Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study
Abstract
:1. Introduction
1.1. Traumatic Brain Injury
1.2. Role of Ketones in Brain Energy and Metabolism after Traumatic Brain Injury
2. Materials and Methods
2.1. IACUC Statement
2.2. Biochemicals
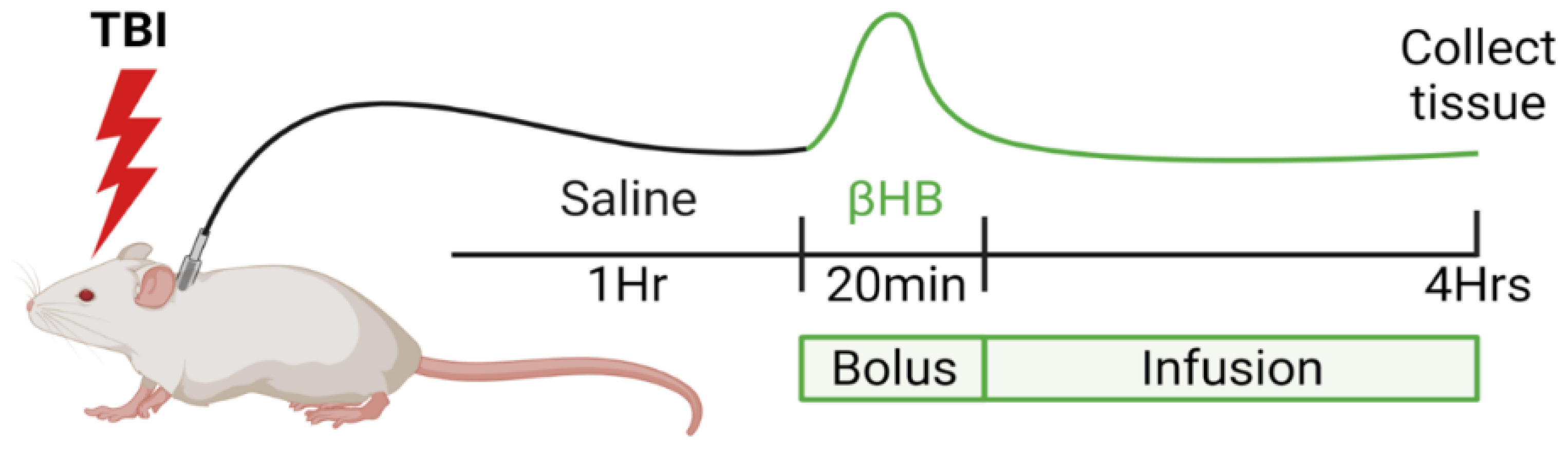
2.3. TBI Model: Controlled Cortical Injury
2.4. Immunohistochemistry
2.5. Tissue and Plasma Extraction
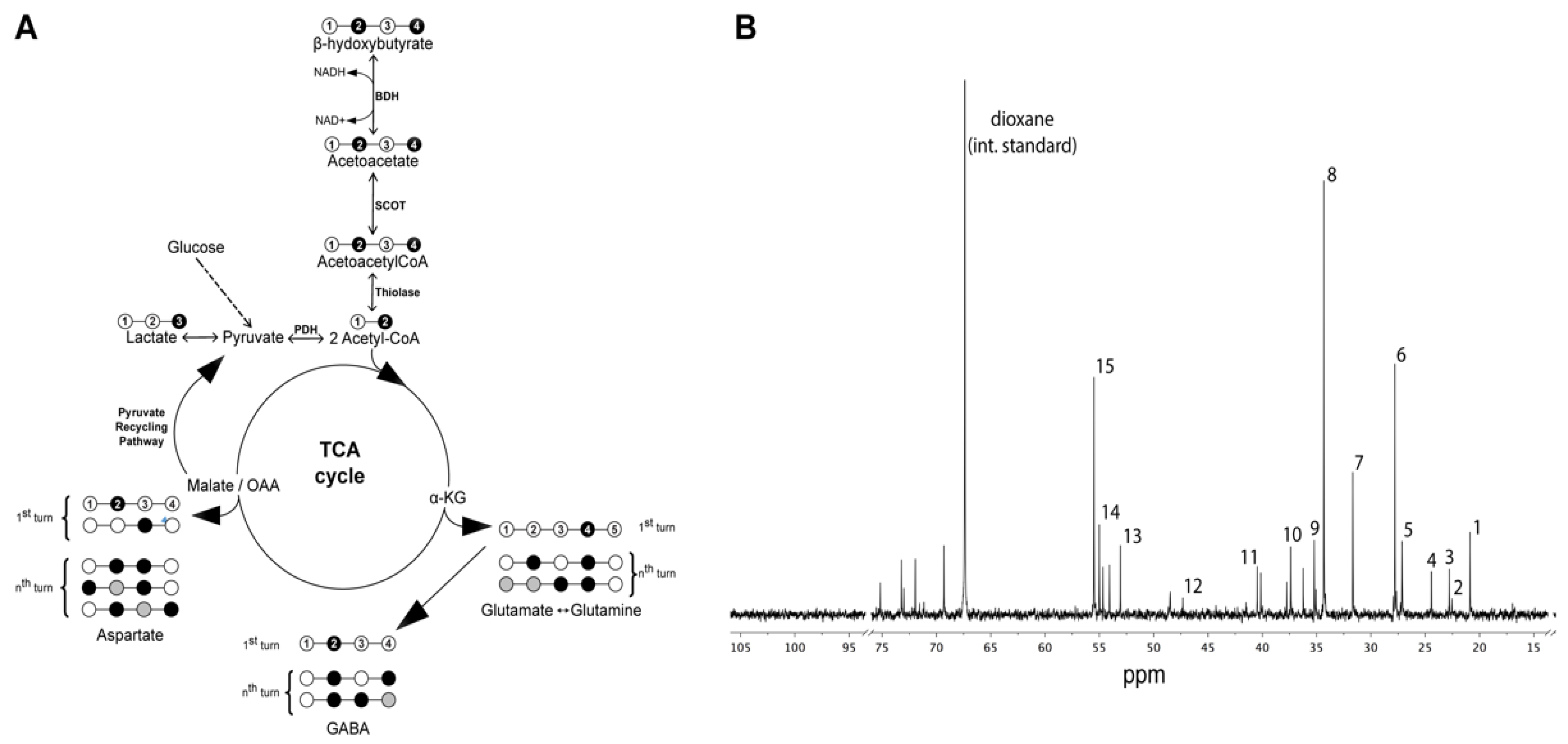
2.6. NMR Spectroscopy
