Abstract
Our understanding of prostate cancer (PCa) has shifted from solely caused by a few genetic aberrations to a combination of complex biochemical dysregulations with the prostate metabolome at its core. The role of metabolomics in analyzing the pathophysiology of PCa is indispensable. However, to fully elucidate real-time complex dysregulation in prostate cells, an integrated approach based on metabolomics and other omics is warranted. Individually, genomics, transcriptomics, and proteomics are robust, but they are not enough to achieve a holistic view of PCa tumorigenesis. This review is the first of its kind to focus solely on the integration of metabolomics with multi-omic platforms in PCa research, including a detailed emphasis on the metabolomic profile of PCa. The authors intend to provide researchers in the field with a comprehensive knowledge base in PCa metabolomics and offer perspectives on overcoming limitations of the tool to guide future point-of-care applications.
1. Introduction
Metabolomics is the newest omic science in systems biology, following genomics, transcriptomics, and proteomics. The four omics are complementary in understanding the interrelated cellular functions of a specific disease phenotype [1]. Metabolomics is currently applied to various disciplines including environmental epidemiology, food technology, ecological restoration, and oncology. It is an analytical profiling technique that measures and compares large numbers of metabolites in a biological sample. Metabolomic analysis is performed to identify (untargeted, global, and top-down approach) and quantify (targeted, specific, and bottom-up approach) metabolites with the goal of understanding the mechanisms by which upstream molecules (genes, RNAs, and proteins) contribute to pathology [2,3,4]. It seeks to investigate how therapeutics affect treatment outcomes [5] by serving as biomarkers via the quantification of these small molecules [6,7]. Metabolites (≤1.5 kD), which include sugars, fatty acids, amino acids, nucleotides, alkaloids, and steroids [1,8] can sometimes be enzymatically transformed into epimetabolites, allowing them to regulate physiological processes [9,10,11]. Because biological matrices are complex with thousands of metabolites in them, the use of analytical methods such as metabolomics allows individual metabolite measurements to be managed [12,13,14,15,16,17,18]. The difference between the two types of metabolomics is that the untargeted approach identifies a single metabolite in a hypothesis-driven manner, while the targeted approach quantifies a metabolite of interest a priori [1,2,19]. In humans, the untargeted approach reveals functional changes to the metabolome as a result of endogenous (diet, exercise) and exogenous (environmental exposures, virus, and genotoxins) agents [20,21,22,23]. Because the untargeted approach deals with a vast number of unknown molecules with disparate physical and chemical characteristics, multiple protocols for sample preparation, data acquisition, and analysis are required including subsequent validation via the targeted approach [24]. Regardless, in both approaches, tools such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) are used to provide insights into the disease mechanisms [3,4,18,25]. For clarity, metabolism refers to the series of biochemical processes that generate energy via ATP, while the metabolome is the collection of metabolites that are produced by cells during metabolism. The number of metabolites (intermediates) in a metabolome depends on the biochemical pathway involved. Moreover, metabolomic and metabolic are distinct from each other in that the former refers to the actual omic approach while the latter is a term that signifies the relationship to metabolism. The process flow for LC-, MS-, and NMR-based metabolomic analysis for disease biomarker research is shown in Figure 1.
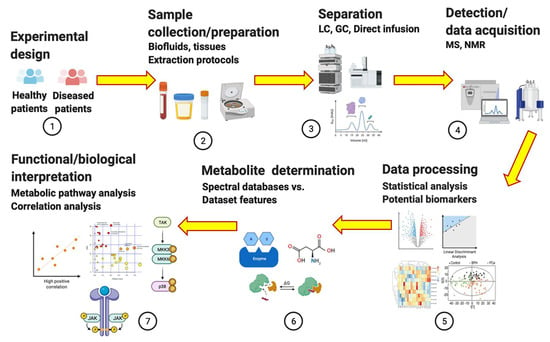
Figure 1.
Process flow for untargeted and targeted metabolomics as applied to disease biomarker research. Figure drawn using BioRender [26].
2. Metabolomics: The “Supra-Omic”
The four omic platforms can be applied complementarily in pathology; however, metabolomics shows remarkable advantages over genomics, transcriptomics, and proteomics. Despite being relatively new, its ‘supra-omic’ nature is due to its ability to provide a real-time snapshot of the physiological state of a cell, tissue, or organism because the measured metabolite concentration accurately reflects infinitesimal biochemical perturbations, both endogenous and exogenous. Metabolomics has advantages over the other omics. First, the metabolome is highly sensitive to functional cellular changes brought about by stimuli including diet, radiation, medications, and stress levels [27]. Metabolites are products or intermediates of a metabolic pathway and their measurement represents a direct and real-time functional readout of physiological status or cellular activity [6]. Second, metabolomic alterations are determined via multiple analyses of biofluids (urine, serum) and tissue extracts in vitro, tissues and organs in vitro, and tissues operando. Samples are conveniently obtained in clinical and point-of-care (POC) settings, making risk assessment, diagnosis, staging, and treatment response evaluation quicker and more accurate. Third, metabolomic procedures can easily be integrated into currently existing clinical infrastructure that utilizes established protocols for a timely, reproducible, and cheap results [28,29,30,31,32]. Fourth, data analysis in metabolomics is easier to handle than those for the other omics because only a small fraction of the human metabolome is associated with key dysregulated metabolic pathways in any disease. In contrast, there are tens of thousands of genes and proteins that are potentially linked to a disease, some of which are yet to be discovered [33,34,35,36,37,38,39]. The biochemical importance of various metabolites is still unknown; although, their number is still relatively small compared to the human genome (~19,000 to 22,000) [40,41,42]. The number includes both polar and non-polar metabolites, present in large (>1 µmol/L) or small (<1 nmol/L) concentrations [6,43]. Fifth, the other omics are partially effective in evaluating cellular functions because no previously defined correlation exists between gene/protein expressions and metabolism, considering that RNA can be spliced or undergo post-translational modification [42]. For example, only a small fraction of transcriptomic alterations correlates with changes in proteomic data [44,45,46]. Even alterations in both genome and proteome are hardly reflective of a diseased cell’s phenotype. However, recent clinical evidence suggests that mutated isocitrate dehydrogenase1/2 (IDH 1/2), the enzyme that converts isocitrate to α-ketoglutarate (αKG) in the tricarboxylic acid (TCA) cycle, causes the conversion of αKG to the oncometabolite D-2-hydroxyglutarate (2-HG), which is responsible for the epigenetic inhibition and cellular differentiation [47,48]. This development establishes the first direct link between gene mutation to metabolic activity and cellular function in hematologic malignancy, providing a promising clinical opportunity for targeting the oncogenic pathway via drugs. Metabolomics is not without challenges, particularly in the use of an untargeted approach and the limiting factor of identifying unknown metabolites [49,50]. Since the approach handles small and diverse metabolic precursors with varying physical and chemical characteristics at unsteady state concentrations, it is necessary to employ sophisticated experimental designs, sample preparations, imaging techniques, and analyses to capture the series of enzyme-mediated catalytic reactions. The other omic platforms typically utilize a single tool. However, metabolomics requires multiple steps [24,50]. Thus, metabolomics is labor intensive requiring excellent techniques; although, it still produces the most meaningful results in disease etiology thus far. Metabolomics in a clinical setting supports the identification of metabolic biomarkers for cancer detection and surveillance [24]. For example, high-resolution metabolomics was used to identify the top 5, 10, and 20 metabolites from plasma using HPLC coupled with a Q-Exactive high-resolution mass spectrometer [51]. The identification and analysis of high-frequency metabolomic biomarkers with tyrosine on top were reported in a review for breast cancer [51] and a recent study by a French cohort utilized untargeted metabolomics in breast cancer to predict disease outcome [52]. Figure 2 depicts the hierarchical interrelationships among the omics.
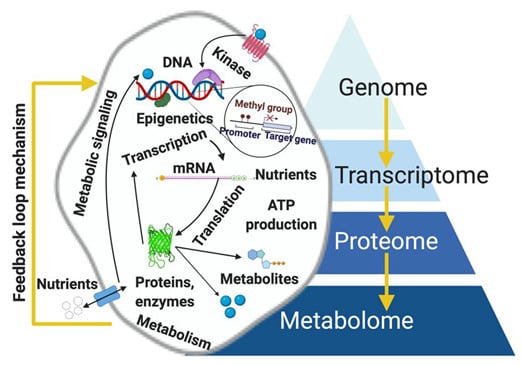
Figure 2.
Hierarchical dimension of the omics reflecting metabolome in the most downstream position, directly linking genotype to the phenotype of a diseased cell. Results of metabolomics serve as inputs for further genomic analysis (i.e., feedback loop mechanism). Figure drawn using BioRender [26].
3. Integration of Metabolomics to Other Omic Platforms
The biological activity of metabolites is a systems biology issue [53,54,55,56,57]. Combining metabolomics with other omics is attractive because the integration elucidates networks of molecular mechanisms in tumorigenesis [58,59,60], and can enhance personalized medicine [61,62,63,64,65,66,67]. For instance, using combined MS and HPLC can obtain information about individual differences in a patient’s metabolome and proteome, something that is difficult to achieve by solely using next-generation sequencing (NGS). NGS strategies as diagnostic solutions can analyze protein-coding regions associated with a patient’s disease, but it is insufficient in terms of adequately predicting temporal cellular states. The integration of all data from these omics is critical insofar as to suitably apply personalized medicine [68] because a metabolite connects a downstream target to a specific annotated gene [69]. The target in turn influences the gene to form a feedback loop mechanism [22], as shown in Figure 2. The importance of integration is seen in some very recent coupled metabolomic–genomic research [70,71,72,73]. The association of metabolites in gene expression, transcription, and translation is more significant than acting as a data sink. The activity of metabolites and associated enzymes is controlled by transcription factors such as androgen receptors (AR) [74,75] or estrogen receptors (ER) [76,77,78,79,80]. For prostate cancer, AR signaling is critical in the growth of prostate tumors given that androgen is required in de novo lipogenesis [81]. This phenomenon enables the tumors to proliferate despite androgen deprivation therapy (ADT) because they generate steroids for sustained ATP production. In other instances, gene expression is controlled by metabolites [82,83,84,85,86,87]. Metabolites are active participants in enzymatic reactions [88,89,90,91,92,93] and they control protein and cellular functions [94,95,96,97,98] so they are essential in comprehensively characterizing disease pathogenesis [99,100,101,102,103,104]. This review article focuses on the metabolomic profile of prostate cancer (PCa) and the current state of metabolomics–diverse omics integration in PCa research. In the first part, current knowledge on biochemical pathway alterations in PCa is discussed, including advances in adapting PCa metabolomics. In the second part, progress in integrating PCa metabolomics with other omics is detailed. In the last part, future directions and concluding remarks are given. To the best of our knowledge, this review article is the first of its kind to focus on metabolomic-based multi-omic data integration. The authors intend to provide researchers in the field with a comprehensive knowledge base in PCa metabolomics applications.
