Pancreatic Cancer Cell-Conditioned, Human-Derived Primary Myotubes Display Increased Leucine Turnover, Increased Lipid Accumulation, and Reduced Glucose Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethical Approval
2.3. Cell Culturing
2.3.1. PANC-1 Cells
2.3.2. hPECs
2.3.3. Skeletal Muscle Cells
2.4. PANC-1- and hPEC-Conditioned Myotubes
2.5. Scintillation Proximity Assay (SPA)
2.6. Substrate Oxidation Assay
2.7. Thin-Layer Chromatography and Measurement of Acid-Soluble Metabolites (ASM)
2.8. Glycogen Synthesis
2.9. Immunoblotting
2.10. RNA Isolation and Analysis of Gene Expression by qPCR
2.11. Proteomics
2.12. Presentation of Data and Statistics
3. Results
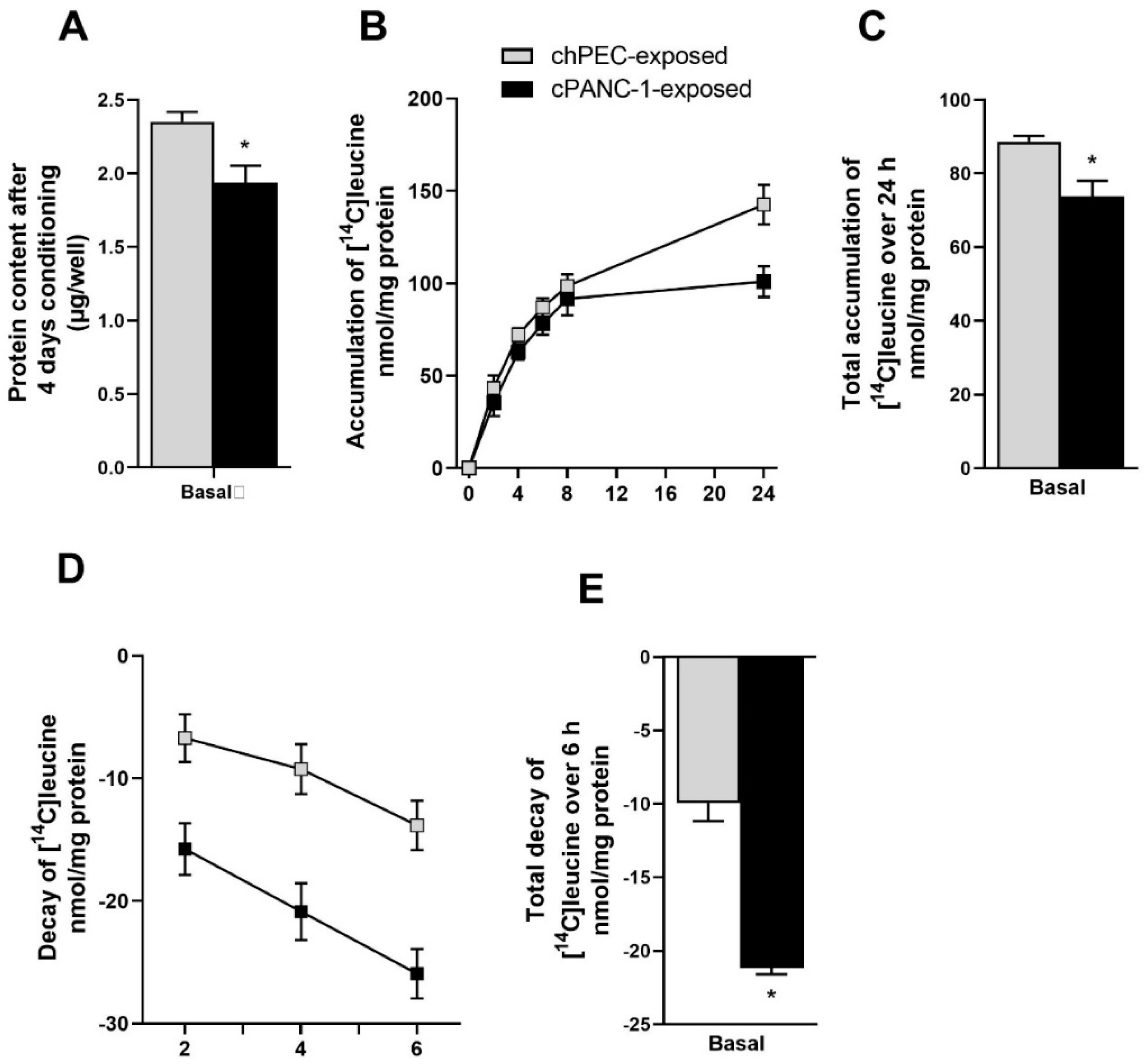
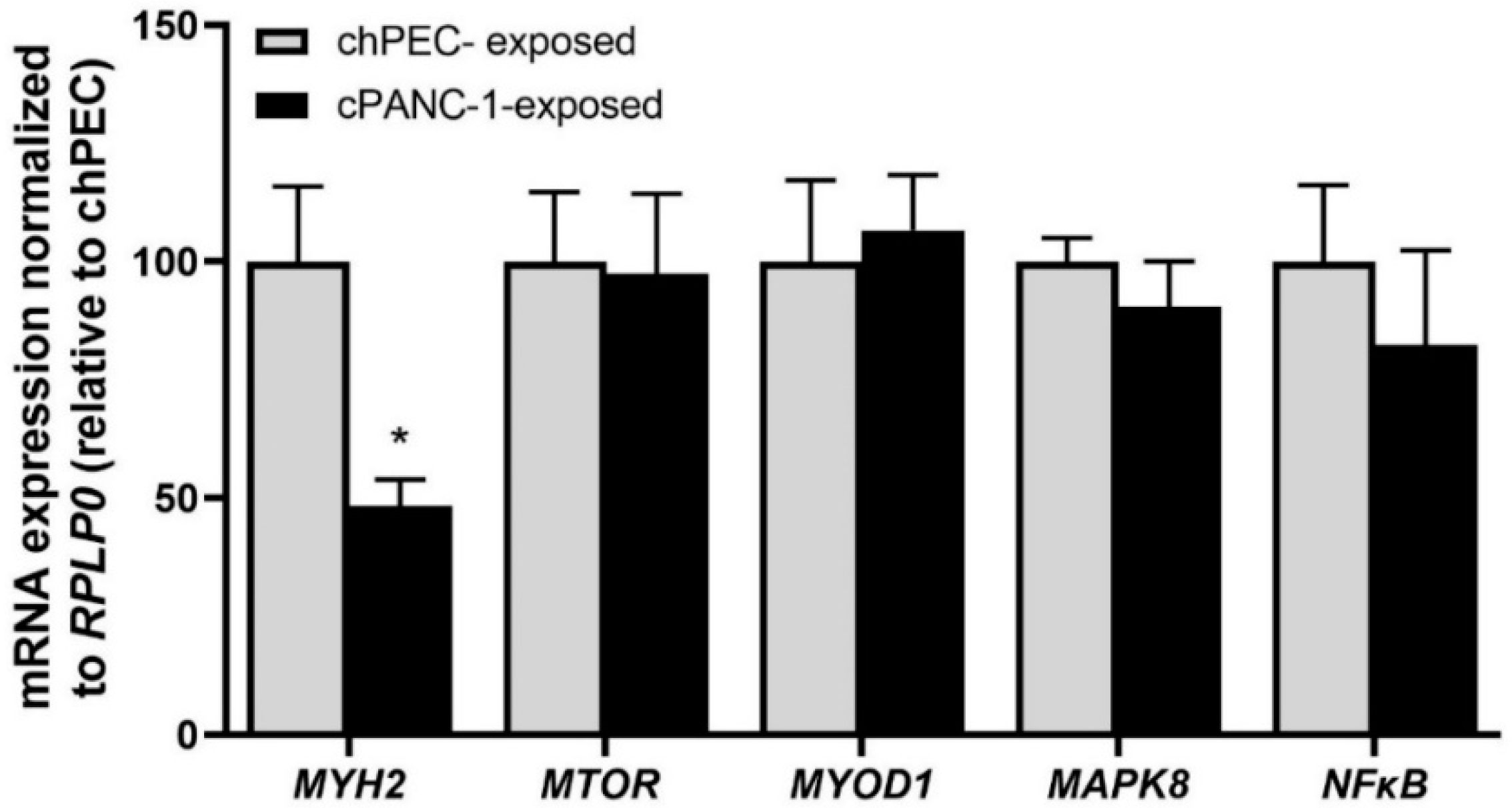
3.1. Measurement of Leucine Incorporation in Myotubes Indicated Decreased Protein Synthesis, Increased Protein Decay, and Lower mRNA Expression of MYH2 after Being Conditioned with the Medium from Pancreatic Cancer Cells
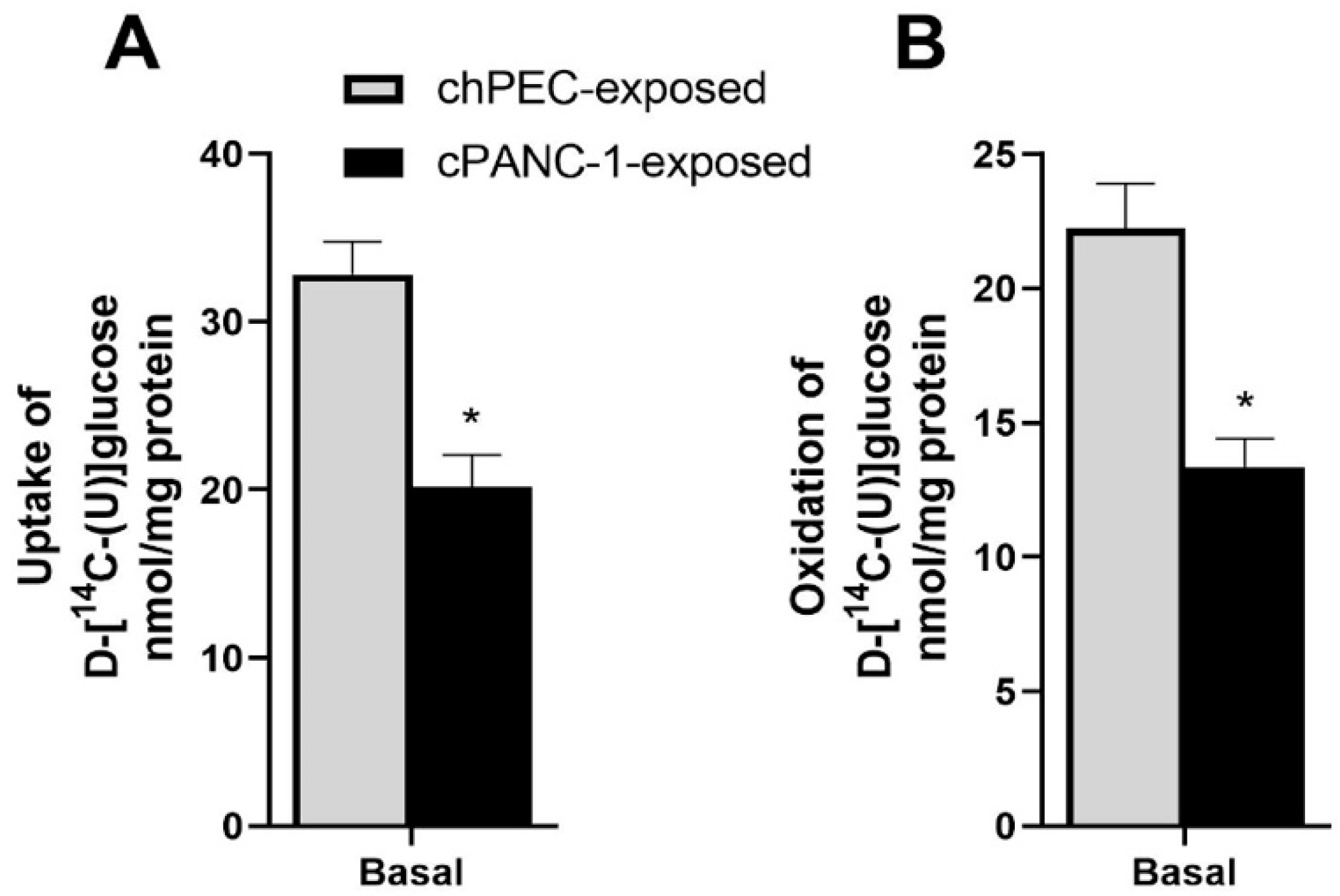
3.2. Myotubes Conditioned with PANC-1 Culture Medium Had Lower Glucose Uptake and Oxidation Compared to hPEC-Conditioned Myotubes
3.3. Akt Phosphorylation Was Higher under Basal Conditions and When Treated with Insulin in Myotubes Conditioned with PANC-1 and hPEC Culture Media
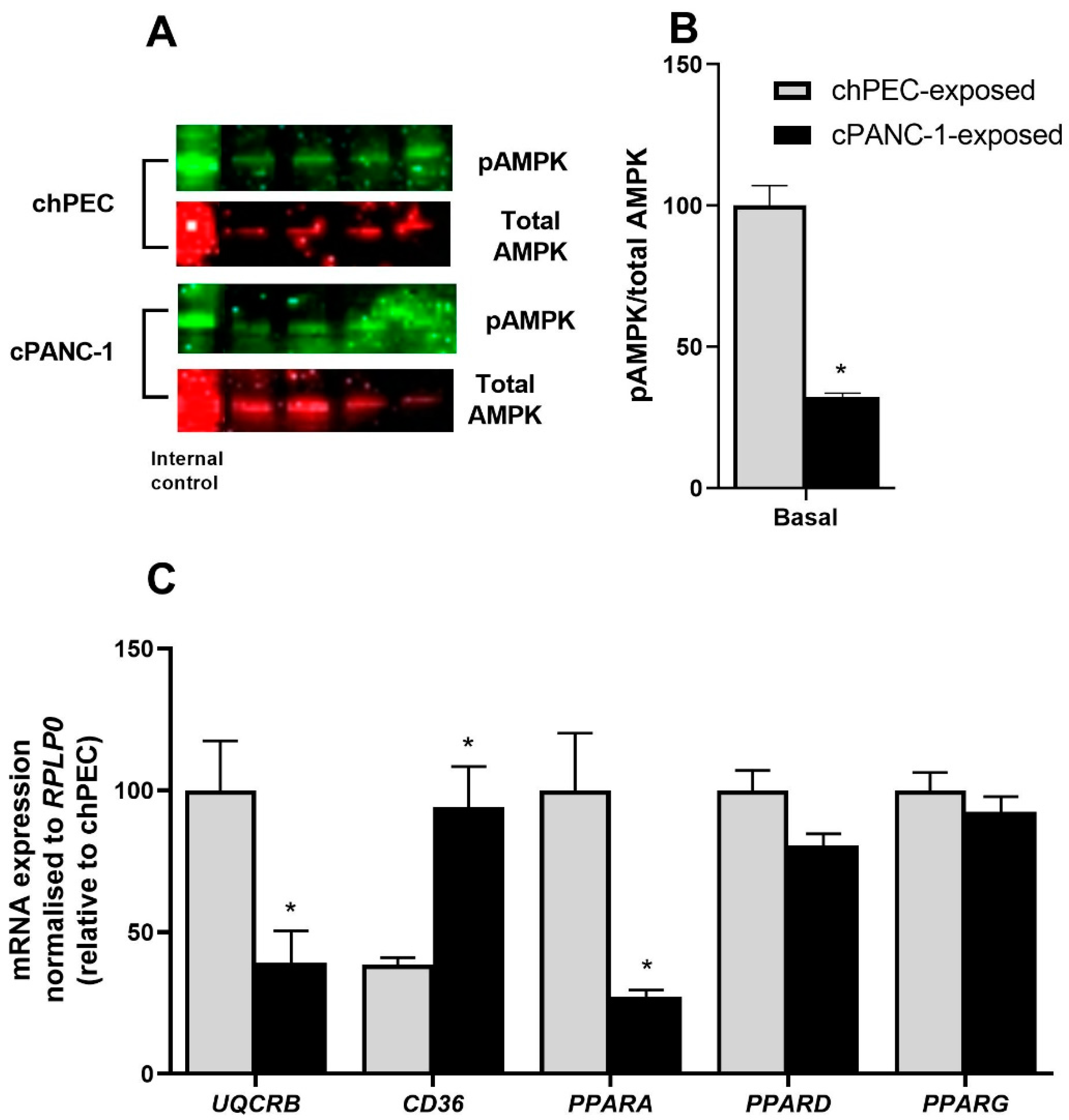
3.4. Myotubes Exposed to PANC-1-Conditioned Medium Showed Increased Lipid Accumulation and Responded Less to Electron Chain Complex Inhibitors Compared to hPEC-Conditioned Myotubes