2.7. Statistical Analysis
3. Results
3.1. Infusion of β-Hydroxybutyrate Results in Increased Blood Levels
3.2. β-Hydroxybutyrate Dehydrogenase Is Found in Both Neurons and Astrocytes
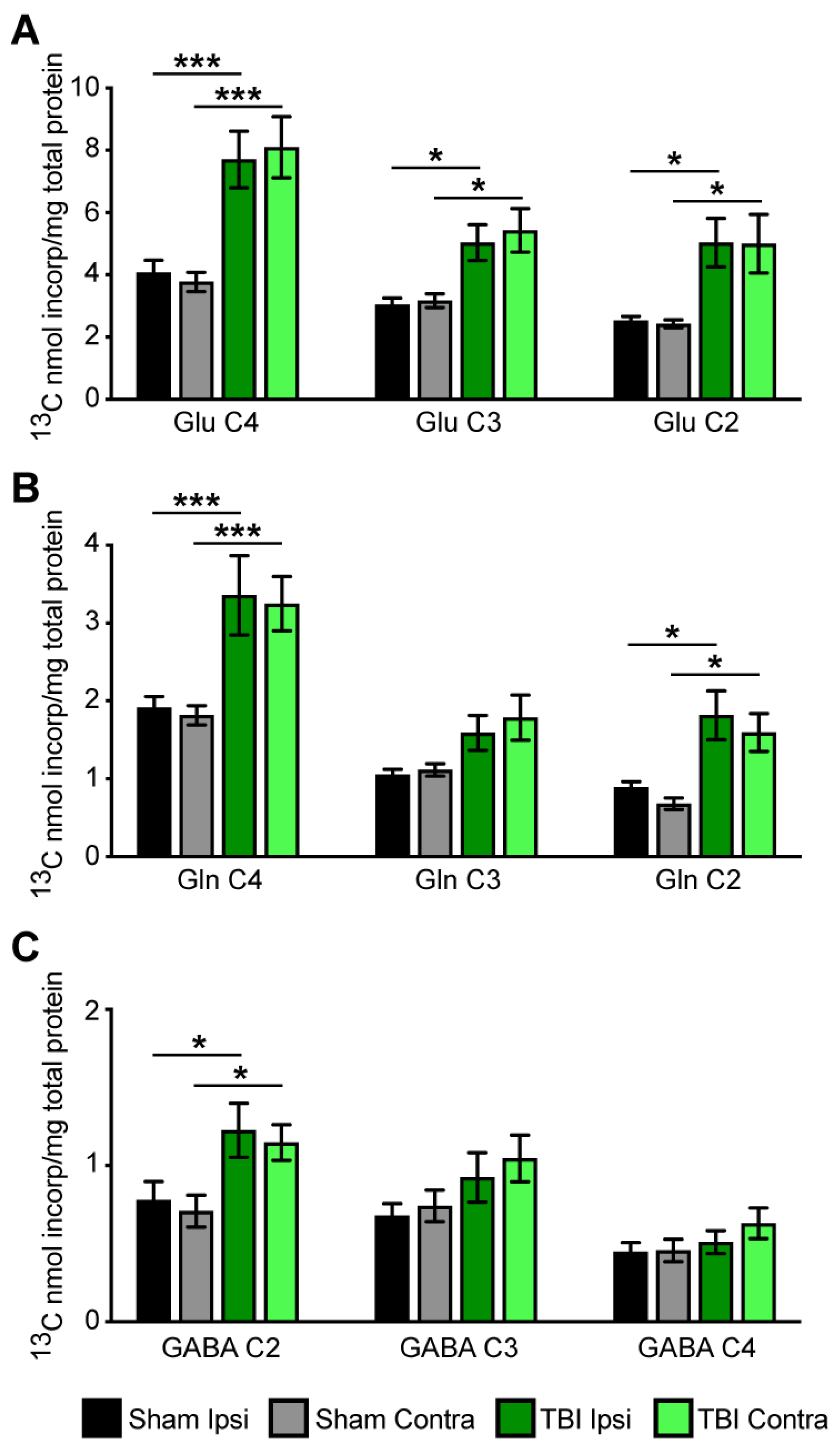
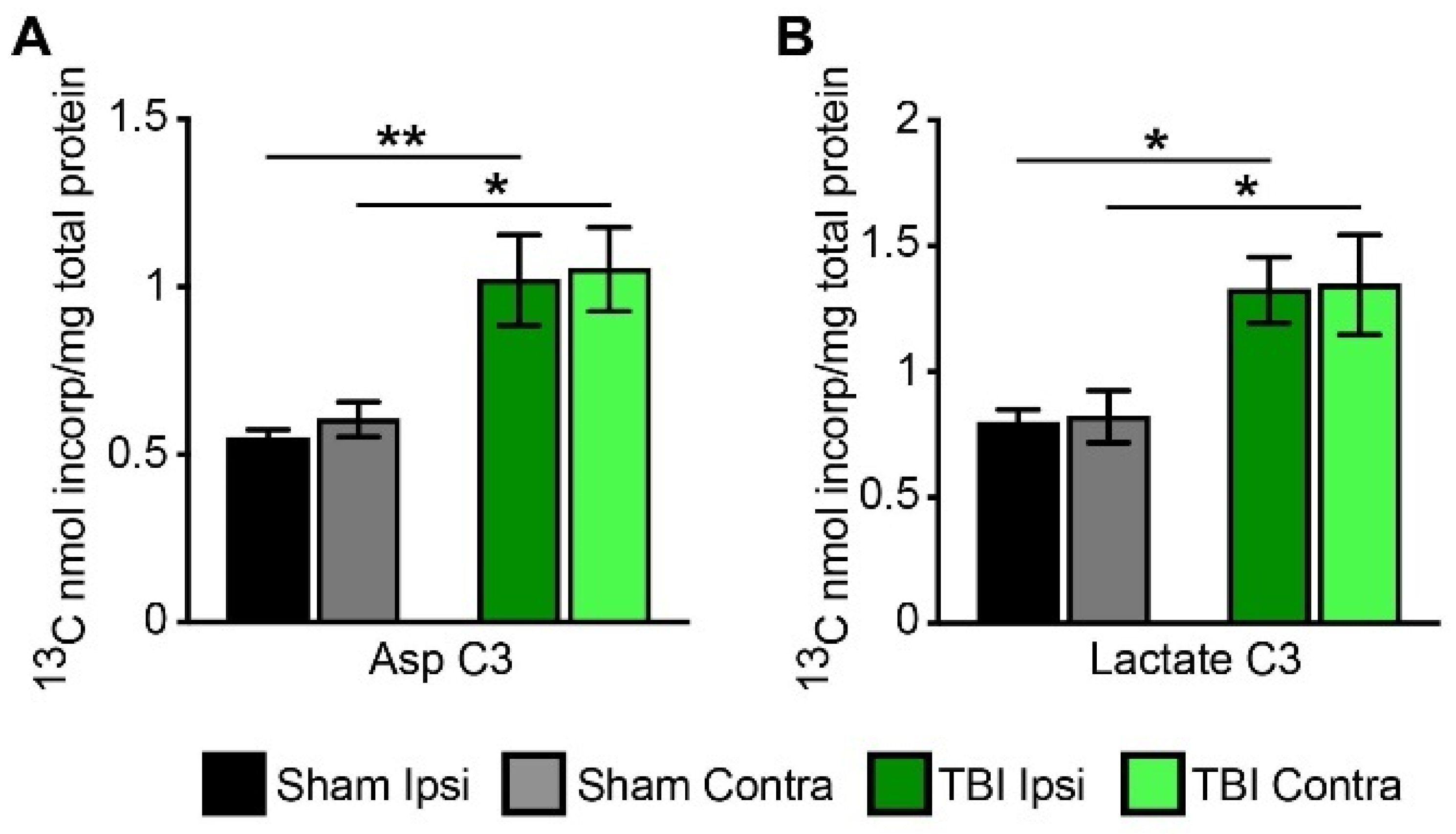
3.3. Metabolism of [2,4-13C] β-Hydroxybutyrate in Brain after TBI
3.4. Cycling Ratios
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faul, M.; Xu, L.; Wald, M.M.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2010.