4. Why Focus on Metabolomics for PCa Cancer Research?
The PCa metabolome contains metabolites that reflect the human body’s reaction to tumor progression. Differentiating the PCa metabolome from the general human metabolome is critical since the complex relationships among these metabolites and how they affect PCa development is still a fairly new research area. As previously mentioned, alterations in genome, transcriptome (blueprints), and proteome (execution) do not directly reflect phenotypic changes. However, accurate identification and measurement of relevant metabolites provide functional PCa information because they are the end products of complex biochemical reactions, which can sensitively monitor any internal and external DNA damaging agents, including environmental factors [105,106]. The number of human PCa metabolites currently being researched is still very low but these few metabolites are highly specific pertinent pathways [107]. PCa metabolites can be conveniently extracted from urine, plasma, blood, and tissue. Traditional human clinical metabolic studies on PCa rely on biofluids because they are convenient and non-invasive to extract. However, researchers and clinicians are moving toward extracting tissues since they are organ specific, which reflects localized biochemical perturbations [108]. Challenges associated with tissue metabolomics, however, involve invasiveness, low patient samples for robust biomarker discovery and validation, and non-standardized protocols for various types of tissues. In terms of the separation of hydrophilic and lipophilic metabolites, whether they are extracted from biofluids or tissue, one challenge is separation and resolution efficiency. Each sample matrix is different, based on source and individual. Thus, equipment in the metabolomic analysis must be optimized, calibrated, and re-optimized to ensure clear separation of the metabolite of interest. Moreover, different mixed solvent standards must be used either as single, combined, or biphasic solvents to ensure increased levels of detection for all biofluid, tissue, and cell line samples. Healthy prostate cells rely on glucose oxidation for ATP production, and they are characterized by low citrate metabolism within the TCA cycle resulting in citrate accumulation [109,110]. Malignant transformation of prostate cells, however, activates the TCA cycle by decreasing zinc levels and the cells rely heavily on lipids for energy [111]. Unlike other cancers, PCa cells are unique in that they are not glucose dependent (non-Warburg). These cells show higher levels of metabolites including choline and sarcosine and lower levels of polyamines and citrate compared to normal prostate epithelial cells [111].
Within the last 10 years, there has been a growing number of purely PCa metabolomic studies, exploiting the various instrumental platforms. Most of these studies focused on biomarkers discovery and therapeutic target identification [1,24,37,109,110,111,112,113,114,115,116,117]. In PCa, sarcosine and choline are the primary metabolites [118]. Urea cycle metabolites such as arginosuccinate, arginine, and proline are elevated in PCa than in benign controls [119]. The study found that the oncogenic pathways HIF1α and NFκB were positively correlated with fumarate levels, inducing low survival rates. The increased plasma concentration of sphingolipids and Cav-1 also positively correlates with PCa aggressiveness [120]. The study determined that Cav-1 alters cell lipid metabolism by increasing the catabolic conversion of sphingomyelins to ceramide derivatives, elevating synthesis and efflux of glycosphingolipid indicative of altered ceramide metabolism and scavenging of exogenous sphingolipid. The landmark study by Sreekumar and colleagues in 2009, although controversial, has garnered further confirmatory studies to validate its results [121]. Although uracil, kynurenine, glycerol-3-phosphate, leucine, and proline were slightly elevated, sarcosine was singularly increased in metastatic PCa, and a localized tumor compared to BPH. An immediate validation study that has conflicting results was from Jentzmik and colleagues [122]. Post-digital rectal exam (DRE) of 106 PCa patients and 33 control patients revealed that the creatine-normalized sarcosine level was not statistically different between the two cohorts, including the absence of correlation between biopsy of prostatectomy Gleason score. Subsequent confirmatory studies ensued, without a conclusion as to the validity of Sreekumar et al., 2009, or Jentzmik et al., 2010. However, in the Cao et al. study investigating sarcosine levels in urine supernatant and sediment, the creatine- and alanine-normalized sarcosine levels were statistically higher in PCa patients than in abnormal prostate without cancer patients or healthy patients, from both sample source and normalization protein [123]. A very recent study using a PCa urine-based 1H-NMR revealed that guanidinoacetate, phenylacetylglycine, and glycine were significantly increased while L-lactate and L-alanine were substantially decreased [124]. In the 20 metabolites identified, sarcosine was not even a player in PCa after employing principal component analysis (PCA), partial least squares-differential analysis (PLS-DA), ortho-PLS-DA (OPLS-DA), and the Wilcoxon test. Another conflicting result among most of these validation studies is that the knockdown of glycine-N-methyltransferase (GNMT), the glycine-producing sarcosine enzyme, inhibits PCa cell proliferation to further abolish malignancy via G1 cell cycle arrest and apoptosis in certain allelic frequencies and ethnicities, with only a few studies finding opposite conclusions [125,126,127,128,129,130,131]. Additionally, the metabolic differences between normal prostate and PCa cells were previously thought to be caused by androgen receptors and that ADT suppresses tumorigenesis. However, the emergence of castration-resistant PCa (androgen-independent) makes androgen targeting by drugs more complicated because of the unique PCa metabolic profile, pointing to the need to identify biomarkers for cancer screening via metabolomics [24,81]. These facts show that the link between current clinical practice and unexplored gaps in using metabolomics is still elusive considering that the metabolites found in various PCa research are non-harmonized and at times contradicting. However, despite limitations and future refinements in analytical technique, metabolomics is suitable and needed in PCa research.
5. Why Merge Metabolomics with Other Omics in PCa?
Merging metabolomics with the three omics provides a more comprehensive PCa analysis [3,132,133,134]. In PCa, integrated metabolomics is utilized in two fashions: individual omics are independently adapted, and their results are co-analyzed for correlation and pattern analysis using statistical means, and multiple omics are integrated into a single model, the results of which then represent a single biological phenomenon [135]. The first case is executed using the functional study approach in which multiple independently generated omics data are plotted into a known metabolic network [106,135]. This visual representation of data is a powerful tool, but the interpretation is subject to errors and bias. Another method is to compare a priori gene ontology (GO) terms to other metabolites genes, enzymes, or proteins that are shown to have differential expressions between normal cells and PCa [135]. Within the last decade, multiple studies have come out pairing metabolomics and genomics. For instance, the overexpression of phosphorylated oncogenes AKT1 and MYC were linked with phenotypic metabolic sets associated with defined metabolic pathways [136]. Information on metabolomic profiles and matched gene expressions provide insight into the function of the gene using gene-metabolite profiles [137]. Correspondingly, metabolites can determine a particular gene target that contributes to the gene annotations [22]. However, integrated metabolomics strategy requires high-throughput computational and mathematical techniques such as Bayesian models [138,139], deep learning models [140,141], and least square models [142,143]. A detailed review of the principles of analytical integration of metabolomics and multi-omics data was made by Jendoubi et al. [132]
Recent PCa studies (2018–2021) in integrated metabolomics and genomics have employed techniques such as LC- or GC- combined with MS, fluorometric assays, and seahorse flux analysis. A study investigated arginine starvation using CWR22Rv1, PC3, and MDA-MB-231 cell lines [144]. Results revealed that deficiency in arginine synthesis (defects in PCa), performed as arginine starvation, resulted in cell death via epigenetic silencing and metabolite depletion. cGAS-STING activation also contributed to cell death. Oxidative phosphorylation, DNA repair pathway, and Type I interferon response were dysregulated, contributing to a decrease in both arginine and αKG. In a 2020 study by Kim and colleagues, withaferin (WA) treatment in 22Rv1, LNCaP, and 22Rv1 for validation employed fluorometric-based metabolomics [145]. In all cell lines, mRNA and protein levels of key fatty acid synthesis enzymes were downregulated. Suppression of a acetyl-coA carboxylase, expression of fatty acid synthase, and PCa cell survival from WA treatment resulted in the expression of c-MYC, not AKT. Glyceraldehyde-3-phosphate (GA3P) and citrate were both decreased. The metabolite-PCa causality was investigated in a study that employed genome-wide association studies (GWAS) in metabolites related to lipid, fatty acid, and amino acid metabolism [146]. Thirty-five metabolites were associated with PCa, and 14 of those were found not to have causality with PCa progression. These research studies that identified key metabolites at the genomic level can then be used as therapeutic targets or directions for further research.
Numerous integrated metabolomics and transcriptomics (2019–2021) have demonstrated the utility of a combined approach. A study in 2021 concluded that per- and polyfluoroalkyl substances (PFAS) exposure led to an increase in xenograft tumor growth and altered metabolic phenotype of PCa, particularly those associated with glucose metabolism via the Warburg effect, involving the transfer of acetyl groups into mitochondria and TCA (pyruvate) [147]. PFAS also increased PPAR signaling and histone acetylation in PCa. Using RWPE-1 and RWPE-kRAS samples and GC-MS, acetyl-coA and pyruvate dehydrogenase complex were both significantly altered. Chen and group evaluated EMT-PCa and epithelial PCa differentiation utilizing ARCaPE and ARCaPM samples in LC-MS and a glucose uptake assay analytical platform [148]. The levels of aspartate, glycolytic enzymes (except for glucose 2 transporters), pyruvate dehydrogenase kinase 1/2, pyruvate dehydrogenase 2, and glutaminase 1/2 were all increased, while succinate dehydrogenase and aconitase 2 were decreased. PCa cells undergoing epithelial–mesenchymal transition (EMT) showed low glucose consumption and glucose metabolism in ARCaPE downregulated. Glucose metabolism in transcription factor- (TF) induced EMT models was also downregulated. ARCaPM cells showed increased aspartate metabolism. The carnitine palmitoyl transferase I (CPT1A) expression was analyzed by a study using the LNCaP-C4-2 and UHPLC-MS platform [149]. Results showed that ER stress, serine biosynthesis, and lipid catabolism were all upregulated, including the overexpression of CPT1A, which showed increased SOD2 when subjected to low fatty acids and no androgen. The implication was that high lipid metabolism and low androgen response resulted in worse progression-free survival. The group of Marin de Mas et al. conducted an aldrin exposure analysis via gene–protein reaction (GPR) associations to determine the effects on carnitine shuttle and prostaglandin biosynthesis [150]. Nineteen metabolites were found to be both consuming and producing. The application of a novel stoichiometric gene–protein reaction (S-GPR) (imbedded in genome-scale metabolic models, GSMM) on the transcriptomic data of Aldrin-exposed DU145 PCa revealed increased metabolite use and production. Carnitine shuttle and prostaglandin biosynthesis were shown to be significantly altered in Aldrin-exposed DU145 PCa.
