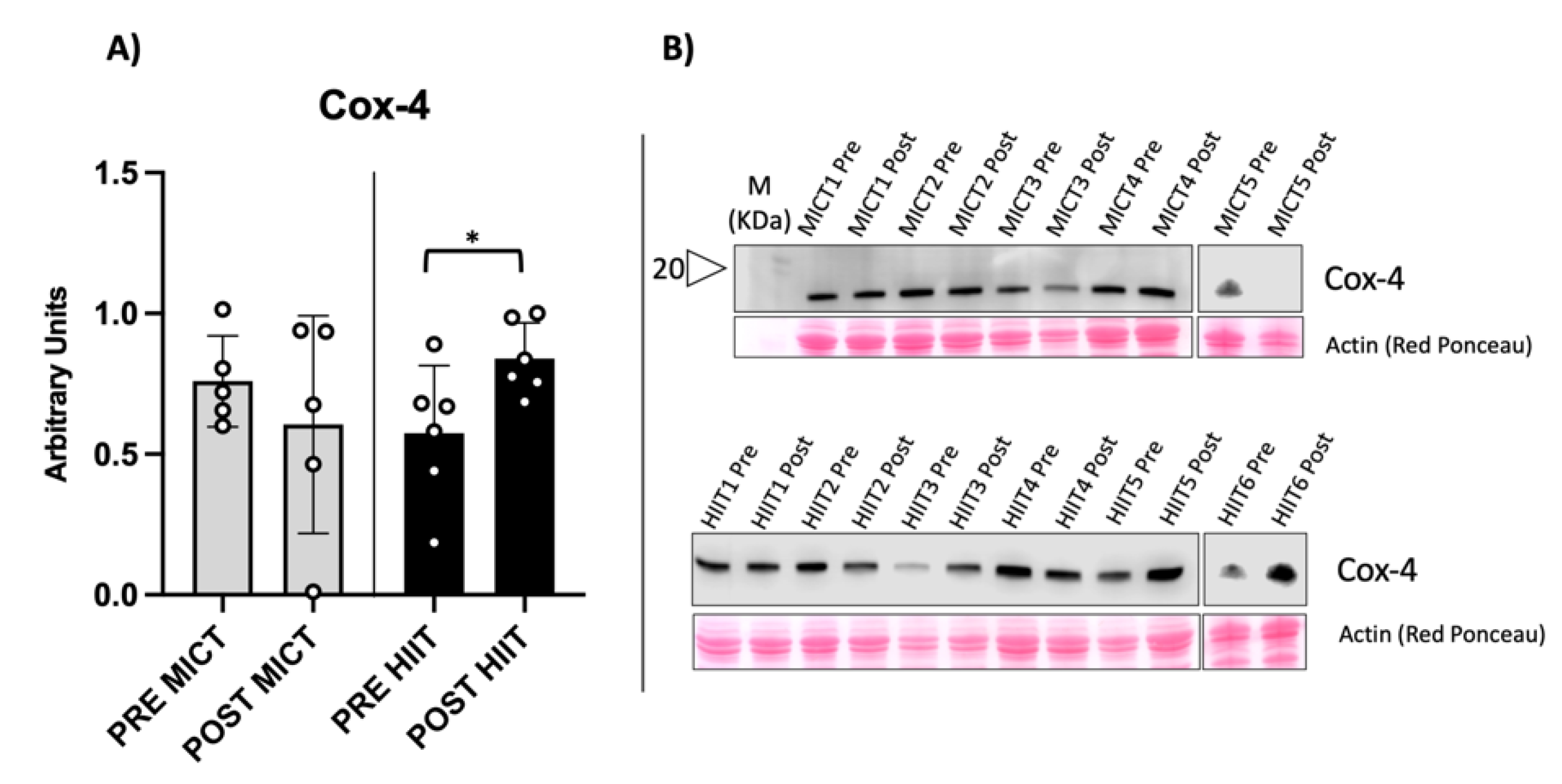
2.1. Evaluation of Mitochondrial Biomarkers
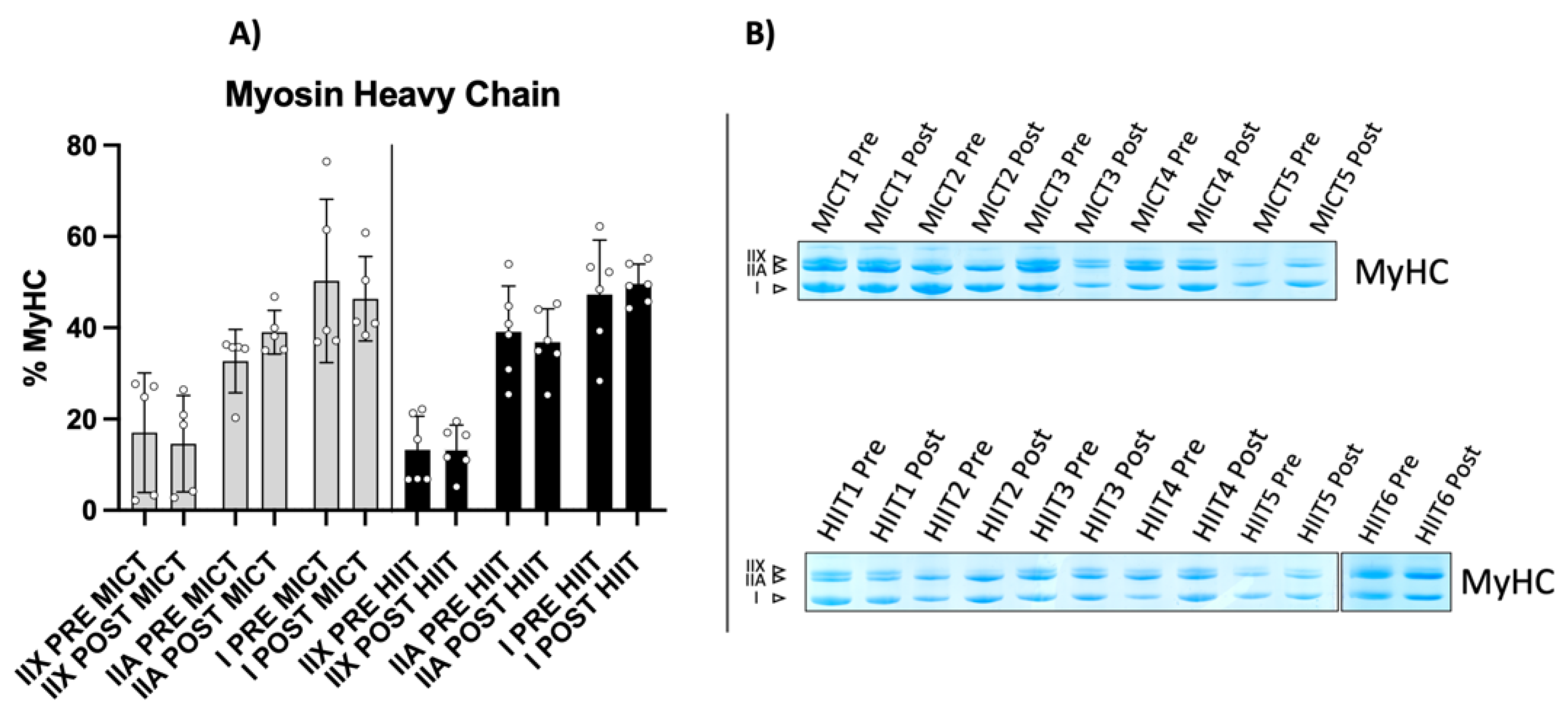
2.2. Myosin Heavy Chain Isoform Characterization
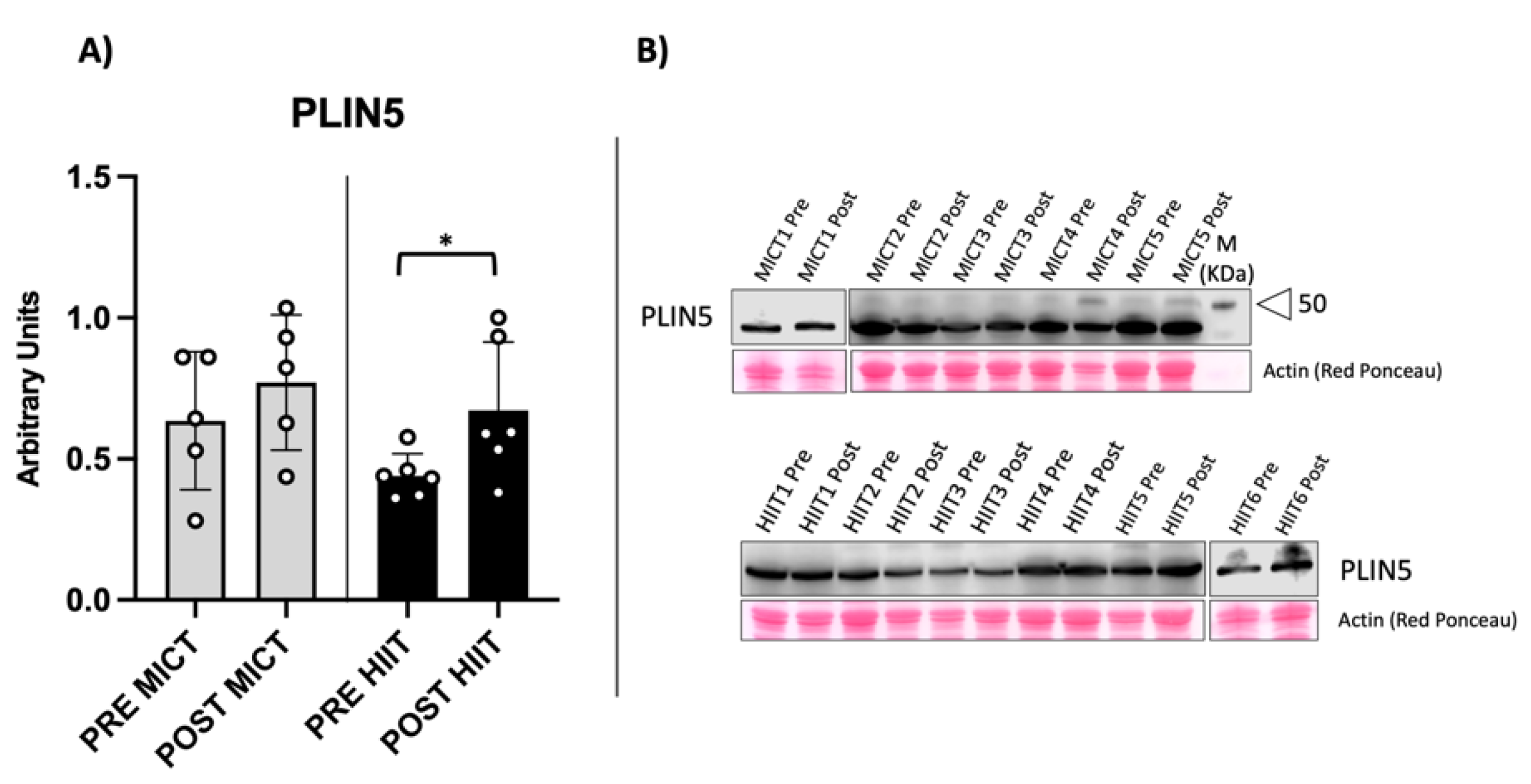
2.3. Evaluation of Muscular Lipid Droplet Biomarkers
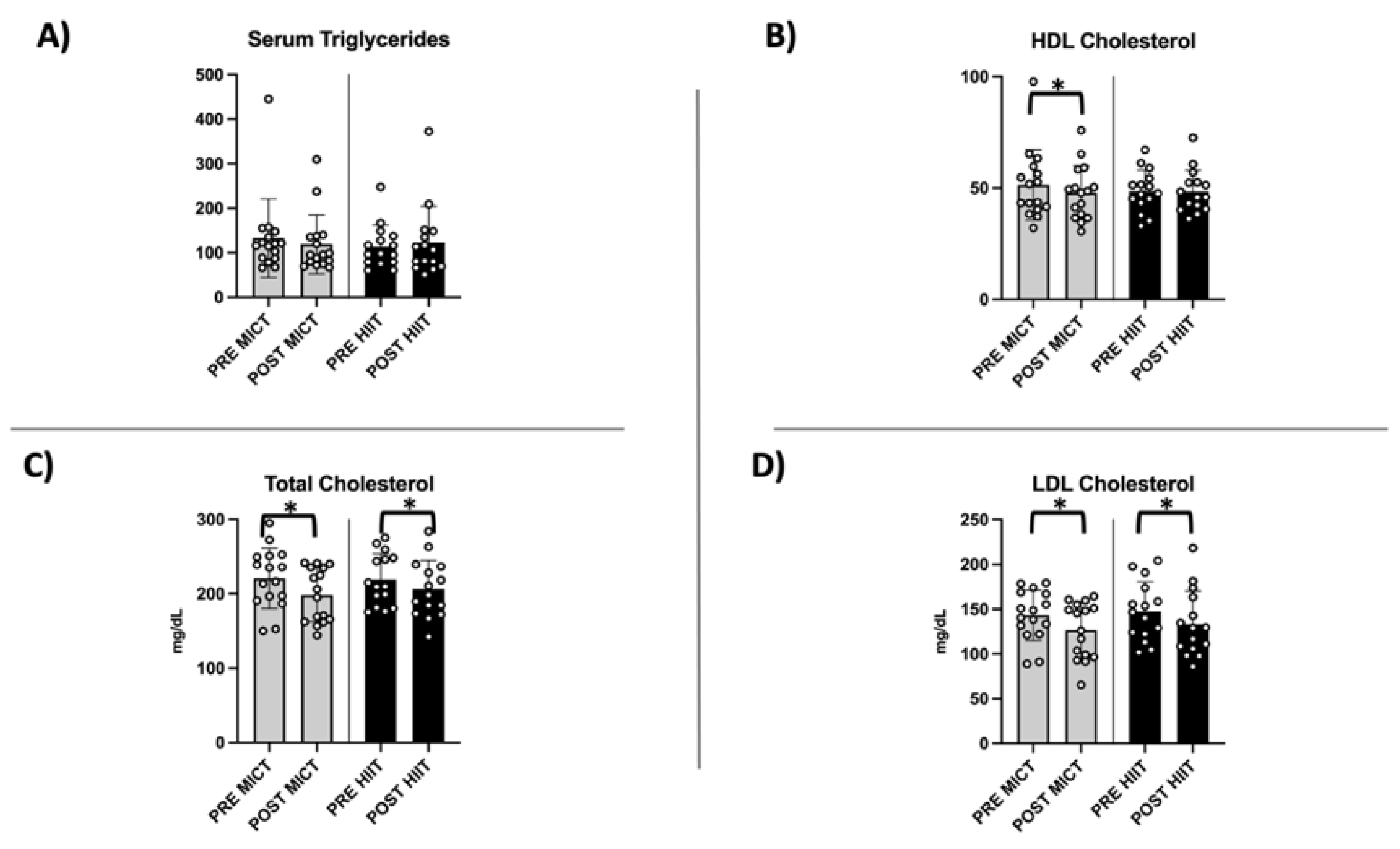
2.4. Evaluation of Circulating Lipid Profile
3. Discussion
4. Methods
4.1. Subjects and Recruitment
4.2. Skeletal Muscle Biopsies and Blood Sampling
4.3. Determination of Circulating Lipid Profile
4.4. Western Blot Analysis
4.5. MyHC Isoforms Analysis
4.6. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. In Obesity and Brain Function; Advances in Neurobiology; Springer Nature: Cham, Switzerland, 2017; Volume 19, pp. 3–31. [Google Scholar]
- Vaccari, F.; Passaro, A.; D’Amuri, A.; Sanz, J.M.; Di Vece, F.; Capatti, E.; Magnesa, B.; Comelli, M.; Mavelli, I.; Grassi, B.; et al. Effects of 3-Month High-Intensity Interval Training vs. Moderate Endurance Training and 4-Month Follow-up on Fat Metabolism, Cardiorespiratory Function and Mitochondrial Respiration in Obese Adults. Eur. J. Appl. Physiol. 2020, 120, 1787–1803. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Hawley, J.A.; Zierath, J.R. SnapShot: Exercise Metabolism. Cell Metab. 2016, 24, 342–342.e1. [Google Scholar] [CrossRef]
- Bosma, M. Lipid Homeostasis in Exercise. Drug Discov. Today 2014, 19, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.S.; Snook, L.A.; Jain, S.S.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Acute Endurance Exercise Increases Plasma Membrane Fatty Acid Transport Proteins in Rat and Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E183–E189. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Howald, H.; Conley, K.; Lindstedt, S.L.; Claassen, H.; Vock, P.; Weibel, E.R. Endurance Training in Humans: Aerobic Capacity and Structure of Skeletal Muscle. J. Appl. Physiol. 1985, 59, 320–327. [Google Scholar] [CrossRef]
