Tissue-Wide Expression of Genes Related to Vitamin D Metabolism and FGF23 Signaling following Variable Phosphorus Intake in Pigs
Abstract
:1. Introduction
| Gene | Ensembl ID (v. 102) | Description | Function |
|---|---|---|---|
| CYP2R1 | ENSSSCG00000013389 | Cytochrome P450, family 2, R1 | Hydroxylation (25-OH) of cholecalciferol (liver) * [23] |
| CYP27A1 | ENSSSCG00000016199 | Cytochrome P450 family 27, A1 | Hydroxylation (25-OH) of cholecalciferol (liver) * [24] |
| CYP27B1 | ENSSSCG00000028637 | Cytochrome P450 family 27, B1 | Hydroxylation (1α-OH) of calcidiol (kidney) * [25] |
| CYP24A1 | ENSSSCG00000007486 | Cytochrome P450 family 24, A1 | Hydroxylation (24-OH) of calcidiol and calcitriol (kidney) * [25] |
| VDR | ENSSSCG00000020864 | Vitamin D receptor | Transcription factor [26] |
| GC | ENSSSCG00000027609 | Vitamin D binding protein | Binding of calcitriol [27] |
| FGF23 | ENSSSCG00000052449 | Fibroblast growth factor 23 | Regulator of P homeostasis [28] |
| FGFR1 | ENSSSCG00000015815 | Fibroblast growth factor receptor 1 | Receptor of FGF23 and other FGFs [29] |
| FGFR2 | ENSSSCG00000010698 | Fibroblast growth factor receptor 2 | Receptor of FGF23 and other FGFs [29] |
| FGFR3 | ENSSSCG00000030827 | Fibroblast growth factor receptor 3 | Receptor of FGF23 and other FGFs [29] |
| FGFR4 | ENSSSCG00000014047 | Fibroblast growth factor receptor 4 | Receptor of FGF23 and other FGFs [29] |
| KL | ENSSSCG00000009347 | Klotho | Co-receptor of FGF23 [30] |
2. Results
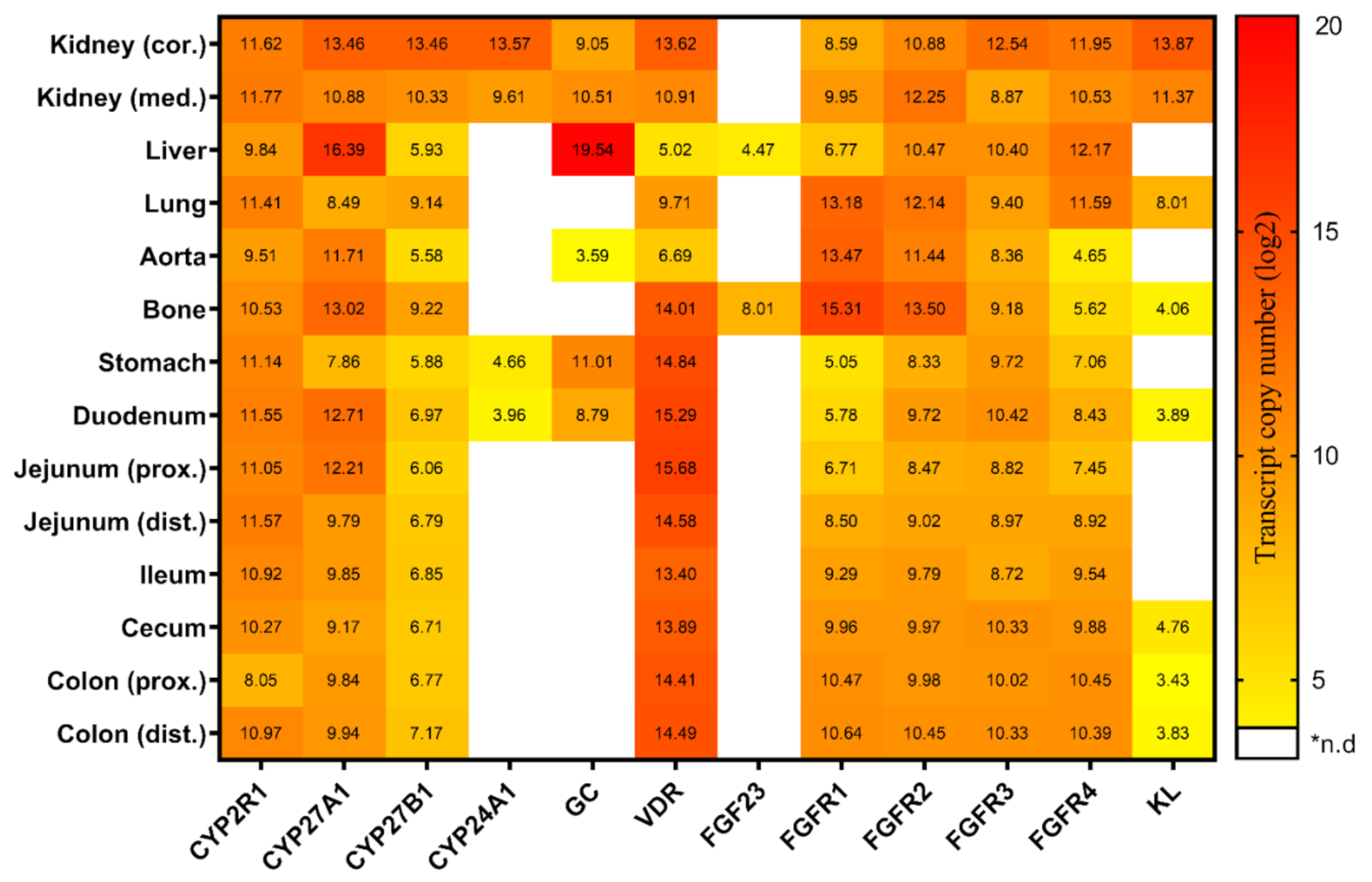
2.1. Tissue-Specific Expression of Genes Linked to Vitamin D Metabolism and FGF23 Signaling under Conventional Standard Dietary P Intake
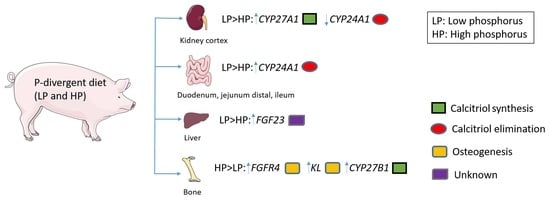
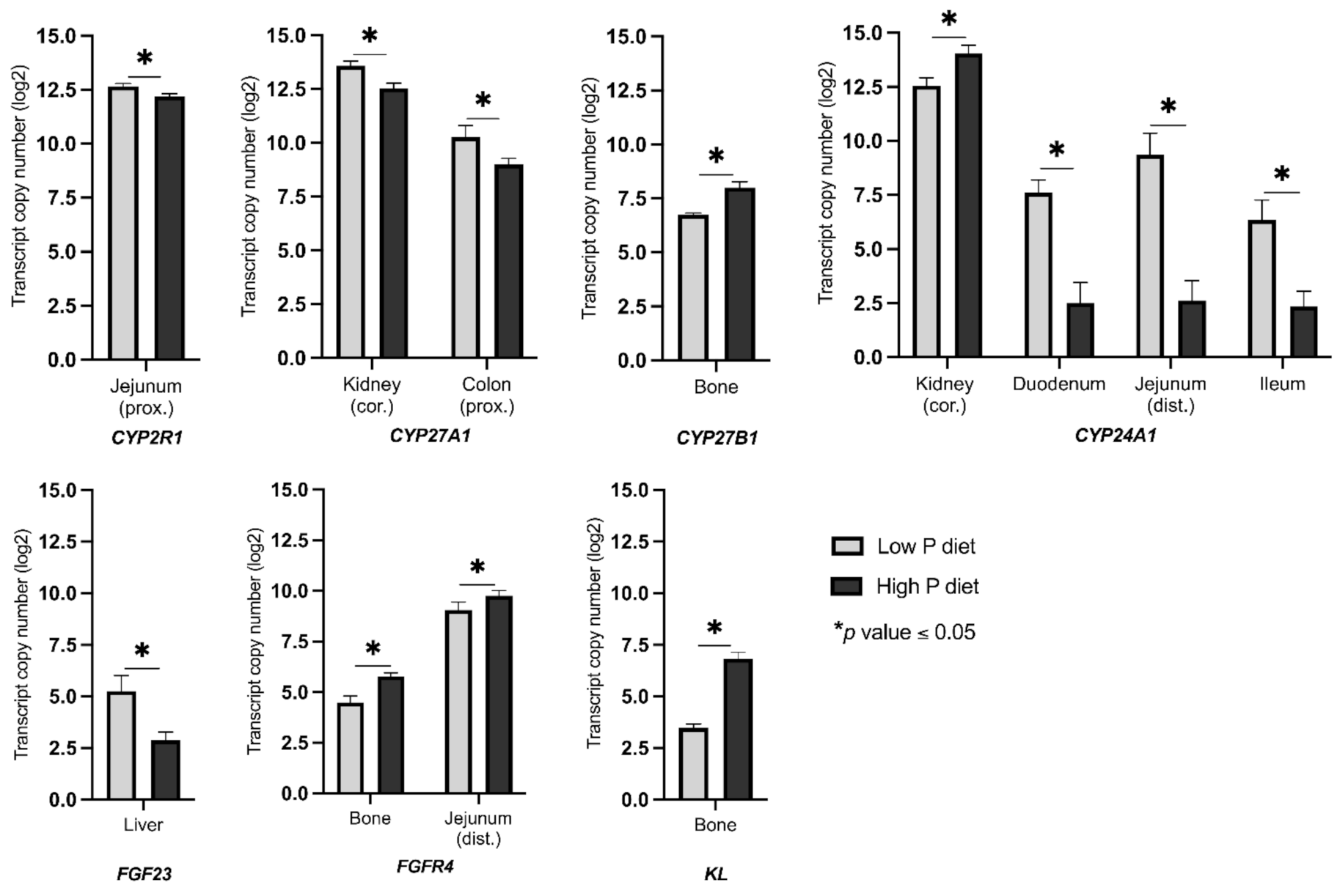
2.2. Changes in the Expression of Genes Linked to Vitamin D Metabolism and FGF23 Signaling as a Result of Divergent Dietary P Intake
3. Discussion
3.1. Status Quo and Reactivity of the Vitamin D System to Maintain Mineral Homeostasis
3.2. Systemic and Autocrine Regulations of FGF23 Signaling
4. Materials and Methods
4.1. Animals and Diets
4.2. Tissue and Serum Sampling
4.3. RNA Isolation and cDNA Synthesis
4.4. Quantitative Real-Time PCR
4.5. Serum Measurement of Calcitriol
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flachowsky, G. Empfehlungen zur Energie-und Nährstoffversorgung von Schweinen; DLG: Frankfurt, Germany, 2006. [Google Scholar]
