Salivary Melatonin Changes in Oncological Patients: A Systematic Review
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Data Extraction
- -
- For PubMed: (melatonin AND saliva) AND (cancer OR carcinoma OR neoplasm OR tumour OR tumor OR oncology);
- -
- For Scopus: TITLE-ABS-KEY((melatonin AND saliva) AND (cancer OR carcinoma OR neoplasm OR tumour OR tumor OR oncology));
- -
- For Web of Science: TS = ((melatonin AND saliva) AND (cancer OR carcinoma OR neoplasm OR tumour OR tumor OR oncology)).
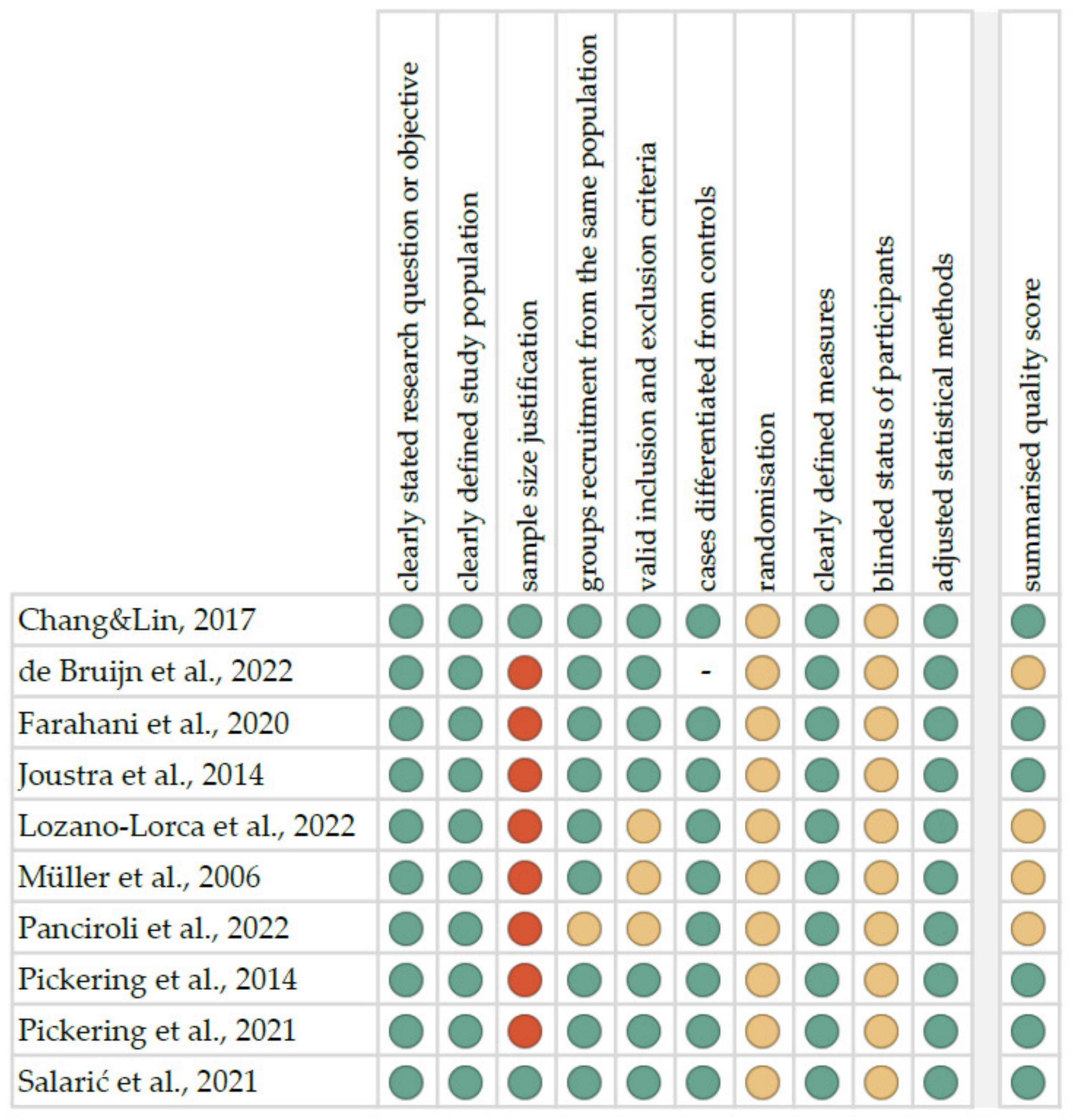
4.2. Quality Assessment and Critical Appraisal for the Systematic Review of Included Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surdacki, M.; Hirnle, A.; Pirogowicz, I. Effects of light pollution on the human body. In Current Challenges of Modern Geriatrics; Wroclaw Medical University: Wroclaw, Poland, 2021; pp. 337–353. ISBN 978-83-7055-635-8. [Google Scholar]
- Tähkämö, L.; Partonen, T.; Pesonen, A.-K. Systematic Review of Light Exposure Impact on Human Circadian Rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, R.G.; Zhu, Y. Electric Light, Particularly at Night, Disrupts Human Circadian Rhythmicity: Is That a Problem? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navara, K.J.; Nelson, R.J. The Dark Side of Light at Night: Physiological, Epidemiological, and Ecological Consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological Effects in Humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.-X. Melatonin: An Established Antioxidant Worthy of Use in Clinical Trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin Anticancer Effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]
- Kandalepas, P.C.; Mitchell, J.W.; Gillette, M.U. Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian Disrupting Exposures and Breast Cancer Risk: A Meta-Analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef]