- Essén, B.; Jansson, E.; Henriksson, J.; Taylor, A.W.; Saltin, B. Metabolic Characteristics of Fibre Types in Human Skeletal Muscle. Acta Physiol. Scand. 1975, 95, 153–165. [Google Scholar] [CrossRef]
- Malenfant, P.; Joanisse, D.R.; Thériault, R.; Goodpaster, B.H.; Kelley, D.E.; Simoneau, J.A. Fat Content in Individual Muscle Fibers of Lean and Obese Subjects. Int. J. Obes. 2001, 25, 1316–1321. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal Muscle Fatty Acid Metabolism in Association with Insulin Resistance, Obesity, and Weight Loss. Am. J. Physiol. Endocrinol. Metab. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid Oxidation Is Reduced in Obese Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef]
- Kimmel, A.R.; Sztalryd, C. Perilipin 5, a Lipid Droplet Protein Adapted to Mitochondrial Energy Utilization. Curr. Opin. Lipidol. 2014, 25, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Grey, J.Y.; Connor, M.K.; Gordon, J.W.; Yano, M.; Mori, M.; Hood, D.A. Tom20-Mediated Mitochondrial Protein Import in Muscle Cells during Differentiation. Am. J. Physiol. Cell Physiol. 2000, 279, C1393–C1400. [Google Scholar] [CrossRef] [PubMed]
- D’Amuri, A.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Passaro, A. Effectiveness of High-Intensity Interval Training for Weight Loss in Adults with Obesity: A Randomised Controlled Non-Inferiority Trial. BMJ Open Sport Exerc. Med. 2021, 7, e001021. [Google Scholar] [CrossRef] [PubMed]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal Muscle and Fiber Type-Specific Intramyocellular Lipid Accumulation in Obese Mice. Bosn. J. Basic Med. Sci. 2021, 21, 730. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sreenevasan, U.; Hu, H.; Saladino, A.; Polster, B.M.; Lund, L.M.; Gong, D.W.; Stanley, W.C.; Sztalryd, C. Perilipin 5, a Lipid Droplet-Associated Protein, Provides Physical and Metabolic Linkage to Mitochondria. J. Lipid Res. 2011, 52, 2159–2168. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Azarbayjani, M.A.; Farzanegi, P.; Moradi, L. High-Intensity Interval Training Has a Greater Effect on Reverse Cholesterol Transport Elements Compared with Moderate-Intensity Continuous Training in Obese Male Rats. Eur. J. Prev. Cardiol. 2021, 28, 692–701. [Google Scholar] [CrossRef]
- Bosma, M.; Minnaard, R.; Sparks, L.M.; Schaart, G.; Losen, M.; De Baets, M.H.; Duimel, H.; Kersten, S.; Bickel, P.E.; Schrauwen, P.; et al. The Lipid Droplet Coat Protein Perilipin 5 Also Localizes to Muscle Mitochondria. Histochem. Cell Biol. 2012, 137, 205–216. [Google Scholar] [CrossRef]
- Gripp, F.; Nava, R.C.; Cassilhas, R.C.; Esteves, E.A.; Magalhães, C.O.D.; Dias-Peixoto, M.F.; de Castro Magalhães, F.; Amorim, F.T. HIIT Is Superior than MICT on Cardiometabolic Health during Training and Detraining. Eur. J. Appl. Physiol. 2021, 121, 159–172. [Google Scholar] [CrossRef]
- Heiat, F.; Heiat, M.; Shojaeifard, M. Changes in Mitochondrial Biogenesis and Fatty Liver Indicators in Rat Following Continuous and High Intensity Interval Training. J. Sports Med. Phys. Fit. 2021, 61, 1416–1422. [Google Scholar] [CrossRef]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M.; Kelly, D.P.; Holloszy, J.O. Adaptations of Skeletal Muscle to Exercise: Rapid Increase in the Transcriptional Coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. An Acute Bout