- McKenna, M.C.; Scafidi, S.; Robertson, C.L. Metabolic Alterations in Developing Brain After Injury: Knowns and Unknowns. Neurochem. Res. 2015, 40, 2527–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernak, I.; Chang, T.; Ahmed, F.A.; Cruz, M.I.; Vink, R.; Stoica, B.; Faden, A.I. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev. Neurosci. 2010, 32, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Kolb, B.; Harris, N.G.; Asarnow, R.F.; Prins, M.L. Hitting a moving target: Basic mechanisms of recovery from acquired developmental brain injury. Dev. Neurorehabil. 2009, 12, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, B.; Kim, H.; Gallagher, P.R.; DiScala, C.; Stineman, M.G. Functional status after childhood traumatic brain injury. J. Trauma 2005, 58, 940–949; discussion 950. [Google Scholar] [CrossRef]
- Yeates, K.O. Social outcomes in pediatric traumatic brain injury: Perspectives from social neuroscience and developmental psychology. J. Int. Neuropsychol. Soc. 2013, 19, 493–496. [Google Scholar] [CrossRef]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.M.; Huang, S.C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Jalloh, I.; Carpenter, K.L.; Grice, P.; Howe, D.J.; Mason, A.; Gallagher, C.N.; Helmy, A.; Murphy, M.P.; Menon, D.K.; Carpenter, T.A.; et al. Glycolysis and the pentose phosphate pathway after human traumatic brain injury: Microdialysis studies using 1,2-(13)C2 glucose. J. Cereb. Blood Flow Metab. 2015, 35, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Bartnik, B.L.; Hovda, D.A.; Lee, P.W. Glucose metabolism after traumatic brain injury: Estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J. Neurotrauma 2007, 24, 181–194. [Google Scholar] [CrossRef]
- Robertson, C.L.; Saraswati, M.; Scafidi, S.; Fiskum, G.; Casey, P.; McKenna, M.C. Cerebral glucose metabolism in an immature rat model of pediatric traumatic brain injury. J. Neurotrauma 2013, 30, 2066–2072. [Google Scholar] [CrossRef] [Green Version]
- Scafidi, S.; O’Brien, J.; Hopkins, I.; Robertson, C.; Fiskum, G.; McKenna, M. Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J. Neurochem. 2009, 109 (Suppl. S1), 189–197. [Google Scholar] [CrossRef] [Green Version]
- Casey, P.A.; McKenna, M.C.; Fiskum, G.; Saraswati, M.; Robertson, C.L. Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats. J. Neurotrauma 2008, 25, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Bartnik, B.L.; Lee, S.M.; Hovda, D.A.; Sutton, R.L. The fate of glucose during the period of decreased metabolism after fluid percussion injury: A 13C NMR study. J. Neurotrauma 2007, 24, 1079–1092. [Google Scholar] [CrossRef]
- Robertson, C.L.; Scafidi, S.; McKenna, M.C.; Fiskum, G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp. Neurol. 2009, 218, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef]
- Thomas, S.; Prins, M.L.; Samii, M.; Hovda, D.A. Cerebral metabolic response to traumatic brain injury sustained early in development: A 2-deoxy-D-glucose autoradiographic study. J. Neurotrauma 2000, 17, 649–665. [Google Scholar] [CrossRef]
- Robertson, C.L.; Saraswati, M.; Fiskum, G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem. 2007, 101, 1248–1257. [Google Scholar] [CrossRef]