There was a total of four recent PCa investigations using integrated metabolomics and proteomics from 2019 to 2021. One of them analyzed mast cell (MC) and cancer-associated fibroblasts (CAF) in PCa tissues from prostatectomy patients [151]. Transcriptomic profiling of MCs isolated from prostate tumor region showed downregulated SAMD14 while proteomic profiling of HMC-1 demonstrated an overexpression of SAMD14. Modified SAMD14 protein was associated with immune regulation and ECM processes. The group of Blomme et al. characterized AR inhibition (ARI) using the wild, bicalutamide-, appalutamide-, and enzalutamide-resistant LNCaP cells via LTQ-OVMS, FT-MS, QEO-MS, and LC-MS [152]. 2,4-dienoyl-coA reductase (DECR1) knockout induced ER stress and stimulated CRPC cells to undergo ferroptosis. DECR1 deletion in vivo, on the other hand, inhibited lipid metabolism, and reduced CRPC tumor growth. Both glucose metabolism and fatty acid β-oxidation were altered. Li et al. analyzed the silencing of FUN14-domain-containing protein-1 (FUNDC1) in PC3, DU145, and C42B cell lines [153]. A decrease in levels of pyruvate, cis-aconitase, α-ketoglutarate, and succinate accompanied by an increase in levels of glutathione and ROS were observed. FUNDC1 was shown to affect cellular plasticity via sustaining oxidative phosphorylation, buffering ROS generation, and supporting cell proliferation. Lastly, the team of Dougan et al. conducted a knockdown of peroxidasin (PDXN) in RWPE1, DU145, PC3, 22Rv1, and LNCaP [154]. PXDN overexpression was positively correlated with PCa progression, while PXDN knockdown increased oxidative stress, ROS, and apoptosis.
6. Clinical Applications of Metabolomics in PCa
The metabolic signature of PCa is used in tumor diagnosis, staging, and continuous assessment of treatment outcomes. The fact that PCa is a metabolic disease makes it suitable for targeted therapeutics. Metabolomics opens tremendous avenues for improving clinical applications. Biomarker discovery is one of metabolomics’ clinical applications. Advances in imaging, such as magnetic resonance imaging (MRI), computed tomography, radionuclide scans, and positron emission tomography (PET), are capitalized for the accurate detection of PCa. Since PCa cells do not rely on the Warburg effect (aerobic glycolysis) like most cancer cells, they are therefore not addicted to glucose (non-glycolytic). Thus, it has low avidity to 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (FDG PET/CT) [155]. It is only in late-stage metastatic PCa does the Warburg effect manifest. Other F-labeled glucose tracers can be employed for glucose-independent PCa. During early-stage PCa, ATP is produced from lipids from androgen signaling to produce energy. In the case of ADT, they utilize de novo lipogenesis. OXPHOS is favored and aerobic glycolysis is downregulated, in contrast to other tumors wherein OXPHOS is evaded to prevent apoptosis. Such a shift is attributed to acidosis in the microenvironment (TME) [24,113,155,156]. FDG PET/CT can be used in this case. Another novel tracer in PCa diagnosis is the [18F]-fluciclovine or the anti–1-amino-3-18F-fluorocyclobutane-1-carboxylic acid [157,158]. Fluciclovine uptake by PCa cells via alanine-serine-cysteine transporter 2 differentiates non-prostatic neoplasms from metastatic PCa [157,159]. Suitable tracers can now be implemented with high diagnostic accuracy considering that this review paper detailed the metabolic differences among normal, benign, and metastatic PCa. Other than metabolic imaging, clinical samples can be directly analyzed using metabolomics. Surgically obtained samples of PCa and the surrounding normal tissues can now be compared using metabolomics. However, this method is least desirable for PCa screening and monitoring. For the purpose of PCa biomarker detection, biofluid samples are adequate. Although sarcosine was recently rejected as a valid PCa biomarker, new clinical evidence using metabolomics suggests that free amino acids such as ethanolamine, arginine, and branched-chain amino acids are potential biomarkers [160,161]. The second clinical application of metabolomics is in identifying PCa risk factors. PCa progression is rooted in oncogenic DNA mutations, such as germline mutations and somatic mutations. These DNA alterations are caused by risk factors including endogenous agents (diet, ROS, macrophage, and neutrophil) and exogenous environmental agents (radiation, metals, and chemicals). Exogenous agents directly interact with DNA while endogenous agents indirectly promote carcinogenesis by promoting TME conducive to mutation. Once damaged, the DNA causes altered metabolism through changes in chromatin accessibility, which in turn modifies the epigenetic landscape. These metabolic risk factors can be accurately determined via untargeted metabolomics in population cohort studies [24]. Lastly, metabolomics can be adapted in a clinical setting in the discovery of advanced therapeutics that target PCa metabolism. For example, a study analyzing AKT and MYC dysregulation in human normal and PCa samples revealed that dysregulation of AKT1 and MYC alters non-glucose-mediated pathways and their downstream targets [136]. Since MYC is one of the leading oncogenes in PCa development, it can serve as a potential drug target. Another study conducted on characterizing urine-enriched mRNA using BPH, PTT, normal, and PCa urine samples in UHPLC-HRMS revealed that glutamate metabolism and TCA aberration contributed to PCa phenotype via GOT1-mediated redox balance [162]. Alanine, aspartate, and glutamate metabolite levels were increased including the level of glutamic-oxaloacetic transaminase 1. GOT11 in this context is an appropriate therapeutic target. Metabolomics can also be combined with immunotherapy and single-cell sequencing to aid in the search for advanced PCa therapeutics [163]. A summary of all recent integrated metabolomic studies on cell lines and in clinical cohorts are summarized in Table 1, Table 2, Table 3 and Table 4.
7. Metabolomic Tools
The most prominent techniques in PCa metabolomics are chromatography coupled to MS (LC-MS and GC-MS) and NMR spectroscopy (mostly proton NMR, 1H-NMR) [5]. NMR is widely used in the screening of patient urine and blood plasma samples because it can be fully automated, reproducible, and metabolites are easily identified from simple one-dimensional spectra [32,164]. It does not require intensive sample preparation and separation, making it ideal to be paired with other tools [164]. However, it is difficult to quantify co-resonant metabolites and it has lower sensitivity compared to MS by up to 100-fold [27,32,165]. Regardless, NMR can detect temporal biochemical changes and monitor real-time alterations in metabolites before and after experimental treatment [32,165]. GC-MS method fractionates mixtures into metabolite components and then uses mass spectrometry to quantitate each metabolite [166]. However, it can only be used for volatile metabolites. It is cheap, reproducible, and has high sensitivity; although, sample preparation takes significant time [166,167]. An alternative to 1H-NMR and GC-MS is LC-MS, in which separation occurs in the liquid phase, which broadens its applicability. It is not time consuming and can identify and quantify hundreds of metabolites in a single extract [168,169]. However, it is costlier than GC-MS and is difficult to control potentially due to the ionization problems when in presence of other ions [168]. Separation using LC-MS can alter the metabolites’ molecular structure. Other PCa techniques include Raman spectroscopy, Fourier-transform infrared (FT-IR) spectrometry, thin-layer chromatography, and metabolite arrays [170,171,172,173].
In the subsequent sections, we will present the current state of knowledge on PCa research, utilizing metabolomics paired with genomics, transcriptomics, and proteomics. Herein, we queried PubMed using keywords such as “genomics, metabolomics, prostate cancer,” “transcriptomics, metabolomics, prostate cancer,” “proteomics, metabolomics, prostate cancer,” and “multi-omics, metabolomics, prostate cancer.” Accompanied by other database searches, we exhaustively compiled all paired and multi-omic studies employing metabolomics.
8. Metabolomics and Genomics
Heterogeneity in PCa tumors and their metastatic form makes functional impact assessment challenging [174,175]. Fundamental mutations in PCa involve tumor suppressors (inactivating mutations) and oncogenes (activating mutations) [176]. To better understand how metabolomic dysregulation and genetic alterations are related to PCa, the main drivers of PCa oncogenic activity must be elucidated: AR expression, PTEN locus mutation, p53 locus mutation, and c-MYC amplification. Detailed PCa genomic reviews were performed elsewhere [101,177]. This review focuses on paired genomic and metabolomic studies performed thus far.
AR expression. Aberrant changes in AR render it sensitive to androgen deprivation therapy (ADT) and AR pharmaco-antagonists (androgen insensitivity syndrome), two mainstream therapies in PCa [178,179]. Alterations in AR genes include point mutations and deletions. Mutations in the second zinc-finger ligand-binding domain of the AR receptor contribute to this insensitivity [176,180,181]. Repeated AR mutations have been associated with resistance to AR-targeted therapy in CRPC [176,180,181,182]. One notable tool used in analyzing AR-mediated biochemical pathways and target genes is 13C-glucose metabolic flux analysis [183,184]. In a study on AR-V7, which correlated to ADT resistance and poor prognosis, the authors intended to validate whether such resistance is caused by AR substitution or potential AR-V7-mediated downstream gene target modifications [185]. Results revealed that AR-V7 promotes PCa growth and enhances glycolysis as with AR, including high dependence on glutaminolysis and reductive carboxylation. However, confirmatory metabolomic flux assay revealed that the ensuing low citrate level in PCa is due to low consumption, not low synthesis [186]. Further, AR targets genes associated with enzymes active in aerobic respiration, fatty acid oxidation, and homeostasis [187,188,189]. Lipid metabolism is an AR-regulated pathway that affects the production of acetyl-coA and modifications in acetylation and glycosylation processes [190].
PTEN locus mutation. PTEN is a tumor suppressor, and the deletion of its gene at the 10q23 location inactivates its protein and lipid phosphatase activities. It is a regulator of the PI3KT/AKT pathway [176,191]. PTEN-deficient PCa cells such as LNCaP are targeted directly or indirectly to restore PTEN function, via the blockade of the PI3KT/AKT pathway in combination with chemotherapy and other drugs [192,193]. Subsequent studies have demonstrated a positive correlation between PTEN mutations and PCa aggressiveness [194,195]. In a recent study, PTEN loss was shown to be positively correlated with fatty acid synthetase (FASN) gene knockdown, the enzyme in de novo lipogenesis. The downregulation of both genes resulted in a decrease in stromal microinvasion [196]. Co-deletion of PTEN with other genes, such as PML1, promoted PCa tumorigenesis in mouse models and activated SREBP, a transcription factor that regulates de novo lipogenesis and adipogenesis [197].
p53 locus mutation. p53 is another tumor suppressor; mutations in its genes lead to PCa development and PCa treatment resistance [198,199]. p53 represses the expression of glucose transporters resulting in the inactivation of glycolysis and PCa cell glucose consumption. p53 expression promotes OXPHOS via the regulation of glutamine uptake via activation of glutaminase 2 (GLS2) [199,200,201]. p53 as a PCa tumor suppressor was first proven in a study linking p53 mutations in PCa cell lines and PCa primary human samples [176]. Consecutive p53 studies validated the functional role of p53 mutation, specifically via loss, on PCa progression [198,199,201]. In a recent study, phenethyl isothiocyanate (PEITC), a dietary compound, inhibits PCa cell growth by inducing apoptosis via rescuing mutant p53 in VCaP and LAPC-4 [202]. Loss in p53 is also associated with enhanced serine one-carbon glycine synthesis (SOG), responsible for DNA methylation [203].
c-MYC amplification. The proto-oncogene and regulator gene c-MYC is a transcription factor encoded by the MYC oncogene on 8q24, shown to be constitutively overexpressed in PCa [204,205,206]. Research indicates that c-MYC alters enzyme expressions associated with glycolytic pathways including HK2, PFK1, ENO1, LDHA, and GLUT1 concentrations [207]. Additionally, GLS1 and its associated transporters are regulated by c-MYC, thereby advancing glutamine metabolism [208]. Amplifying c-MYC activates the PI3K/AKT axis. A study demonstrated that in localized and metastatic PCa, there is a correlation between c-MYC amplification with PI3K-associated dysregulation, including PTEN and all AKT homologs [209]. Activities of c-MYC and AKT1 stimulate the increase in glycolytic and lipogenic-associated metabolites in all PCa cell models [210,211]. It is found that c-MYC expression is positively correlated with AR activity [212,213,214], as shown in a recent study [212]. However, in another study, c-MYC overexpression exhibited an antagonistic effect on AR activity and transcription in PCa cell lines due to both proteins co-occupying similar enhancer binding sites [215]. The AR target genes KLK3 (PSA) and GNMT were inversely correlated with c-MYC in advanced PCa [215].