of High-Intensity Interval Training Increases the Nuclear Abundance of PGC-1α and Activates Mitochondrial Biogenesis in Human Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Za’don, N.H.A.; Kamal, A.F.M.; Ismail, F.; Othman, S.I.T.; Appukutty, M.; Salim, N.; Fauzi, N.F.M.; Ludin, A.F.M. High-Intensity Interval Training Induced PGC-1α and Adipor1 Gene Expressions and Improved Insulin Sensitivity in Obese Individuals. Med. J. Malays. 2019, 74, 461–467. [Google Scholar]
- Khalafi, M.; Mohebbi, H.; Symonds, M.E.; Karimi, P.; Akbari, A.; Tabari, E.; Faridnia, M.; Moghaddami, K. The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients 2020, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- McAinch, A.J.; Steinberg, G.R.; Mollica, J.; O’Brien, P.E.; Dixon, J.B.; Kemp, B.E.; Cameron-Smith, D. Leptin Stimulation of COXIV is Impaired in Obese Skeletal Muscle Myotubes. Obes. Res. Clin. Pract. 2007, 1, 53–60. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.F.; Little, J.P.; Renner, K.; Bishop, D.J. Training Intensity Modulates Changes in PGC-1α and P53 Protein Content and Mitochondrial Respiration, but Not Markers of Mitochondrial Content in Human Skeletal Muscle. FASEB J. 2016, 30, 959–970. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic Control through the PGC-1 Family of Transcription Coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Lee, S.J.; Zhang, J.; Choi, A.M.K.; Kim, H.P. Mitochondrial Dysfunction Induces Formation of Lipid Droplets as a Generalized Response to Stress. Oxid. Med. Cell. Longev. 2013, 2013, 327167. [Google Scholar] [CrossRef]