3.5. Secretomes from hPECs and PANC-1 Cells Indicate Increased Secretion of Certain Cytokines, Growth Factors, and Growth-Factor-Binding Proteins Potentially Affecting Metabolism in Skeletal Muscle Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef] [PubMed]
- Kunzke, T.; Buck, A.; Prade, V.M.; Feuchtinger, A.; Prokopchuk, O.; Martignoni, M.E.; Heisz, S.; Hauner, H.; Janssen, K.P.; Walch, A.; et al. Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2020, 11, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am. J. Clin. Nutr. 2008, 89, 463S–466S. [Google Scholar] [CrossRef]
- Permert, J.; Adrian, T.E.; Jacobsson, P.; Jorfelt, L.; Fruin, A.B.; Larsson, J. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am. J. Surg. 1993, 165, 61–67. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Wang, F.; Herrington, M.; Larsson, J.; Permert, J. The relationship between diabetes and pancreatic cancer. Mol. Cancer 2003, 2, 4. [Google Scholar] [CrossRef]
- Krapf, S.A.; Lund, J.; Lundkvist, M.; Dale, M.G.; Nyman, T.A.; Thoresen, G.H.; Kase, E.T. Pancreatic cancer cells show lower oleic acid oxidation and their conditioned medium inhibits oleic acid oxidation in human myotubes. Pancreatology 2020, 20, 676–682. [Google Scholar] [CrossRef]
- Wensaas, A.J.; Rustan, A.C.; Lovstedt, K.; Kull, B.; Wikstrom, S.; Drevon, C.A.; Hallen, S. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J. Lipid Res. 2007, 48, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gaster, M.; Rustan, A.C.; Aas, V.; Beck-Nielsen, H. Reduced Lipid Oxidation in Skeletal Muscle From Type 2 Diabetic Subjects May Be of Genetic Origin. Diabetes 2004, 53, 542–548. [Google Scholar] [CrossRef]