In these paired approaches, genomic data preceded metabolomic data; although, it is unclear as to the time-sensitive effect of genetic aberration on downstream metabolite levels [105]. There has been an increase in metabolomic genome-wide association studies (GWAS) that seek to quantify the extent to which genetic manipulations affect metabolite levels. In humans, GWAS and exome sequencing revealed that genetic variations account for roughly 10–76% of metabolic aberrations in blood metabolome [216]. Chu et al. published an epidemiological-based multi-omic study [105] and Jendoubi et al. published a review article on metabolomics and multi-omics integration [132]. These papers focused on methodological paradigms non-specific to PCa pathology, which emphasizes computational/mathematical approaches. Our literature search within the last decade (2011–2021) resulted in 91 exclusive paired studies and was trimmed to 14 pertinent PCa studies. These are listed in Table 1.

Table 1.
Summary of genomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
Table 1.
Summary of genomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
| Reference | Experimental Condition | Sample/ n Samples | Analytical Tool for Metabolites | Altered Metabolites (+/−) | Dysregulated Metabolic Pathways | Main Findings |
|---|---|---|---|---|---|---|
| Hsu et al., 2021 [144] | Arginine starvation | Cell lines: CWR22Rv1, PC3, MDA-MB-231 | LC-MS Seahorse flux analysis | Arginine metabolites (−) α-ketoglutarate (−) | Oxidative phosphorylation DNA repair pathway Type I interferon response | Deficiency in arginine synthesis (defects in PCa), performed as arginine starvation resulted in cell death via epigenetic silencing and metabolite depletion. cGAS-STING activation contributed to cell death. |
| Cai et al., 2020 [217] | Citrate synthase (CS) down- regulation | 71 = adenocarcinoma 2 = leiomyo-sarcoma 1 = hyperplasia 6 = normal | UPHPLC-MS/MS Seahorse assay | Glyceraldehyde 3-phosphate (−) Citrate (−) | Lipid metabolism Mitochondrial function | CS expression: PCa > normal prostate. Decreased CS expression resulted in inhibited PCa proliferation, colony formation, migration, invasion, cell cycle in vitro, and low tumor growth in vivo. CS downregulation lowers lipid metabolism and mitochondrial function. |
| Kim et al., 2020 [145] | Withaferin (WA) treatment | 22Rv1 LNCaP, 22Rv1 (validation) Hi-MYC | Fluorometric assay | ATP citrase lyase, acetyl-coA carboxylase 1, fatty acid synthase, carnitine palmitoyltransferase (−) | Fatty acid synthesis | WA treatment in all cell lines downregulated mRNA and protein levels of key fatty acid synthesis enzymes. Suppression of a acetyl-coA carboxylase, expression of fatty acid synthase, and PCa cell survival from WA treatment → expression of c-MYC, not AKT. |
| Adams et al., 2018 [146] | Metabolite-PCa causality | 24,925 = GWAS metabolites 44,825 = GWAS PCa 27,904 control | Data mining and statistical analysis, no experimental tool | Lipids and lipoproteins Fatty acids and ratios Amino acids Fluids 35 metabolites association w/ PCa, 14 has no causality | Lipid metabolism Fatty acid metabolism Amino acid metabolism | 35 metabolites were associated w/ PCa, and 14 of those were found not to have causality w/ PCa progression. |
| Khodayari-Moez et al., 2018 [136] | AKT and MYC dysregulation | 60 = human PCa samples 16 = normal prostate | Data analysis, no experimental tool | Metabolites related to dysregulated metabolic pathways | D-glutamine and D-glutamate metabolism Fatty acid biosynthesis Fructose and mannose Metabolism Nitrogen metabolism Pyrimidine metabolism | Dysregulation of AKT1 and MYC alters non-glucose-mediated pathways and their downstream targets. MYC is one of the leading oncogenes in PCa development. |
| Heger et al., 2016 [128] | Sarcosine dehydro- genase (SDH) supplementation | PC3, LNCaP PCa murine xenograft (validation) | IEC | Glycine, serine, sarcosine (+) dimethylglycine and glycine-N-methyltransferase (slight +) | Sarcosine metabolism | SDH supplementation significantly increased levels of glycine, serine, and sarcosine, but slight increase in dimethylglycine and glycine-N-methyltransferase levels. PC-3 → 25, LNCaP → 32, overlapping → 18 differentially expressed genes. |
| Liu et al., 2015 [137] | Gene-metabolite association | 16 = benign 12 = PCa 14 = metasta- sized | Mathematical, no experimental tool, second-hand LC/GC-MS from Sreekumar et al. | 1353 genes 1489 metabolites | Non-applicable | Directed random walk global gene-metabolite graph (DRW-GM) = from integrated matched gene and matched metabolomic profiles →accurate evaluation of gene importance and pathway activities in PCa. Use of method in three independent datasets → accurate evaluation of risk pathways. |
| Shafi et al., 2015 [186] | Androgen receptor variant 7 (AR-V7) | LNCaP | Seahorse assay LC-MS | Glucose/fructose (−) 3-phosphoglycerate, 2-phosphoglycerate (−) Pyruvate (+) Citrate (−) α-ketoglutarate (+) Malate (−) Oxaloacetate (+) Glutamine (+) Citrate (−) | Glycolysis via extracellular acidification rate (ECAR) Glutamine metabolism via reductive carboxylation Tricarboxylic acid (TCA) cycle Glutaminolysis | AR-V7 stimulated growth, migration, and glycolysis measured by ECAR (extracellular acidification rate) similar to AR. AR → increase citrate, AR-V7 → reduce citrate mirroring metabolic shifts (castration-resistant PCa). AR-V7 is highly dependent on glutaminolysis and reductive carboxylation → produce metabolites consumed by TCA cycle. |
| Gilbert et al., 2014 [218] | SNPs of vitamin D-PCa association | 1275 = PCa 2062 = healthy controls | MS | 25-hydroxyvitamin-D (25(OH)D) 1,25-dihydroxyvitamin, (1,25(OH)2D) | 25(OH)D synthesis 25(OH)D metabolism | Vitamin D-binding protein SNPs were associated with prostate cancer. Low 25(OH)D metabolism score was associated with high grade. |
| Zecchini et al., 2014 [219] | Beta-arrestin 1 (ARB1) | C4-2 786-O | 1,2-13C2 glucose assay GC-MS | Succinate dehydrogenase Fumarate hydratase | Oxidative phosphorylation Aerobic glycolysis | ARB1 contributes to PCa metabolic shift via regulation of hypoxia-inducible factor 1A (HIF1A) transcription through regulation of succinate dehydrogenase and fumarate hydratase in normoxic conditions. ARB1 was directly linked in PCa as a promoter by altering metabolic pathways. Survival of PCa cells in harsh conditions due to ARB1. |
| Hong et al., 2013 [220] | Metabolic quantitative trait loci (mQTLs) via genome-wide association study (GWAS) | 214 = PCa 188 = control 489 = PCa (replication) | UPLC-MS w/ XCMS | Caprolactam Glycerolphosphocholine 2,6-dimethylheptanoylcarnitine Glycerolphosphocholine Bilirubin C9H14Ona Glycerophospho-N-palmitoyl ethanolamine Stearoylcarnitine Glycochenodeoxycholic acid 3-glucuronide | Fatty acid β-oxidation via acyl-CoA dehydrogenase | Seven genes (PYROXD2, FADS1, PON1, CYP4F2, UGT1A8, ACADL, and LIPC) and their variants contributed significantly to trait variance for one or more metabolites. Enrichment of 6 genes was associated w/ increased ACAD activity. mQTL SNPs and mQTL-harboring genes over-represented in GWAS → implications in PCa. |
| Poisson et al., 2012 [221] | Gene expression mapping | 402 = original 488 = replication | Statistical and mathematical, no experimental tool | Non-applicable | Non-applicable | Convert gene information to p-value weight via 4 enrichment tests and 4 weight functions. Used p weights on PCa metabolomic dataset. Disjoint pathways → higher capability to differentiate metabolites than enriched pathways. |
| Lu et al., 2011 [222] | Single-minded homolog 2 (SIM2) expression | PC3 LNCaP VCaP DU145 | LC-MS-MS | 38 dysregulated metabolites | PTEN signaling PI3K/AKT signaling Toll-like receptor signaling | Lenti-shRNA in PC3 → downregulates SIM2 gene and protein → affects key signaling and metabolic pathways. |
| Massie et al., 2011 [223] | AR regulatory effects | LNCaP | NMR 1,2-13C2 glucose assay GC-MS | Calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) | Glycolysis via activating 5’ AMP-activated protein kinase (AMPK)- phosphofructokinase (PFK) signaling | AR regulates aerobic glycolysis and anabolism in PCa. CAMKK2, a direct AR target gene, regulates downstream metabolic processes. CAMKK2 is important in androgen-dependent and castration-resistant PCa. |
1 The list is non-exhaustive, tabulated as of the writing of this review article. 2 Total of 91 queries trimmed down to 14 integrated genomic-metabolomic PCa studies.
9. Metabolomics and Transcriptomics
The PCa’s genome has limited somatic mutations, but its gene expression profiles, as recorded in the transcriptome, are varied in both localized and metastatic PCa. Integrating transcriptomic data with metabolomic data reveals levels of known and unknown metabolites indicative of genetic aberrations or protein/enzyme expression. Table 2 summarizes a comprehensive decade-long study on paired transcriptomics and metabolomics. We scoured the literature and found 17 relevant publications.

Table 2.
Summary of transcriptomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
Table 2.
