Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation
Abstract
:1. Introduction
2. Results
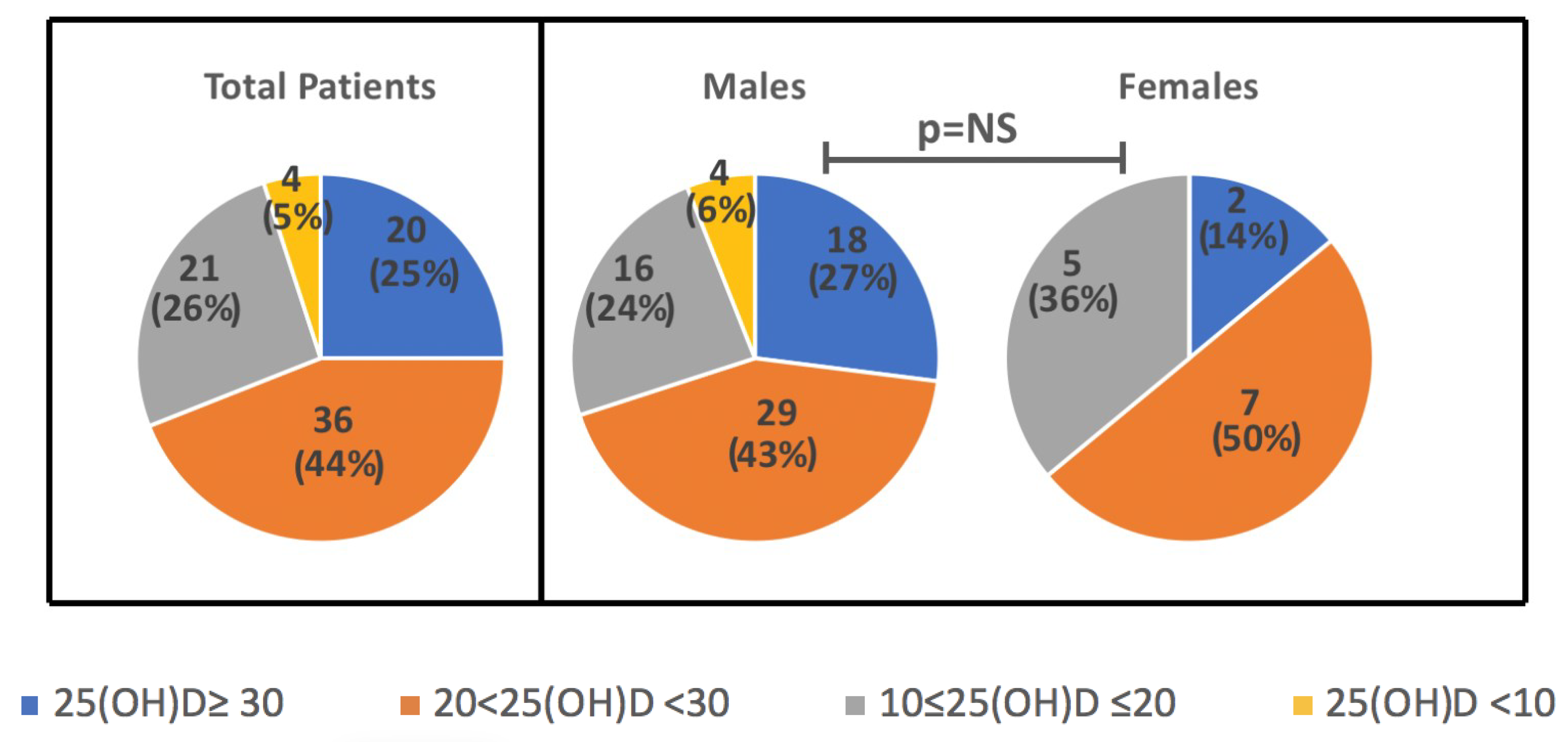
2.1. Characteristics of the Population
2.2. Annual Rhythm Cycle and Anthropometric Characteristics
2.3. 25(OH)D According to Blood Parameters and BMI
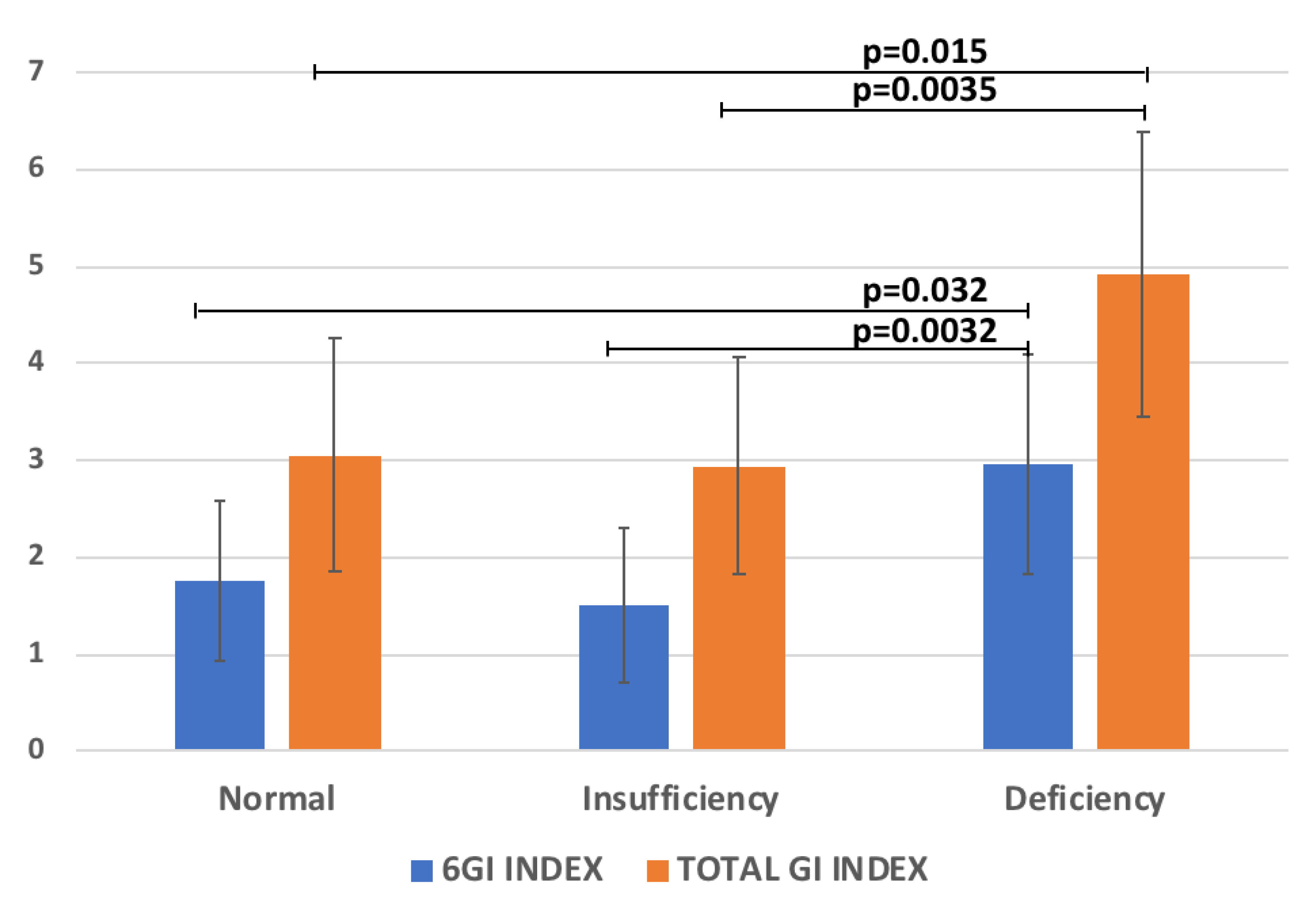
2.4. 25(OH)D According to GI and ADOS
2.5. Multivariate Regression Analysis for 25(OH)D
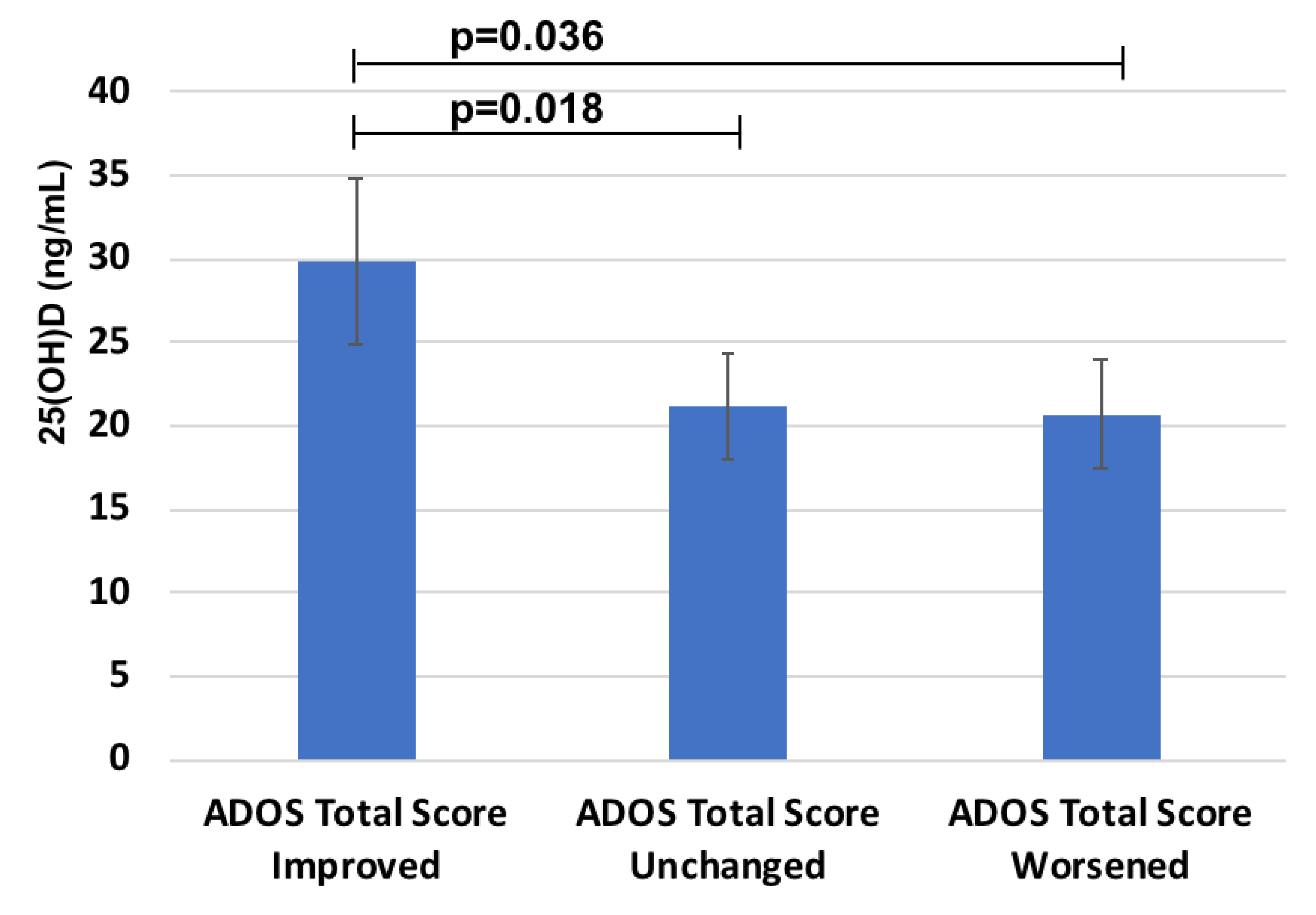
2.6. 25(OH)D According to ADOS Total Score Improvement Due to Probiotics
2.7. 25(OH)D According to GI Improvement Due to Probiotics
3. Discussion
3.1. Population Characteristics
3.2. 25(OH)D, Anthropometric Data, and Annual Rhythm Cycle
3.3. 25(OH)D, Blood Biomarkers, and BMI
3.4. 25(OH)D, ADOS, and GI
3.5. 25(OH)D and the Effects of Probiotic Supplementation
3.6. Strengths and Limitations
4. Materials and Methods
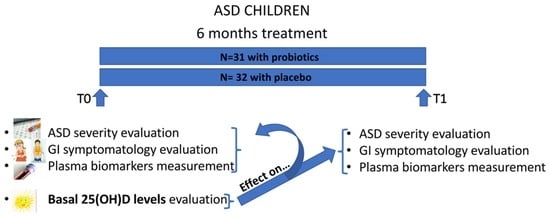
4.1. Participants
4.2. ASD Severity
4.3. GI Symptoms
4.4. Blood Sample Collection and Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub.: Washington, DC, USA, 2013. [Google Scholar]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Mayne, P.E.; Burne, T.H. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, M.; Riccioni, A.; Abate, R.; Benvenuto, A.; Curatolo, P.; Mazzone, L. Vitamin D deficiency and autism spectrum disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Kurutaş, E.B.; Şahin, N.; Güngör, O.; Fındıklı, E. The Levels of Vitamin D, Vitamin D Receptor, Homocysteine and Complex B Vitamin in Children with Autism Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2018, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Dehbokri, N.; Noorazar, G.; Ghaffari, A.; Mehdizadeh, G.; Sarbakhsh, P.; Ghaffary, S. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. World J. Pediatrics 2019, 15, 78–84. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am. J. Physiol.-Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef] [Green Version]
- Alzghoul, L. Role of vitamin D in autism spectrum disorder. Curr. Pharm. Des. 2019, 25, 4357–4367. [Google Scholar] [CrossRef]
