Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review
Abstract
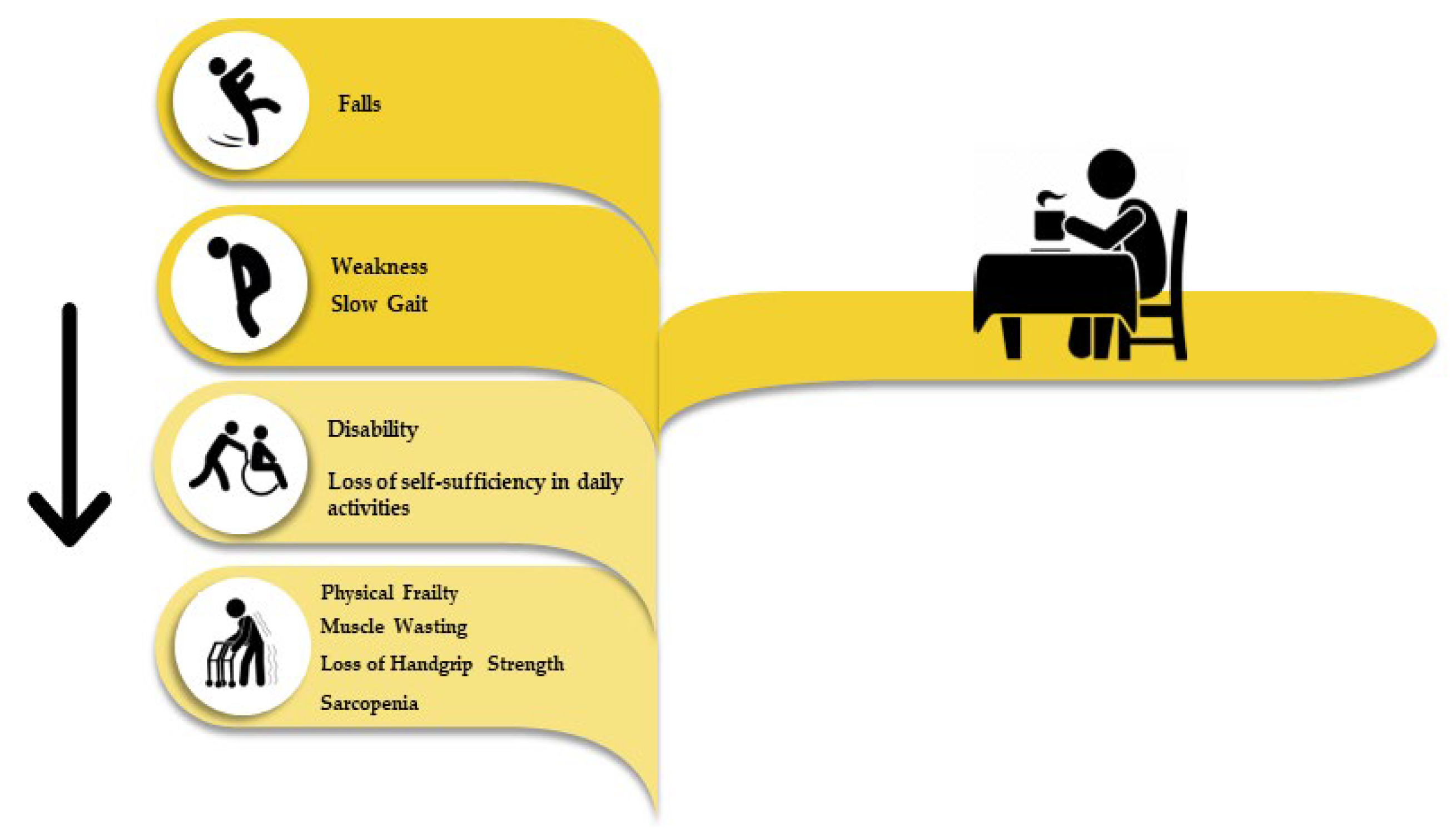
1. Introduction
2. Results
3. Discussion
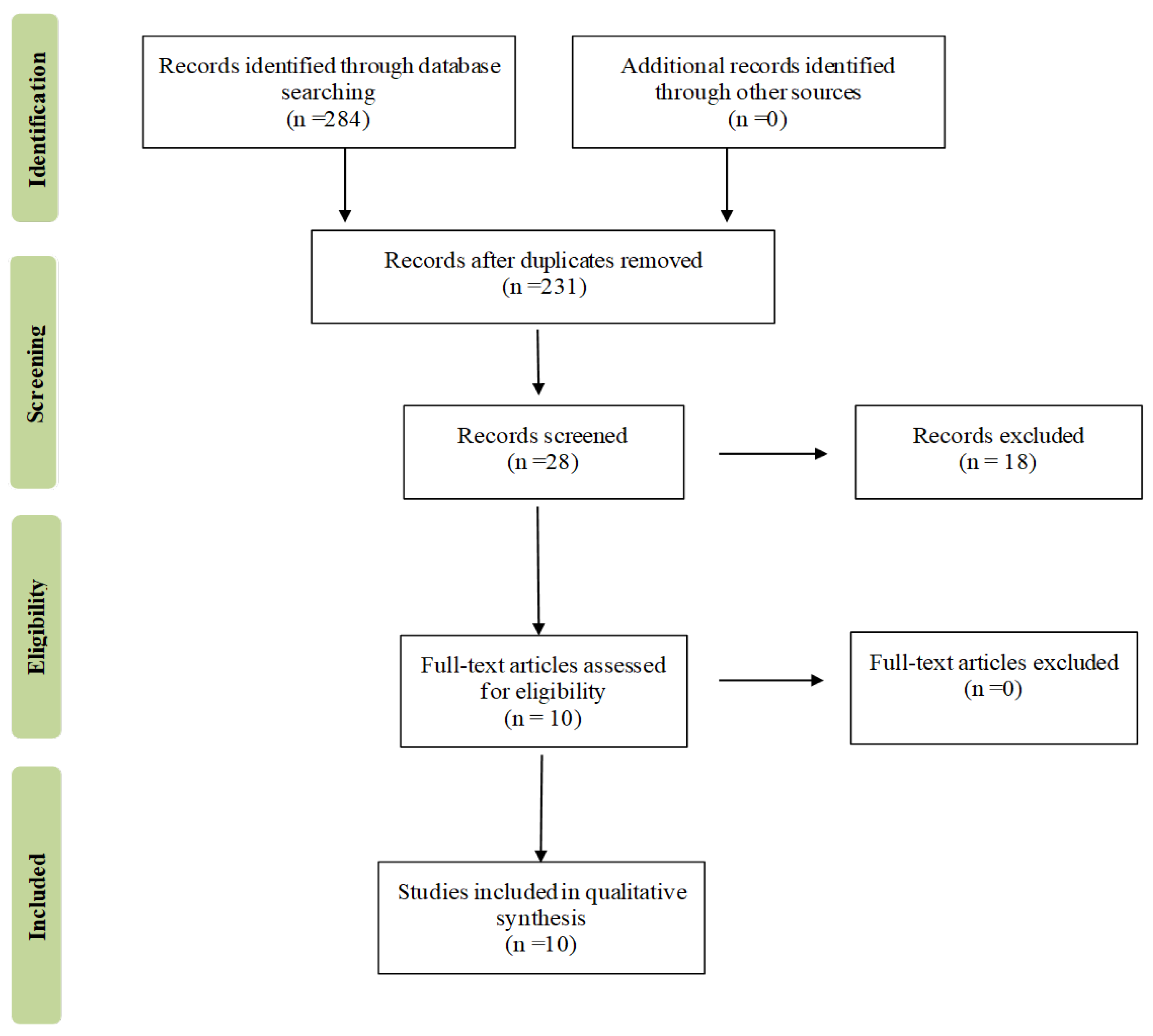
4. Methods
4.1. Search Strategy and Data Extraction
4.2. Inclusion Criteria, Data Extraction, and Registration
4.3. Quality Assessment within and across Studies and Overall Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- World Health Organization Global Age-Friendly Cities: A Guide; World Health Organization: Geneva, Switzerland, 2007; ISBN 9789241547307.
- Miller, E.A.; Weissert, W.G. Predicting Elderly People’s Risk for Nursing Home Placement, Hospitalization, Functional Impairment, and Mortality: A Synthesis. Med. Care Res. Rev. 2000, 57, 259–297. [Google Scholar] [CrossRef] [PubMed]
- Castellana, F.; Lampignano, L.; Bortone, I.; Zupo, R.; Lozupone, M.; Griseta, C.; Daniele, A.; De Pergola, G.; Giannelli, G.; Sardone, R.; et al. Physical Frailty, Multimorbidity, and All-Cause Mortality in an Older Population From Southern Italy: Results from the Salus in Apulia Study. J. Am. Med. Dir. Assoc. 2021, 22, 598–605. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Brayne, C.; Jagger, C. Limitations in Physical Functioning among Older People as a Predictor of Subsequent Disability in Instrumental Activities of Daily Living. Age Ageing 2011, 40, 463–469. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Seeman, T.E.; Merkin, S.S.; Crimmins, E.M.; Karlamangla, A.S. Disability Trends among Older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am. J. Public Health 2010, 100, 100–107. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; De Nucci, S.; Sila, A.; Aresta, S.; Buscemi, C.; Randazzo, C.; Buscemi, S.; Triggiani, V.; De Pergola, G.; et al. Role of Dietary Carotenoids in Frailty Syndrome: A Systematic Review. Biomedicines 2022, 10, 632. [Google Scholar] [CrossRef]
- Pilleron, S.; Ajana, S.; Jutand, M.-A.; Helmer, C.; Dartigues, J.-F.; Samieri, C.; Féart, C. Dietary Patterns and 12-Year Risk of Frailty: Results From the Three-City Bordeaux Study. J. Am. Med. Dir. Assoc. 2017, 18, 169–175. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Guerra, V.; Donghia, R.; Bortone, I.; Griseta, C.; Lampignano, L.; Dibello, V.; Lozupone, M.; Coelho-Júnior, H.J.; et al. Associations between Nutritional Frailty and 8-Year All-Cause Mortality in Older Adults: The Salus in Apulia Study. J. Intern. Med. 2021, 290, 1071–1082. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Blanco-Rojo, R.; Graciani, A.; López-García, E.; Moreno-Franco, B.; Laclaustra, M.; Donat-Vargas, C.; Ordovás, J.M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption and Incident Frailty: A Prospective Cohort Study of Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, M.B.; Avgerinou, C.; Fukushima, F.B.; Vidal, E.I.O. Nutritional Interventions for the Management of Frailty in Older Adults: Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutr. Rev. 2021, 79, 889–913. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kühn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee Drinking and Mortality in 10 European Countries: A Multinational Cohort Study. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef]
