Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages
Abstract
:1. Introduction
2. Results
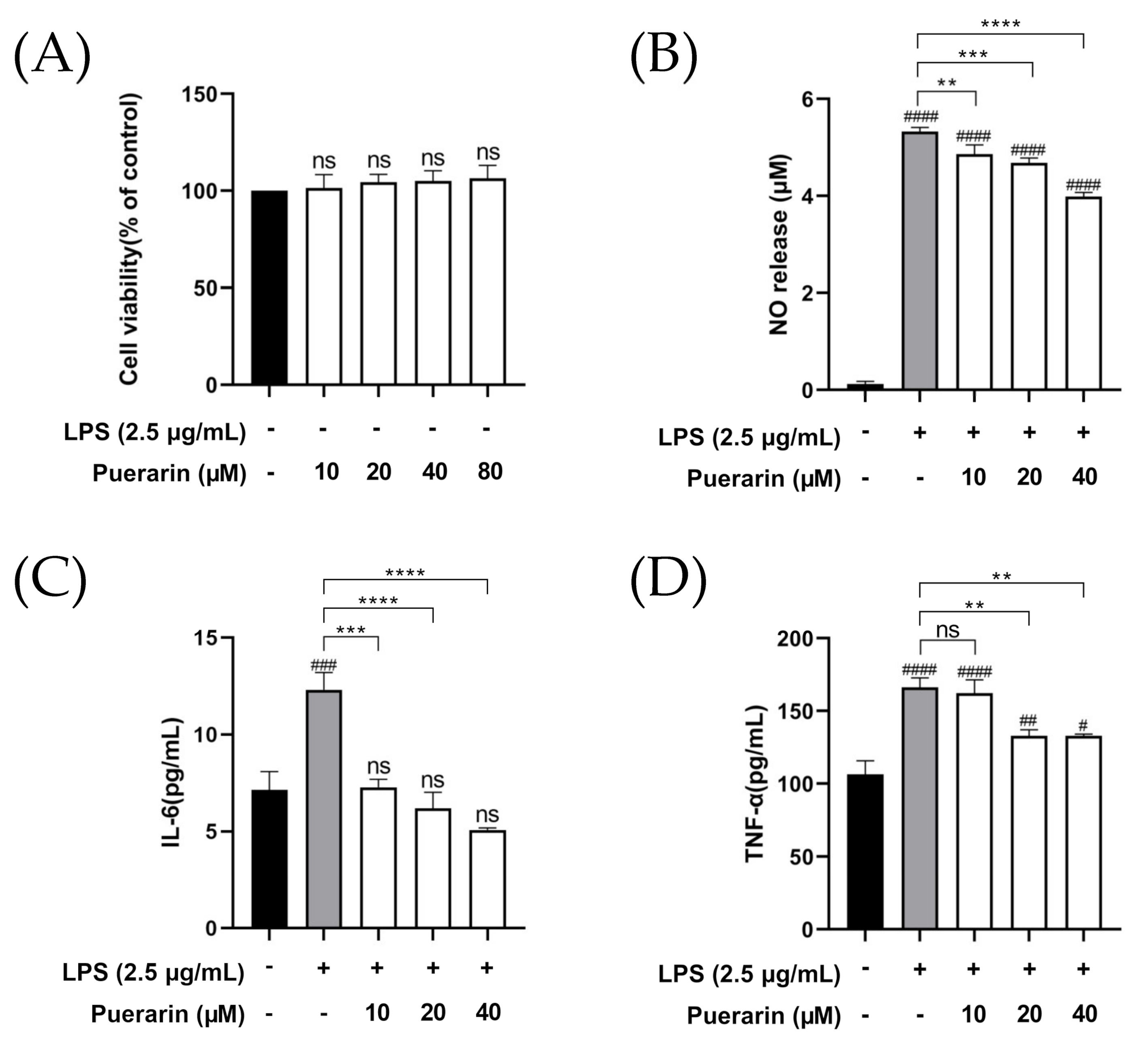
2.1. The Anti-Inflammatory Effect of Puerarin on RAW264.7
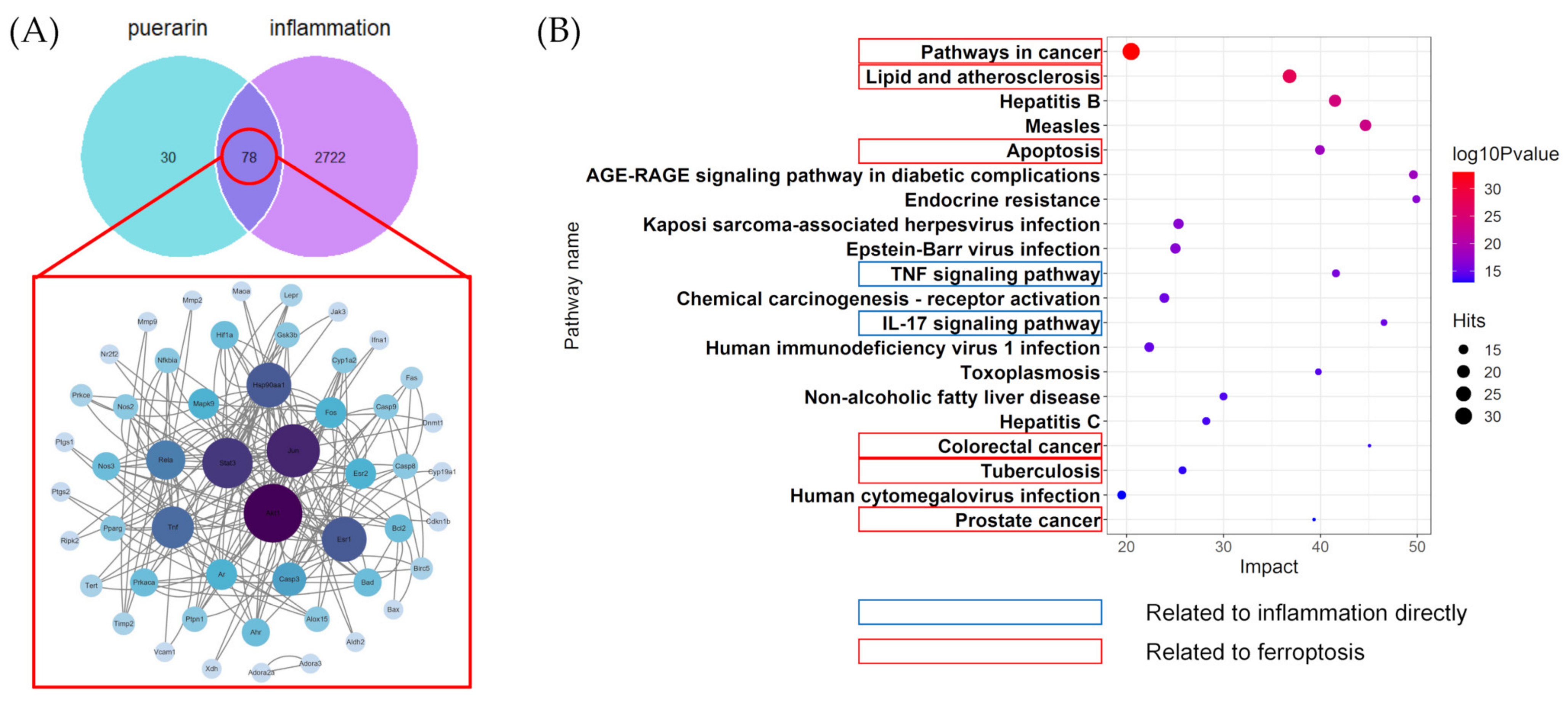
2.2. Result of Network Pharmacology
2.3. Result of Metabolites
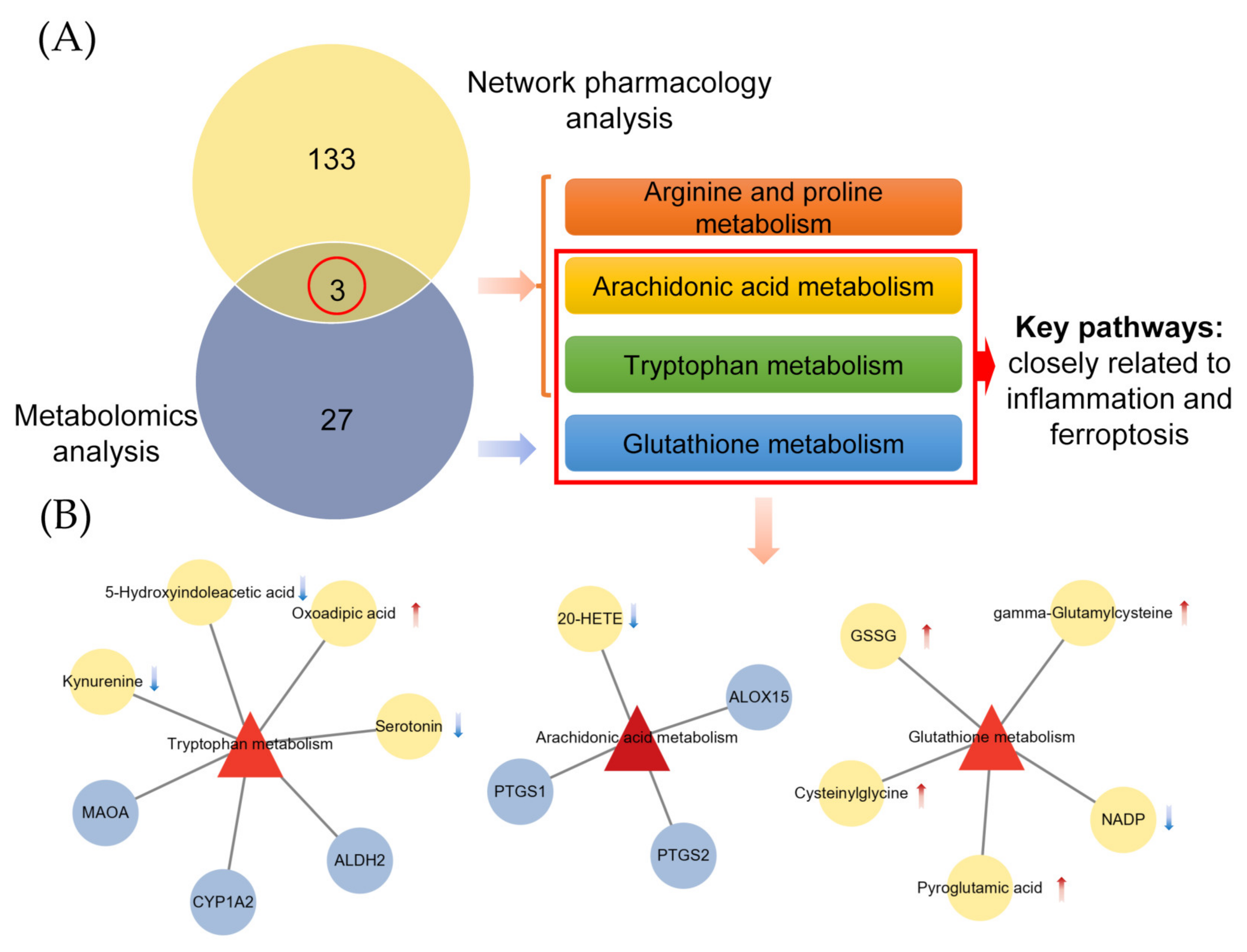
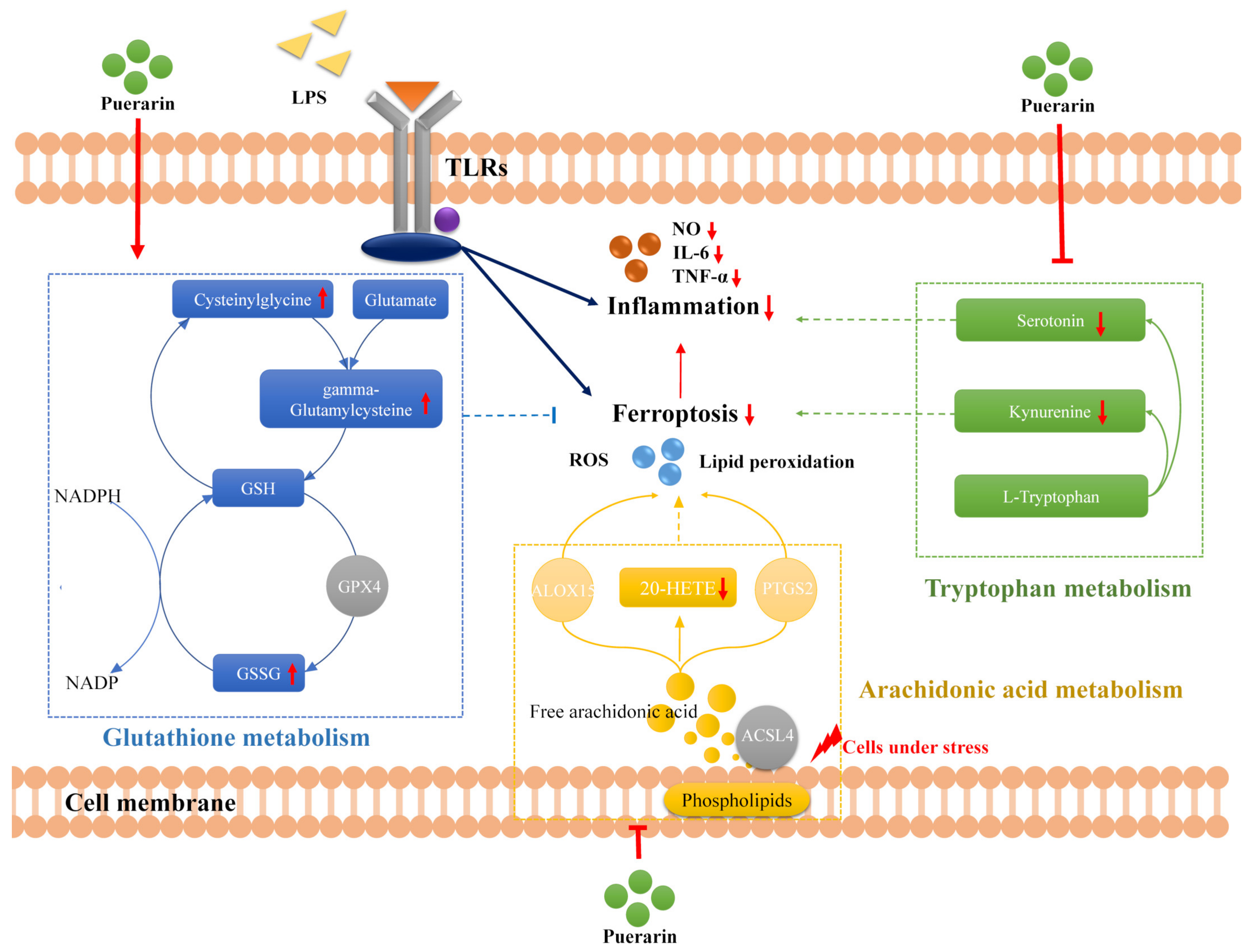
2.4. Combined Analysis of Metabolomics and Network Pharmacology
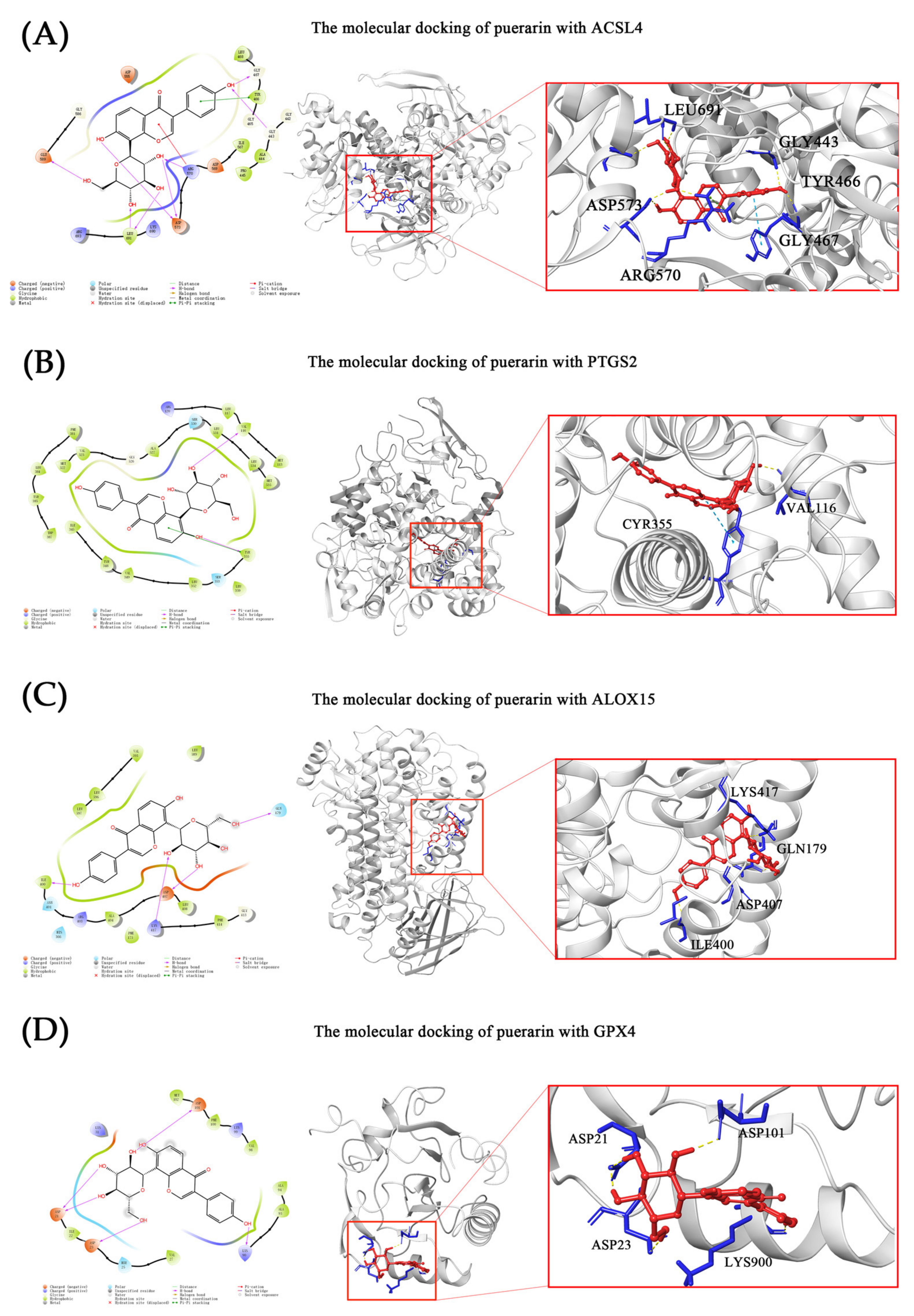
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Detection of Cell Viability
4.4. Detection of NO and Cytokine Production
4.5. Network Pharmacology Analysis
4.6. Metabolites Analysis
4.7. Combined Analysis of Metabolomics and Network Pharmacology
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.L.; Deng, H.D.; Cui, H.M.; Fang, J.; Zuo, Z.C.; Deng, J.L.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.Y.; Chen, P.; Zhai, B.T.; Zhang, M.M.; Xiang, Y.; Fang, J.H.; Xu, S.N.; Gao, Y.F.; Chen, X.; Sui, X.B.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, J.Y.; Xing, R.X.; Sha, T.; Sun, B. Molecular mechanisms of ferroptosis and their role in inflammation. Int. Rev. Immunol. 2021, 1–11. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Salazar, G.; Huang, J.; Feresin, R.G.; Zhao, Y.; Griendling, K.K. Zinc regulates Nox1 expression through a NF-κB and mitochondrial ROS dependent mechanism to induce senescence of vascular smooth muscle cells. Free Radic. Biol. Med. 2017, 108, 225–235. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.B.; Kang, R.; Klionsky, D.J.; Tang, D.L. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Flohe, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Duan, J.; Yin, M.; Shao, Y.; Zheng, J.; Nie, S. Puerarin induces platinum-resistant epithelial ovarian cancer cell apoptosis by targeting SIRT1. J. Int. Med. Res. 2021, 49, 1410580551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Y.; Li, X.; Yang, B.; He, P.; Zhang, H.; Duan, M. Investigation of the anti-diabetic nephropathy activity of puerarin. Mater. Express 2020, 10, 1846–1853. [Google Scholar] [CrossRef]
- Liu, S.; Cao, X.L.; Liu, G.Q.; Zhou, T.; Yang, X.L.; Ma, B.X. The in silico and in vivo evaluation of puerarin against Alzheimer’s disease. Food Funct. 2019, 10, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Peng, C. Effects of puerarin on the prevention and treatment of cardiovascular diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef]
- Xing, Z.; Ma, Y.; Li, X.; Zhang, B.; Zhang, M.; Wan, S.; Yang, X.; Yang, T.; Jiang, J.; Bao, R. Research progress of puerarin and its derivatives on anti-inflammatory and anti-gout activities. Zhongguo Zhong Yao Za Zhi 2017, 42, 3703–3708. [Google Scholar]
- Xu, B.; Wang, H.; Chen, Z. Puerarin inhibits ferroptosis and inflammation of lung injury caused by sepsis in LPS induced lung epithelial cells. Front. Pediatr. 2021, 9, 706327. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, C.; Li, H.; Chen, X.; Ding, Y.; Xu, S. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 2018, 497, 233–240. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.; Chen, Y.; Liu, Y.; Tang, X.; Xia, P.; Yu, P.; Yu, S. Puerarin protects against sepsis-induced myocardial injury through AMPK-mediated ferroptosis signaling. Aging 2022, 14, 3617–3632. [Google Scholar] [CrossRef]
- Lyons, C.L.; Roche, H.M. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.M.; Chen, J.; Wang, M.; Zheng, C.; Zhou, X.L. Puerarin attenuates cadmium-induced hepatic lipid metabolism disorder by inhibiting oxidative stress and inflammation in mice. J. Inorg. Biochem. 2021, 222, 111521. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, Y.; Li, K.; Yuan, D.; Yang, S.; Zhou, L.; Zhao, Y.; Miao, S.; Lv, C.; Zhao, J. COX-2/PGE2 pathway inhibits the ferroptosis induced by cerebral ischemia reperfusion. Mol. Neurobiol. 2022, 59, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Golfinopoulos, S.; Liakopoulos, V.; Stefanidis, I. Role of indoleamine 2,3-dioxygenase in ischemia-reperfusion injury of renal tubular epithelial cells. Mol. Med. Rep. 2021, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Theiss, A.L.; Venuprasad, K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021, 42, 1037–1050. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Tang, P.; Cao, J.; Song, Q.; Zhu, L.; Ma, S.; Zhang, J. Lipid peroxidation aggravates anti-tuberculosis drug-induced liver injury: Evidence of ferroptosis induction. Biochem. Biophys. Res. Commun. 2020, 533, 1512–1518. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kalimuthu, K.; Park, Y.S.; Luo, X.; Choudry, M.; Bartlett, D.L.; Lee, Y.J. BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. Apoptosis 2020, 25, 625–631. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, S.; Li, C.; Ai, Z.; Shen, W.; Ren, W.; Yang, X. Discovery of a novel ferroptosis inducer-talaroconvolutin A—Killing colorectal cancer cells in vitro and in vivo. Cell Death Dis. 2020, 11, 988. [Google Scholar] [CrossRef]
- Meunier, E.; Neyrolles, O. Die another way: Ferroptosis drives tuberculosis pathology. J. Exp. Med. 2019, 216, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Ghoochani, A.; Hsu, E.; Aslan, M.; Rice, M.A.; Nguyen, H.M.; Brooks, J.D.; Corey, E.; Paulmurugan, R.; Stoyanova, T. Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Res. 2021, 81, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.; Castermans, T.M.; Hommen, M.P.; Meesters, D.M.; Poeze, M. Arginine and citrulline and the immune response in sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, M. Tryptophan-kynurenine pathway is dysregulated in inflammation and immune activation. Front. Biosci. 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, A.; Zeitler, L.; Russier, M.; Groß, A.; Hiller, M.; Parker, J.L.; Stier, L.; Köcher, T.; Newstead, S.; Murray, P.J. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell 2022, 82, 920–932. [Google Scholar] [CrossRef]