- Gjelstad, I.M.F.; Haugen, F.; Gulseth, H.L.; Norheim, F.; Jans, A.; Bakke, S.S.; Raastad, T.; Tjønna, A.E.; Wisløff, U.; Blaak, E.E.; et al. Expression of Perilipins in Human Skeletal Muscle in Vitro and in Vivo in Relation to Diet, Exercise and Energy Balance. Arch. Physiol. Biochem. 2012, 118, 22–30. [Google Scholar] [CrossRef]
- Shaw, C.S.; Shepherd, S.O.; Wagenmakers, A.J.M.; Hansen, D.; Dendale, P.; van Loon, L.J.C. Prolonged Exercise Training Increases Intramuscular Lipid Content and Perilipin 2 Expression in Type I Muscle Fibers of Patients with Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1158–E1165. [Google Scholar] [CrossRef]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.M.; Shaw, C.S. Sprint Interval and Traditional Endurance Training Increase Net Intramuscular Triglyceride Breakdown and Expression of Perilipin 2 and 5. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef]
- Peters, S.J.; Samjoo, I.A.; Devries, M.C.; Stevic, I.; Robertshaw, H.A.; Tarnopolsky, M.A. Perilipin Family (PLIN) Proteins in Human Skeletal Muscle: The Effect of Sex, Obesity, and Endurance Training. Appl. Physiol. Nutr. Metab. 2012, 37, 724–735. [Google Scholar] [CrossRef]
- Bosma, M.; Hesselink, M.K.C.; Sparks, L.M.; Timmers, S.; Ferraz, M.J.; Mattijssen, F.; Van Beurden, D.; Schaart, G.; De Baets, M.H.; Verheyen, F.K.; et al. Perilipin 2 Improves Insulin Sensitivity in Skeletal Muscle despite Elevated Intramuscular Lipid Levels. Diabetes 2012, 61, 2679–2690. [Google Scholar] [CrossRef] [Green Version]
- Bosma, M.; Sparks, L.M.; Hooiveld, G.J.; Jorgensen, J.A.; Houten, S.M.; Schrauwen, P.; Kersten, S.; Hesselink, M.K.C. Overexpression of PLIN5 in Skeletal Muscle Promotes Oxidative Gene Expression and Intramyocellular Lipid Content without Compromising Insulin Sensitivity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 844–852. [Google Scholar] [CrossRef]