- Bakke, S.S.; Moro, C.; Nikolić, N.; Hessvik, N.P.; Badin, P.-M.; Lauvhaug, L.; Fredriksson, K.; Hesselink, M.K.; Boekschoten, M.V.; Kersten, S. Palmitic acid follows a different metabolic pathway than oleic acid in human skeletal muscle cells; lower lipolysis rate despite an increased level of adipose triglyceride lipase. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 1323–1333. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Bakke, S.S.; Fredriksson, K.; Boekschoten, M.V.; Fjorkenstad, A.; Koster, G.; Hesselink, M.K.; Kersten, S.; Kase, E.T.; Rustan, A.C.; et al. Metabolic switching of human myotubes is improved by n-3 fatty acids. J. Lipid Res. 2010, 51, 2090–2104. [Google Scholar] [CrossRef]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef]
- Poulia, K.A.; Sarantis, P.; Antoniadou, D.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic Cancer and Cachexia-Metabolic Mechanisms and Novel Insights. Nutrients 2020, 12, 1543. [Google Scholar] [CrossRef]
- Madigan, N.N.; Polzin, M.J.; Cui, G.; Liewluck, T.; Alsharabati, M.H.; Klein, C.J.; Windebank, A.J.; Mer, G.; Milone, M. Filamentous tangles with nemaline rods in MYH2 myopathy: A novel phenotype. Acta Neuropathol. Commun. 2021, 9, 79. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Nikolić, N.; Bakke, S.S.; Kase, E.T.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef]