- Colakoglu, M.; Tuncer, S.; Banerjee, S. Emerging cellular functions of the lipid metabolizing enzyme 15-Lipoxygenase-1. Cell Prolif. 2018, 51, e12472. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef] [PubMed]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Ni, L.; He, K.; Su, K.; Deng, Y.; Li, Y.; Xia, H. The role of arachidonic acid metabolism in myocardial ischemia-reperfusion injury. Cell Biochem. Biophys. 2020, 78, 255–265. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378. [Google Scholar] [CrossRef]
- Liu, C.M.; Ma, J.Q.; Liu, S.S.; Feng, Z.J.; Wang, A.M. Puerarin protects mouse liver against nickel-induced oxidative stress and inflammation associated with the TLR4/p38/CREB pathway. Chem. Biol. Interact. 2016, 243, 29–34. [Google Scholar] [CrossRef]
- Ren, P.; Cao, J.L.; Lin, P.L.; Cao, B.Y.; Chen, J.L.; Gao, K.; Zhang, J. Molecular mechanism of luteolin regulating lipoxygenase pathway against oxygen-glucose deprivation/reperfusion injury in H9c2 cardiomyocytes based on molecular docking. Zhongguo Zhong Yao Za Zhi 2021, 46, 5665–5673. [Google Scholar] [CrossRef]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Leipnitz, G.; Schumacher, C.; Dalcin, K.B.; Scussiato, K.; Solano, A.; Funchal, C.; Dutra-Filho, C.S.; Wyse, A.T.S.; Wannmacher, C.M.D.; Latini, A.; et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem. Int. 2007, 50, 83–94. [Google Scholar] [CrossRef]
- Song, C.; Lin, A.; Bonaccorso, S.; Heide, C.; Verkerk, R.; Kenis, G.; Bosmans, E.; Scharpe, S.; Whelan, A.; Cosyns, P.; et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J. Affect. Disord. 1998, 49, 211–219. [Google Scholar] [CrossRef]