- Koves, T.R.; Sparks, L.M.; Kovalik, J.P.; Mosedale, M.; Arumugam, R.; DeBalsi, K.L.; Everingham, K.; Thorne, L.; Phielix, E.; Meex, R.C.; et al. PPARγ Coactivator-1α Contributes to Exercise-Induced Regulation of Intramuscular Lipid Droplet Programming in Mice and Humans. J. Lipid Res. 2013, 54, 522–534. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid Method for the Isolation of Lipoproteins from Human Serum by Precipitation with Polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bamman, M.M.; Clarke, M.S.F.; Talmadge, R.J.; Feeback, D.L. Enhanced Protein Electrophoresis Technique for Separating Human Skeletal Muscle Myosin Heavy Chain Isoforms. Electrophoresis 1999, 20, 466–468. [Google Scholar] [CrossRef]
- Kang, D.; Gho, Y.S.; Suh, M.; Kang, C. Highly Sensitive and Fast Protein Detection with Coomassie Brilliant Blue in Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis. Bull. Korean Chem. Soc. 2002, 23, 1511–1512. [Google Scholar]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved Staining of Proteins in Polyacrylamide Gels Including Isoelectric Focusing Gels with Clear Background at Nanogram Sensitivity Using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef]

| Variable | MICT | HIIT | p-Value |
|---|---|---|---|
| Number | Number | ||
| Subjects | 16 | 16 | |
| Sex, Men/Women | 9/7 | 8/8 | 0.723 |
| Smoker, never/in the past/current | 12/4/0 | 9/4/1 | 0.553 |
| Hypertension medication, no/yes | 16/0 | 16/0 | |
| Hyperlipidemia medication, no/yes | 16/0 | 16/0 | |
| Diabetes medication, no/yes | 16/0 | 16/0 | |
| Means ± SD | Means ± SD | ||
| Age (years) | 37 ± 9 | 40 ± 7 | 0.335 |
| Weight (kg) | 107.1 ± 17.4 | 103.4 ± 10.9 | 0.479 |
| BMI (kg/m2) | 36.1 ± 5.1 | 35.1 ± 3.6 | 0.514 |
| VO2peak (mL) | 3016.3 ± 782.7 | 2888.7 ± 706.2 | 0.632 |