- Cannell, J.J. Autism and vitamin D. Med. Hypotheses 2008, 70, 750–759. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Niu, Q.; Eyles, D.W.; Hansen, R.L.; Iosif, A.M. Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case–control study. Autism Res. 2019, 12, 976–988. [Google Scholar] [CrossRef]
- Garcia-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 2468–2481. [Google Scholar] [CrossRef]
- Meguid, N.A.; Hashish, A.F.; Anwar, M.; Sidhom, G. Reduced serum levels of 25-hydroxy and 1, 25-dihydroxy vitamin D in Egyptian children with autism. J. Altern. Complement. Med. 2010, 16, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Camargo, C.A., Jr.; Conlon, C.; Beck, K.L.; Kruger, M.C.; von Hurst, P.R. Vitamin D and Autism Spectrum Disorder: A Literature Review. Nutrients 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Alzghoul, L.; Al-Eitan, L.N.; Aladawi, M.; Odeh, M.; Abu Hantash, O. The association between serum vitamin D3 levels and autism among Jordanian boys. J. Autism Dev. Disord. 2020, 50, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottiere, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and microbiota: Is there a link with allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Prosperi, M.; Guiducci, L.; Peroni, D.G.; Narducci, C.; Gaggini, M.; Calderoni, S.; Tancredi, R.; Morales, M.A.; Gastaldelli, A.; Muratori, F.; et al. Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder. Brain Sci. 2019, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J. Psychiatr. Res. 2019, 115, 90–102. [Google Scholar] [CrossRef]
- Rodrigues, D.H.; Rocha, N.P.; Sousa, L.F.; Barbosa, I.G.; Kummer, A.; Teixeira, A.L. Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology 2014, 69, 6–10. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Loomes, R.; Hull, L.; Mandy, W. ¿ Cuál es la proporción entre hombres y mujeres en el trastorno del espectro autista? Una revisión sistemática y meta-análisis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobjova, T.; Tagoma, A.; Oras, A.; Alnek, K.; Kisand, K.; Talja, I.; Uibo, O.; Uibo, R. Celiac disease in children, particularly with accompanying type 1 diabetes, is characterized by substantial changes in the blood cytokine balance, which may reflect inflammatory processes in the small intestinal mucosa. J. Immunol. Res. 2019, 2019, 6179243. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, E.; Foraita, R.; Pigeot, I.; Barba, G.; Veidebaum, T.; Tornaritis, M.; Michels, N.; Eiben, G.; Ahrens, W.; Moreno, L.A. Reference values for leptin and adiponectin in children below the age of 10 based on the IDEFICS cohort. Int. J. Obes. 2014, 38, S32–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bener, A.; Khattab, A.O.; Al-Dabbagh, M.M. Is high prevalence of Vitamin D deficiency evidence for autism disorder?: In a highly endogamous population. J. Pediatric Neurosci. 2014, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, K.; Abdel-Rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; Cannell, J.J.; Bjørklund, G.; Abdel-Reheim, M.K.; Othman, H.A.; El-Houfey, A.A.; Abd El-Aziz, N.H. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr. Neurosci. 2016, 19, 346–351. [Google Scholar] [CrossRef]
- Arastoo, A.A.; Khojastehkia, H.; Rahimi, Z.; Khafaie, M.A.; Hosseini, S.A.; Mansouri, M.T.; Yosefyshad, S.; Abshirini, M.; Karimimalekabadi, N.; Cheraghi, M. Evaluation of serum 25-Hydroxy vitamin D levels in children with autism Spectrum disorder. Ital. J. Pediatrics 2018, 44, 1–5. [Google Scholar] [CrossRef]
- Şengenç, E.; Kıykım, E.; Saltik, S.; Vitamin, D. Levels in Children and Adolescents with Autism. J. Int. Med. Res. 2020, 48, 300060520934638. [Google Scholar] [CrossRef]
- Cannell, J.J. Vitamin D and autism, what’s new? Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef]
- Fan, X.; Warner, M.; Gustafsson, J.-Å. Estrogen receptor β expression in the embryonic brain regulates development of calretinin-immunoreactive GABAergic interneurons. Proc. Natl. Acad. Sci. USA 2006, 103, 19338–19343. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Cui, X.; Eyles, D. Developmental vitamin D deficiency and autism: Putative pathogenic mechanisms. J. Steroid Biochem. Mol. Biol. 2018, 175, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Cui, X.; Pertile, R.A.N.; Li, X.; Medley, G.; Alexander, S.A.; Whitehouse, A.J.