- Zeitler, L.; Fiore, A.; Meyer, C.; Russier, M.; Zanella, G.; Suppmann, S.; Gargaro, M.; Sidhu, S.S.; Seshagiri, S.; Ohnmacht, C.; et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife 2021, 10, e64806. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, C.; Zhang, G.; Tong, X.; Zhang, H.; Ding, J.; Ma, Y.; Cheng, R.; Hou, S.; An, S.; et al. Crucial roles of 5-HT and 5-HT2 receptor in diabetes-related lipid accumulation and pro-inflammatory cytokine generation in hepatocytes. Cell. Physiol. Biochem. 2018, 48, 2409–2428. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriadis, T.; Pissas, G.; Filippidis, G.; Liakopoulos, V.; Stefanidis, I. Reoxygenation induces reactive oxygen species production and ferroptosis in renal tubular epithelial cells by activating aryl hydrocarbon receptor. Mol. Med. Rep. 2021, 23, 41. [Google Scholar] [CrossRef]
- Chen, Y.C.; He, X.L.; Qi, L.; Shi, W.; Yuan, L.W.; Huang, M.Y.; Xu, Y.L.; Chen, X.; Gu, L.; Zhang, L.L.; et al. Myricetin inhibits interferon-γ-induced PD-L1 and IDO1 expression in lung cancer cells. Biochem. Pharmacol. 2022, 197, 114940. [Google Scholar] [CrossRef]
- Kwon, M.; Ko, S.K.; Jang, M.; Kim, G.H.; Ryoo, I.J.; Son, S.; Ryu, H.W.; Oh, S.R.; Lee, W.K.; Kim, B.Y.; et al. Inhibitory effects of flavonoids isolated from Sophora flavescens on indoleamine 2,3-dioxygenase 1 activity. J. Enzyme Inhib. Med. Chem. 2019, 34, 1481–1488. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; MacNee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Friedmann, A.J.