- Acharyya, S.; Butchbach, M.E.R.; Sahenk, Z.; Wang, H.; Saji, M.; Carathers, M.; Ringel, M.D.; Skipworth, R.J.E.; Fearon, K.C.H.; Hollingsworth, M.A.; et al. Dystrophin glycoprotein complex dysfunction: A regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 2005, 8, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chen, Y.-L.; Chou, T.-C. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016, 7, 51608–51618. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.P.; Jeanneret, C.; Santoro, T.; Dormond, O. mTOR and Tumor Cachexia. Int. J. Mol. Sci. 2018, 19, 2225. [Google Scholar] [CrossRef]
- Liu, J.; Knezetic, J.A.; Strömmer, L.; Permert, J.; Larsson, J.; Adrian, T.E. The Intracellular Mechanism of Insulin Resistance in Pancreatic Cancer Patients. J. Clin. Endocrinol. Metab. 2000, 85, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Fuster, G.; Busquets, S.l.; Ametller, E.; Olivan, M.; Almendro, V.; Fontes de Oliveira, C.C.; Figueras, M.; López-Soriano, F.J.; Argilés, J.M. Are Peroxisome Proliferator-Activated Receptors Involved in Skeletal Muscle Wasting during Experimental Cancer Cachexia? Role of β2-Adrenergic Agonists. Cancer Res. 2007, 67, 6512–6519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Jana, B.A.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Wadhwani, A.; Mohankumar, S.K. Cytosolic lipid excess-induced mitochondrial dysfunction is the cause or effect of high fat diet-induced skeletal muscle insulin resistance: A molecular insight. Mol. Biol. Rep. 2019, 46, 957–963. [Google Scholar] [CrossRef]
- Pala, F.; Di Girolamo, D.; Mella, S.; Yennek, S.; Chatre, L.; Ricchetti, M.; Tajbakhsh, S. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 2018, 131, jcs212977. [Google Scholar] [CrossRef]
