Prognostic Value of Salivary Biochemical Indicators in Primary Resectable Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Overall Survival Rates Depending on the Clinicopathological Characteristics of Patients and Type of Treatment
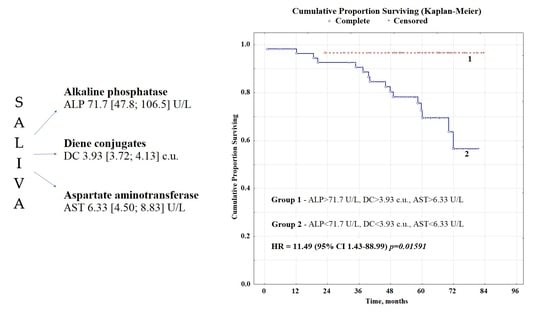
2.2. Overall Survival Rates Depending on the Biochemical Composition of Saliva
2.3. Analysis of the Risk of Relapse in Patients with Primary Operable Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Study Design and Group Description
4.2. Determination of the Expression of the Receptors for Estrogen, Progesterone, and HER2
4.3. Collection and Analysis of Saliva
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabel, A.M.; Baali, F.H. Breast cancer: Insights into risk factors, pathogenesis, diagnosis and management. J. Cancer Res. Treat. 2015, 3, 28–33. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Uygur, M.M.; Gümüş, M. The utility of serum tumor markers CEA and CA 15–3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat. Res. Commun. 2021, 28, 100402. [Google Scholar] [CrossRef]
- Kabel, A.M.; Elkhoely, A.A. Ameliorative potential of fluoxetine/raloxifene combination on experimentally induced breast cancer. Tissue Cell 2016, 48, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Rosso, K.; Nathanson, S.D. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J. Clin. Oncol. 2014, 5, 283–298. [Google Scholar] [CrossRef]
- Tyulyandin, S.A.; Zhukova, L.G.; Koroleva, I.A.; Parokonnaya, A.A.; Semiglazova, T.Y.; Stenina, M.B.; Frolova, M.A. Practical recommendations for the drug treatment of breast cancer. Malig. Tumors Russco. Pract. Guidel. 2021, 11, 119–157. [Google Scholar]
- Soerjomataram, I.; Louwman, M.W.; Ribot, J.G.; Roukema, J.A.; Coebergh, J.W. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res. Treat. 2008, 107, 309–330. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Sahoo, C.R.; Padhy, R.N. Role of hormone receptors and HER2 as prospective molecular markers for breast cancer: An update. Genes Dis. 2022, 9, 648–658. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Tian, B.; Wang, Y.; Du, L.; Sun, T.; Shi, Y.; Zhao, X.; Jing, J. The Diagnostic Value of Serum Tumor Markers CEA, CA19-9, CA125, CA15-3, and TPS in Metastatic Breast Cancer. Clin. Chim. Acta 2017, 470, 51–55. [Google Scholar] [CrossRef]
- Greten, F.; Grivennikov, S. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Hing, J.X.; Mok, C.W.; Tan, P.T.; Sudhakar, S.S.; Seah, C.M.; Lee, W.P.; Tan, S.M. Clinical utility of tumour marker velocity of cancer antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA) in breast cancer surveillance. Breast 2020, 52, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Prabhu, J.S.; Ts, S. High expression of ACE2 in HER2 subtype of breast cancer is a marker of poor prognosis. Cancer Treat. Res. Commun. 2021, 27, 100321. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, M.; Zhou, H.; Chen, K.; Jin, J.; Wu, Y.; Ying, L.; Ding, X.; Su, D.; Zou, D. A Nomogram to Predict the Pathologic Complete Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Based on Simple Laboratory Indicators. Ann. Surg. Oncol. 