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hambright, W.S.; Na, R.; Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015, 290, 28097–28106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Song, P.; Chen, Y.; Liu, Z.; Lai, L. Allosteric type and pathways are governed by the forces of protein-ligand binding. J. Phys. Chem. Lett. 2021, 12, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-kappaB signaling pathway. Cns Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Fan, Z.; Cai, L.; Wang, S.; Wang, J.; Chen, B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front. Pharmacol. 2021, 12, 628988. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin yellow improves cerebral ischemiareperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef]
- The Integrative Pharmacology-Based Research Platform of Traditional Chinese Medicine. Available online: http://www.tcmip.cn/TCMIP/index.php/Home/Login/login.html (accessed on 6 January 2022).
- Traditional Chinese Medicine Systems Pharmacology. Available online: https://old.tcmsp-e.com/tcmsp.php (accessed on 6 January 2022).
- The Swiss Target Prediction. Available online: http://www.swisstargetprediction.ch/ (accessed on 6 January 2022).
- The TargetNet Database. Available online: http://targetnet.scbdd.com/ (accessed on 6 January 2022).
- The Pharmacogenetics and Pharmacogenomics Knowledge Base. Available online: https://www.pharmgkb.org (accessed on 6 January 2022).
- GeneCards. Available online: https://www.genecards.org/ (accessed on 6 January 2022).
- Online Mendelian Inheritance in Man. Available online: https://www.omim.org (accessed on 6 January 2022).