- Castellana, F.; De Nucci, S.; De Pergola, G.; Di Chito, M.; Lisco, G.; Triggiani, V.; Sardone, R.; Zupo, R. Trends in Coffee and Tea Consumption during the COVID-19 Pandemic. Foods 2021, 10, 2458. [Google Scholar] [CrossRef]
- Torres-Collado, L.; García-de la Hera, M.; Navarrete-Muñoz, E.M.; Compañ-Gabucio, L.M.; Gonzalez-Palacios, S.; Vioque, J. Coffee Drinking and Associated Factors in an Elderly Population in Spain. Int. J. Environ. Res. Public Health 2018, 15, 1661. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage Caffeine Intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; van Dam, R.M.; Rajpathak, S.; Willett, W.C.; Manson, J.E.; Hu, F.B. Changes in Caffeine Intake and Long-Term Weight Change in Men and Women. Am. J. Clin. Nutr. 2006, 83, 674–680. [Google Scholar] [CrossRef]
- Wang, A.; Wang, S.; Zhu, C.; Huang, H.; Wu, L.; Wan, X.; Yang, X.; Zhang, H.; Miao, R.; He, L.; et al. Coffee and Cancer Risk: A Meta-Analysis of Prospective Observational Studies. Sci. Rep. 2016, 6, 33711. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef]
- Loopstra-Masters, R.C.; Liese, A.D.; Haffner, S.M.; Wagenknecht, L.E.; Hanley, A.J. Associations between the Intake of Caffeinated and Decaffeinated Coffee and Measures of Insulin Sensitivity and Beta Cell Function. Diabetologia 2011, 54, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-Term Coffee Consumption and Risk of Cardiovascular Disease: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, Y.; Wang, W.; Zhang, D. Association between Coffee Consumption and Functional Disability in Older US Adults. Br. J. Nutr. 2021, 125, 695–702. [Google Scholar] [CrossRef]
- Chung, H.; Moon, J.H.; Kim, J.I.; Kong, M.H.; Huh, J.S.; Kim, H.J. Association of Coffee Consumption with Sarcopenia in Korean Elderly Men: Analysis Using the Korea National Health and Nutrition Examination Survey, 2008–2011. Korean J. Fam. Med. 2017, 38, 141–147. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, Y.S. Light Coffee Consumption Is Protective against Sarcopenia, but Frequent Coffee Consumption Is Associated with Obesity in Korean Adults. Nutr. Res. 2017, 41, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Groups Inverse Association between Dietary Habits with High Total Antioxidant Capacity and Prevalence of Frailty among Elderly Japanese Women: A Multicenter Cross-Sectional Study. J. Nutr. Health Aging 2014, 18, 827–839. [Google Scholar] [CrossRef]
- Iwasaka, C.; Yamada, Y.; Nishida, Y.; Hara, M.; Yasukata, J.; Miyoshi, N.; Shimanoe, C.; Nanri, H.; Furukawa, T.; Koga, K.; et al. Association between Habitual Coffee Consumption and Skeletal Muscle Mass in Middle-Aged and Older Japanese People. Geriatr. Gerontol. Int. 2021, 21, 950–958. [Google Scholar] [CrossRef]