- Berndt, T.; Kumar, R. Novel Mechanisms in the Regulation of Phosphorus Homeostasis. Physiology 2009, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chande, S.; Bergwitz, C. Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 2018, 14, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The unsettled science of nonrenal calcitriol production and its clinical relevance. J. Clin. Invest. 2020, 130, 4519–4521. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.K.; Kelly, A.K.; Rajauria, G.; Jakobsen, J.; Clarke, L.C.; Monahan, F.J.; Dowling, K.G.; Hull, G.; Galvin, K.; Cashman, K.D.; et al. The use of synthetic and natural vitamin D sources in pig diets to improve meat quality and vitamin D content. Meat Sci. 2018, 143, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Barnkob, L.L.; Argyraki, A.; Petersen, P.M.; Jakobsen, J. Investigation of the effect of UV-LED exposure conditions on the production of vitamin D in pig skin. Food Chem. 2016, 212, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirams, M.; Robinson, B.G.; Mason, R.S.; Nelson, A.E. Bone as a source of FGF23: Regulation by phosphate? Bone 2004, 35, 1192–1199. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Bacchetta, J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Wesseling-Perry, K.; Gales, B.; Adams, J.S.; Salusky, I.B.; Hewison, M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2013, 28, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Gattineni, J.; Bates, C.; Twombley, K.; Dwarakanath, V.; Robinson, M.L.; Goetz, R.; Mohammadi, M.; Baum, M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal Physiol. 2009, 297, F282–F291. [Google Scholar] [CrossRef] [Green Version]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Shi, M.; Moe, O.W. Role of αKlotho and FGF23 in regulation of type II Na-dependent phosphate co-transporters. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 99–108. [Google Scholar] [CrossRef]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Vervloet, M. Renal and extrarenal effects of fibroblast growth factor 23. Nat. Rev. Nephrol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Staude, H.; Jeske, S.; Schmitz, K.; Warncke, G.; Fischer, D.C. Cardiovascular Risk and Mineral Bone Disorder in Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2013, 37, 68–83. [Google Scholar] [CrossRef]
- Gerlinger, C.; Oster, M.; Reyer, H.; Polley, C.; Vollmar, B.; Muráni, E.; Wimmers, K.; Wolf, P. Effects of excessive or restricted phosphorus and calcium intake during early life on markers of bone architecture and composition in pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Wubuli, A.; Gerlinger, C.; Reyer, H.; Oster, M.; Muráni, E.; Trakooljul, N.; Ponsuksili, S.; Wolf, P.; Wimmers, K. Reduced phosphorus intake throughout gestation and lactation of sows is mitigated by transcriptional adaptations in kidney and intestine. BMC Genom. 2020, 21, 626. [Google Scholar] [CrossRef]
- Oster, M.; Reyer, H.; Gerlinger, C.; Trakooljul, N.; Siengdee, P.; Keiler, J.; Ponsuksili, S.; Wolf, P.; Wimmers, K. mRNA Profiles of Porcine Parathyroid Glands Following Variable Phosphorus Supplies throughout Fetal and Postnatal Life. Biomedicines 2021, 9, 454. [Google Scholar] [CrossRef]