- Comparative Toxicogenomics Database. Available online: https://ctdbase.org/ (accessed on 6 January 2022).
- Therapeutic Target Database. Available online: http://db.idrblab.net/ttd/ (accessed on 6 January 2022).
- STRING11.5. Available online: https://cn.string-db.org/ (accessed on 6 January 2022).
- Database for Annotation, Visualization, and Integrated Discovery. Available online: https://david.ncifcrf.gov/home.jsp (accessed on 6 January 2022).
- Xia, X.; Hao, H.; Zhang, X.; Wong, I.N.; Chung, S.K.; Chen, Z.; Xu, B.; Huang, R. Immunomodulatory sulfated polysaccharides from Caulerpa racemosa var. peltata induces metabolic shifts in NF-κB signaling pathway in RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 182, 321–332. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC−MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- MetaboAnalyst 5.0. Available online: https://www.metaboanalyst.ca/ (accessed on 10 April 2022).
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Future J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 16 April 2022).
- AlphaFold Protein Structure Database. Available online: https://alphafold.ebi.ac.uk (accessed on 16 April 2022).
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 April 2022).

| Gene Symbol | Uniprot ID | Protein Name | Degree |
|---|---|---|---|
| Akt1 | P31750 | RAC-alpha serine/threonine-protein kinase | 30 |
| Jun | P05627 | Transcription factor Jun | 26 |
| Stat3 | P42227 | Signal transducer and activator of transcription 3 | 24 |
| Esr1 | P19785 | Estrogen receptor | 20 |
| Hsp90aa1 | P07901 | Heat shock protein HSP 90-alpha | 20 |
| Tnf | P06804 | Tumor necrosis factor | 18 |
| Rela | Q04207 | Transcription factor p65 | 16 |
| Casp3 | P70677 | Caspase-3 | 12 |
| Ar | P19091 | Androgen receptor | 10 |
| Mapk9 | Q9WTU6 | Mitogen-activated protein kinase 9 | 10 |
| Fos | P01101 | Protein c-Fos | 10 |
| Esr2 | O08537 | Estrogen receptor beta | 10 |
| Ahr | P30561 | Aryl hydrocarbon receptor | 8 |
| Bcl2 | P10417 | Apoptosis regulator Bcl-2 | 8 |
| Hif1a | Q61221 | Hypoxia-inducible factor 1-alpha | 8 |
| Bad | Q61337 | Bcl2-associated agonist of cell death | 8 |
| Nos3 | P70313 | Nitric oxide synthase, endothelial | 8 |
| Prkaca | P05132 | cAMP-dependent protein kinase catalytic subunit alpha | 8 |
| Proteins | Interaction | Gilde Gscore (kcal/mol) | ΔG (kcal/mol) | ||
|---|---|---|---|---|---|
| H-Bond | Pi–Pi Stacking | Pi–cation | |||
| ACSL4 | GLY443, GLY467, ARG570, ASP 573, LEU691 and GLU589 | TYR466 | ARG570 | −7.808 | −55.41 |
| PTGS2 | VAL116, TYR355 | TYR355 | - | −8.414 | −24.62 |
| ALOX15 | GLN179, ILE400, ASP407 and LYS417 | - | - | −6.858 | −27.73 |
| GPX4 | ASP21, ASP23, LYS90 and ASP101 | - | - | −4.872 | −17.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Zhao, N.; Yang, J.; Kuang, W.; Xia, X.; Chen, X.; Liu, Z.; Huang, R. Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages. Metabolites 2022, 12, 653. https://doi.org/10.3390/metabo12070653
Zeng J, Zhao N, Yang J, Kuang W, Xia X, Chen X, Liu Z, Huang R. Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages. Metabolites. 2022; 12(7):653. https://doi.org/10.3390/metabo12070653
Chicago/Turabian StyleZeng, Jinzi, Ning Zhao, Jiajia Yang, Weiyang Kuang, Xuewei Xia, Xiaodan Chen, Zhiyuan Liu, and Riming Huang. 2022. "Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages" Metabolites 12, no. 7: 653. https://doi.org/10.3390/metabo12070653
APA StyleZeng, J., Zhao, N., Yang, J., Kuang, W., Xia, X., Chen, X., Liu, Z., & Huang, R. (2022). Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages. Metabolites, 12(7), 653. https://doi.org/10.3390/metabo12070653