- Huang, W.-C.; Huang, Y.-C.; Lee, M.-S.; Chang, H.-Y.; Doong, J.-Y. Frailty Severity and Cognitive Impairment Associated with Dietary Diversity in Older Adults in Taiwan. Nutrients 2021, 13, 418. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Struijk, E.A.; Graciani, A.; Guallar-Castillon, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Coffee Consumption and Risk of Physical Function Impairment, Frailty and Disability in Older Adults. Eur. J. Nutr. 2019, 58, 1415–1427. [Google Scholar] [CrossRef]
- Verlinden, V.J.A.; Maksimovic, A.; Mirza, S.S.; Ikram, M.A.; Kiefte-de Jong, J.C.; Hofman, A.; Franco, O.H.; Tiemeier, H.; van der Geest, J.N. The Associations of Alcohol, Coffee and Tobacco Consumption with Gait in a Community-Dwelling Population. Eur. J. Clin. Nutr. 2016, 70, 116–122. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Struijk, E.A.; Ballesteros, J.-M.; Ortolá, R.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Habitual Coffee Consumption and Risk of Falls in 2 European Cohorts of Older Adults. Am. J. Clin. Nutr. 2019, 109, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Jyväkorpi, S.K.; Urtamo, A.; Kivimäki, M.; Strandberg, T.E. Associations of Coffee Drinking with Physical Performance in the Oldest-Old Community-Dwelling Men The Helsinki Businessmen Study (HBS). Aging Clin. Exp. Res. 2021, 33, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Sardone, R.; Castellana, F.; Bortone, I.; Lampignano, L.; Zupo, R.; Lozupone, M.; Griseta, C.; Dibello, V.; Seripa, D.; Guerra, V.; et al. Association Between Central and Peripheral Age-Related Hearing Loss and Different Frailty Phenotypes in an Older Population in Southern Italy. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Bortone, I.; Griseta, C.; Sardone, R.; Lampignano, L.; Lozupone, M.; Solfrizzi, V.; Castellana, M.; Giannelli, G.; et al. Nutritional Domains in Frailty Tools: Working towards an Operational Definition of Nutritional Frailty. Ageing Res. Rev. 2020, 64, 101148. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; De Nucci, S.; Dibello, V.; Lozupone, M.; Giannelli, G.; De Pergola, G.; Panza, F.; Sardone, R.; Boeing, H. Beverages Consumption and Oral Health in the Aging Population: A Systematic Review. Front. Nutr. 2021, 8, 762383. [Google Scholar] [CrossRef]
- Tatoli, R.; Lampignano, L.; Donghia, R.; Castellana, F.; Zupo, R.; Bortone, I.; De Nucci, S.; Campanile, G.; Lofù, D.; Vimercati, L.; et al. Dietary Customs and Social Deprivation in an Aging Population From Southern Italy: A Machine Learning Approach. Front. Nutr. 2022, 9, 811076. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, K.; Okazaki, T.; Wu, H.; Yoshikawa, T.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Arai, H.; et al. Coffee Treatment Prevents the Progression of Sarcopenia in Aged Mice in Vivo and in Vitro. Exp. Gerontol. 2014, 50, 1–8. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Kim, J.-S.; Jung, C.H.; Ahn, J.; Hur, J.; Ha, T.Y. Coffee Consumption Promotes Skeletal Muscle Hypertrophy and Myoblast Differentiation. Food Funct. 2018, 9, 1102–1111. [Google Scholar] [CrossRef]