Summary of transcriptomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
| Reference | Experimental Condition | Sample/ n Samples | Analytical Tool for Metabolites | Altered Metabolites (+/−) | Dysregulated Metabolic Pathways | Main Findings |
|---|---|---|---|---|---|---|
| Imir et al., 2021 [147] | Perfluoroalkyl sulfonate (PFAS) exposure | RWPE-1 RWPE-kRAS | GC-MS | Acetyl-coA Pyruvate dehydrogenase complex (PDC) | Glycolysis via Warburg effect and transfer of acetyl group into mitochondria TCA cycle Threonine and 2-oxobutanoate degradation Phosphatidylethanol-amine biosynthesis Lysine degradation Pentose phosphate pathway (PPP) | PFAS exposure led to increase in xenograft tumor growth and altered metabolic phenotype of PCa, particularly those associated w/ glucose metabolism via the Warburg effect, involving the transfer of acetyl groups into mitochondria and TCA (pyruvate). PFAS increased PPAR signaling and histone acetylation in PCa. |
| Tilborg and Saccenti 2021 [224] | Gene expression-metabolic dysregulation relationships | 14 metabolic data sets, one of those is for PCa. 7 = tissue PCa 7 = tissue normal | Statistical, no experimental tool | Out of 72 metabolites investigated in PCa, 0 significantly differentially abundant metabolites were found (padj < 0.05) | No enriched or dysregulated pathways for PCa | Topological analysis of Gaussian networks → PCa more defined by genetic networks than metabolic ones. PCa-related metabolites were not significantly altered between controls and PCa samples. |
| Wang et al., 2021 [225] | Differential metabolites between PCa and BHP | 41 = PCa 38 = BPH | GC-MS GC/Q-TOF-MS Multivariate and univariate statistical analysis | 12 metabolites (+/−) including L-serine, myo-inositol, and decanoic acid | L-serine, myo-inositol, and decanoic acid metabolism | L-serine, myo-inositol, and decanoic acid → potential biomarkers for discriminating PCa from BHP. The 3 metabolites → increased area under the curve (AUC) of cPSA and tPSA from 0.542 and 0.592 to 0.781, respectively. |
| Gómez-Cebrián et al., 2020 [226] | Dysregulated PCa metabolic pathway mapping | 73 using serum and urine | NMR | 36 metabolites (+/−) including glucose, glycine, 1-methylnicotinamide | Energy metabolism Nucleotide synthesis | 36 metabolic pathways were dysregulated in PCa based on Gleason score (GS) (low-GS (GS < 7), high-GS PCa (GS ≥ 7) groups). Levels of glucose, glycine, and 1-methylnicotinamide → significantly altered between Gleason groups. |
| Chen et al., 2020 [148] | EMT-PCa and epithelial PCa differentiation | ARCaPE ARCaPM | LC-MS Glucose uptake assay | Aspartate (+) Glycolytic enzymes (+) except for glucose 2 transporter (−) TCA cycle: pyruvate dehydrogenase kinase 1/2, pyruvate dehydrogenase 2 (+) Succinate dehydrogenase A, aconitase 2 (−) Glutaminase 1/2 (+) | Glucose uptake Aspartate metabolism Glycolysis TCA cycle Glutamine–glutamate conversion | PCa cells undergoing epithelial-mesenchymal transition (EMT) showed low glucose consumption. Glucose metabolism in ARCaPE downregulated. Glucose metabolism in transcription factor- (TF) induced EMT models downregulated. ARCaPM cells showed increased aspartate metabolism. |
| Joshi et al., 2020 [149] | Carnitine palmitoyl transferase I (CPT1A) expression | LNCaP-C4-2 | UPHLC-MS | Acyl-carnitines Mitochondrial reactive oxygen species Superoxide dismutase 2 | ER stress Serine biosynthesis Lipid catabolism Androgen response | Upregulated pathways via transcriptomic analysis → ER stress, serine biosynthesis, lipid catabolism. Overexpressed (OE) of CPT1A showed increased SOD2 when subjected to low fatty acids and no androgen → better antioxidant defense w/ CPT1A OE. High lipid metabolism, low androgen response → worse progression-free survival. |
| Lee et al., 2020 [162] | Urine-enriched mRNA characteriza-tion | Urine: 20 = BPH 11 = PTT 20 = PCa 20 = normal 65 = PCa (validation) | UHPLC-HRMS | Alanine, aspartate, and glutamate (+) Glutamic-oxaloacetic transaminase 1 (+) | 14 metabolic pathways including aminoacyl-tRNA biosynthesis TCA cycle Pyruvate metabolism Amino acid pathways | Integrated gene expression-metabolite signature analysis → glutamate metabolism and TCA aberration contributed to PCa phenotype via GOT1-mediated redox balance. |
| Marin de Mas et al., 2019 [150] | Aldrin exposure analysis via gene-protein-reactions (GPR) associations | DU145 | Dataset processing, no experimental tool | 19 metabolites, both consuming and producing | Carnitine shuttle Prostaglandin biosynthesis | The application of novel stoichiometric gene–protein reaction (S-GPR) (imbedded in genome-scale metabolic models, GSMM) on the transcriptomic data of Aldrin-exposed DU145 PCa revealed increased metabolite use/production. Carnitine shuttle and prostaglandin biosynthesis → significantly altered in Aldrin-exposed DU145 PCa. |
| Andersen et al., 2018 [227] | Differential genes and metabolites | 158 tissue samples from 43 patients | HR-MAS MRS | 23 metabolites differentially expressed between high RSG and low RSG, including spermine, taurine, scyllo-inositol, and citrate | Immunity and ECM remodeling DNA repair pathway Type I interferon signaling | High RSG (≥16%) was associated w/ PCa biochemical recurrence (BCR). These high reactive stromata → upregulated genes and metabolites involved in immune functions and ECM remodeling. |
| Shao et al., 2018 [228] | Metabolomics-RNA-seq analysis | Tissue: 21 = PCa 21 = normal 50 = PCa and normal each (validation) | GC-MS | Fumarate Malate Branched-chain amino acid (+) Glutaminase, glutamate dehydrogenase ½ (+) Pyruvate dehydrogenase (+) | TCA cycle BCAA degradation Glutamine catabolism Pyruvate catabolism | Fumarate and malate levels → highly correlated w/ Gleason score, tumor stage, and expression of genes involved in BCAA degradation. BCAA degradation, glutamine catabolism, and pyruvate catabolism replenished TCA cycle metabolites. |
| Al Khadi et al., 2017 [229] | Peripheral and transitional zone differentiation | 20 PCa patients undergoing prostatectomy | Network-based integrative analysis, no experimental tool | 23 metabolites (+) including fatty acid synthase (FC = 2.9) and ELOVL fatty acid elongase 2 (FC = 2.8) | 15 KEGG pathways including de novo lipogenesis and fatty acid β-oxidation | RNA sequencing and high-throughput metabolic analyses (non-cancerous tissue, prostatectomy patients) → genes involved in de novo lipogenesis: peripheral > transitional. Peripheral zone induced lipo-rich priming → PCa oncogenesis. |
| Sandsmark et al., 2017 [230] | CWP, NCWP, EMT evaluation | 129 1519 samples (validation) | HR-MAS MRS MRSI | Citrate (−) Spermine (−) | TCA cycle | Increased NCWP activation via Wnt5a/Fzd2 Wnt activation mode → common in PCa. NCWP activation is associated w/ high EMT expression and high Gleason score. NCWP-EMT → significant predictor of PCa metastasis and biochemical recurrence. |
| Ren et al., 2016 [231] | Paired approach for altered pathways determination | 25 = PCa and adjacent non-cancerous tissues each 51 = PCa and 16 = BHP (validation) | LC-MS TOF-MS | Sphingosine (+) Sphingosine-1-phosphate receptor 2 (−) Choline, S-adenosylhomoserine, 5- methylthioadensine, S-adenosylmethionine, Nicotinamide mononucleotide, Nicotinamide adenine dinucleotide, and Nicotinamide adenine dinucleotide phosphate (+) Adenosine, uric acid (−) | Cysteine metabolism Methionine metabolism Nicotinamide adenine dinucleotide metabolism Hexosamine biosynthesis | Cysteine, methionine, and nicotinamide adenine dinucleotide metabolisms and hexosamine biosynthesis were aberrantly altered in PCT vs. ANT. Sphingosine was able to distinguish PCa from BHP cells for patients w/ low PSA levels. The loss of sphingosine-1-phosphate receptor 2 signaling → loss of TSG (oncogenic pathway). |
| Torrano et al., 2016 [232] | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) assessment | 150 = PCa 29 = control LNCaP DU145 PC3 | LCHR-MS Stable isotope 13C-U6-glucose labeling |
PGC1α (−) PGC1β Histone deacetylase 1 |
PGC1α pathway Estrogen-related receptor α (ERRα) pathway | PGC1α was a co-regulator and inhibits PCa progression and metastasis. Its deletion in murine prostate epithelium confirmed the finding. PGC1α dictates PCa oncogenic metabolic wiring, and its tumor-suppressive ability was mediated by the ERRα pathway. |
| Zhang et al., 2016 [233] | Angelica gigas Nakai (AGN) evaluation | 5 mice per group | UHPLC-MS-MS | 11 metabolites (+) including glutathione disulfide and taurine 11 metabolites (−) including lysine, tyrosine, and lactate | Methionine-cysteine metabolism Purine metabolism Citrate metabolism | Dosing w/ AGN → detectable decursinol, little decursin decursinol angelate. |
| Cerasuolo et al., 2015 [234] | Neuro- Endocrine transdifferen-tiation | LNCaP | H-NMR, Mathematical modeling | Creatinine + phosphor-creatinine (+) Glycine (+) Proline (+) Alanine (+) Fatty acids (+) Phospholipids (+) Glutathione (+) Glutamine (+) | Glucose oxidation Arginine and proline metabolism Glycine, serine, and threonine metabolism Glutamine and glutamate metabolism Glutathione metabolism | Hormone-deprived LNCaP cells were transdifferentiated to non-malignant neuroendocrine phenotype. Initially, LNCaP cells dwindled, neuroendocrine-type cells proliferated → later, neuroendocrine-type cells sustained LNCaP cells making them androgen-independent. |
| Meller et al., 2015 [235] | Metabolites analysis | 106 = PCa | GC-MS LC-MS MRM | Malignant vs. non-malignant: 156 metabolites (+) 17 metabolites (−) Gleason score: 11 metabolites (+) 4 metabolites (−) ERG translocation: 53 metabolites (+) 17 metabolites (−) | Fatty acid β-oxidation Sphingolipids metabolism Polyamines metabolism Cholesterol metabolism | Fatty acid β-oxidation and sphingolipids metabolism were dysregulated in PCa relative to non-malignant tumors. TMPRSS-ERG translocated was positively correlated (causality) w/ metabolites from PCa samples. Advanced PCA tumors exhibited increased cholesterol metabolism → energy storage. |
1 The list is non-exhaustive, tabulated as of the writing of this review article. 2 Total of 50 queries trimmed down to 17 integrated transcriptomic–metabolomic PCa studies.
10. Metabolomics and Proteomics
The proteome’s phenotype is closest to the metabolome’s [105]. Kim et al. identified proteins encoded by 17,294 genes [236] and Schroeder estimated that there are about 80,000–400,000 since one gene can encode multiple proteins [237]. In PCa, proteomics is applied to determine proteasomal degradation and aberrant metabolic processes. Most PCa studies focused on protein profiles and protein expression aberrations resulting from localized or metastatic PCa. A proteome sample is separated into components via gel- and liquid-based approaches. The gel-based method includes gel electrophoresis while the liquid-based method involves LC or LC-MS [101]. Implementing proteomics is expensive so integrated proteomics–metabolomics study is limited in the literature compared to genomics–metabolomics or transcriptomics–metabolomics studies. However, recent mapping development of the proteome and the emergence of top-down proteomics have made its use more manageable [105]. The integration of proteomic and metabolomic data has been focused on profiling, pathway mapping, and association studies. For example, PCa versus normal prostate cell differentiation is achieved via proteomics–metabolomics. The approach analyzes dysregulation in lipid metabolism and increases in protein phosphorylation [238]. Advancement in computing enables the coupled approach to move beyond simple pathway mapping. Herein, we summarized seven integrated proteomic–metabolomic PCa studies, presented in Table 3, within the last decade (2011–2021). The list was extracted from 86 online queries from PubMed and multiple databases.