- Wubuli, A.; Reyer, H.; Muráni, E.; Ponsuksili, S.; Wolf, P.; Oster, M.; Wimmers, K. Tissue-Wide Gene Expression Analysis of Sodium/Phosphate Co-Transporters in Pigs. Int. J. Mol. Sci. 2019, 20, 5576. [Google Scholar] [CrossRef] [Green Version]
- Strushkevich, N.; Usanov, S.A.; Plotnikov, A.N.; Jones, G.; Park, H.-W. Structural Analysis of CYP2R1 in Complex with Vitamin D3. J. Mol. Biol. 2008, 380, 95–106. [Google Scholar] [CrossRef]
- Hosseinpour, F.; Ibranovic, I.; Tang, W.; Wikvall, K. 25-Hydroxylation of vitamin D3 in primary cultures of pig hepatocytes: Evidence for a role of both CYP2D25 and CYP27A1. Biochem. Biophys. Res. Commun. 2003, 303, 877–883. [Google Scholar] [CrossRef]
- Ohyama, Y.; Yamasaki, T. Eight cytochrome P450s catalyze vitamin D metabolism. Front. Biosci.-Landmark 2004, 9, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Omdahl, J.L.; Morris, H.A.; May, B.K. Hydroxylase enzymes of the vitamin D pathway: Expression, function, and regulation. Annu. Rev. Nutr. 2002, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P.; Schanfield, M.S.; Cavalli-Sforza, L.L. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc. Natl. Acad. Sci. USA 1975, 72, 2076–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Forster, R.; Saini, R.; Hsieh, J.-C.; Haussler, C.A.; Jurutka, P.W. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev. Endocr. Metab. Disord. 2012, 13, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho signaling axis in the kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Seo, H.; Shim, J.; Hyun, S.-H.; Lee, E.; Ka, H. Klotho: Expression and regulation at the maternal-conceptus Interface in pigs. J. Embryo Transf. 2014, 29, 375–383. [Google Scholar] [CrossRef]
- Schook, L.; Beattie, C.; Beever, J.; Donovan, S.; Jamison, R.; Zuckermann, F.; Niemi, S.; Rothschild, M.; Rutherford, M.; Smith, D. Swine in biomedical research: Creating the building blocks of animal models. Anim. Biotechnol. 2005, 16, 183–190. [Google Scholar] [CrossRef]
- Oster, M.; Gerlinger, C.; Heide, K.; Just, F.; Borgelt, L.; Wolf, P.; Polley, C.; Vollmar, B.; Muráni, E.; Ponsuksili, S. Lower dietary phosphorus supply in pigs match both animal welfare aspects and resource efficiency. Ambio 2018, 47, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-orphanization of Cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef] [Green Version]
- Elkhwanky, M.-S.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues. JBMR Plus 2020, 4, e10397. [Google Scholar] [CrossRef]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase-Four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Sawada, N.; Sakaki, T.; Ohta, M.; Inouye, K. Metabolism of vitamin D(3) by human CYP27A1. Biochem. Biophys. Res. Commun. 2000, 273, 977–984. [Google Scholar] [CrossRef]