- Dirks-Naylor, A.J. The Benefits of Coffee on Skeletal Muscle. Life Sci. 2015, 143, 182–186. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Panza, F.; Imbimbo, B.P.; D’Introno, A.; Galluzzo, L.; Gandin, C.; Misciagna, G.; Guerra, V.; Osella, A.; Baldereschi, M.; et al. Coffee Consumption Habits and the Risk of Mild Cognitive Impairment: The Italian Longitudinal Study on Aging. J. Alzheimers Dis. 2015, 47, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jaccaud, E.; Huggett, A.C. Analysis of the Content of the Diterpenes Cafestol and Kahweol in Coffee Brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef]
- Ferré, S.; Orrú, M.; Guitart, X. Paraxanthine: Connecting Caffeine to Nitric Oxide Neurotransmission. J. Caffeine Res. 2013, 3, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine Supplementation Increases Muscle Mass, Strength, and Endurance in Mice. Nutrients 2022, 14, 893. [Google Scholar] [CrossRef]
- Stavric, B. Methylxanthines: Toxicity to Humans. 3. Theobromine, Paraxanthine and the Combined Effects of Methylxanthines. Food Chem. Toxicol. 1988, 26, 725–733. [Google Scholar] [CrossRef]
- Aubier, M. Effect of Theophylline on Diaphragmatic and Other Skeletal Muscle Function. J. Allergy Clin. Immunol. 1986, 78, 787–792. [Google Scholar] [CrossRef]
- Ohnishi, A.; Kato, M.; Kojima, J.; Ushiama, H.; Yoneko, M.; Kawai, H. Differential Pharmacokinetics of Theophylline in Elderly Patients. Drugs Aging 2003, 20, 71–84. [Google Scholar] [CrossRef]
- Evans, W.V. Plasma Theophylline Concentrations, Six Minute Walking Distances, and Breathlessness in Patients with Chronic Airflow Obstruction. Br. Med. J. 1984, 289, 1649–1651. [Google Scholar] [CrossRef]
- Eaton, M.L.; MacDonald, F.M.; Church, T.R.; Niewoehner, D.E. Effects of Theophylline on Breathlessness and Exercise Tolerance in Patients with Chronic Airflow Obstruction. Chest 1982, 82, 538–542. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Belur, J.; Tompson, L.; Thornton, A.; Simon, M. Interrater Reliability in Systematic Review Methodology: Exploring Variation in Coder Decision-Making. Sociol. Methods Res. 2021, 50, 837–865. [Google Scholar] [CrossRef]
- Koren-Hakim, T.; Gumieiro, D.N.; Drevet, S. Others Quality of the Selected Observational Study Was Assessed Using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Criteria1. Was the Research Question or Objective in This Paper Clearly Stated? Criteria 2. Was the Study Population Clearly Specified and Defined? Criteria 3. Was the Participation Rate of Eligible Persons at Least 50%? Criteria 4. Were All the Subjects Selected or Recruited from the Same or Similar Populations (including the Same Time Period)? Were Inclusion and Exclusion. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 May 2022).