Table 3.
Summary of proteomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
Table 3.
Summary of proteomic–metabolomic integration studies for PCa within the last decade (2011–2021) 1,2.
| Reference | Experimental Condition | Sample/ n Samples | Analytical Tool for Metabolites | Altered Metabolites (+/−) | Dysregulated Metabolic Pathways | Main Findings |
|---|---|---|---|---|---|---|
| Kopylov et al., 2021 [239] | Schizophrenia-PCa association | 52 = PCa | Q-TOF MS UPLC | Cer(d18:1/14:0) 3Cholesta-3,5-dien-7-one 1α,25-dihydroxy-19-nor-22-oxavitamin D312:0 Cholesteryl ester24-hydroxy-cholesterol11-cis-RetinolElaidolinoleic acid14-hydroxy palmitic acid12-amino-dodecanoic acidL-Leucine | Sphingolipid metabolism 3 CholestanoidSteroid biosynthesisSteroid biosynthesis Bile acid biosynthesis Retinol metabolism Linoleic acid metabolismFatty acid biosynthesisFatty acid biosynthesis Valine, leucine and isoleucine degradation | Proteomic and metabolic data → input to approach employing systems biology and one-dimensional convolutional neural network (1DCNN) machine learning. Systems biology + 1DCNN → efficiently discriminate between: Unrelated pathologies = 0.90 (SCZ and oncophenotypes) Oncophenotypes/gender specific diseases = 0.93 (PCa). 1DCNN → high efficiency in PCa diagnosis. |
| Shen et al., 2021 [240] | Laser-capture-micro-dissection (LCM) androgen quantification | 16 = PCa | LC-SRM-MS | Androsterone 4 Androstenedione Dehydroepiandrosterone Testosterone | Interleukin signaling 4 IGF signaling NOTCH4 signaling Wnt signaling PDGF signaling Steroid metabolism ECM signaling, RAF/MAPK signaling by integrins | Coupled parallel LC-MS-based global proteomics and targeted metabolomics → ultrasensitive and robust quantification of androgen from low sample quantity. LC-MS-based method → robust and reliable protein quantification in LCM, including highly accurate profiling of stroma and epithelial LCM of PCa patients. |
| Teng et al., 2021 [151] | Mast cell (MC) and cancer-associated fibroblasts (CAF) profiling | PCa tissue from prostatectomy patients BPH-1 HMC-1 | SAMD14 (+) 5 | Immune signaling ECM processes | Transcriptomic profiling of MCs isolated from prostate tumor region → downregulated SAMD14. Proteomic profiling of HMC-1 → overexpression of SAMD14 → modified proteins associated w/ immune regulation and ECM processes. Add HMC-1-SAMD14+ medium to culture of (CAF + prostate epithelium) → reduced deposition and alignment of ECM generated by CAF; suppressed tumorigenic morphology of prostate epithelium. | |
| Blomme et al., 2020 [152] | Androgen receptor inhibitor (ARI)-based LNCaP characterization | LNCaP WT 6 LNCaP bicalut-res LNCaP apalut-res LNCaP enzalut-res | LTQ-OVMS FT-MS QEO-MS LC-MS | Metabolites associated w/ glucose metabolism (citrate, acetyl-coA) and lipid metabolism (+) for DECR1 overexpression Dihydroxyacetone phosphate and glycerol 3-phosphate (−) for DECR1 knockout | Glucose metabolism Fatty acid β-oxidation | 2,4-dienoyl-coA reductase (DECR1) knockout → induced ER stress, and stimulated CRPC cells to undergo ferroptosis. DECR1 deletion in vivo → inhibited lipid metabolism, and reduced CRPC tumor growth. |
| Felgueiras et al., 2020 [238] | PCa-normal prostate differentiation | Tissue: 8 = PCa 8 = normal | FT-IR | Polysaccharide and glycogen (−) Nucleic acid (+) | Lipid metabolism Protein phosphorylation | FT-IR (spectroscopic profiling) and antibody microarray (signaling proteins) → dysregulation in lipid metabolism and increased protein phosphorylation. |
| Li et al., 2020 [153] | FUN14-domain-containing protein-1 (FUNDC1) silencing | PC3 DU145 C42B | LC-MS UPHLC | AAA+ protease LonP1 Complex V (ATP synthase) TCA intermediates: pyruvate, cis-aconitase, α-ketoglutarate, succinate (−) Glutathione, ROS (+) | TCA cycle Oxidative phosphorylation | FUNDC1 affects cellular plasticity via sustaining oxidative phosphorylation, buffering ROS generation, and supporting cell proliferation. FUNDC1 expression → facilitated LonP1 proteostasis → preserved complex V function and decreased ROS generation. |
| Dougan et al., 2019 [154] | Peroxidasin (PXDN) knockdown | RWPE1 DU145 PC3 22Rv1 LNCaP | LC-MS-MS | Metabolites that prevent oxidative stress and promote nucleotide biosynthesis (−) (i.e., desirable to increase oxidative stress and decrease nucleotide biosynthesis → apoptosis of PCa cells) | Oxidative stress response Phagosome maturation Eukaryotic initiation factor 2 (eIF2) signaling Mitochondrial bioenergetics Gluconeogenesis I | Increased PXDN expression positively correlated w/ PCa progression. PXDN knockdown → increased oxidative stress and decreased nucleotide synthesis. PXDN knockdown → increased ROS → decreased cell viability, increased apoptosis. PXDN knockdown → decreased colony formation. |
1 The list is non-exhaustive, tabulated as of the writing of this review article. 2 Total of 86 queries trimmed down to 7 integrated proteomic–metabolomic PCa studies. 3 Altered metabolite indicates corresponding dysregulated metabolic pathway. 4 Enumerated metabolites are presented for quantification purposes using the coupled parallel LC-MS-based global proteomics and targeted metabolomics of LCM. The associated potential biochemical pathways are also listed. These pathways are not dysregulated since there are no experimental conditions applied. 5 Tumor-suppressor gene whose protein counterpart potentially induces regulation in immune signaling and ECM processes. 6 LCaP cell lines: LNCaP WT = LNCaP wild type; LNCaP bicalut-res = LNCaP bicalutamide-resistant; LNCaP apalut-res = LNCaP apalutamide-resistant; LNCaP enzalut-res = LNCaP enzalutamide-resistant.
11. Integrated Omic Analysis
Thus far, there are numerous studies combining multiple types of omic approaches and data within the last decade; however, there are few investigations in the literature that have employed metabolomics with other multiple omics. The excellent review by Zhang et al. showed PCa studies with few metabolomic-based omic combinations [177]. An example of a three-tier approach was performed by Oberhuber et al., in which they analyzed the effects of the expression of the signal transducer and activator of transcription 3 (STAT3) on PCa tumor growth, metabolite level, and PCa-associated metabolic pathways [241]. With transcriptomics, the group determined that high STAT3 expression corresponded to downregulation in OXPHOS. Similarly, proteomics revealed that STAT3 expression inhibits OXPHOS-TCA cycle activity. Nonetheless, the upregulation of pyruvate dehydrogenase kinase 4 (PDK4), an enzyme that lowers metabolism by inhibiting pyruvate-to-acetyl-coA conversion, resulted in the suppression of tumor growth [241]. These and other metabolomic-based multi-omic integration PCa studies are summarized in Table 4. It is important to note that omic science has expanded into new forms including epigenomics, lipidomics, volatilomics, and phosphoproteomics.

Table 4.
Summary of metabolomic-based multi-omic integration studies for PCa within the last decade (2011–2021) 1,2.
Table 4.
Summary of metabolomic-based multi-omic integration studies for PCa within the last decade (2011–2021) 1,2.
| Reference | Experimental Condition | Sample/ n Samples | Analytical Tool | Altered Metabolites (+/−) | dysregulated Metabolic Pathways | Combined Modality/Main Findings |
|---|---|---|---|---|---|---|
| Kiebish et al., 2020 [100] | PCa prognostic markers identification | 382 pre-surgical serum samples from PCa patients 267 = training set (validation) 115 = testing set (validation) | MS-MS HILC-MS LC-MS GC-TOF-MS | 1-methyladenosine (+) | Cholesterol metabolism | Proteomics + Lipidomics + Metabolomics: Linear regression + Bayesian method + multi-omics → Tenascin C (TNC) and Apolipoprotein A1V (Apo-AIV), 1-Methyladenosine (1-MA), and phosphatidic acid (PA) 18:0–22:0, AUC = 0.78 (OR (95% CI) = 6.56 (2.98–14.40), P < 0.05) → high differentiating ability w/ and w/o BCR. |
| Oberhuber et al., 2020 [241] | Signal transducer and activator of transcription 3 (STAT3) expression | 84 = PCa from prostatectomy patients | LC-MS-MS LC-HRMS | Pyruvate dehydrogenase kinase 4 (+) | Oxidative phosphorylation TCA cycle Pyruvate oxidation | Transcriptomics + Proteomics + Metabolomics: High STAT3 expression → OXPHOS downregulated (Transcriptomics). High STAT3 expression → TCA cycle/OXPHOS downregulated (Proteomics). High PDK4 expression → inhibited PCa tumor growth. |
| Itkonen et al., 2019 [242] | Cyclin-dependent kinase 9 (CDK9) inhibition | LNCaP PC3 | Seahorse metabolic flux analysis | Acyl-carnitines (+) | Oxidative phosphorylation ATP synthesis AMP-activated protein kinase (AMPK) phosphorylation | Lipidomics + Fluxomics + Metabolomics: CDK9 inhibition → acute metabolic stress in PCa cells. CDK9 inhibition → downregulated oxidative phosphorylation, ATP depletion, and sustained AMPK phosphorylation. CDK9 inhibition → increased levels of acyl-carnitines |
| Gao et al., 2019 [243] | LASCPC-01 and LNCaP differentiation | LASCPC-01 LNCaP | GC-TOF-MS LC-MS | 25 metabolites altered from control Carnitine (−) | Glycolysis One-carbon metabolism | Transcriptomics + Lipidomics + Metabolomics: 62 genes upregulated in LSCPC-01, 112 genes upregulated in LNCaP (Transcriptomics). 25 genes significantly altered from control (Lipidomics + Metabolomics). LASCPC-01: high glycolytic rate, low-level triglycerides. LNCaP: high 1C metabolism rate, low carnitine. |
| Kregel et al., 2019 [244] | Bromodomain/ extraterminal (BET)- containing proteins (BRD2/3/4) inhibitor analysis | 22RV1 LNCaP VCaP PC3 DU145 | LC-MS | Polyunsaturated fatty acids (+) Thioredoxin-interacting protein Interferon regulatory transcription factor (−) | Cyclin-dependent kinase 9 inhibition CDK9 hyperphosporylation Polycomb repressive complex 2 activity | Proteomics + Lipidomics + Metabolomics: BET inhibitors: affected AR+ PCa (22RV1, LNCaP, VCaP) more than AR- PCa (PC3, DU145). BET inhibitors → disrupted AR and MYC signaling at concentrations: (BET) < (BET inhibitors) (Proteomics). |
| Zadra et al., 2019 [245] | Fatty acid synthase (FASN) suppression via IPI-9119 | LNCaP 22RV1 HeK293T RWPE-1 | UPLC-MS-MS LC-MS GC-MS 14C-labeling | 91 of the 418 metabolites modulated Malonyl-coA carnitine (+) Carnitine palmitoyltransferase 1 (−) | De novo fatty acid synthesis and neutral lipid accumulation ER stress response signaling Amino acid synthesis TCA cycle Carbohydrate metabolism Nucleotide metabolism | Lipidomics + Metabolomics: IPI-9119, a selective inhibitor of FASN altered the PCa metabolome by inhibiting fatty acid oxidation via accumulating malonyl-coA carnitine. Malonyl-coA carnitine accumulation → inhibited carnitine palmitoyltransferase 1 → FAO suppression. FA synthesis suppression → inhibited AR and AR-V7 expression. IPI-9119 → induced ER stress, inhibited AR/AR-V7 translation. |
| Murphy et al., 2018 [246] | PCa biomarker identification | 158 = PCa prostatectomy patients | LC-MS-MS Statistical modeling | 13 glycosylation metabolites (+) including tetraantennary tetrasialylated structures and A3G3S3 | Glycosylation | Genomics + Transcriptomics + Proteomics +Lipidomics + Metabolomics: Integration of data across 5 omic platforms from tissue and serum → single AUC value that better differentiates aggressive PCa from the indolent type compared to AUCs obtained from single omics. |
| Hansen et al., 2016 [247] | TMPRSS2-ERG expression | 129 = PCa samples from 41 patients 40 = PCa samples from 40 patients | HR-MAS-MRSI | Out of 23 metabolites, citrate and spermine (−) | TCA cycle Nucleic acid synthesis Citrate metabolism Polyamines metabolism | Transcriptomics + Metabolomics: ERGhigh = low citrate and spermine concentrations → increased PCa aggressiveness (Metabolomics). Metabolomic alterations for ERGhigh vs. ERGlow → more pronounced in low Gleason samples → implication: potential risk stratification tool. |
1 The list is non-exhaustive, tabulated as of the writing of this review article. 2 Total of 82 queries trimmed down to 8 metabolomic-based integrated multi-omic PCa studies.
