Energy Metabolism in Mouse Sciatic Nerve A Fibres during Increased Energy Demand
Abstract
:1. Introduction
2. Results
2.1. Glycogen Supports the CAP in Substrate-Free aCSF
2.2. Increasing aCSF Glucose Restores the CAP
2.3. Lactate Supports the CAP
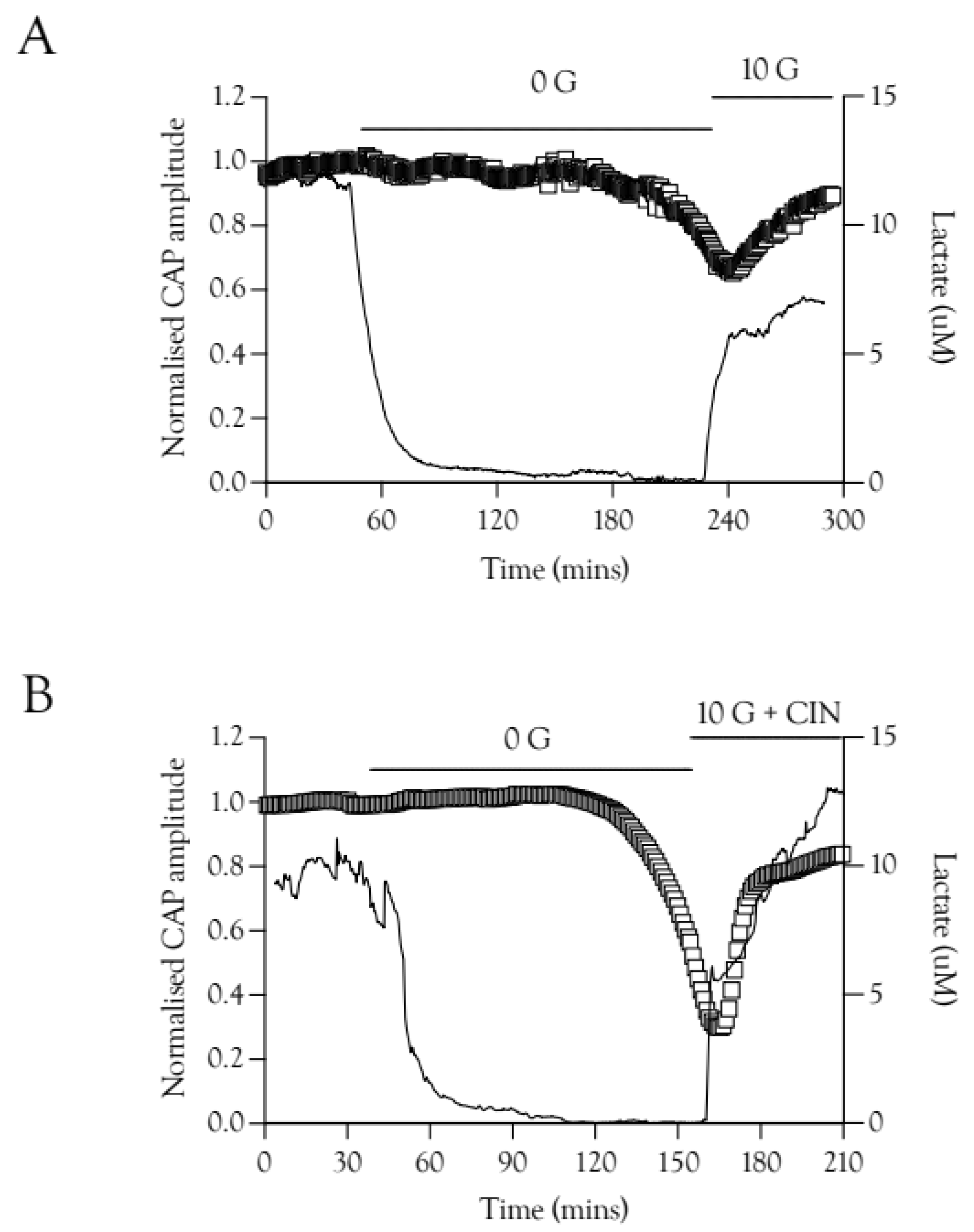
2.4. Pre-Incubation with High-Frequency Stimulus Causes CAP Failure
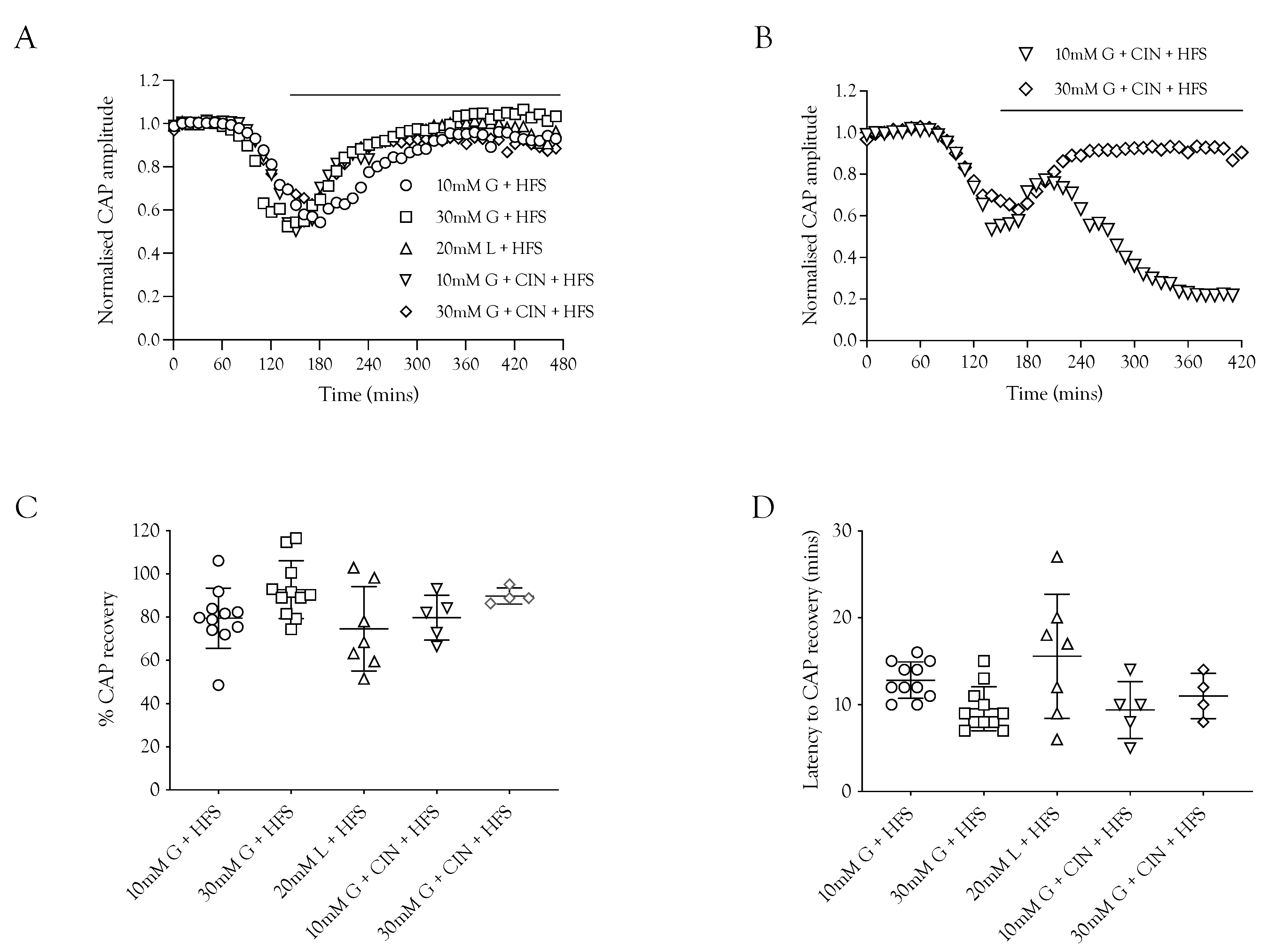
2.5. CAP Rescue
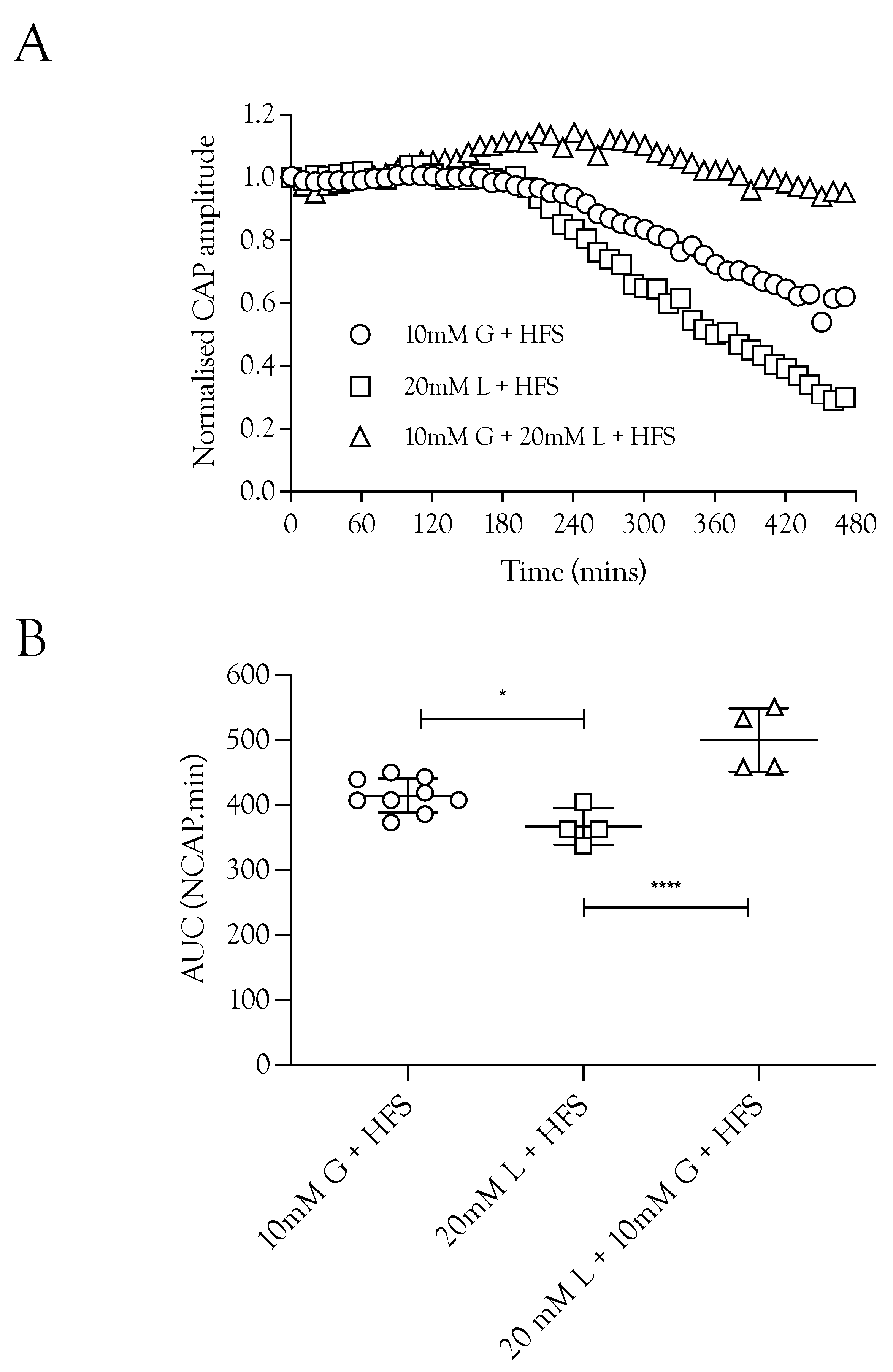
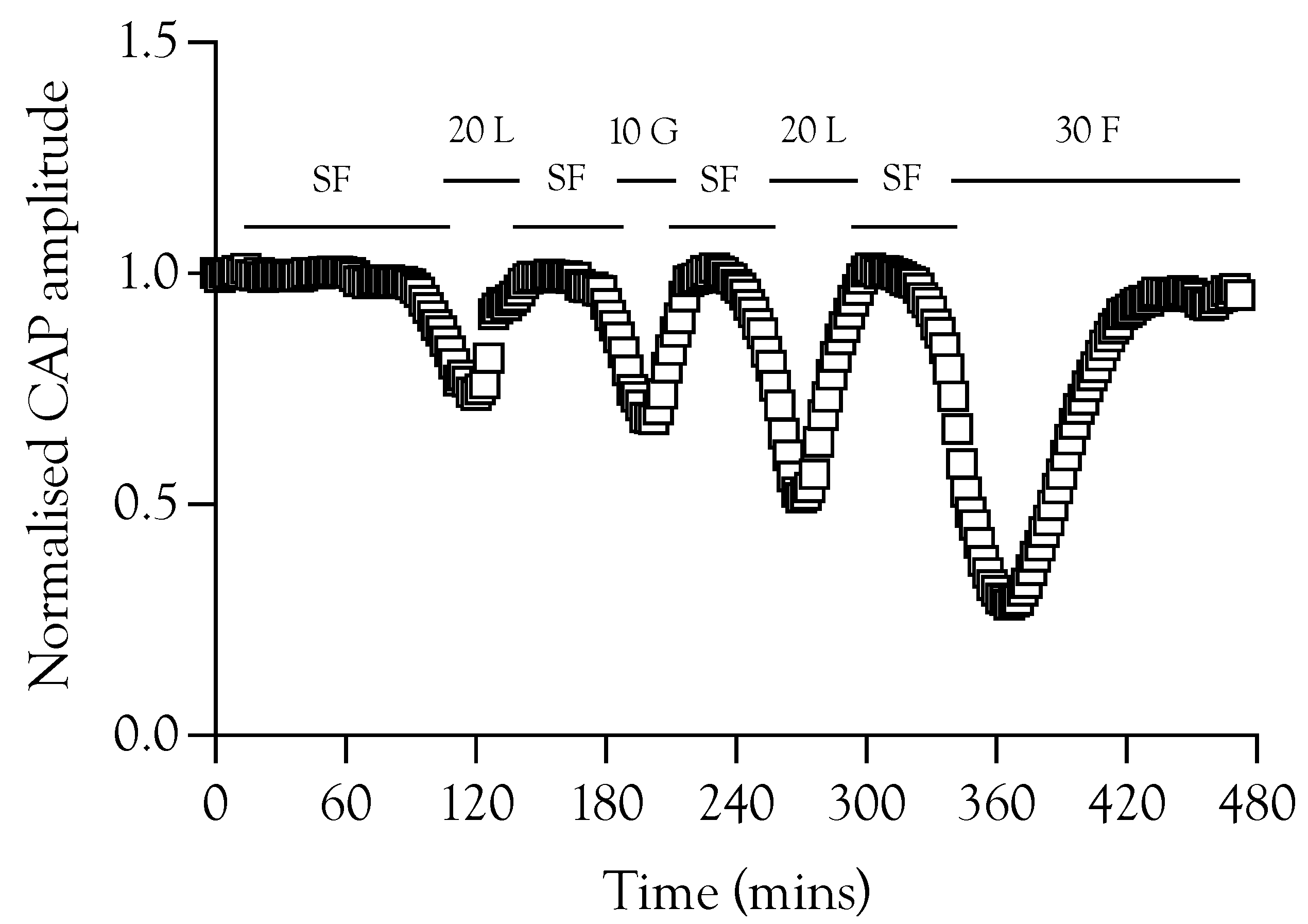
2.6. Substrate Restoration of the CAP during High-Frequency Stimulus
2.7. Lactate Elevations Precede CAP Recovery
3. Discussion
3.1. CAP Failure Signals Depletion of Utilisable Glycogen
3.2. CAP Restoration
4. Methods
4.1. Ethical Approval
4.2. Electrophysiology
4.3. Lactate Biosensors