- Schwingshackl, L.; Rüschemeyer, G.; Meerpohl, J.J. [How to interpret the certainty of evidence based on GRADE (Grading of Recommendations, Assessment, Development and Evaluation)]. Urologe A 2021, 60, 444–454. [Google Scholar] [CrossRef] [PubMed]

| Authors, Year | Sample Size | Country | Age (Mean) | Study Design | Study Setting | Outcome(s) | Outcome(s) Assessment | Exposure Assessment | Major Findings |
|---|---|---|---|---|---|---|---|---|---|
| Wang T., 2020 [24] | 7704 | USA (America) | >60 | Cross-sectional | Community US population (NHANES) | LEM | Physical Functioning Questionnaire | 24-h dietary recall | Coffee consumption was inversely associated with the lower odds of functional disability in older American adults |
| GPA | Physical Functioning Questionnaire | 24-h dietary recall | |||||||
| LSA | Physical Functioning Questionnaire | 24-h dietary recall | |||||||
| ADL disability | Physical Functioning Questionnaire | 24-h dietary recall | |||||||
| IADL disability | Physical Functioning Questionnaire | 24-h dietary recall | |||||||
| Chung H., 2017 [25] | 1781 (100% M) | Korea (Asia) | >60 | Cross-sectional | Community Korean population (KNHANES) | Sarcopenia | ASMI less than two standard deviations below the gender-specific mean of this value | FFQ | Consuming at least 3 cups of coffee per day was associated with a lower prevalence of sarcopenia in elderly Korean elderly men |
| Sarcopenia | ASMI less than two standard deviations below the gender-specific mean of this value | FFQ | |||||||
| Sarcopenia | ASMI less than two standard deviations below the gender-specific mean of this value | FFQ | |||||||
| Kim J.H., 2017 [26] | 6906 (41% M, 59% F) | Korea (Asia) | ≥40 | Cross-sectional | Community Korean population (KNHANES) | Sarcopenia | ASMI below the lower quintile of the study population | FFQ | Light coffee consumption was protective against sarcopenia in men |
| Sarcopenia | ASMI below the lower quintile of the study population | FFQ | |||||||
| Sarcopenia | ASMI below the lower quintile of the study population | FFQ | |||||||
| Kobayashi S., 2014 [27] | 2121 (100% F) | Northern and western Japan (Asia) | 65 + (74.7 ± 5.0) | Cross-sectional | Institutions (universities, colleges, and technical schools) | Frailty | Frailty phenotype | FFQ | Coffee intake was associated with lower odds of frailty |
| Frailty | Frailty phenotype | FFQ | |||||||
| Frailty | Frailty phenotype | FFQ | |||||||
| Frailty | Frailty phenotype | FFQ | |||||||
| Iwasaka C., 2021 [28] | 6369 (37% M, 63% F) | Japan (Asia) | 45–74 years | Cross-sectional | Community | Sarcopenia dimension | SMI (bioimpedance) | FFQ | A significant positive association was found between coffee consumption and SMI. The relationship between coffee consumption and grip strength did not reach statistical significance; however, a positive trend was observed |
| Sarcopenia dimension | SMI (bioimpedance) | FFQ | |||||||
| Sarcopenia dimension | SMI (bioimpedance) | FFQ | |||||||
| Sarcopenia dimension | Grip Strength (dynamometry) | FFQ | |||||||
| Sarcopenia dimension | Grip Strength (dynamometry) | FFQ | |||||||
| Sarcopenia dimension | Grip Strength (dynamometry) | FFQ | |||||||
| Huang W.C., 2021 [29] | 1115 | Taiwan (Asia) | 65+ | Cross-sectional | Community | Frailty | FRAIL scale | FFQ | Frail subjects had significantly lower daily consumption of coffee |
| Machado-Fragua M., 2018 [30] | 2073 | Spain (Europe) | 60+ | Longitudinal, 7-year | Community (Seniors-ENRICA cohort) | Impaired agility | Single question from the Rosow and Breslau scale: “On an average day with your current health, would you be limited in bending and kneeling?” | FFQ | Habitual coffee consumption was not associated with increased risk of functional impairment, and it might even be beneficial in women and those with hypertension, obesity or diabetes |
| Impaired agility | Single question from the Rosow and Breslau scale: “On an average day with your current health, would you be limited in bending and kneeling?” | FFQ | |||||||
| 2062 | Impaired mobility | Responding “a lot” to any of the following questions also from the Rosow and Breslau scale: “On an average day with your current health, would you be limited in the following activities: (1) picking up or carrying a shopping bag?; (2) climbing one flight of stairs?; (3) walking several city blocks (a few 100 m)?” | FFQ | ||||||
| Impaired mobility | Responding “a lot” to any of the following questions also from the Rosow and Breslau scale: “On an average day with your current health, would you be limited in the following activities: (1) picking up or carrying a shopping bag?; (2) climbing one flight of stairs?; (3) walking several city blocks (a few 100 m)?” | FFQ | |||||||
| 1653 | Impaired overall physical function | ≥10-point decrease from baseline to follow-up in the physical component summary score of the 12-item short-form health survey (SF-12) | FFQ | ||||||