12. Metabolomic Profile of Prostate Cancer
In the U.S., PCa incidence and mortality is around 270,000 and 35,000, respectively, by 2022 [248]. It is the second leading cancer death in American men and the fifth leading cancer death among men worldwide [109,248,249,250]. PCa cells undergo substantial metabolic changes that define their unique phenotype [110,251]. The primary driver of PCa development is genetic alterations, but neoplastic transformations can occur, which further supplies energy to tumors [1,111,252,253,254,255,256,257,258,259]. Metabolic reprogramming is one of the hallmarks of PCa development [260,261,262,263,264]. PCa cells, unlike other cancers, do not depend on aerobic glycolysis for ATP production [81,265]. Instead, they obtain energy primarily from lipids via the activation of the TCA cycle [188,261,266]. Only in advanced metastatic PCa do cells favor lactate production in the presence of oxygen [267,268,269]. Although PCa cells do not exhibit the Warburg effect, they still produce lactate, which aids in immune escape, cell mobility, angiogenesis, and PCa development [270,271]. In normal prostate, citrate is accumulated [81] with glucose as the main source of energy [272]. In PCa, citrate is decreased [273]. The decrease in citrate lowers NADH production [81,273]. As a result, PCa cells produce energy less efficiently [274,275,276,277,278]. The accretion of zinc in normal prostate inhibits m-aconitase (m-ACO), the enzyme that catalyzes the isomerization of citrate to isocitrate in the TCA cycle [81,273]. Zinc is key in prostate malignancy since it dictates the tumor’s metabolic and energy consumption preference, growth and proliferation, and invasiveness. Simultaneously reducing citrate levels and preventing zinc accumulation drives PCa progression and metastasis [273,279,280]. PCa tissues have low levels of spermine in the prostatic fluid [279,280], contributing to their aggressiveness [281,282]. They are characterized by high levels of taurine [1,283,284], choline [285,286,287], sarcosine [121,288], myo-inositol [1,283,284], and pyruvate kinase M2 [1,283,284]. Androgen is the primary driver of PCa via AR signaling. Non-metastatic PCa is androgen dependent, with AR affecting the one-carbon metabolism and other transcription factors in PCa-related catabolic pathways [289]. Metastatic PCa is androgen independent, able to resist ADT by switching from one steroid receptor to another [290,291]. Glucocorticoids are often used in conjunction with antiandrogen agents and their effects are dependent on glucocorticoid receptors (GR) [290,292]. Research efforts have aimed at increasing glucocorticoid metabolism and GR responsiveness via hexose-6-phosphate dehydrogenase as a means of reversing metastatic PCa cells’ resistance to ADT [290]. In the succeeding sections, the canonical pathways associated with PCa progression are discussed: glycolysis, OXPHOS via the TCA cycle, de novo lipogenesis, and glycogenesis/glycogenolysis. Pentose phosphate pathway (PPP) and amino acid metabolism are included as non-canonical pathways. The metabolic profile between normal prostate and PCa cells is shown in Figure 3.
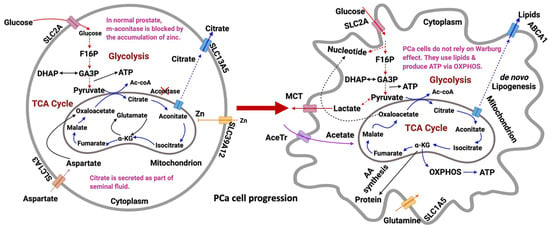
Figure 3.
Metabolic profile of epithelial prostate cell during tumorigenesis. In the normal type (left), zinc inactivates m-aconitase (ACO), which accumulates citrate to prostatic fluid. In the malignant type, cells do not rely on the Warburg effect; although, they produce lactate. Instead, they consume lipids (generated via de novo lipogenesis), activate the TCA cycle, and stimulate OXPHOS for ATP generation. Enhanced glutamine metabolism and acetate consumption were also observed in PCa cells. Dashed lines indicate abridged pathways, and solid lines indicate direct pathways. Transporters for each species are indicated. Figure drawn using BioRender [26].
12.1. Glycolysis
The metabolism of healthy prostate epithelial cells and acinar epithelial cells are regulated by glycolysis [293,294]. In normal prostate, pyruvate in cytosol enters the mitochondria to be converted into acetyl-coA. Because glucose oxidation is incomplete in normal prostate, the bioenergetic balance is lower than the glycolysis–TCA tandem. Citrate accumulates in normal prostate due to the action of zinc, which inhibits m-ACO [113,114,295,296]. In essence, m-ACO compromises TCA, lowers citrate oxidation, and amasses citrate (produced from glucose and aspartate) in mitochondria, cytosol, and prostatic fluid. To sustain the energy requirement of the compromised aerobic respiration, non-essential biochemical pathways are limited [189]. However, in PCa, glycolysis is upregulated and reprogrammed, providing ATP energy for tumor proliferation [297,298]. Early-stage PCa limits glycolysis but stimulates enhanced OXPHOS [113]. Nonetheless, when it becomes metastatic and castration resistant, glycolysis is reinforced, including de novo lipogenesis, [299] amino acid metabolism, and nucleic acid synthesis [300]. Both benign and metastatic PCa cells exhibit some form of Warburg effect because ATP comes from aerobic glycolysis, not OXPHOS [267,301]. In fact, early-stage PCa cells derive their ATPs from lipids and other biomolecules, and when the cells have metastasized into late-stage PCa cells, they become wholly glycolytic.
Under anaerobic conditions, glycolysis is favored, and very little pyruvate is presented to the aerobic mitochondria [267,294]. Regardless of oxygen availability, PCa cells favor glycolysis [114,267,271,294]. The Warburg effect was initially associated with dysfunction in mitochondria but is now associated with the cell’s quick consumption of glucose, even for those pathways that are outside of mitochondria [302]. Because of the disregard for OXPHOS, PCa cells produce less ATP, but they efficiently convert glucose into lipids, amino acids, or nucleotides [303]. Glycolysis is regulated by AMP-activated protein kinase (AMPK) [304], which in turn activates the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) [305]. Mouse models have revealed that PI3K/AKT/mTOR signaling pathways cause PTEN-deprived tumorigenesis in PCa [297,306,307,308]. This loss in PTEN results in the activation of pyruvate kinase M-2 (PKM-2), a key enzyme in aerobic glycolysis [309]. Another correlation exists between PTEN/p53 loss and elevated levels of hexokinases (HK2). The increase in HK2 has been attributed to the deletion of PTEN and p53 tumor suppressor genes in mouse models [310,311,312]. PTEN loss is associated with the activation of the AKT/mTORC1/4EBP1 signaling pathway [297,306,307,308], while p53 deletion is caused by the inhibition of miR143 synthesis [313,314,315,316]. PTEN/p53-mediated HK2 overexpression drives aerobic glycolysis, which promotes PCa metastasis. Another gene implicated in PCa cells’ survival is 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4). The gene has demonstrated control over glycolysis and its associated mRNA; it is higher in metastatic PCa than in the localized version [317].
12.2. OXPHOS via the TCA cycle
Like glycolysis, AMPK controls the TCA cycle and is triggered when there is not enough ATP produced (e.g., high levels of AMP/ADP) [305]. It is a heterotrimeric protein encoded by the 5′-AMP-activated protein kinase gene (PRKA). AMPK protects cells from ATP decrease by regulating ATP consumption pathways. AMPK1 controls PCa oncogenes with its association with PI3K, mTOR, and MAPK pathways [318]. Activation results in reducing anabolic processes to limit energy use; however, AMPK controls lipid homeostasis [319] and mitochondrial homeostasis [320].
Acetyl-coA is produced in the cytosol from the β-oxidation of free fatty acids, oxidation of pyruvate, deamination and oxidation of amino acids, and oxidation of ketone (acetoacetate and β-hydroxybutyrate) [109,321,322,323]. Although the Warburg effect is crucial to PCa, OXPHOS via TCA provides additional energy in tumorigenesis. The normal and benign prostate epithelium promotes citrate synthesis over citrate oxidation [324]. In PCa, zinc is lost, m-ACO activity is enhanced, and citrate oxidation is activated [298]. The process ensures efficient and fast ATP consumption [325,326]. Rapid energy consumption guarantees PCa cells survival despite the limited availability of acetyl co-A. The production of oxaloacetate is also elevated to ensure sustained citrate oxidation [327]. For both upregulated glycolysis and the TCA cycle, the levels of glucose, lactate, and citrate are monitored using 13C isotope labeling metabolomics.