- McKenna, M.C.; Dienel, G.A.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A. Energy metabolism of the brain. In Basic Neurochemistry: Principles of Molecular, Cellular and Medical Neurobiology, 8th ed.; Brady, S., Siegel, G.J., Albers, W.R., Price, D., Eds.; Academic Press: Burlington, MA, USA, 2012; pp. 200–231. [Google Scholar]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Lim, S.; Chesser, A.S.; Grima, J.C.; Rappold, P.M.; Blum, D.; Przedborski, S.; Tieu, K. D-beta-hydroxybutyrate is protective in mouse models of Huntington’s disease. PLoS ONE 2011, 6, e24620. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.M.; Ploug, K.B.; Iversen, A.; Jensen, A.A.; Jansen-Olesen, I. The metabolic impact of beta-hydroxybutyrate on neurotransmission: Reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J. Neurochem. 2015, 132, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.Q.; Zhao, Y.; Chen, G.Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616. [Google Scholar] [CrossRef]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [Green Version]
- Prins, M.L. Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 2008, 28, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Prins, M.L.; Matsumoto, J.H. The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. J. Lipid Res. 2014, 55, 2450–2457. [Google Scholar] [CrossRef] [Green Version]
- Greco, T.; Glenn, T.C.; Hovda, D.A.; Prins, M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- Davis, L.M.; Pauly, J.R.; Readnower, R.D.; Rho, J.M.; Sullivan, P.G. Fasting is neuroprotective following traumatic brain injury. J. Neurosci. Res. 2008, 86, 1812–1822. [Google Scholar] [CrossRef]
- Prins, M.L.; Fujima, L.S.; Hovda, D.A. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 2005, 82, 413–420. [Google Scholar] [CrossRef]
- Deng-Bryant, Y.; Prins, M.L.; Hovda, D.A.; Harris, N.G. Ketogenic diet prevents alterations in brain metabolism in young but not adult rats after traumatic brain injury. J. Neurotrauma 2011, 28, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Greco, T.; Vespa, P.M.; Prins, M.L. Alternative substrate metabolism depends on cerebral metabolic state following traumatic brain injury. Exp. Neurol. 2020, 329, 113289. [Google Scholar] [CrossRef]
- Pan, J.W.; de Graaf, R.A.; Petersen, K.F.; Shulman, G.I.; Hetherington, H.P.; Rothman, D.L. [2,4-13 C2 ]-beta-Hydroxybutyrate metabolism in human brain. J. Cereb. Blood Flow Metab. 2002, 22, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, T.M.; Nehlig, A.; Sonnewald, U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem. Int. 2006, 48, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Kunnecke, B.; Cerdan, S.; Seelig, J. Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed. 1993, 6, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Cerdan, S.; Kunnecke, B.; Seelig, J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J. Biol. Chem. 1990, 265, 12916–12926. [Google Scholar] [CrossRef]
- Cerdan, S. Twenty-seven Years of Cerebral Pyruvate Recycling. Neurochem. Res. 2017, 42, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Osier, N.; Dixon, C.E. The Controlled Cortical Impact Model of Experimental Brain Trauma: Overview, Research Applications, and Protocol. Methods Mol. Biol. 2016, 1462, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischiutta, F.; Micotti, E.; Hay, J.R.; Marongiu, I.; Sammali, E.; Tolomeo, D.; Vegliante, G.; Stocchetti, N.; Forloni, G.; De Simoni, M.G.; et al. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp. Neurol. 2018, 300, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mao, H.; Yang, K.H.; Abel, T.; Meaney, D.F. A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front. Neurol. 2014, 5, 100. [Google Scholar] [CrossRef] [Green Version]
- Selwyn, R.; Hockenbury, N.; Jaiswal, S.; Mathur, S.; Armstrong, R.C.; Byrnes, K.R. Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDG PET study. J. Neurotrauma 2013, 30, 1943–1953. [Google Scholar] [CrossRef]