- Postlind, H.; Wikvall, K. Purification of a cytochrome P-450 from pig kidney microsomes catalysing the 25-hydroxylation of vitamin D3. Biochem. J. 1988, 253, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.B.; Benkusky, N.A.; Kaufmann, M.; Lee, S.M.; Redfield, R.R.; Jones, G.; Pike, J.W. Targeted genomic deletions identify diverse enhancer functions and generate a kidney-specific, endocrine-deficient Cyp27b1 pseudo-null mouse. J. Biol. Chem. 2019, 294, 9518–9535. [Google Scholar] [CrossRef]
- Chanakul, A.; Zhang, M.Y.H.; Louw, A.; Armbrecht, H.J.; Miller, W.L.; Portale, A.A.; Perwad, F. FGF-23 Regulates CYP27B1 Transcription in the Kidney and in Extra-Renal Tissues. PLoS ONE 2013, 8, e72816. [Google Scholar] [CrossRef] [Green Version]
- Atkins, G.J.; Anderson, P.H.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.C.W.; O’Loughlin, P.D.; Morris, H.A. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Dusso, A.; Brown, A.; Slatopolsky, E. Extrarenal production of calcitriol. Semin. Nephrol. 1994, 14, 144–155. [Google Scholar]
- Bikle, D.D. Extrarenal Synthesis of 1,25-Dihydroxyvitamin D and Its Health Implications. In Vitamin D: Physiology, Molecular Biology, and Clinical Applications; Holick, M.F., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 277–295. [Google Scholar]
- Petkovich, M.; Jones, G. CYP24A1 and kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 337–344. [Google Scholar] [CrossRef]
- Vilaça, T.; Lazaretti-Castro, M. Vitamin D-binding protein. In Vitamin D in Clinical Medicine; Karger Publishers: Basel, Switzerland, 2018; Volume 50, pp. 31–41. [Google Scholar]
- Feldman, D.; Malloy, P.J.; Krishnan, A.V.; Balint, E.V.A. Chapter 13—Vitamin D: Biology, Action, and Clinical Implications. In Osteoporosis, 3rd ed.; Marcus, R., Feldman, D., Nelson, D.A., Rosen, C.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 317–382. [Google Scholar]
- Schiødt, F.V. Gc-globulin in liver disease. Dan. Med. Bull. 2008, 55, 131–146. [Google Scholar] [PubMed]
- Cooke, N.E.; McLeod, J.F.; Wang, X.; Ray, K. Vitamin D binding protein: Genomic structure, functional domains, and mRNA expression in tissues. J. Steroid Biochem. Mol. Biol. 1991, 40, 787–793. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; Burton MacLeod, N.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-Scale in Silico and Microarray-Based Identification of Direct 1,25-Dihydroxyvitamin D3 Target Genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Fleet, J.C. Intestinal Vitamin D Receptor Is Required for Normal Calcium and Bone Metabolism in Mice. Gastroenterology 2009, 136, 1317–1327. [Google Scholar] [CrossRef] [Green Version]