| VO2peak/weight (mL/kg) | 28.3 ± 6.5 | 27.8 ± 5.6 | 0.824 |
| Outcome Measures | Intervention Type | BDC | PTDC | p-Value Paired Samples t-Test | Intervention x Intervention Type Effects MD (95% CI) | p-Value GLM Change Between Groups | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | MICT | 107.1 ± 17.4 | 101.1 ± 17.9 | 0.001 | −6.0 (−9.0–−3.0) | 0.860 | |
| HIIT | 102.8 ± 11.0 | 97.1 ± 10.2 | <0.001 | −5.7 (−8.3–−3.1) | |||
| BMI (kg/m2) | MICT | 36.1 ± 5.1 | 33.9 ± 4.8 | 0.001 | −2.1 (−3.2–−1.1) | 0.696 | |
| HIIT | 35.0 ± 3.7 | 33.1 ± 4.2 | <0.001 | −1.9 (−2.7–−1.0) | |||
| Waist (cm) | Men | MICT | 116.5 ± 17.1 | 109.6 ± 17.7 | 0.369 | −1.8 (−6.1–2.5) | 0.039 |
| HIIT | 117.1 ± 9.5 | 101.2 ± 9.0 | <0.001 | −8.5 (−10.9–−6.1) | |||
| Women | MICT | 108.6 ± 8.1 | 90.1 ± 11.5 | 0.054 | −4.4 (−8.9–0.1) | 0.774 | |
| HIIT | 110.6 ± 7.6 | 93.4 ± 10.4 | 0.043 | −2.4 (−4.7–−0.1) | |||
| Hip (cm) | Men | MICT | 120.3 ± 10.4 | 118.7 ± 12.8 | 0.280 | −1.5 (−4.6– 1.5) | 0.102 |
| HIIT | 116.4 ± 6.8 | 111.9 ± 5.7 | 0.001 | −4.5 (−6.4–−2.5) | |||
| Women | MICT | 126.7 ± 11.6 | 118.0 ± 9.9 | 0.011 | −8.7 (−14.7–−2.8) | 0.155 | |
| HIIT | 124.4 ± 6.4 | 119.9 ± 10.0 | 0.020 | −4.5 (−8.1–−1.0) | |||
| FM (kg) | MICT | 37.7 ± 10.9 | 32.4 ± 9.1 | <0.001 | −5.3(−7.8–−2.8) | 0.919 | |
| HIIT | 38.4 ± 8.2 | 32.9 ± 10.1 | 0.001 | −5.5(−8.3–−2.6) | |||
| FM (%) | MICT | 35.4 ± 8.9 | 32.5 ± 8.3 | 0.001 | −2.9(−4.4 –−1.4 ) | 0.619 | |
| HIIT | 37.3 ± 7.7 | 33.7 ± 9.2 | 0.006 | −3.6(−5.9 –−1.2 ) | |||
| FFM (kg) | MICT | 69.4 ± 15.5 | 65.1 ± 11.7 | 0.127 | −5.3 (−7.8–−2.8) | 0.735 | |
| HIIT | 68.6 ± 16.3 | 64.7 ± 11.0 | 0.641 | −5.5 (−8.3–−2.6) | |||
| FFM (%) | MICT | 64.6 ± 8.9 | 67.4 ± 8.3 | 0.002 | 2.8 (1.3– 4.4) | 0.554 | |