| Impaired overall physical function | ≥10-point decrease from baseline to follow-up in the physical component summary score of the 12-item short-form health survey (SF-12) | FFQ | |||||||
| 2262 | Impaired lower extremity function | Short Physical Performance Battery (SPPB) | FFQ | ||||||
| Impaired lower extremity function | Short Physical Performance Battery (SPPB) | FFQ | |||||||
| 1714 | Frailty | Frailty phenotype by Fried | FFQ | ||||||
| Frailty | Frailty phenotype by Fried | FFQ | |||||||
| 1564 | IADL disability | Lawton and Brody Scale | FFQ | ||||||
| IADL disability | Lawton and Brody Scale | FFQ | |||||||
| 1756 | ADL disability | Katz Scale | FFQ | ||||||
| ADL disability | Katz Scale | FFQ | |||||||
| Verlinden V.J.A., 2016 [31] | 2546 (1128 M, 1418 F) | Netherlands (Europe) | 45+ | Cross-sectional | Community | Global Gait | Average of seven gait domains: Rhythm, Phases, Variability, Pace, Tandem, Turning, and Base of Support | FFQ | In a community-dwelling population, consuming more than 1 cup of coffee and 1–3 glasses of alcohol relate to better gait |
| Global Gait | Average of seven gait domains: Rhythm, Phases, Variability, Pace, Tandem, Turning, and Base of Support | FFQ | |||||||
| Gait speed (m/s) | 5.79-m-long electronic walkway | FFQ | |||||||
| Gait speed (m/s) | 5.79-m-long electronic walkway | FFQ | |||||||
| Machado-Fragua M.D., 2019 [32] | 2964 | Spain (Europe) | 60+ | Cross-sectional | Community (Seniors-ENRICA cohort) | Falls | Asking participants: “How many times have you fallen down since the last interview?” and using the following outcomes in our analyses: ≥1 fall, injurious fall, and ≥1 fall with fracture | FFQ | Habitual coffee consumption was associated with lower risk of falling in older adults in Spain and the United Kingdom |
| Falls | Asking participants: “How many times have you fallen down since the last interview?” and using the following outcomes in our analyses: ≥1 fall, injurious fall, and ≥1 fall with fracture | FFQ | |||||||
| UK (Europe) | Community (UK Biobank study) | Falls | Asking the participants “In the last year have you had any falls?” The possible answers were “no falls”, “only one fall”, and “more than one fall”. | FFQ | |||||
| Falls | Asking the participants “In the last year have you had any falls?” The possible answers were “no falls”, “only one fall”, and “more than one fall”. | FFQ | |||||||
| Jyvakorpi S.K., 2020 [33] | 126 | Finland (Europe) | 60+ | Cross-sectional | Hospital | Gait speed (m/s) | 4-m walk, m/s | 3-day food diaries | Coffee consumption was positively associated with higher gait speed, grip strength, SPPB score, and chair rise points |
| Gait speed (m/s) | 4-m walk, m/s | 3-day food diaries | |||||||
| Gait speed (m/s) | 4-m walk, m/s | 3-day food diaries | |||||||
| SPPB | SPPB. Ability to stand for 10 sec with feet in 3 different positions: (1) together side-by-side, semi-tandem, and tandem; (2) two timed trials of a 3-m or 4-m walk (fastest recorded); (3) time to rise from a chair five times | 3-day food diaries | |||||||
| SPPB | SPPB. Ability to stand for 10 sec with feet in 3 different positions: (1) together side-by-side, semi-tandem, and tandem; (2) two timed trials of a 3-m or 4-m walk (fastest recorded); (3) time to rise from a chair five times | 3-day food diaries | |||||||
| SPPB | SPPB. Ability to stand for 10 sec with feet in 3 different positions: (1) together side-by-side, semi-tandem, and tandem; (2) two timed trials of a 3-m or 4-m walk (fastest recorded); (3) time to rise from a chair five times | 3-day food diaries | |||||||
| Chair rise | Chair rise test (points): stand up repeatedly from a chair for 30 s | 3-day food diaries | |||||||
| Chair rise | Chair rise test (points): stand up repeatedly from a chair for 30 s | 3-day food diaries | |||||||
| Chair rise | Chair rise test (points): stand up repeatedly from a chair for 30 s | 3-day food diaries | |||||||
| Sarcopenia dimension | Grip Strength (dynamometry) | 3-day food diaries |
| Authors, Year | Sample Size (M/F) | Outcome | Level of Exposure | Strength of the Association | Major Findings |
|---|---|---|---|---|---|
| Wang T., 2020 [24] | 7704 | LEM | <1 cups/day | Lower extremity mobility levels across three categories of increasing coffee consumption (0 to <1, 1 to <2, and >2) versus no consumption: adj OR: 0.74 (95% CI 0.57–0.96), adj OR: 0.79 (95% CI 0.59–1.05), and adj OR: 0.67 (95% CI 0.50–0.91). p for trend: 0.084 | Coffee consumption is inversely associated with the lower odds of functional disability in older American adults |
| <2 cups/day | |||||
| >2 cups/day | |||||
| GPA | <1 cups/day | General physical activity levels across three categories of increasing coffee consumption (0 to <1, 1 to <2, and >2) versus no consumption: adj OR: 0.86 (95% CI 0.67–1.10), adj OR: 0.82 (95% CI 0.62–1.08), adj OR: 0.65 (95% CI 0.47–0.88). p for trend: 0.015 | |||