- Hendy, G.N.; Hruska, K.A.; Mathew, S.; Goltzman, D. New insights into mineral and skeletal regulation by active forms of vitamin D. Kidney Int. 2006, 69, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, 116–124. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Tanaka, Y.; Deluca, H.F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch. Biochem. Biophys. 1973, 154, 566–574. [Google Scholar] [CrossRef]
- Meyer, M.B.; Lee, S.M.; Carlson, A.H.; Benkusky, N.A.; Kaufmann, M.; Jones, G.; Pike, J.W. A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues. J. Biol. Chem. 2019, 294, 14467–14481. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, M.; Lee, S.M.; Pike, J.W.; Jones, G. A High-Calcium and Phosphate Rescue Diet and VDR-Expressing Transgenes Normalize Serum Vitamin D Metabolite Profiles and Renal Cyp27b1 and Cyp24a1 Expression in VDR Null Mice. Endocrinology 2015, 156, 4388–4397. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.B.; Benkusky, N.A.; Kaufmann, M.; Lee, S.M.; Onal, M.; Jones, G.; Pike, J.W. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J. Biol. Chem. 2017, 292, 17541–17558. [Google Scholar] [CrossRef] [Green Version]
- Naja, R.P.; Dardenne, O.; Arabian, A.; Arnaud, R.S. Chondrocyte-Specific Modulation of Cyp27b1 Expression Supports a Role for Local Synthesis of 1,25-Dihydroxyvitamin D3 in Growth Plate Development. Endocrinology 2009, 150, 4024–4032. [Google Scholar] [CrossRef] [Green Version]
- Shinki, T.; Jin, C.H.; Nishimura, A.; Nagai, Y.; Ohyama, Y.; Noshiro, M.; Okuda, K.; Suda, T. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J. Biol. Chem. 1992, 267, 13757–13762. [Google Scholar] [CrossRef]
- Christakos, S.; Lieben, L.; Masuyama, R.; Carmeliet, G. Vitamin D endocrine system and the intestine. Bonekey Rep. 2014, 3, 496. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.H.E.; Apalset, E.M.; Nordbø, Y.; Varhaug, J.E.; Mellgren, G.; Lien, E.A. 1,25-Dihydroxyvitamin D and the Vitamin D Receptor Gene Polymorphism Apa1 Influence Bone Mineral Density in Primary Hyperparathyroidism. PLoS ONE 2013, 8, e56019. [Google Scholar] [CrossRef]
- Björkhem-Bergman, L.; Torefalk, E.; Ekström, L.; Bergman, P. Vitamin D binding protein is not affected by high-dose vitamin D supplementation: A post hoc analysis of a randomised, placebo-controlled study. BMC Res. Notes 2018, 11, 619. [Google Scholar] [CrossRef]
- Guha, C.; Osawa, M.; Werner, P.A.; Galbraith, R.M.; Paddock, G.V. Regulation of human Gc (vitamin D—Binding) protein levels: Hormonal and cytokine control of gene expression in vitro. Hepatology 1995, 21, 1675–1681. [Google Scholar] [CrossRef]