- Debashree, B.; Kumar, M.; Keshava Prasad, T.S.; Natarajan, A.; Christopher, R.; Nalini, A.; Bindu, P.S.; Gayathri, N.; Srinivas Bharath, M.M. Mitochondrial dysfunction in human skeletal muscle biopsies of lipid storage disorder. J. Neurochem. 2018, 145, 323–341. [Google Scholar] [CrossRef]
- Taylor, R.W.; Birch-Machin, M.A.; Bartlett, K.; Lowerson, S.A.; Turnbull, D.M. The control of mitochondrial oxidations by complex III in rat muscle and liver mitochondria. Implications for our understanding of mitochondrial cytopathies in man. J. Biol. Chem. 1994, 269, 3523–3528. [Google Scholar] [CrossRef]
- Jørgensen, S.B.; Richter, E.A.; Wojtaszewski, J.F.P. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J. Physiol. 2006, 574, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: A patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.; Schrauwen, P.; Broekhuizen, R.; Willems, J.; Kersten, S.; Gosker, H.R.; Schols, A.M. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 2007, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Atherton, H.J.; Gulston, M.K.; Bailey, N.J.; Cheng, K.K.; Zhang, W.; Clarke, K.; Griffin, J.L. Metabolomics of the interaction between PPAR-alpha and age in the PPAR-alpha-null mouse. Mol. Syst. Biol. 2009, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, W.-S.; Dong, F.; Sun, H.-M.; Wu, B.; Tan, J.; Zou, W.-J.; Zhou, D.-S. KITLG is a novel target of miR-34c that is associated with the inhibition of growth and invasion in colorectal cancer cells. J. Cell. Mol. Med. 2014, 18, 2092–2102. [Google Scholar] [CrossRef]