| <2 cups/day | |||||
| >2 cups/day | |||||
| LSA | <1 cups/day | Leisure and social activities levels across three categories of increasing coffee consumption (0 to <1, 1 to <2, and >2) versus no consumption: adj OR: 0.93 (95% CI 0.68–1.26), adj OR: 0.96 (95% CI 0.69–1.34), adj OR: 0.61 (95% CI 0.45–0.83). p for trend 0.006 | |||
| <2 cups/day | |||||
| >2 cups/day | |||||
| ADL disability | <1 cups/day | Activities of daily living across three categories of increasing coffee consumption (0 to <1, 1 to <2, and >2) versus no consumption: adj OR: 0.88 (95% CI 0.65–1.19), adj OR: 0.95 (95% CI 0.69–1.30), adj OR: 0.70 (95% CI 0.50–1.01). p for trend 0.088 | |||
| <2 cups/day | |||||
| >2 cups/day | |||||
| IADL disability | <1 cups/day | Instrumental activities of daily living across three categories of increasing coffee consumption (0 to <1, 1 to <2, and >2) versus no consumption: adj OR: 0.77 (95% CI 0.57–1.03), adj OR: 0.72 (95% CI 0.54–0.95), adj OR: 0.59 (95% CI 0.44–0.78). p for trend 0.001 | |||
| <2 cups/day | |||||
| >2 cups/day | |||||
| Chung H., 2017 [25] | 1781 (100% M) | Sarcopenia | 1 cup/day | Logistic regression analysis between categories of increasing daily coffee consumption (1, 2, and 3 or more cups/day) versus fewer than 1 cup/day and risk of sarcopenia: adj OR: 0.69 (95% CI 0.39–1.24), adj OR: 0.60 (95% CI 0.32–1.12), adj OR: 0.44 (95% CI 0.21–0.94). p for trend: 0.026 | Consuming at least 3 cups of coffee per day was associated with a lower prevalence of sarcopenia in elderly Korean men |
| 2 cups/day | |||||
| ≥3 cups/day | |||||
| Kim J.H., 2017 [26] | 6906 (41% M, 59% F) | Sarcopenia | 1 cup/day | Logistic regression analysis between categories of increasing daily coffee consumption (1, 2, and 3 or more cups/day) versus less than 1 cup/day and risk of sarcopenia: adj OR 0.69 (95% CI 0.50–0.94), adj OR: 1.07 (95% CI 0.79–1.45), adj OR: 0.85 (95% CI 0.60–1.22) in males, and adj OR: 0.87 (95% CI 0.69–1.10), adj OR: 0.88 (95% CI 0.68–1.15), adj OR: 0.77 (95% CI 0.56–1.06) in males | Light coffee consumption was protective against sarcopenia in men |
| 2 cups/day | |||||
| ≥3 cups/day | |||||
| Kobayashi S., 2014 [27] | 2121 (100% F) | Physical Frailty | 2nd quintile of consumption (11.3–44.6 g/day) | Linear regression analysis between quintiles of increasing daily coffee consumption (grams/day) versus the lowest quintile and risk of physical frailty: adj OR: 0.66 (95% CI 0.46–0.96) for the 2nd quintile, adj OR: 0.77 (95% CI 0.54, 1.10) for the 3rd quintile, adj OR: 0.60 (95% CI 0.41, 0.87) for the 4th quintile, and adj OR: 0.48 (95% CI 0.32, 0.72) for the highest quintile | Coffee intake was associated with lower odds of frailty |
| 3rd quintile of consumption (44.6–140 g/day) | |||||
| 4th quintile of consumption (140–174 g/day) | |||||
| 5th quintile of consumption (>174 g/day) | |||||
| Iwasaka C., 2021 [28] | 6369 (37% M, 63% F) | Sarcopenia dimension (SMI) | <1 cup/day | Adjusted means and their 95% confidence intervals of skeletal muscle mass index according to increasing daily coffee consumption (<1, 1–2, 3 or more cups/day) compared to no consumption: adj mean 7.07 (95% CI 7.08–7.14), adj mean 7.12 (95% CI 7.09–7.14), and adj mean 7.14 (95% CI 7.11–7.17) in males, and adj mean 23.9 (95% CI 23.7–24.1), adj mean 23.8 (95% CI 23.6–24), and adj mean 23.7 (95% CI 23.4–23.9) in females | A significant positive association was found between coffee consumption and SMI. The relationship between coffee consumption and grip strength did not reach statistical significance; however, a positive trend was observed |
| 1–2 cups/day | |||||
| ≥3 cups/day | |||||
| Sarcopenia dimension (HGS) | <1 cup/day | Adjusted means and their 95% confidence intervals of hand grip strength according to increasing daily coffee consumption (<1, 1–2, 3 or more cups/day) compared to no consumption: adj mean 38.1 (95% CI 37.7–38.6), adj mean 38.3 (95% CI 37.9–38.6), adj mean 38.7 (95% CI 38.2–39.1) in males, and adj mean: 23.9 (95% CI 23.7–24.1), adj mean: 23.8 (95% CI 23.6–24), adj mean: 23.7 (95% CI 23.4–23.9) in females | |||
| 1–2 cups/day | |||||
| ≥3 cups/day | |||||
| Huang W.C., 2021 [29] | 1115 | Physical Frailty | Daily frequency | Significant difference in daily frequency of coffee consumption across frailty categories: 0.27 ± 0.16 (frail) vs. 0.30 ± 0.05 (pre-frail) vs. 0.34 ± 0.04 (robust) times/day. p < 0.05 | Frail subjects had significantly lower daily consumption of coffee |
| Machado-Fragua M., 2018 [30] | 2073 | Impaired agility | 1 cup/day | Hazard ratio (95% CI) of impaired agility according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 0.91 (95% CI 0.77–1.09) and HR: 0.86 (95% CI 0.67–1.10). p for trend 0.19 | Habitual coffee consumption was not associated with increased risk of functional impairment |
| ≥2 cups/day | |||||