- González-González, A.; García Nieto, E.; González, A.; Sánchez-Fernández, C.; Alonso-González, C.; Menéndez-Menéndez, J.; Gómez-Arozamena, J.; Cos, S.; Martínez-Campa, C. Melatonin Modulation of Radiation and Chemotherapeutics-Induced Changes on Differentiation of Breast Fibroblasts. Int. J. Mol. Sci. 2019, 20, 3935. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin Is an Appropriate Candidate for Breast Cancer Treatment: Based on Known Molecular Mechanisms. J. Cell. Biochem. 2019, 120, 12208–12215. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.R.; Haneuse, S.; Fall, K.; Schernhammer, E.S.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary Melatonin Levels, Sleep Disruption, and Risk of Prostate Cancer in Elderly Men. Eur. Urol. 2015, 67, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light Exposure at Night Disrupts Host/Cancer Circadian Regulatory Dynamics: Impact on the Warburg Effect, Lipid Signaling and Tumor Growth Prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef] [Green Version]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef] [Green Version]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef]
- Chang, W.-P.; Lin, C.-C. Relationships of Salivary Cortisol and Melatonin Rhythms to Sleep Quality, Emotion, and Fatigue Levels in Patients with Newly Diagnosed Lung Cancer. Eur. J. Oncol. Nurs. 2017, 29, 79–84. [Google Scholar] [CrossRef]
- de Bruijn, L.; Starreveld, D.E.J.; Schaapveld, M.; van Leeuwen, F.E.; Bleiker, E.M.A.; Berentzen, N.E. Single-Item chronotype is associated with dim light melatonin onset in Lymphoma survivors with fatigue. J. Sleep Res. 2022, e13577. [Google Scholar] [CrossRef]
- Farahani, H.; Alaee, M.; Amri, J.; Baghinia, M.-R.; Rafiee, M. Serum and Saliva Concentrations of Biochemical Parameters in Men with Prostate Cancer and Benign Prostate Hyperplasia. Lab. Med. 2020, 51, 243–251. [Google Scholar] [CrossRef]
- Joustra, S.D.; Thijs, R.D.; van den Berg, R.; van Dijk, M.; Pereira, A.M.; Lammers, G.J.; van Someren, E.J.W.; Romijn, J.A.; Biermasz, N.R. Alterations in Diurnal Rhythmicity in Patients Treated for Nonfunctioning Pituitary Macroadenoma: A Controlled Study and Literature Review. Eur. J. Endocrinol. 2014, 171, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Lorca, M.; Olmedo-Requena, R.; Rodríguez-Barranco, M.; Redondo-Sánchez, D.; Jiménez-Pacheco, A.; Vázquez-Alonso, F.; Arana-Asensio, E.; Sánchez, M.-J.; Fernández-Martínez, J.; Acuña-Castroviejo, D.; et al. Salivary Melatonin Rhythm and Prostate Cancer: CAPLIFE Study. J. Urol. 2022, 207, 565–572. [Google Scholar] [CrossRef]