- Miura, S.; Mitsuhashi, N.; Shimizu, H.; Kimura, F.; Yoshidome, H.; Otsuka, M.; Kato, A.; Shida, T.; Okamura, D.; Miyazaki, M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 2012, 12, 56. [Google Scholar] [CrossRef]
- Pan, S.; Chen, R.; Crispin, D.A.; May, D.; Stevens, T.; McIntosh, M.W.; Bronner, M.P.; Ziogas, A.; Anton-Culver, H.; Brentnall, T.A. Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J. Proteome Res. 2011, 10, 2359–2376. [Google Scholar] [CrossRef]
- Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018, 18, 128. [Google Scholar] [CrossRef]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2013, 59, 240–249. [Google Scholar] [CrossRef]
- Barton-Davis, E.R.; Shoturma, D.I.; Sweeney, H.L. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 1999, 167, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, H.; Chen, Y.; Huang, T.; Mi, Y.; Zou, Z. Cancer cachexia: Molecular mechanism and pharmacological management. Biochem. J. 2021, 478, 1663–1688. [Google Scholar] [CrossRef] [PubMed]


| Protein | log10 Fold-Change PANC-1 Cells vs. hPECs | |
|---|---|---|
| VGF | Neurosecretory protein VGF | 3.175 |
| KITLG | Kit ligand | 2.381 |
| AREG | Amphiregulin | 1.810 |
| LTBP3 | Latent-transforming growth factor beta-binding protein 3 | 1.779 |
| IGFBP2 | Insulin-like growth-factor-binding protein 2 | 1.694 |
| LTBP4 | Latent-transforming growth factor beta-binding protein 4 | 1.594 |
| FGF19 | Fibroblast growth factor 19 | 1.510 |
| TIMP1 | Metalloproteinase inhibitor 1 | 1.392 |
| GMFB | Glia maturation factor beta | 1.338 |
| BMP1 | Bone morphogenetic protein 1 | 1.362 |