- Kolek, O.I.; Hines, E.R.; Jones, M.D.; LeSueur, L.K.; Lipko, M.A.; Kiela, P.R.; Collins, J.F.; Haussler, M.R.; Ghishan, F.K. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: The final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1036–G1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onal, M.; Carlson, A.H.; Thostenson, J.D.; Benkusky, N.A.; Meyer, M.B.; Lee, S.M.; Pike, J.W. A Novel Distal Enhancer Mediates Inflammation-, PTH-, and Early Onset Murine Kidney Disease-Induced Expression of the Mouse Fgf23 Gene. JBMR Plus 2018, 2, 31–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daryadel, A.; Ruiz, P.A.; Gehring, N.; Stojanovic, D.; Ugrica, M.; Bettoni, C.; Sabrautzki, S.; Pastor-Arroyo, E.-M.; Frey-Wagner, I.; Lorenz-Depiereux, B.; et al. Systemic Jak1 activation provokes hepatic inflammation and imbalanced FGF23 production and cleavage. FASEB J. 2021, 35, e21302. [Google Scholar] [CrossRef]
- Farrow, E.G.; Davis, S.I.; Summers, L.J.; White, K.E. Initial FGF23-Mediated Signaling Occurs in the Distal Convoluted Tubule. J. Am. Soc. Nephrol. 2009, 20, 955. [Google Scholar] [CrossRef]
- Erben, R.G. Physiological Actions of Fibroblast Growth Factor-23. Front. Endocrinol. 2018, 9, 267. [Google Scholar] [CrossRef]
- Saito, Y.; Yamagishi, T.; Nakamura, T.; Ohyama, Y.; Aizawa, H.; Suga, T.; Matsumura, Y.; Masuda, H.; Kurabayashi, M.; Kuro-o, M.; et al. Klotho Protein Protects against Endothelial Dysfunction. Biochem. Biophys. Res. Commun. 1998, 248, 324–329. [Google Scholar] [CrossRef]
- Jacob, A.L.; Smith, C.; Partanen, J.; Ornitz, D.M. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev. Biol. 2006, 296, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Xu, J.; Liu, Z.; Sosic, D.; Shao, J.; Olson, E.N.; Towler, D.A.; Ornitz, D.M. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 2003, 130, 3063–3074. [Google Scholar] [CrossRef] [Green Version]
- Hubert, F.; Payan, S.M.; Rochais, F. FGF10 Signaling in Heart Development, Homeostasis, Disease and Repair. Front. Genet. 2018, 9, 599. [Google Scholar] [CrossRef]
- Ruiz-Camp, J.; Morty, R.E. Divergent fibroblast growth factor signaling pathways in lung fibroblast subsets: Where do we go from here? Am. J. Physiol. Lung Cell. Mol.Physiol. 2015, 309, L751–L755. [Google Scholar] [CrossRef] [Green Version]
- Takashi, Y.; Fukumoto, S. FGF23 beyond Phosphotropic Hormone. Trends Endocrinol. Metab. 2018, 29, 755–767. [Google Scholar] [CrossRef]
- Liu, S.; Vierthaler, L.; Tang, W.; Zhou, J.; Quarles, L.D. FGFR3 and FGFR4 Do not Mediate Renal Effects of FGF23. J. Am. Soc. Nephrol. 2008, 19, 2342. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M. Endocrine FGFs and Klothos: Emerging concepts. Trends Endocrinol. Metab. 2008, 19, 239–245. [Google Scholar] [CrossRef]