- Müller, H.L.; Handwerker, G.; Gebhardt, U.; Faldum, A.; Emser, A.; Kolb, R.; Sörensen, N. Melatonin Treatment in Obese Patients with Childhood Craniopharyngioma and Increased Daytime Sleepiness. Cancer Causes Control 2006, 17, 583–589. [Google Scholar] [CrossRef]
- Panciroli, C.; Esteve, A.; Muñoz-Ferrer, A.; Abad, J.; Hernandez, J.M.; Balaña, C.; Lucente, G.; Comas, S.; Villà, S. Prospective Pilot Study to Explore the Melatonin Level in Brain Tumor Patients Undergoing Radiotherapy. Sleep Breath. 2022, 26, 469–475. [Google Scholar] [CrossRef]
- Pickering, L.; Jennum, P.; Gammeltoft, S.; Poulsgaard, L.; Feldt-Rasmussen, U.; Klose, M. Sleep-Wake and Melatonin Pattern in Craniopharyngioma Patients. Eur. J. Endocrinol. 2014, 170, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Pickering, L.; Main, K.M.; Feldt-Rasmussen, U.; Klose, M.; Sehested, A.; Mathiasen, R.; Jennum, P. Brain Tumours in Children and Adolescents May Affect the Circadian Rhythm and Quality of Life. Acta Paediatr. 2021, 110, 3376–3386. [Google Scholar] [CrossRef]
- Salarić, I.; Karmelić, I.; Lovrić, J.; Baždarić, K.; Rožman, M.; Čvrljević, I.; Zajc, I.; Brajdić, D.; Macan, D. Salivary Melatonin in Oral Squamous Cell Carcinoma Patients. Sci. Rep. 2021, 11, 13201. [Google Scholar] [CrossRef]
- Morin, D.; Simon, N.; Deprés-Brummer, P.; Lévi, F.; Tillement, J.P.; Urien, S. Melatonin High-Affinity Binding to Alpha-1-Acid Glycoprotein in Human Serum. Pharmacology 1997, 54, 271–275. [Google Scholar] [CrossRef]
- Kennaway, D.J. A Critical Review of Melatonin Assays: Past and Present. J. Pineal Res. 2019, 67, e12572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Faassen, M.; Bischoff, R.; Kema, I.P. Relationship between Plasma and Salivary Melatonin and Cortisol Investigated by LC-MS/MS. Clin. Chem. Lab. Med. CCLM 2017, 55, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 August 2020).
- OCEBM Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 22 August 2020).

| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | patients with oncological diseases—aged from 0 to 99 years, both sexes | patients with other diseases |
| Intervention | not applicable | |
| Comparison | not applicable | |
| Outcomes | salivary melatonin concentrations or changes | only other forms of melatonin (e.g., serum) |
| Study design | case-control, cohort, and cross-sectional studies | literature reviews, case reports, expert opinion, letters to the editor, conference reports |
| published after 2000 | not published in English |
| Author, Year | Setting | Study Group (F/M) * | Control Group (F/M) * | Oncological Diagnosis | Inclusion Criteria | Exclusion Criteria | Sleep Parameters | Other Parameters |
|---|---|---|---|---|---|---|---|---|
| Chang&Lin, 2017 [17] | Taiwan | 40 (9/31), 66.92 ± 11.01 | 40 (9/31), 66.68 ± 10.84 | lung cancer | patients whose diagnosis was based on their first tissue biopsy; those who had not received lung-cancer-related treatment and were able to communicate | patients who were shift workers before hospitalisation because shift work can negatively impact the circadian clock, who were too weak to complete a questionnaire interview or submit a salivary sample, or who had a mental disorder | Pittsburgh Sleep Quality Index (PSQI) | Hospital Anxiety and Depression Scale (HADS), Brief Fatigue Inventory (BFI) |
| de Bruijn et al., 2022 [18] | the Netherlands | 47 (31/16), 44.6 ± 12.8 | NA | lymphoma | primary diagnosis of Hodgkin lymphoma or diffuse large B-cell lymphoma ≥2 years before study entry; moderate-to-severe fatigue since diagnosis and/or treatment; aged 18–70 years | other factors that could have affected acute fatigue or circadian rhythms | Pittsburgh Sleep Quality Index (PSQI) | visual analogue scale for fatigue (VAS-Fatigue) |
| Farahani et al., 2020 [19] | Iran | 20 (0/20), 50.95 ± 2.76 | 20 (0/20), 50.45 ± 2.61; with benign prostatic hyperplasia | prostate cancer | age between 50 and 55 years; histologically confirmed diagnosis; absence of malignant neoplasms and infections; no history of oral or dental diseases | metastatic disease or already treated tumors, previous chemotherapy or radiotherapy, systemic diseases, oral or salivary-gland diseases | - | - |
| Joustra et al., 2014 [20] | the Netherlands | 17 (8/9), 54 (26–65) | 17 (6/11), 52 (30–63) | nonfunctioning pituitary macroadenoma | stable and adequate substitution of pituitary insufficiencies for at least 6 months; age of 18–65 years | patients using hypnotics or psychotropic medication; patients suffering from conditions that may alter circadian rhythmicity, i.e., sleep disorders, depression, hypertension, dyslipidemia, and diabetes mellitus | Total sleep time, Sleep efficiency; Berlin Questionnaire, Clinical symptom score, Epworth Sleepiness Scale, | Hospital Anxiety and Depression Scale, Short Form-36; skin and core body temperature, 24-h blood pressure |
| Lozano-Lorca et al., 2022 [21] | Spain | 40 (0/40), 67.0 ± 7.3 | 38 (0/38), 67.5 ± 5.5 | prostate cancer | newly diagnosed with histological confirmation, between 40 and 80 years old and resided in the coverage area of the reference hospitals for 6 months before recruitment in CAPLIFE study, before the first treatment for the disease | NR | Pittsburgh Sleep Quality Index (PSQI) | - |
| Müller et al., 2006 [22] | Germany | craniopharyngioma: BMI < 4SD: 49 (24/25), 16.1 (6.0–33.2), BMI ≥ 4SD: 30 (15/15), 16.6 (5.8–33.0); hypothalamic tumour: BMI < 4SD: 15 (5/10), 10.5 (4.8–25.6), BMI ≥ 4SD: 4 (1/3), 7.4 (6.3–8.5) | BMI < 4SD: 16 (8/8), 10.1 (4.4–24.0), BMI ≥ 4SD: 14 (3/11), 14.9 (7.4–15.5) | craniopharyngioma or hypothalamic pilocytic astrocytoma | NR | patients with missing data on ESS and melatonin secretion due to non-compliance or technical problems in collecting nocturnal saliva samples | German version of the Epworth Sleepiness Scale | BMI |