2019, 26, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, S.; Rilla, K.; Hämäläinen, K.; Oikari, S.; Auvinen, P. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio and the monocyte-to-lymphocyte ratio in early breast cancer, especially in the HER2+ subtype. Breast Cancer Res. Treat. 2021, 185, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Park, J.M.; Ahn, J.H.; Go, J.; Kim, J.; Park, H.S.; Kim, S.I.; Park, B.W.; Park, S. Ki-67 and breast cancer prognosis: Does it matter if Ki-67 level is examined using preoperative biopsy or postoperative specimen? Breast Cancer Res. Treat. 2022, 192, 343–352. [Google Scholar] [CrossRef]
- Kourea, H.P.; Zolota, V.; Scopa, C.D. Targeted pathways in breast cancer: Molecular and protein markers guiding therapeutic decisions. Curr. Mol. Pharmacol. 2014, 7, 4–21. [Google Scholar] [CrossRef]
- Bertheau, P.; Lehmann-Che, J.; Varna, M.; Dumay, A.; Poirot, B.; Porcher, R.; Turpin, E.; Plassa, L.F.; de Roquancourt, A.; Bourstyn, E.; et al. P53 in breast cancer subtypes and new insights into response to chemotherapy. Breast 2013, 22, S27–S29. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef]
- Krüger, J.M.; Wemmert, C.; Sternberger, L.; Bonnas, C.; Dietmann, G.; Gançarski, P.; Feuerhake, F. Combat or surveillance? Evaluation of the heterogeneous inflamatory breast cancer microenvironment. J. Pathol. 2013, 229, 569–578. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef]
- Cavaco, C.; Pereira, J.A.M.; Taunk, K.; Taware, R.; Rapole, S.; Nagarajaram, H.; Câmara, J.S. Screening of salivary volatiles for putative breast cancer discrimination: An exploratory study involving geographically distant populations. Anal. Bioanal. Chem. 2018, 410, 4459–4468. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaseb, S.I. Serum and saliva protein levels in females with breast cancer. Oncol. Lett. 2014, 8, 2752–2756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Yu, H.; Qiao, Y.; Yang, J.; Shu, J.; Zhang, J.; Zhang, Z.; He, J.; Li, Z. Salivary glycopatterns as potential biomarkers for screening of early-stage breast cancer. EBioMedicine 2018, 28, 70–79. [Google Scholar] [CrossRef]
- Takayama, T.; Tsutsui, H.; Shimizu, I.; Toyama, T.; Yoshimoto, N.; Endo, Y.; Inoue, K.; Todoroki, K.; Min, J.Z.; Mizuno, H.; et al. Diagnostic approach to breast cancer patients based on target metabolomics in saliva by liquid chromatography with tandem mass spectrometry. Clin. Chim. Acta 2016, 452, 18–26. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Chou, J.; Yu, J.; Yang, T.; Liu, L.; Zhang, F. Taurine, glutamic acid and ethylmalonic acid as important metabolites for detecting human breast cancer based on the targeted metabolomics. Cancer Bio. 2018, 23, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Yanagisawa, T.; Kurihara, T.; Kaneko, M.; Ota, S.; Enomoto, A.; Tomita, M.; Sugimoto, M.; Sunamura, M.; Hayashida, T.; et al. Salivary metabolomics with alternative decision tree-based machine learning methods for breast cancer discrimination. Breast Cancer Res. Treat. 2019, 177, 591–601. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Takkouche, B.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Salivary biomarkers for cancer diagnosis: A meta-analysis. Ann. Med. 2020, 52, 131–144. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Metabolic Features of Saliva in Breast Cancer Patients. Metabolites 2022, 12, 166. [Google Scholar] [CrossRef]