| 2062 | Impaired mobility | 1 cup/day | Hazard ratio (95% CI) of impaired mobility according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 0.82 (95% CI 0.66–1.01) and HR: 0.82 (95% CI 0.61–1.09). p for trend 0.07 | ||
| ≥2 cups/day | |||||
| 1653 | Impaired overall physical function | 1 cup/day | Hazard ratio (95% CI) of impaired overall physical function according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 0.98 (95% CI 0.81–1.18) and HR: 1.03 (95% CI 0.80–1.33). p for trend 0.88 | ||
| ≥2 cups/day | |||||
| 2262 | Impaired lower extremity function | 1 cup/day | Hazard ratio (95% CI) of impaired lower extremity function according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 1.21 (95% CI 0.97–1.50) and HR: 1.02 (95% CI 0.75–1.38). p for trend 0.45 | ||
| ≥2 cups/day | |||||
| 1714 | Physical Frailty | 1 cup/day | Hazard ratio (95% CI) of frailty according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 1.16 (95% CI 0.85–1.60) and HR: 1.23 (95% CI 0.80–1.90). p for trend 0.25 | ||
| ≥2 cups/day | |||||
| 1564 | IADL disability | 1 cup/day | Hazard ratio (95% CI) of IADL disability according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 0.79 (95% CI 0.55–1.13) and HR: 0.93 (95% CI 0.56–1.53). p for trend 0.46 | ||
| ≥2 cups/day | |||||
| 1756 | ADL disability | 1 cup/day | Hazard ratio (95% CI) of ADL disability according to increasing coffee consumption (1 and 2 or more cups/day) compared to non-coffee drinkers: HR: 0.78 (95% CI 0.62–0.99) and HR: 1.07 (95% 0.78–1.45). p for trend 0.66 | ||
| ≥2 cups/day | |||||
| Verlinden V.J.A., 2016 [31] | 2546 (1128 M, 1418 F) | Global Gait | 1–3 cups/day | Differences in standard deviation of global gait (95% CI) for increasing categories of coffee consumption (1 to 3, and 3 or more cups/day) compared to 1 or fewer cup/day: 0.13 SD (95% CI 0.01–0.25) and 0.18 SD (95% CI 0.08–0.28) | In a community-dwelling population, consuming >1 cup of coffee relate to better gait |
| >3 cups/day | |||||
| Gait speed (m/s) | 1–3 cups/day | Differences in cm/s of gait speed (95% CI) for increasing categories of coffee consumption (1 to 3, and 3 or more cups/day) compared to 1 or fewer cup/day: 2.74 cm/s (95% CI 0.67–4.80) and 2.63 cm/s (95% CI 0.80–4.45) | |||
| >3 cups/day | |||||
| Machado-Fragua M.D., 2019 [32] | 2964 | Falls | 1 cup/day | Hazard ratios (95% CIs) for the association between increasing coffee consumption (1 and 2 or more cups/day) and the risk of ≥1 fall compared to <1 cup/day: HR: 0.88 (95% CI 0.73–1.07) and HR: 0.79 (95% CI 0.63, 0.98). p for trend 0.03 | Habitual coffee consumption was associated with lower risk of falling in older adults in Spain and the United Kingdom |
| ≥2 cups/day | |||||
| 1 cup/day | Hazard ratios (95% CIs) for the association between increasing coffee consumption (1 and 2 or more cups/day) and the risk of ≥1 fall compared to <1 cup/day: HR: 0.61 (95% CI 0.37–0.98) and HR: 0.64 (95% CI 0.39–1.03). p for trend 0.13 | ||||
| ≥2 cups/day | |||||
| Jyvakorpi S.K. 2020 [33] | 126 | Gait speed (m/s) | <110 g/day | Linear association between coffee consumption and gait speed (p = 0.003) | Coffee consumption was positively associated with higher gait speed, handgrip strength, SPPB score, and chair rise points |
| 110–130 g/day | |||||
| >330 g/day | |||||
| SPPB | <110 g/day | Linear association between coffee consumption and SPPB-test scores (p = 0.035) | |||
| 110–130 g/day | |||||
| >330 g/day | |||||
| Chair rise | <110 g/day | Linear association between coffee consumption and chair rise points (p = 0.043) | |||
| 110–130 g/day | |||||
| >330 g/day | |||||
| Sarcopenia dimension (HGS) | <110 g/day | Linear, non-significant association between coffee consumption and handgrip strength (p = 0.856) | |||
| 110–130 g/day | |||||
| >330 g/day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazeaud, S.; Castellana, F.; Coelho-Junior, H.J.; Panza, F.; Rondanelli, M.; Fassio, F.; De Pergola, G.; Zupo, R.; Sardone, R. Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review. Metabolites 2022, 12, 654. https://doi.org/10.3390/metabo12070654
Mazeaud S, Castellana F, Coelho-Junior HJ, Panza F, Rondanelli M, Fassio F, De Pergola G, Zupo R, Sardone R. Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review. Metabolites. 2022; 12(7):654. https://doi.org/10.3390/metabo12070654
Chicago/Turabian StyleMazeaud, Simon, Fabio Castellana, Hélio José Coelho-Junior, Francesco Panza, Mariangela Rondanelli, Federico Fassio, Giovanni De Pergola, Roberta Zupo, and Rodolfo Sardone. 2022. "Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review" Metabolites 12, no. 7: 654. https://doi.org/10.3390/metabo12070654
APA StyleMazeaud, S., Castellana, F., Coelho-Junior, H. J., Panza, F., Rondanelli, M., Fassio, F., De Pergola, G., Zupo, R., & Sardone, R. (2022). Coffee Drinking and Adverse Physical Outcomes in the Aging Adult Population: A Systematic Review. Metabolites, 12(7), 654. https://doi.org/10.3390/metabo12070654