| Panciroli et al., 2022 [23] | Spain | 12 (2/10), median = 48.5 | 8 (5/3), median = 38.5 | brain tumors treated with radiotherapy | patients with brain tumors ≥ 18 years old before and after having received radiotherapy close to the pineal gland region | NR | Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS) | European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaires (QLQ) C30 and BN20, Distress Thermometer, Mini-Mental State Examination (MMSE) |
| Pickering et al., 2014 [24] | Denmark | 15 (6/9), 51.4 (18.2–70.2) | 15 (6/9), 51.6 (22.6–70.0) | craniopharyngioma | patients treated for former craniopharyngiomas aged 18–70 years | insufficient substitution of pituitary hormone deficiencies within 6 months before inclusion, total blindness with complete lack of perception of light and form, clinically significant liver or renal disease, use of non-steroidal anti-inflammatory drugs, b-receptor antagonists, antidepressants that affect serotonin, active cancer, epileptic seizures, working night shift, breast feeding, pregnancy and alcohol, travelling across time zones or drug abuse | Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS) | Multidimensional Fatigue Inventory, Medical Outcomes Study 36-Item Short-Form Health Survey |
| Pickering et al., 2021 [25] | Denmark | 48 (19/29), 12.2 (7.6–16.2) | 20 (7/13), 11.9 (8.0–16.3); with other brain tumours | brain tumours categorised by location involving the circadian regulatory system, defined as involving the diencephalon, pineal gland, brain stem, posterior fossa tumours compressing the brain stem or cervical medulla, or involving other locations | patients aged 0–18 years at the time of inclusion and with a previous diagnosis of a tumour of the brain or cervical medulla | tumour diagnosis, surgical tumour intervention or irradiation within six months of enrolment, and insufficient substitution of pituitary hormone deficiencies within three months of enrolment | sleep latency, duration of night sleep, total duration of day and night sleep, and sleep efficiency | Pediatric Quality of Life Inventory Multidimensional Fatigue Scale, Pediatric Quality of Life Inventory Generic Core Scales |
| Salarić et al., 2021 [26] | Croatia | 34 (9/25), 60.6 ± 11.1 | 33 (10/23), 63.0 ± 13.3 | oral squamous cell carcinoma (T1N0M0, n = 14, T2N0M0, n = 20) | patients with histologically verified T1N0M0 and T2N0M0 OSCC; no history of radiation therapy of the head and neck; absence of salivary, jaw and oral mucosal tissue diseases and conditions | OSCC located on the tongue root and epiglottis | Pittsburgh Sleep Quality Index (PSQI) | - |
| Type of Tumour | Study | Type of Saliva | Method of Collection | Centrifugation and Storing | Method of Analysis | Other Markers |
|---|---|---|---|---|---|---|
| lung cancer | [17] | unstimulated whole saliva | collected through expectoration into a sterile container over a 10-min period, three times daily (9 a.m., 2 p.m. and 9 p.m.), and at least 3 mL each time | immediately stored at −20 °C | radioimmunoassay (RIA) | salivary cortisol |
| lymphoma | [18] | unstimulated whole saliva | collected 5 h before usual bedtime followed by one sample every sequential hour | stored at −80 °C until the analysis | liquid chromatography tandem mass spectrometry | - |
| prostate cancer | [19] | unstimulated whole saliva | collected for 5 min | centrifuged at 3000 rpm for 10 min and stored at −70 °C until the analysis | sandwich enzyme-linked immunosorbent assay (ELISA) | serum and salivary prostate-specific antigen (PSA), beta-2 microglobulin, creatine kinase BB, creatinine, urea and zinc; serum melatonin |
| prostate cancer | [21] | unstimulated whole saliva | using Salivettes on 6 timepoints: (1) 4 h before bedtime, (2) 2 h before bedtime, (3) at bedtime, (4) 2 h after bedtime, (5) 4 h after bedtime, and (6) when getting up in the morning | centrifuged and stored at −80 °C until the analysis | ultra-performance liquid chromatography-electrospray ionisation-mass spectrometry | - |