| MIF | Macrophage migration inhibitory factor | 0.887 |
| IGF2 | Insulin-like growth factor II | 0.885 |
| TGFB2 | Transforming growth factor beta-2 | −1.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapf, S.A.; Lund, J.; Saqib, A.U.R.; Bakke, H.G.; Rustan, A.C.; Thoresen, G.H.; Kase, E.T. Pancreatic Cancer Cell-Conditioned, Human-Derived Primary Myotubes Display Increased Leucine Turnover, Increased Lipid Accumulation, and Reduced Glucose Uptake. Metabolites 2022, 12, 1095. https://doi.org/10.3390/metabo12111095
Krapf SA, Lund J, Saqib AUR, Bakke HG, Rustan AC, Thoresen GH, Kase ET. Pancreatic Cancer Cell-Conditioned, Human-Derived Primary Myotubes Display Increased Leucine Turnover, Increased Lipid Accumulation, and Reduced Glucose Uptake. Metabolites. 2022; 12(11):1095. https://doi.org/10.3390/metabo12111095
Chicago/Turabian StyleKrapf, Solveig A., Jenny Lund, Awais Ur Rehman Saqib, Hege G. Bakke, Arild C. Rustan, G. Hege Thoresen, and Eili T. Kase. 2022. "Pancreatic Cancer Cell-Conditioned, Human-Derived Primary Myotubes Display Increased Leucine Turnover, Increased Lipid Accumulation, and Reduced Glucose Uptake" Metabolites 12, no. 11: 1095. https://doi.org/10.3390/metabo12111095
APA StyleKrapf, S. A., Lund, J., Saqib, A. U. R., Bakke, H. G., Rustan, A. C., Thoresen, G. H., & Kase, E. T. (2022). Pancreatic Cancer Cell-Conditioned, Human-Derived Primary Myotubes Display Increased Leucine Turnover, Increased Lipid Accumulation, and Reduced Glucose Uptake. Metabolites, 12(11), 1095. https://doi.org/10.3390/metabo12111095