| HIIT | 62.3 ± 7.7 | 66.0 ± 9.4 | 0.008 | 3.7 (1.2– 6.3) | |||
| Antibody | Code | Saturation | Ab Dilution |
|---|---|---|---|
| Cox-4 | sc-517553 | 5% BSA | 1:1.000 |
| TOMM20 | sc-11415 | 5% BSA | 1:1.000 |
| PLIN-5 | LS-B5964-100 | 5% BSA | 1:40.000 |
| PLIN-2 | NB110-40877 | 5% BSA | 1:2.000 |
| Anti-Mouse | ab205719 | 3% Milk | 1:10.000 |
| Anti-Rabbit | ab205718 | 3% Milk | 1:10.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirago, G.; Vaccari, F.; Lazzer, S.; D’Amuri, A.; Sanz, J.M.; Narici, M.V.; Reggiani, C.; Passaro, A.; Toniolo, L. Skeletal Muscle Mitochondrial and Perilipin Content in a Cohort of Obese Subjects Undergoing Moderate and High Intensity Training. Metabolites 2022, 12, 855. https://doi.org/10.3390/metabo12090855
Sirago G, Vaccari F, Lazzer S, D’Amuri A, Sanz JM, Narici MV, Reggiani C, Passaro A, Toniolo L. Skeletal Muscle Mitochondrial and Perilipin Content in a Cohort of Obese Subjects Undergoing Moderate and High Intensity Training. Metabolites. 2022; 12(9):855. https://doi.org/10.3390/metabo12090855
Chicago/Turabian StyleSirago, Giuseppe, Filippo Vaccari, Stefano Lazzer, Andrea D’Amuri, Juana M. Sanz, Marco V. Narici, Carlo Reggiani, Angelina Passaro, and Luana Toniolo. 2022. "Skeletal Muscle Mitochondrial and Perilipin Content in a Cohort of Obese Subjects Undergoing Moderate and High Intensity Training" Metabolites 12, no. 9: 855. https://doi.org/10.3390/metabo12090855
APA StyleSirago, G., Vaccari, F., Lazzer, S., D’Amuri, A., Sanz, J. M., Narici, M. V., Reggiani, C., Passaro, A., & Toniolo, L. (2022). Skeletal Muscle Mitochondrial and Perilipin Content in a Cohort of Obese Subjects Undergoing Moderate and High Intensity Training. Metabolites, 12(9), 855. https://doi.org/10.3390/metabo12090855