| brain tumour | [20] | unstimulated whole saliva | using Salivettes on two subsequent evenings (at 3, 2, and 1 h before and at habitual bedtime, and upon waking up spontaneously at night) and on the day in between (at awakening, 1 and 2 h after awakening, noon, and 3 p.m.) | kept in the dark at 4 °C until the end of assessment and stored at −20 °C until analysis; centrifuged at 1800× g for 15 min | radioimmunoassay (RIA) | - |
| brain tumour | [22] | unstimulated whole saliva | collected at different time points (morning: 6–8 h; midday: 11–14 h; evening: 18–21 h; and night time: 23–3 h) using special tubes | centrifuged and stored at −20 °C until the analysis | radioimmunoassay (RIA) | salivary cortisol |
| brain tumour | [24] | unstimulated whole saliva | during the 2 weeks of actigraphy, eight samples with a volume of 3 mL each collected over a 24-h period (at 12 p.m., 4 p.m., 8 p.m., 10 p.m., 12 a.m., 4 a.m., 8 a.m. and 12 p.m.) | during collection kept at 3–5 °C, then centrifuged and stored at −22 °C until the analysis | radioimmunoassay (RIA) | salivary cortisol |
| brain tumour | [23] | unstimulated whole saliva | collected at midnight and at 6 a.m. in the next morning, using Salivettes | NR | LC/MS/MS (liquid chromatography mass spectrometry) using ISD-MS (isotope dilute mass spectrometry) | salivary cortisol and cortisone; urinary metabolite sulfatoxi-melatonine (STM), cortisol and cortisone |
| brain tumour | [25] | unstimulated whole saliva | collected with Salivettes at 12 p.m., 4 p.m., 8 p.m., 10 p.m., 12 a.m., 4 a.m., 8 a.m. and 12 p.m. | during collection kept at 3–5 °C, then centrifuged at 2100× g for 5 min and stored at −20 °C until the analysis | enzyme-linked immunosorbent assay (ELISA) | salivary cortisol |
| oral cancer | [26] | unstimulated whole saliva | collected before any surgical procedure between 7 and 9 a.m. in a dark room (<3 lx) by using the specially designed saliva collecting apparatus | stored at −80 °C until the analysis | enzyme-linked immunosorbent assay (ELISA) | serum melatonin |
| Type of Tumour | Study | Salivary Melatonin Findings |
|---|---|---|
| lung cancer | [17] | The patient group had a lower salivary melatonin level and flatter slope (p-value < 0.001 and < 0.001), higher salivary cortisol level and steeper slope (p-value < 0.001 and < 0.001), higher sleep disturbance level (p-value = 0.004), and higher depression level (p-value = 0.001). The multivariate linear regression analysis indicated that the cortisol slope (p-value = 0.005) and fatigue score (p-value = 0.032) predicted the sleep quality score (p-value = 0.011). |
| lymphoma | [18] | The mean (SD) dim light melatonin onset was at 8:42 (1:19) p.m. and the most common chronotype was more evening than morning person (29.2%). A gradual increase in dim light melatonin onset with later chronotype (i.e., evening preference) was observed, with a mean ranging from 7:45 p.m. in definite morning persons to 9:16 p.m. in definite evening persons. |
| prostate cancer | [19] | Serum and salivary concentrations of melatonin were significantly lower in patients with PC, compared with BPH group (p-value < 0.05). In both groups, salivary concentrations were lower (p-value < 0.05), compared with those values in serum. It was observed positive correlation between serum and salivary concentrations (p-value < 0.05). |
| prostate cancer | [21] | Melatonin levels were always lower in PC cases than in controls. On average, melatonin levels in cases were −64.0% (95% CI −73.4, −51.4) than controls. PC cases had lower amplitude, 26.0 pg/mL (SD 27.8) vs 46.3 pg/mL (SD 28.2; p-value < 0.001). A high amplitude was associated with a decreased risk of PC, aOR = 0.31 (95% CI 0.11, 0.86), while a late acrophase could be increased risk of PC, aOR = 2.36 (95% CI 0.88, 6.27). |