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D deficiency increases foetal exposure to testosterone. Mol. Autism 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Cannell, J.J. Autism prevalence in the United States with respect to solar UV-B doses: An ecological study. Dermato-endocrinology 2013, 5, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspini, B.; Prosperi, M.; Guiducci, L.; Santocchi, E.; Tancredi, R.; Calderoni, S.; Morales, M.A.; Morelli, M.; Simione, M.; Fiechtner, L. Dietary Patterns and Weight Status in Italian Preschoolers with Autism Spectrum Disorder and Typically Developing Children. Nutrients 2021, 13, 4039. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Kwong, C.; Hansen, R.; Hertz-Picciotto, I.; Croen, L.; Krakowiak, P.; Walker, W.; Pessah, I.N.; Van de Water, J. Brief Report: Plasma Leptin Levels are Elevated in Autism: Association with Early Onset Phenotype? J. Autism Dev. Disord. 2008, 38, 169–175. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Chełchowska, M. Comparison of body composition and adipokine levels between thin and normal-weight prepubertal children. J. Pediatr. 2017, 93, 428–435. [Google Scholar] [CrossRef]
- Castro, K.; Faccioli, L.S.; Perry, I.S.; dos Santos Riesgo, R. Leptin and adiponectin correlations with body composition and lipid profile in children with Autism Spectrum Disorder. bioRxiv 2019, 621003. [Google Scholar] [CrossRef]
- Blardi, P.; de Lalla, A.; Ceccatelli, L.; Vanessa, G.; Auteri, A.; Hayek, J. Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci. Lett. 2010, 479, 54–57. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Shab-Bidar, S.; Neyestani, T. Vitamin D and serum leptin: A systematic review and meta-analysis of observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 1144–1153. [Google Scholar] [CrossRef]
- Van Doorn, C.; Macht, V.A.; Grillo, C.A.; Reagan, L.P. Leptin resistance and hippocampal behavioral deficits. Physiol. Behav. 2017, 176, 207–213. [Google Scholar] [CrossRef]