- López-Jornet, P.; Aznar, C.; Ceron, J.; Asta, T. Salivary biomarkers in breast cancer: A cross-sectional study. Support. Care Cancer 2021, 29, 889–896. [Google Scholar] [CrossRef]
- Porto-Mascarenhas, E.C.; Assad, D.X.; Chardin, H.; Gozal, D.; De Luca Canto, G.; Acevedo, A.C.; Guerra, E.N. Salivary biomarkers in the diagnosis of breast cancer: A review. Crit. Rev. Oncol./Hematol. 2017, 110, 62–73. [Google Scholar] [CrossRef]
- Koopaie, M.; Kolahdooz, S.; Fatahzadeh, M.; Manifar, S. Salivary biomarkers in breast cancer diagnosis: A systematic review and diagnostic meta-analysis. Cancer Med. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Survival Rates of Patients with Non-Small Cell Lung Cancer Depending on Lymph Node Metastasis: A Focus on Saliva. Diagnostics 2021, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K. A new field of application of saliva tests for prognostic purpose: Focus on lung cancer. Biomedical Chemistry: Res. Methods 2020, 3, e00133. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K.; Gundyrev, I.A. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, Á.; Aguiar, P.; Del Rio, M.C.; Arias, J.I.; Menéndez-Rodríguez, P.; Gude, F.; Herranz, M. Histological grade (HG) in invasive ductal carcinomas of the breast of less than 1 cm: Clinical and biological assosiations during progression from HG1 to HG3. Anticancer Res. 2015, 35, 569–573. [Google Scholar]
- Gurleyik, G.; Gurleyik, E.; Aker, F.; Aktekin, A.; Emir, S.; Gungor, O.; Saglam, A. Lymphovascular invasion, as a prognostic marker in patients with invasive breast cancer. Acta Chir. Belg. 2007, 107, 284–287. [Google Scholar] [CrossRef]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline Phosphatase: A Novel Treatment Target for Cardiovascular Disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef]
- Damera, S.; Raphael, K.; Baird, B.; Cheung, A.; Greene, T.; Beddhu, S. Serum Alkaline Phosphatase Levels Associate with Elevated Serum C-Reactive Protein in Chronic Kidney Disease. Kidney Int. 2011, 79, 228–233. [Google Scholar] [CrossRef]
- Rao, S.R.; Snaith, A.E.; Marino, D.; Cheng, X.; Lwin, S.T.; Orriss, I.R.; Hamdy, F.C.; Edwards, C.M. Tumour-Derived Alkaline Phosphatase Regulates Tumour Growth, Epithelial Plasticity and Disease-Free Survival in Metastatic Prostate Cancer. Br. J. Cancer 2017, 116, 227–236. [Google Scholar]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP Drives Systemic Inflammation, Tissue Damage and Mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef]