- Zhou, J.; Burns, M.P.; Huynh, L.; Villapol, S.; Taub, D.D.; Saavedra, J.M.; Blackman, M.R. Temporal Changes in Cortical and Hippocampal Expression of Genes Important for Brain Glucose Metabolism Following Controlled Cortical Impact Injury in Mice. Front. Endocrinol. 2017, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Mannino, C.; Glenn, T.C.; Hovda, D.A.; Vespa, P.M.; McArthur, D.L.; Van Horn, J.D.; Wright, M.J. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J. Neurosci. Res. 2018, 96, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.J.; McArthur, D.L.; Alger, J.R.; Van Horn, J.; Irimia, A.; Filippou, M.; Glenn, T.C.; Hovda, D.A.; Vespa, P. Early metabolic crisis-related brain atrophy and cognition in traumatic brain injury. Brain Imaging Behav. 2013, 7, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.M.; Risa, O.; Sonnewald, U.; Schousboe, A.; Waagepetersen, H.S. Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured neurons. J. Neurochem. 2009, 110, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Edmond, J.; Robbins, R.A.; Bergstrom, J.D.; Cole, R.A.; de Vellis, J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res. 1987, 18, 551–561. [Google Scholar] [CrossRef]
- Edmond, J.; Auestad, N.; Robbins, R.A.; Bergstrom, J.D. Ketone body metabolism in the neonate: Development and the effect of diet. Fed. Proc. 1985, 44, 2359–2364. [Google Scholar]
- Eloqayli, H.; Melo, T.M.; Haukvik, A.; Sonnewald, U. [2,4-(13)C]beta-hydroxybutyrate metabolism in astrocytes and C6 glioblastoma cells. Neurochem. Res. 2011, 36, 1566–1573. [Google Scholar] [CrossRef] [Green Version]
- Achanta, L.B.; Rowlands, B.D.; Thomas, D.S.; Housley, G.D.; Rae, C.D. beta-Hydroxybutyrate Boosts Mitochondrial and Neuronal Metabolism but is not Preferred Over Glucose Under Activated Conditions. Neurochem. Res. 2017, 42, 1710–1723. [Google Scholar] [CrossRef]
- McKenna, M.C.; Tildon, J.T.; Stevenson, J.H.; Hopkins, I.B. Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: Evidence for multiple compartments of tricarboxylic acid cycle activity. Dev. Neurosci. 1994, 16, 291–300. [Google Scholar] [CrossRef]
- McKenna, M.C. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem. Res. 2012, 37, 2613–2626. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.Y.; Streuli, V.L.; Zee, P. Ketone bodies serve as important precursors of brain lipids in the developing rat. Lipids 1977, 12, 957–964. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Simpson, I.A. Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1127–E1134. [Google Scholar] [CrossRef]
- Blomqvist, G.; Alvarsson, M.; Grill, V.; Von Heijne, G.; Ingvar, M.; Thorell, J.O.; Stone-Elander, S.; Widen, L.; Ekberg, K. Effect of acute hyperketonemia on the cerebral uptake of ketone bodies in nondiabetic subjects and IDDM patients. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E20–E28. [Google Scholar] [CrossRef] [Green Version]
- Nehlig, A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 265–275. [Google Scholar] [CrossRef]
- Tildon, J.T.; Cone, A.L.; Cornblath, M. Coenzyme A transferase activity in rat brain. Biochem. Biophys. Res. Commun. 1971, 43, 225–231. [Google Scholar] [CrossRef]
- Middleton, B. The acetoacetyl-coenzyme A thiolases of rat brain and their relative activities during postnatal development. Biochem. J. 1973, 132, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Chechik, T.; Roeder, L.M.; Tildon, J.T.; Poduslo, S.E. Ketone body enzyme activities in purified neurons, astrocytes and oligodendroglia. Neurochem. Int. 1987, 10, 95–99. [Google Scholar] [CrossRef]
- Prins, M.L.; Giza, C.C. Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev. Neurosci. 2006, 28, 447–456. [Google Scholar] [CrossRef]