- Morrison, C.D. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2009, 1792, 401–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.-E.; Hetherington, C.J.; Gonzalez, D.A.; Chen, H.-M.; Tenen, D.G. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha, 25-dihydroxyvitamin D3. J. Immunol. 1994, 153, 3276–3284. [Google Scholar] [PubMed]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.C.; Haussier, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Godar, D.E.; Merrill, S.J. Untangling the most probable role for vitamin D3 in autism. Dermato-endocrinology 2017, 9, e1387702. [Google Scholar] [CrossRef] [Green Version]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 944. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Scarpa, M.; Brun, P.; Bernabe, G.; Sagheddu, V.; Elli, M.; Fiore, W.; De Vitis, V.; Guglielmetti, S. Co-administration of vitamin D3 and Lacticaseibacillus paracasei DG increase 25-hydroxyvitamin D serum levels in mice. Ann. Microbiol. 2021, 71, 1–9. [Google Scholar] [CrossRef]
- Abboud, M.; Rizk, R.; AlAnouti, F.; Papandreou, D.; Haidar, S.; Mahboub, N. The health effects of vitamin D and probiotic co-supplementation: A systematic review of randomized controlled trials. Nutrients 2020, 13, 111. [Google Scholar] [CrossRef]
- Prosperi, M.; Santocchi, E.; Guiducci, L.; Frinzi, J.; Morales, M.A.; Tancredi, R.; Muratori, F.; Calderoni, S. Interventions on Microbiota: Where Do We Stand on a Gut–Brain Link in Autism? A Systematic Review. Nutrients 2022, 14, 462. [Google Scholar] [CrossRef]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Chen, X.; Kaboff, A.; Sadiq, F.; Hanauer, S.B. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-G.; Wu, S.; Lu, R.; Zhou, D.; Zhou, J.; Carmeliet, G.; Petrof, E.; Claud, E.C.; Sun, J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 2015, 5, 1–12. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, (ADOS-2) Modules 1–4; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Wiggins, L.D.; Barger, B.; Moody, E.; Soke, G.; Pandey, J.; Levy, S. Brief Report: The ADOS Calibrated Severity Score Best Measures Autism Diagnostic Symptom Severity in Pre-School Children. J. Autism. Dev. Disord. 2019, 49, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism. Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.K.; Melmed, R.D.; Barstow, L.E.; Enriquez, F.J.; Ranger-Moore, J.; Ostrem, J.A. Oral human immunoglobulin for children with autism and gastrointestinal dysfunction: A prospective, open-label study. J. Autism. Dev. Disord. 2006, 36, 1053–1064. [Google Scholar] [CrossRef]