- Peters, E.; Geraci, S.; Heemskerk, S.; Wilmer, M.J.; Bilos, A.; Kraenzlin, B.; Gretz, N.; Pickkers, P.; Masereeuw, R. Alkaline Phosphatase Protects Against Renal Inflammation Through Dephosphorylation of Lipopolysaccharide and Adenosine Triphosphate. Br. J. Pharm. 2015, 172, 4932–4945. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Li, Z.; Lai, S.; Zhong, X.; Fu, X.; Huang, X.; Li, Q.; Liu, S.; Li, H. Construction and Validation of a Serum Albumin-to-Alkaline Phosphatase Ratio-Based Nomogram for Predicting Pathological Complete Response in Breast Cancer. Front. Oncol. 2021, 11, 681905. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, J.; Tao, Y.L.; Xu, B.Q.; Tu, Z.W.; Liu, Z.G.; Zeng, M.S.; Xia, Y.F. Increased Pretreatment Levels of Serum LDH and ALP as Poor Prognostic Factors for Nasopharyngeal Carcinoma. Chin. J. Cancer 2012, 31, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Pond, G.R.; Berry, W.R.; de Wit, R.; Armstrong, A.J.; Eisenberger, M.A.; Tannock, I.F. Serum Alkaline Phosphatase Changes Predict Survival Independent of PSA Changes in Men with Castration-Resistant Prostate Cancer and Bone Metastasis Receiving Chemotherapy. Urol. Oncol. 2012, 30, 607–613. [Google Scholar] [CrossRef]
- Maisano, R.; Azzarello, D.; Del Medico, P.; Maisano, M.; Bottari, M.; Egitto, G.; Nardi, M. Alkaline Phosphatase Levels as a Prognostic Factor in Metastatic Colorectal Cancer Treated with the FOLFOX 4 Regimen: A Monoinstitutional Retrospective Study. Tumori 2011, 97, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dai, D.; Tang, H.; Chen, X.; Ai, X.; Huang, X.; Wei, W.; Xie, X. Pre-Treatment Serum Alkaline Phosphatase and Lactate Dehydrogenase as Prognostic Factors in Triple Negative Breast Cancer. J. Cancer 2016, 7, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, A.; Tewari, M.; Kumar, R.; Sharma, A.; Singh, K.A.; Pandey, H.P.; Shukla, H.S. Advanced stage of breast cancer hoist alkaline phosphatase activity: Risk factor for females in India. 3 Biotech 2013, 3, 517–520. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Kosenok, V.K. Age and gender characteristics of the biochemical composition of saliva: Correlations with the composition of blood plasma. J. Oral Biol. Craniofacial Res. 2020, 10, 59–65. [Google Scholar] [CrossRef]
- Chen, S.-L.; Xue, N.; Wu, M.-T.; Chen, H.; He, X.; Li, J.-P.; Liu, W.-L.; Dai, S.-Q. Influence of Preoperative Serum Aspartate Aminotransferase (AST) Level on the Prognosis of Patients with Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1474. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Elf, S.E.; Chen, J. Targeting glucose metabolism in patients with cancer. Cancer 2014, 120, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, J.M.; Nelson, K.K.; Clem, B.F.; Lane, A.N.; Arumugam, S.; Simmons, A.; Eaton, J.W.; Telang, S.; Chesney, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008, 10, R84. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, Y.H.; Sung, H.H.; Han, D.H.; Jeon, H.G.; Chang Jeong, B.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y. De Ritis Ratio (AST/ALT) as a Significant Prognostic Factor in Patients with Upper Tract Urothelial Cancer Treated with Surgery. Clin. Genitourin. Cancer 2017, 15, e379–e385. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G.; Zav’yalov, A.A. Status Indicators of Lipid Peroxidation and Endogenous Intoxication in Lung Cancer Patients. Ann. Russ. Acad. Med. Sci. 2016, 71, 313–322. [Google Scholar]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Gȩgotek, A.; Nikliński, J.; Žarković, N.; Žarković, K.; Waeg, G.; Łuczaj, W.; Charkiewicz, R.; Skrzydlewska, E. Lipid mediators involved in the oxidative stress and antioxidant defense of human lung cancer cells. Redox Biol. 2016, 9, 210–219. [Google Scholar] [CrossRef]