- Andrukhova, O.; Zeitz, U.; Goetz, R.; Mohammadi, M.; Lanske, B.; Erben, R.G. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2–SGK1 signaling pathway. Bone 2012, 51, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Agoro, R.; Ni, P.; Noonan, M.L.; White, K.E. Osteocytic FGF23 and Its Kidney Function. Front. Endocrinol. 2020, 11, 592. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Pastor, J.J.; Mereu, A.; Villarroya, F.; Ipharraguerre, I.R. Developmental regulation of the intestinal FGF19 system in domestic pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G647–G654. [Google Scholar] [CrossRef]
- Dalton, G.D.; Xie, J.; An, S.-W.; Huang, C.-L. New Insights into the Mechanism of Action of Soluble Klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Goetz, R.; Nakada, Y.; Hu, M.C.; Kurosu, H.; Wang, L.; Nakatani, T.; Shi, M.; Eliseenkova, A.V.; Razzaque, M.S.; Moe, O.W.; et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl. Acad. Sci. USA 2010, 107, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Komaba, H.; Fukagawa, M. Jury still out on whether FGF23 is a direct contributor, a useful biomarker, or neither. Kidney Int. 2021, 100, 989–993. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues—With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Ravikumar, P.; Ye, J.; Zhang, J.; Pinch, S.N.; Hu, M.C.; Kuro-o, M.; Hsia, C.C.W.; Moe, O.W. α-Klotho protects against oxi-dative damage in pulmonary epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L566–L575. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.L.; Bonjour, J.-P.; Rizzoli, R. Fibroblast Growth Factor-23 Relationship to Dietary Phosphate and Renal Phosphate Handling in Healthy Young Men. J. Clin. Endocrinol. Metab. 2005, 90, 1519–1524. [Google Scholar] [CrossRef] [Green Version]
- Cool, S.; Jackson, R.; Pincus, P.; Dickinson, I.; Nurcombe, V. Fibroblast growth factor receptor 4 (FGFR4) expression in newborn murine calvaria and primary osteoblast cultures. Int. J. Dev. Biol. 2004, 46, 519–523. [Google Scholar]
- Kawaguchi, H.; Manabe, N.; Chikuda, H.; Nakamura, K.; Kuroo, M. Cellular and molecular mechanism of low-turnover osteopenia in the klotho-deficient mouse. Cell. Mol. Life Sci. 2000, 57, 731–737. [Google Scholar] [CrossRef]
- Komaba, H.; Kaludjerovic, J.; Hu, D.Z.; Nagano, K.; Amano, K.; Ide, N.; Sato, T.; Densmore, M.J.; Hanai, J.I.; Olauson, H.; et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017, 92, 599–611. [Google Scholar] [CrossRef] [Green Version]
| Tissue Labeling | Description | Trial |
|---|---|---|
| Kidney cortex | Cortex of left kidney | 1, 2 |
| Kidney medulla | Medulla of left kidney | 1, 2 |
| Liver | Lobulus spigelii | 1, 2 |
| Lung | Lower tip of the left lung lobe | 1 |
| Aorta | Aorta, descending thoracic aorta | 1 |
| Bone | Calvarial bone along the sagittal suture | 1, 2 |
| Stomach | Fundus mucosa | 1 |
| Duodenum | Mucosa 30–40 cm distal of pylorus | 1, 2 |