- Bianchi, S.; Maffei, S.; Prontera, C.; Battaglia, D.; Vassalle, C. Preanalytical, analytical (DiaSorin LIAISON) and clinical variables potentially affecting the 25-OH vitamin D estimation. Clin. Biochem. 2012, 45, 1652–1657. [Google Scholar] [CrossRef]
| Anthropometric Characteristics | |
|---|---|
| Number | 81 |
| Males | 67 (83) |
| Females | 14 (17) |
| Age (years) | 4.1 ± 1.1 |
| N-GI patients | 54 (67) |
| GI patients | 27 (33) |
| BMI (Kg/m2) | 16.0 ± 1.7 |
| GI and ADOS values | |
| Total GI severity score | 3.6 ± 2.6 |
| 6GI-Severity score | 2.0 ± 1.9 |
| Social Affect (SA) ADOS CSS | 6.5 ± 2.0 |
| Restricted Repetitive Behaviours (RRB) ADOS_CSS | 8.2 ± 1.4 |
| Total ADOS_CSS | 7.1 ± 1.8 |
| Blood parameters | |
| 25(OH)D (ng/mL) | 24.8 ± 9.9 |
| TNF-alfa (pg/mL) | 6.1 ± 2.4 |
| IL-6 (pg/mL) | 6.2 ± 16.5 |
| Leptin (ng/mL) | 1.15 ± 0.9 |
| Insulin (mU/L) | 22.6 ± 10.5 |
| Resistin (ng/mL) | 23.4 ± 13.7 |
| PAI-1 (ng/mL) | 26.2 ± 19.2 |
| MCP-1 (pg/mL) | 127.8 ± 60.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiducci, L.; Vassalle, C.; Prosperi, M.; Santocchi, E.; Morales, M.A.; Muratori, F.; Calderoni, S. Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation. Metabolites 2022, 12, 611. https://doi.org/10.3390/metabo12070611
Guiducci L, Vassalle C, Prosperi M, Santocchi E, Morales MA, Muratori F, Calderoni S. Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation. Metabolites. 2022; 12(7):611. https://doi.org/10.3390/metabo12070611
Chicago/Turabian StyleGuiducci, Letizia, Cristina Vassalle, Margherita Prosperi, Elisa Santocchi, Maria Aurora Morales, Filippo Muratori, and Sara Calderoni. 2022. "Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation" Metabolites 12, no. 7: 611. https://doi.org/10.3390/metabo12070611
APA StyleGuiducci, L., Vassalle, C., Prosperi, M., Santocchi, E., Morales, M. A., Muratori, F., & Calderoni, S. (2022). Vitamin D Status in Children with Autism Spectrum Disorders: Determinants and Effects of the Response to Probiotic Supplementation. Metabolites, 12(7), 611. https://doi.org/10.3390/metabo12070611