- Barsukov, V.Y.; Plokhov, V.N.; Tchesnokova, N.P. Activation of lipid peroxidation as typical cellular disintegration process in neoplastic area in breast cancer. Vestn. VolGMU 2007, 3, 3–6. [Google Scholar]
- Cossetti, R.J.; Tyldesley, S.K.; Speers, C.H.; Zheng, Y.; Gelmon, K.A. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J. Clin. Oncol. 2015, 33, 65–73. [Google Scholar] [CrossRef]
- Morrow, M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast 2013, 22, S106–S109. [Google Scholar] [CrossRef]
- Pilewskie, M.; King, T.A. Age and molecular subtypes: Impact on surgical decisions. J. Surg. Oncol. 2014, 110, 8–14. [Google Scholar] [CrossRef]
- Mersin, H.; Gülben, K.; Berberoğlu, U.; Yazi, M.; Acun, G.; Kinaş, V.; Erdoğan, S. Prognostic factors affecting postmastectomy locoregional recurrence in patients with early breast cancer: Are intrinsic subtypes effective? World J. Surg. 2011, 35, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Finetti, P.; Cervera, N.; Tallet, A.; Benchalal, M.; Houvenaeghel, G.; Jacquemier, J.; Birnbaum, D.; Bertucci, F. Gene expression profiling and its utility in prediction of local relapse after breast-conserving therapy in early breast cancer. Cancer Genom. Proteom. 2011, 8, 199–209. [Google Scholar]
- Roka, S.; Rudas, M.; Taucher, S.; Dubsky, P.; Bachleitner-Hofmann, T.; Kandioler, D.; Gnant, M.; Jakesz, R. High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur. J. Surg. Oncol. 2004, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ilić, I.R.; Stojanović, N.M.; Radulović, N.S.; Živković, V.V.; Randjelović, P.J.; Petrović, A.S.; Božić, M.; Ilić, R.S. The Quantitative ER Immunohistochemical Analysis in Breast Cancer: Detecting the 3 + 0, 4 + 0, and 5 + 0 Allred Score Cases. Medicina 2019, 55, 461. [Google Scholar] [CrossRef] [PubMed]
- Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Sarf, E.A. Chronophysiological features of the normal mineral composition of human saliva. Arch. Oral Biol. 2017, 82, 286–292. [Google Scholar] [CrossRef]


| Category | OS, Months | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age, years | 30–39, n = 34 | 56.7 | 1 | 0.06851 |
| 40–49, n = 68 | 59.3 | 1.24 (0.40–3.83) | ||
| 50–59, n = 117 | 65.4 | 1.12 (0.39–3.25) | ||
| 60–69, n = 105 | 61.0 | 1.04 (0.35–3.07) | ||
| 70+, n = 27 | 48.1 | 3.41 (1.00–11.47) * | ||
| Menopause | No, n = 106 | 57.2 | 1 | 0.95402 |
| Yes, n = 248 | 61.8 | 1.32 (0.71–2.43) | ||
| pT | 1, n = 133 | 62.3 | 1 | 0.00000 |
| 2, n = 172 | 60.9 | 2.73 (1.32–5.56) * | ||
| 3, n = 47 | 56.3 | 6.88 (2.91–15.84) * | ||
| pN | 0, n = 245 | 60.7 | 1 | 0.00000 |
| 1, n = 110 | 60.5 | 3.93 (2.22–6.82) * | ||
| Grade | 1, n = 28 | 64.3 | 1 | 0.06269 |
| 2, n = 58 | 59.5 | 3.71 (0.44–30.98) | ||
| 3, n = 88 | 60.8 | 9.00 (1.15–68.17) * | ||
| Histological type | Ductal, n = 171 | 65.2 | 1 | 0.61737 |
| Lobular, n = 58 | 59.6 | 1.18 (0.56–2.47) | ||
| Subtype | Luminal A-like, n = 50 | 68.0 | 1 | 0.00652 |