- Roy, M.; Beauvieux, M.C.; Naulin, J.; El Hamrani, D.; Gallis, J.L.; Cunnane, S.C.; Bouzier-Sore, A.K. Rapid adaptation of rat brain and liver metabolism to a ketogenic diet: An integrated study using (1)H- and (13)C-NMR spectroscopy. J. Cereb. Blood Flow Metab. 2015, 35, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Mason, G.F.; Rothman, D.L.; de Graaf, R.A.; Behar, K.L. Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J. Cereb. Blood Flow Metab. 2011, 31, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, G.M.; Jiang, L.; Rothman, D.L.; Behar, K.L. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow Metab. 2014, 34, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Perez-Liebana, I.; Casarejos, M.J.; Alcaide, A.; Herrada-Soler, E.; Llorente-Folch, I.; Contreras, L.; Satrustegui, J.; Pardo, B. betaOHB Protective Pathways in Aralar-KO Neurons and Brain: An Alternative to Ketogenic Diet. J. Neurosci. 2020, 40, 9293–9305. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Scott, S.R.; Barroso, I.; Santisteban, P.; Cerdan, S. Ontogeny and cellular localization of the pyruvate recycling system in rat brain. J. Neurochem. 1998, 70, 2613–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scafidi, S.; Fiskum, G.; Lindauer, S.L.; Bamford, P.; Shi, D.; Hopkins, I.; McKenna, M.C. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J. Neurochem. 2010, 114, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Time | Group | Glucose (mmol/L) | BHB (mmol/L) |
|---|---|---|---|
| 0 h | Sham + NS | 14.87 ± 1.12 | 0.43 ± 0.11 |
| Sham + BHB | 11.28 ± 0.86 | 0.45 ± 0.12 | |
| TBI + NS | 16.53 ± 3.46 | 0.70 ± 0.19 | |
| TBI + BHB | 14.85 ± 1.73 | 0.80 ± 0.21 | |
| 1 h | Sham + NS | 22.03 ± 3.54 | 0.37 ± 0.04 |
| Sham + BHB | 13.70 ± 2.67 | 0.57 ± 0.12 | |
| TBI + NS | 22.33 ± 2.76 | 0.80 ± 0.25 | |
| TBI + BHB | 14.37 ± 6.45 | 0.87 ± 0.26 | |
| 1 h 20 min | Sham + NS | 13.55 ± 4.05 | 0.60 ± 0.28 |
| Sham + BHB | 10.73 ± 1.25 | 1.76 ± 0.25 | |
| TBI + NS | 16.80 ± 1.80 | 0.83 ± 0.21 | |
| TBI + BHB | 14.93 ± 6.53 | 1.87 ± 0.54 | |
| 4 h | Sham + NS | 9.83 ± 2.21 | 0.57 ± 0.11 |
| Sham + BHB | 10.08 ± 3.08 | 2.15 ± 0.76 | |
| TBI + NS | 10.03 ± 1.21 | 0.40 ± 0.10 | |
| TBI + BHB | 8.10 ± 1.92 | 2.65 ± 1.20 |
| Ratios | Sham Ipsi | Sham Contra | TBI Ipsi | TBI Contra |
|---|---|---|---|---|
| Metabolite ratios | ||||
| Glu C4/Gln C4 | 2.12 ± 0.09 | 2.08 ± 0.1 | 2.36 ± 0.09 | 2.50 ± 0.15 |
| Asp C3/Glu C4 | 0.14 ± 0.01 | 0.17 ±0.02 | 0.13 ± 0.01 | 0.14 ± 0.01 |
| Total GABA/Total Glu | 0.20 ± 0.02 | 0.20 ± 0.03 | 0.15 ± 0.01 | 0.16 ± 0.02 |
| Cycling ratios | ||||
| Glu C3/Glu C4 | 0.77 ± 0.07 | 0.87 ± 0.08 | 0.66 ± 0.03 | 0.67 ± 0.15 |
| GABA C3/GABAC2 | 0.91 ± 0.06 | 1.06 ± 0.06 | 0.76 ± 0.08 | 0.90 ± 0.09 |
| Gln C3/Gln C4 | 0.57 ± 0.04 | 0.62 ± 0.03 | 0.48 ± 0.03 | 0.54 ± 0.05 |
| TCA cycling | 1.43 ± 0.13 | 1.55 ± 0.14 | 1.30 ± 0.06 | 1.28 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scafidi, S.; Jernberg, J.; Fiskum, G.; McKenna, M.C. Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study. Metabolites 2022, 12, 710. https://doi.org/10.3390/metabo12080710
Scafidi S, Jernberg J, Fiskum G, McKenna MC. Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study. Metabolites. 2022; 12(8):710. https://doi.org/10.3390/metabo12080710
Chicago/Turabian StyleScafidi, Susanna, Jennifer Jernberg, Gary Fiskum, and Mary C. McKenna. 2022. "Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study" Metabolites 12, no. 8: 710. https://doi.org/10.3390/metabo12080710
APA StyleScafidi, S., Jernberg, J., Fiskum, G., & McKenna, M. C. (2022). Metabolism of Exogenous [2,4-13C]β-Hydroxybutyrate following Traumatic Brain Injury in 21-22-Day-Old Rats: An Ex Vivo NMR Study. Metabolites, 12(8), 710. https://doi.org/10.3390/metabo12080710