| Jejunum (prox.) | Mucosa 2 m distal of pylorus | 1, 2 |
| Jejunum (dist.) | Mucosa 2 m proximal of the ileocecal junction | 1, 2 |
| Ileum | Mucosa 20 cm proximal of the ileocecal junction | 1, 2 |
| Caecum | Mucosa | 1, 2 |
| Colon (prox.) | Mucosa 50–60 cm distal of cecolic junction | 1, 2 |
| Colon (dist.) | Mucosa 50–60 cm proximal of rectum | 1, 2 |
| Sl. No. | Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | AT * (°C) | FS ** (bp) |
|---|---|---|---|---|---|
| 1 | CYP2R1 | TTGCTTCAGCGATTTCACTTG | TGTGCATTTTCAGCGTCTTTC | 60 | 123 |
| 2 | CYP27A1 | CAAGTACCCAGTACGGAACGAC | AGCATCCGCTGGTTCAGAG | 60 | 132 |
| 3 | CYP27B1 | CCATCAGCCACTGTTCTATCC | TCCCTTGAAGTGGCATAGTGAC | 60 | 179 |
| 4 | CYP24A1 | GGAATTGTATGCGTCTGTGAC | CATCTGATTCTCAGGCAGTACAC | 60 | 154 |
| 5 | GC | AAGTTGCCCACAAACAAAGATG | TCAGGGTTGGCTCAAGTATTTTAC | 60 | 130 |
| 6 | VDR | CTTCTGTGACCCTGGACCTG | GCACTTGACTTCAGCAGCAC | 60 | 157 |
| 7 | FGF23 | CAGGCTTCGTGGTCATAACAG | CTGACGAGGAAGCGGTAGTG | 60 | 172 |
| 8 | FGFR1 | GACTCCTAACCCCACCTTGC | GTGTAGTTGCCCTTGTCGGA | 60 | 141 |
| 9 | FGFR2 | CCTCACAGAGACCCACCTTC | GTTCGAGAGGCTGACTGAGG | 60 | 212 |
| 10 | FGFR3 | TCATAGGCGTGGCTGAGAAG | CACCACCAGGATGAAGAGGAG | 60 | 187 |
| 11 | FGFR4 | AGAGTACCTTGACCTCCGCT | CTCATGGCTGAAGACCGAGT | 60 | 213 |
| 12 | KL | ACTGGCTGAGGTCCAAGTACG | GGAGCTGTGCGATCATTAAATG | 60 | 199 |
| 13 | RPL32 *** | AGCCCAAGATCGTCAAAAAG | TGTTGCTCCCATAACCAATG | 60 | 165 |
| 14 | ACTB | GAGAAGCTCTGCTACGTCGC | CCTGATGTCCACGTCGCACT | 60 | 231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Oster, M.; Reyer, H.; Ponsuksili, S.; Murani, E.; Wolf, P.; Fischer, D.-C.; Wimmers, K. Tissue-Wide Expression of Genes Related to Vitamin D Metabolism and FGF23 Signaling following Variable Phosphorus Intake in Pigs. Metabolites 2022, 12, 729. https://doi.org/10.3390/metabo12080729
Hasan M, Oster M, Reyer H, Ponsuksili S, Murani E, Wolf P, Fischer D-C, Wimmers K. Tissue-Wide Expression of Genes Related to Vitamin D Metabolism and FGF23 Signaling following Variable Phosphorus Intake in Pigs. Metabolites. 2022; 12(8):729. https://doi.org/10.3390/metabo12080729
Chicago/Turabian StyleHasan, Maruf, Michael Oster, Henry Reyer, Siriluck Ponsuksili, Eduard Murani, Petra Wolf, Dagmar-Christiane Fischer, and Klaus Wimmers. 2022. "Tissue-Wide Expression of Genes Related to Vitamin D Metabolism and FGF23 Signaling following Variable Phosphorus Intake in Pigs" Metabolites 12, no. 8: 729. https://doi.org/10.3390/metabo12080729
APA StyleHasan, M., Oster, M., Reyer, H., Ponsuksili, S., Murani, E., Wolf, P., Fischer, D.-C., & Wimmers, K. (2022). Tissue-Wide Expression of Genes Related to Vitamin D Metabolism and FGF23 Signaling following Variable Phosphorus Intake in Pigs. Metabolites, 12(8), 729. https://doi.org/10.3390/metabo12080729