| Luminal B-like (HER2−), n = 41 | 55.1 | 2.72 (1.01–7.26) * | ||
| Luminal B-like (HER2+), n = 181 | 60.5 | 0.80 (0.34–1.91) | ||
| Non-Luminal (HER2+), n = 22 | 68.6 | 0.83 (0.20–3.45) | ||
| Basal-like, n = 20 | 58.8 | 1.75 (0.50–6.11) | ||
| HER2-status | (−), n = 112 | 60.1 | 1 | 0.03198 |
| (+), n = 98 | 61.5 | 0.63 (0.32–1.24) | ||
| (++), n = 62 | 60.0 | 0.38 (0.16–0.94) * | ||
| (+++), n = 48 | 60.1 | 0.35 (0.13–0.97) * | ||
| ER-status | (−), n = 49 | 61.7 | 1 | 0.09137 |
| (+), n = 41 | 60.4 | 1.27 (0.49–3.29) | ||
| (++), n = 57 | 64.9 | 0.83 (0.33–2.10) | ||
| (+++), n = 173 | 60.3 | 0.56 (0.25–1.23) | ||
| PR-status | (−), n = 85 | 60.4 | 1 | 0.79103 |
| (+), n = 44 | 60.1 | 0.89 (0.35–2.25) | ||
| (++), n = 60 | 62.6 | 0.71 (0.29–1.71) | ||
| (+++), n = 131 | 60.2 | 0.85 (0.43–1.70) | ||
| Category | OS, Months | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Operation status | BCS, n = 61 | 59.0 | 1 | 0.37724 |
| TM, n = 286 | 61.3 | 1.50 (0.64–3.47) | ||
| Radiation therapy | Done, n = 191 | 60.5 | 1 | 0.00452 |
| Not done, n = 163 | 60.9 | 0.34 (0.19–0.64) * | ||
| Chemotherapy | Done, n = 181 | 59.5 | 1 | 0.00005 |
| Not done, n = 173 | 61.8 | 0.33 (0.18–0.60) * | ||
| Endocrine therapy | Done, n = 241 | 61.3 | 1 | 0.00413 |
| Not done, n = 113 | 58.3 | 2.18 (1.24–3.77) * | ||
| Category | OS, Months | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| ALP, U/L | >71.7, n = 175 | 61.4 | 1 | 0.00243 |
| <71.7, n = 179 | 58.5 | 2.60 (1.44–4.62) * | ||
| AST, U/L | >6.33, n = 174 | 61.5 | 1 | 0.36144 |
| <6.33, n = 163 | 58.5 | 1.13 (0.64–1.97) | ||
| DC, c.u. | >3.93, n = 176 | 58.7 | 1 | 0.08518 |
| <3.93, n = 178 | 60.2 | 1.78 (1.02–3.08) * | ||
| ALP + AST | >71.7, >6.33, n = 64 | 59.6 | 1 | 0.02068 |
| >71.7, <6.33, n = 98 | 63.3 | 1.80 (0.61–5.27) | ||
| <71.7, >6.33, n = 76 | 60.5 | 3.15 (1.08–9.01) * | ||
| <71.7, <6.33, n = 97 | 56.7 | 4.10 (1.47–11.18) * | ||
| ALP + DC | >71.7, >3.93, n = 87 | 61.7 | 1 | 0.00580 |
| >71.7, <3.93, n = 87 | 61.0 | 3.15 (1.08–9.02) * | ||
| <71.7, >3.93, n = 88 | 56.5 | 4.22 (1.49–11.74) * | ||
| <71.7, <3.93, n = 91 | 58.9 | 6.21 (2.24–16.79) * | ||
| ALP + AST + DC | Favorable, n = 55 | 62.8 | 1 | 0.01591 |
| Unfavorable, n = 29 | 58.9 | 11.49 (1.43–88.99) * | ||
| Prognostic Factors | β | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Age group | 0.2421 | 0.1363 | 1.7761 | 0.0757 |
| pT | 0.8087 | 0.2213 | 3.6550 | 0.0003 |
| pN | 0.9110 | 0.2749 | 3.3138 | 0.0009 |
| Grade | 0.7704 | 0.3017 | 2.5538 | 0.0107 |
| Molecular biological subtype | 0.1252 | 0.1184 | 1.0577 | 0.2902 |
| HER2-status | −1.1583 | 0.3460 | −3.3477 | 0.0008 |
| ALP, U/L | −1.0105 | 0.3821 | −2.6446 | 0.0082 |
| AST, U/L | 0.1803 | 0.3829 | 0.4709 | 0.6377 |
| DC, c.u. | −0.4967 | 0.3822 | −1.2997 | 0.1937 |
| Category | Relapse, n = 59 | No Relapse, n = 292 | HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Clinicopathological characteristics of patients | |||||
| Age group | 30–39 | 9 | 28 | 1 | 0.69523 |
| 40–49 | 16 | 51 | 0.98 (0.38–2.48) | ||
| 50–59 | 19 | 98 | 0.60 (0.25–1.48) | ||
| 60–69 | 13 | 91 | 0.44 (0.17–1.15) | ||
| 70+ | 2 | 25 | 0.25 (0.05–1.26) | ||