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.; Brown, A.M. Glycogen: Multiple roles in the CNS. Neuroscientist 2017, 23, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Evans, R.D.; Black, J.; Ransom, B.R. Schwann cell glycogen selectively supports myelinated axon function. Ann. Neurol. 2012, 72, 406–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.M.; Tekkok, S.B.; Ransom, B.R. Glycogen regulation and functional role in mouse white matter. J. Physiol. 2003, 549 Pt 2, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Cater, H.L.; Benham, C.D.; Sundstrom, L.E. Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus. J. Physiol. 2001, 531, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Machler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; von Faber-Castell, A.; Kaelin, V.; Zuend, M.; Martín, A.S.; Romero-Gómez, I.; et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, L.R.; Brown, A.M. Fibre sub-type specific conduction reveals metabolic function in mouse sciatic nerve. J. Physiol. 2018, 596, 1795–1812. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Kennedy, W.R.; Fries, T.J. Small nerve fiber dysfunction in diabetic neuropathy. Muscle Nerve 1989, 12, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Passonneau, J.V.; Gatfield, P.D.; Schulz, D.W.; Lowry, O.H. An enzymic method for measurement of glycogen. Anal. Biochem. 1967, 19, 315–326. [Google Scholar] [CrossRef]
- Walls, A.B.; Sickmann, H.M.; Brown, A.; Bouman, S.D.; Ransom, B.; Schousboe, A.; Waagepetersen, H.S. Characterization