| brain tumour | [20] | Out of 17 NFMA patients, 7 (41%) showed at least one of the three possible abnormal parameters during daytime, i.e., either an increased distal–proximal gradient (n = 4), increased core body temperature (n = 2), or increased melatonin values (n = 4). Lower daytime proximal skin temperatures in NFMA patients were associated with increased daytime melatonin values (r = −0.527, p-value = 0.036). |
| brain tumour | [22] | Morning salivary melatonin levels were related to BMI (p-value = 0.004) and tumor diagnosis (p-value = 0.032). Also for nighttime salivary melatonin levels, significant relations with BMI (p-value < 0.001) and tumor diagnosis (p-value = 0.025) were detectable. Melatonin concentrations in saliva of craniopharyngioma patients collected at nighttime or in the morning showed a negative correlation (Spearman’s rho: −0.42; p-value = 0.001; Spearman’s rho: −0.31; p-value = 0.020) with the patient’s ESS score. Severely obese craniopharyngioma patients and severely obese hypothalamic tumor patients had similar patterns of melatonin secretion. Differences in terms of diurnal salivary cortisol concentrations were not detectable when patient groups and controls were compared. |
| brain tumour | [24] | Low midnight melatonin was associated with reduced sleep time and efficiency (p-value < 0.03) and a tendency for increased sleepiness, impaired sleep quality, and physical health. Midnight melatonin remained independently related to sleep time after adjustment for cortisol. Three different patterns of melatonin profiles were observed: normal (n = 6), absent midnight peak (n = 6), and phase-shifted peak (n = 2). Only patients with absent midnight peak had impaired sleep quality, increased daytime sleepiness, and general and mental fatigue. |
| brain tumour | [23] | No statistically significant differences in morning and evening melatonin levels were found according to the radiotherapy dose delivered throughout the study. |
| brain tumour | [25] | Children with tumours involving the circadian regulatory system typically had a lower melatonin peak (p-value = 0.06) and experienced significantly more fatigue and poorer quality of life. Low melatonin profiles were observed in 31% and 4% had a phase-shifted daytime peak, compared with 14% and 0%, respectively, in children with tumours located elsewhere. Children with low melatonin profiles had significantly lower inter-daily stability than those with normal profiles. |
| oral cancer | [26] | Melatonin levels in both unstimulated and stimulated whole saliva were significantly higher in the OSCC group. Sleep quality was significantly lower in patients with OSCC (p-value = 0.0001). ROC analysis was found to be significant (p-value < 0.001) in evaluating melatonin concentration limit in diagnosing OSCC. The expected relationship between sleep quality and salivary melatonin levels in OSCC patients was not observed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijakowski, K.; Surdacki, M.; Sobieszczańska, M. Salivary Melatonin Changes in Oncological Patients: A Systematic Review. Metabolites 2022, 12, 439. https://doi.org/10.3390/metabo12050439
Nijakowski K, Surdacki M, Sobieszczańska M. Salivary Melatonin Changes in Oncological Patients: A Systematic Review. Metabolites. 2022; 12(5):439. https://doi.org/10.3390/metabo12050439
Chicago/Turabian StyleNijakowski, Kacper, Michał Surdacki, and Małgorzata Sobieszczańska. 2022. "Salivary Melatonin Changes in Oncological Patients: A Systematic Review" Metabolites 12, no. 5: 439. https://doi.org/10.3390/metabo12050439
APA StyleNijakowski, K., Surdacki, M., & Sobieszczańska, M. (2022). Salivary Melatonin Changes in Oncological Patients: A Systematic Review. Metabolites, 12(5), 439. https://doi.org/10.3390/metabo12050439