| Menopause | No | 40 | 84 | 1 | 0.00526 |
| Yes | 19 | 208 | 0.19 (0.11–0.35) * | ||
| pT | 1 | 10 | 123 | 1 | 0.00032 |
| 2 | 30 | 141 | 2.62 (1.23–5.51) * | ||
| 3 | 19 | 28 | 8.35 (3.47–19.50) * | ||
| pN | 0 | 26 | 216 | 1 | 0.00079 |
| 1 | 33 | 76 | 3.61 (2.02–6.34) * | ||
| Grade | 1 | 4 | 24 | 1 | 0.46751 |
| 2 | 8 | 50 | 0.96 (0.27–3.48) | ||
| 3 | 16 | 71 | 1.35 (0.41–4.40) | ||
| Histological type | Ductal | 32 | 137 | 1 | 0.98523 |
| Lobular | 14 | 44 | 1.36 (0.67–2.76) | ||
| Molecular biological subtype | Luminal A-like | 11 | 39 | 1 | 0.72153 |
| Luminal B-like (HER2−) | 8 | 33 | 0.86 (0.31–2.37) | ||
| Luminal B-like (HER2+) | 30 | 149 | 0.71 (0.33–1.55) | ||
| Non-Luminal (HER2+) | 2 | 20 | 0.35 (0.07–1.75) | ||
| Basal-like | 4 | 15 | 0.95 (0.26–3.41) | ||
| HER2-status | (−) | 24 | 87 | 1 | 0.12697 |
| (+) | 16 | 81 | 0.72 (0.36–1.44) | ||
| (++) | 10 | 52 | 0.70 (0.31–1.57) | ||
| (+++) | 7 | 39 | 0.65 (0.26–1.63) | ||
| ER-status | (−) | 8 | 39 | 1 | 0.56214 |
| (+) | 11 | 30 | 1.79 (0.64–4.94) | ||
| (++) | 9 | 48 | 0.91 (0.32–2.57) | ||
| (+++) | 29 | 142 | 1.00 (0.42–2.34) | ||
| PR-status | (−) | 16 | 69 | 1 | 0.64852 |
| (+) | 6 | 36 | 0.72 (0.26–1.99) | ||
| (++) | 10 | 50 | 0.86 (0.36–2.05) | ||
| (+++) | 25 | 105 | 1.03 (0.51–2.05) | ||
| Type of treatment | |||||
| Operation status | BCS | 5 | 56 | 1 | 0.16325 |
| TM | 51 | 235 | 2.43 (0.93–6.29) | ||
| Radiation therapy | Done | 41 | 150 | 1 | 0.00289 |
| Not done | 18 | 145 | 0.45 (0.25–0.83) * | ||
| Chemotherapy | Done | 42 | 139 | 1 | 0.00117 |
| Not done | 17 | 156 | 0.36 (0.20–0.66) * | ||
| Endocrine therapy | Done | 38 | 203 | 1 | 0.62547 |
| Not done | 21 | 92 | 1.22 (0.68–2.18) | ||
| Biochemical indicators of saliva | |||||
| ALP, U/L | >71.7 | 22 | 163 | 1 | 0.00524 |
| <71.7 | 36 | 129 | 2.07 (1.16–3.66) * | ||
| AST, U/L | >6.33 | 29 | 142 | 1 | 0.69441 |
| <6.33 | 28 | 133 | 1.03 (0.58–1.82) | ||
| DC, c.u. | >3.93 | 26 | 148 | 1 | 0.45597 |
| <3.93 | 33 | 144 | 1.30 (0.74–2.28) | ||
| ALP + AST | >71.7, >6.33 | 16 | 86 | 1 | 0.14965 |
| <71.7, <6.33 | 23 | 67 | 1.85 (0.90–3.73) | ||
| ALP + DC | >71,7, >3.93 | 10 | 81 | 1 | 0.00124 |
| <71.7, <3.93 | 21 | 61 | 2.79 (1.22–6.27) * | ||
| ALP + AST + DC | Favorable | 7 | 45 | 1 | 0.08963 |
| Unfavorable | 14 | 37 | 2.43 (0.89–6.57) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel’skaya, L.V.; Sarf, E.A. Prognostic Value of Salivary Biochemical Indicators in Primary Resectable Breast Cancer. Metabolites 2022, 12, 552. https://doi.org/10.3390/metabo12060552
Bel’skaya LV, Sarf EA. Prognostic Value of Salivary Biochemical Indicators in Primary Resectable Breast Cancer. Metabolites. 2022; 12(6):552. https://doi.org/10.3390/metabo12060552
Chicago/Turabian StyleBel’skaya, Lyudmila V., and Elena A. Sarf. 2022. "Prognostic Value of Salivary Biochemical Indicators in Primary Resectable Breast Cancer" Metabolites 12, no. 6: 552. https://doi.org/10.3390/metabo12060552
APA StyleBel’skaya, L. V., & Sarf, E. A. (2022). Prognostic Value of Salivary Biochemical Indicators in Primary Resectable Breast Cancer. Metabolites, 12(6), 552. https://doi.org/10.3390/metabo12060552