of 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) as an inhibitor of brain glycogen shunt activity. J. Neurochem. 2008, 105, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Sickmann, H.M.; Fosgerau, K.; Lund, T.M.; Schousboe, A.; Waagepetersen, H.S.; Ransom, B.R. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 2005, 79, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hamner, M.A.; Brown, A.M.; Evans, R.D.; Ye, Z.C.; Chen, S.; Ransom, B.R. Novel hypoglycemic injury mechanism: N-methyl-D-aspartate receptor-mediated white matter damage. Ann. Neurol. 2014, 75, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, D.; Chvatal, A.; Kettenmann, H.; Orkand, R.K.; Ransom, B.R. Characteristics of activity-dependent potassium accumulation in mammalian peripheral nerve in vitro. Brain Res. 1991, 552, 106–112. [Google Scholar] [CrossRef]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 2015, 593, 2547–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, L.R.; Patrick, J.A.; Hamner, M.A.; Ransom, B.R.; Brown, A.M. A method for reducing animal use whilst maintaining statistical power in electrophysiological recordings from rodent nerves. Heliyon 2020, 6, e04143. [Google Scholar] [CrossRef]
- Stys, P.K.; Ransom, B.R.; Waxman, S.G. Compound action potential of nerve recorded by suction electrode: A theoretical and experimental analysis. Brain Res. 1991, 546, 18–32. [Google Scholar] [CrossRef]


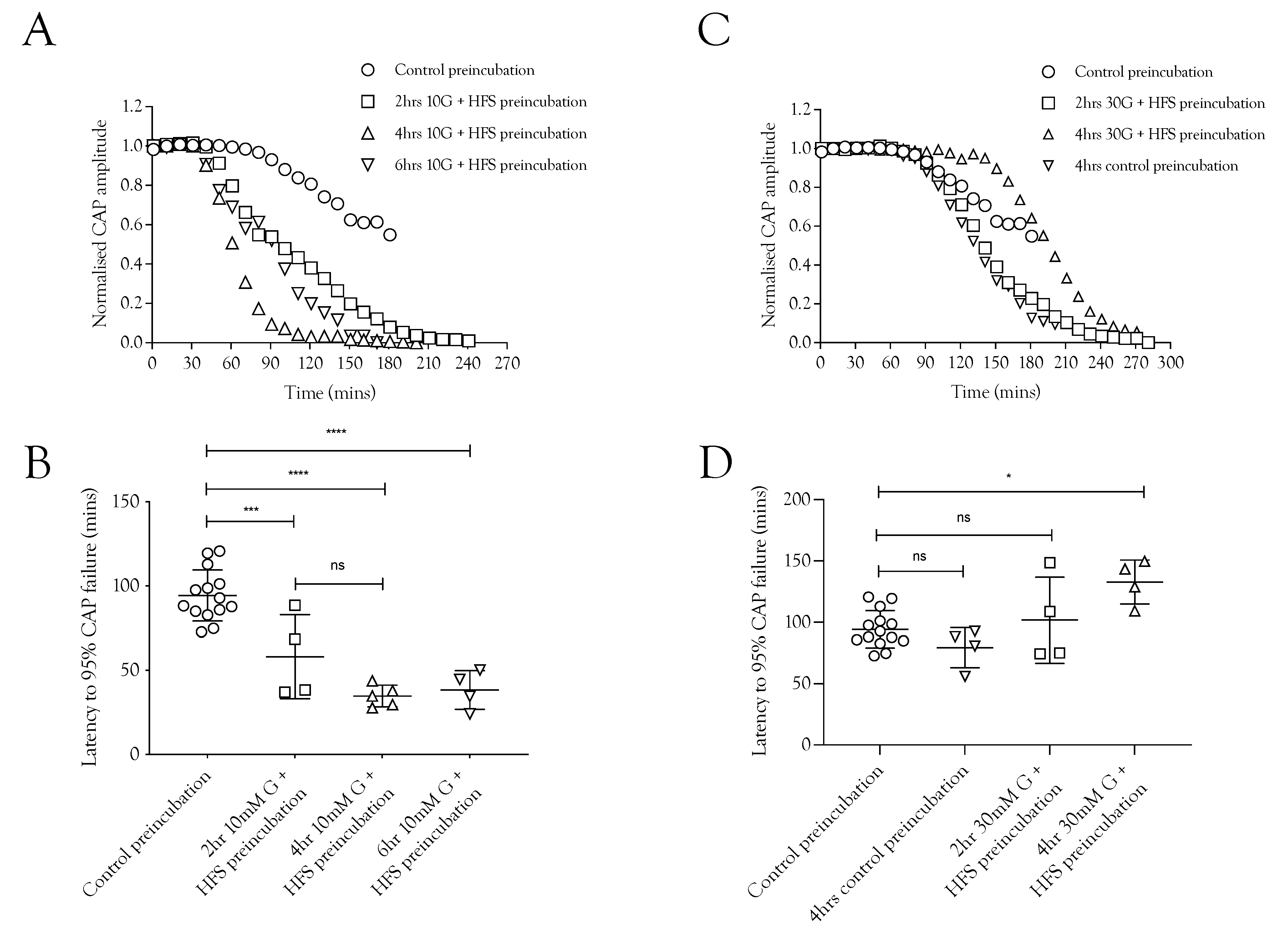
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rich, L.R.; Ransom, B.R.; Brown, A.M. Energy Metabolism in Mouse Sciatic Nerve A Fibres during Increased Energy Demand. Metabolites 2022, 12, 505. https://doi.org/10.3390/metabo12060505
Rich LR, Ransom BR, Brown AM. Energy Metabolism in Mouse Sciatic Nerve A Fibres during Increased Energy Demand. Metabolites. 2022; 12(6):505. https://doi.org/10.3390/metabo12060505
Chicago/Turabian StyleRich, Laura R., Bruce R. Ransom, and Angus M. Brown. 2022. "Energy Metabolism in Mouse Sciatic Nerve A Fibres during Increased Energy Demand" Metabolites 12, no. 6: 505. https://doi.org/10.3390/metabo12060505
APA StyleRich, L. R., Ransom, B. R., & Brown, A. M. (2022). Energy Metabolism in Mouse Sciatic Nerve A Fibres during Increased Energy Demand. Metabolites, 12(6), 505. https://doi.org/10.3390/metabo12060505