There are two zinc transporters relevant to PCa: SLC39 protein (Zrt- and Irt-like proteins ZIP) and SLC30 protein (ZnT) [328]. ZIP increases zinc levels in the cytoplasm by importing extracellular and vesicular zinc, while ZnT exports zinc out of the cell of moves them into mitochondria or lysosomes [328,329,330]. ZIP1-ZIP4 proteins have been shown to be downregulated in PCa [331,332]. ZIP1 (encoded by SLC39A1) was found to be absent in the TRAMP PCa model and was lower in RWPE2 human tumorigenic cells compared to RWPE2 non-tumorigenic cells [298]. ZIP1 was shown as the major zinc transporter because it is expressed in LNCaP and PC-3 cell lines, proving that its absence in some PCa studies is not due to mutation but rather transport [328]. ZIP2 (encoded by SLC39A2) reabsorbs zinc from prostatic fluid and is shown to be significantly downregulated in PCa compared to normal or benign prostate [331,333,334]. ZIP3 (encoded by SLC39A3) acts similarly to ZIP2; its protein expression changes with zinc status, but its mRNA expression is unchanged indicating post-translational modification [328,332]. Mutations in SLC39A4 gene-encoding ZIP4 were shown to be related to acrodermatitis enteropathica and its expression is decreased [328,335,336]. The knockdown of both ZIP1 and ZIP4 contributes to cell invasiveness [332]. Similarly, the knockdown of ZnT-1 as per their function, increases cell proliferation [337,338]. High levels of zinc were shown to induce apoptosis because zinc activates caspase-9, caspase-3, the release of cytochrome c from mitochondria, and the cleavage of poly(ADP-ribose) polymerase [339]. Low levels of zinc, on the other hand, reduce p53 and p21 concentrations in the nucleus and have been connected to high levels of PKB/AKT and Mdm2 phosphorylation. The importance of zinc in the TCA cycle is further emphasized by studies that reduce PCa invasiveness by inhibiting aminopeptidase N activity [340]. Reducing zinc in PCa cells elevated the expression of cytokines responsible for metastasis [337,338]. Zinc inhibits the activity of NF-kB, a transcription factor that regulates genes associated with PCa metastasis as well as reducing expressions of MMP-9, IL-6, IL-8, and VEGF genes [337,341]. Besides m-ACO, high-throughput mass spectrometry has revealed high levels of TCA enzymes such as citrate synthase, fumarase, and malate dehydrogenase in PCa cells [342].
12.3. De Novo Lipogenesis
Apart from serving as energy storage and directing intracellular signaling, lipids guide tumorigenesis because alterations in lipid or choline metabolites have ramifications in PCa cell proliferation [273,343]. Cholesterol inside the lipid droplets found in the cytosol of PTEN-deprived PCa cells proves the relationship between tumor development and lipid metabolism [344]. A major metabolic reprogramming in PCa cells is the upregulation of lipid synthesis for cell membrane formation, cell signaling, and cellular proliferation [114]. Early-stage PCa is characterized by the expression of lipogenic enzymes, but late-stage aggressive PCa shows the buildup of phospholipids (phosphatidylcholine), cholesterol esters, and triglycerides [273]. The letter type can also ingest exogenous lipids for synthesis. It is also observed that in late-stage metastatic PCa, acetyl-CoA is produced from acetate using acetyl-CoA synthetase 2 instead of being generated from glucose and glutamine [343].
Fatty acids. Studies show that the generated fatty acids are deposited in PCa cells [345]. However, no accumulation of lipids is observed despite an increase in de novo lipogenesis [346]. This may be due to the equilibrium between lipogenesis (cell membrane synthesis) and lipid oxidation (energy for survival and growth), wherein the elevated rate of fatty acid synthesis supplies energy to PCa cells while concurrently oxidizing lipids [113,278]. Evidence of such equilibrium in PCa can be seen by the overexpression of α-methylacyl-CoA racemase (AMCR), an enzyme that catalyzes lipid oxidation [345]. PCa is characterized by the presence of PRKAB1 and PFKFB4 genes required for cell proliferation, proving that like glycolysis and the TCA cycle, lipogenesis is AMPK regulated [317]. More proof of lipogenesis reprogramming in PCa is the high levels of phosphocholine, phosphoethanolamine, and glycerophosphocholine, responsible for cell membrane reconstruction and cell proliferation [347]. Lipogenic enzymes are increased in PCa due to the activation of the oncogenic-signaling pathway PI3K/AKT [277] while fatty acid enzymes are also elevated due to nuclear localization of AKT [348]. AR regulates fatty acids and cholesterol synthesis enzymes used in lipogenesis [323,349,350]. However, the elevated expression of a lipogenesis transcription factor sterol regulatory element-binding protein-1 (SREBP-1) in PCa alters the expression of fatty acid synthase/fatty acids by serving as a transcription factor for AR in a feedback loop fashion [351]. SREBP-1 further activates lipogenesis by increasing the production of reactive oxygen species (ROS) and NADPH oxidase 5—two species that promote PCa cell proliferation [352]. Fatty acid synthase also reprograms androgen-dependent and castration-resistant AR+ PCa models; thus, it can serve as a target that can potentially affect tumor aggressiveness [353].
Cholesterol. The increase in cholesterol synthesis, especially in the PCa cell membrane, is accompanied by high levels of choline and creatine [354]. PCa growth is mediated by AR, which can be addressed via AR antagonists such as enzalutamide. In PCa, cholesterol homeostasis is perturbed, and lipogenesis is upregulated. Studies suggest that a high level of circulating cholesterol and active cholesteryl ester synthesis in the blood increases the risk for PCa development [355,356,357,358,359]; although, some studies indicate that lower LDL and lower total cholesterol are associated with PCa at the time of diagnosis [360]. Recent evidence suggests that modulation of cholesterol metabolism or inhibition of its biosynthesis enzymes potentially suppresses tumor proliferation and metastasis [358,361,362,363,364]. Correspondingly, cholesterol esterification enzyme sterol-o-acyl transferases (SOAT) 1/2 or acyl-coenzyme A: cholesterol acyltransferase (ACAT) 1/2 are associated with PCa proliferation and invasion [359,365,366,367,368]. The homeostasis transcription factor SREBP 1/2 accumulates cellular cholesterol by increasing uptake and synthesis while the liver X (LXR) receptor promotes efflux [369,370]. SREBP increases ROS generation and NADPH oxidase overexpression causing PCa cells’ invasion and proliferation [352]. Blocking the SREBP-regulated metabolic pathway using statins has shown anti-tumor activity and, consequently, lowers AR signaling, which also controls cholesterol enzyme synthesis [371,372]. The downregulation of SREBP mediated by the inactivation of the PI3K/AKT/mTOR pathway (i.e., increase PTEN signaling) inhibits cholesteryl ester accumulation and aberrant SREBP-dependent lipogenesis [344,373,374,375]. The activation of the PI3K/AKT signaling pathway activates MDM2 (inhibitor of tumor suppressor p53), inhibits apoptotic genes BAX and GSK3, downregulates cell survival gene BAD, and inhibits cell cycle progression genes p21 and p27 [376]. An example strategy consisting of coordinated lipogenesis and AR signaling blockade is the use of fatostatin, which not only inhibited cholesterol biosynthesis but also caused G2-M cell cycle arrest and apoptosis [245,377,378]. The inverse relationship between statin use and PCa antitumor action is exemplified by several studies [363,379,380,381,382,383], which target major oncogenic/metabolic pathways such as AR-AKT complex and molecular mediators such as MK167 and cMYC [380]. Synergism between PI3K/AKT/mTOR dysregulation and PTEN-p53 inhibition in lipogenesis causes the Warburg effect and promotes PCa aggressiveness.
12.4. Glycogenesis/Glycogenolysis
Specific to PCa, a study added R1881 to androgen dependent PC3 cell lines expressing AR (i.e., androgen abscission), determining that in 5 days, cells were reduced (via G1 cell cycle arrest) and glycogen content was increased up to five times [384]. In addition, G6P was increased three times and both GS and GP were increased two times, providing evidence of enhanced glycogenesis. Moreover, glycogenolysis was inhibited by subjecting LNCaP cells to GP inhibitor CP-91149 and further validated that cell growth was curtailed [384]. This combined approach in targeting the glycogenesis pathway has since proven an efficacious PCa therapy. The metabolic reprogramming effects of glycogenesis in PCa were validated in another study; although, the authors employed CCL39 lung fibroblasts [385]. The authors showed that under low O2 levels, HF1/2 induced glycogenesis, as evidenced by increased glycogen stores and increased PGM1′s mRNA and protein levels. The generated glycogen served as feed to glucose-starved cells (hypoxia preconditioned cells), allowing them to survive via glycogenolysis (i.e., glycogen as glucose substitute) [385]. Such results parallel the Schnier study in that in order to combat PCa cell growth, invasion, and proliferation, glycogenolysis must be terminated through pharmacologic targeting of its intermediates and enzymes. Further, the approach opens a potential to also halt glycogenesis by AR deprivation therapy, which invariably stops glycogen as an alternative food.
12.5. Pentose Phosphate Pathway
PPP is a parallel glucose-degrading mechanism to glycolysis, with an interlink through fructose-6-phosphate (F6P) and GA3P [386]. The interlink with glycolysis is seen after isomerization of ribulose-5-phosphate (R5P) using transketolase and transaldolase. PPP is controlled by G6PDH wherein studies have indicated that this enzyme is increased in PCa [387,388,389,390,391]. G6PDH, NAPDH, and ribose synthesis were all upregulated in PCa through the action of AR signaling [343,388]. Further, upregulation of G6PDH through mTOR increased AR flux within PPP, as evidenced by the removal of the G6PDH-AR regulation mechanism following rapamycin treatment. While PPP’s role in PCa is only beginning to be understood, the results demonstrate its significant role in tumorigenesis [388].
12.6. Amino Acid Metabolism
Recent investigations have elucidated the role of amino acids in cancer metabolism [160]. Because the basis for amino acid metabolism is the generation of intermediates for the synthesis of nucleobases required for growing cells [392], depriving PCa cells with these intermediates can serve as PCa therapy [393]. Amino acids, like glucose, also fuel PCa progression. Glutamine, for example, is an important amino acid in human plasma shown to be associated with PCa [81]. It has an anaplerotic function in the human metabolism because it supports the TCA cycle by being transformed into glutamate and then to the intermediate α-ketoglutarate (glutaminolysis) [81,110,160,268]. PCa cells proliferate by maintaining glutamine metabolism through upregulating the glutamine transporter ASCT2 (encoded by the gene SLC1A5) and glutaminase, the enzyme in glutamine-glutamate conversion [394,395]. Glutamine in PCa is also responsible for acetyl-coA production; nitrogen donor for protein, nucleotide, lipid synthesis, and lipogenesis [110,113]. Glutamate is used in glutathione synthesis, which protects the cell from stress and PCa cell oxidation [396]. The two-prong role of glutamine in sustaining lipogenesis and glutaminolysis in PCa is highlighted in studies where both glutamine and the glutaminase transporter are overexpressed in tumor cells [397,398,399]. Whereas citrate is generated from OXPHOS via the TCA cycle, the same citrate is produced from α-ketoglutarate via the reverse TCA cycle (reductive carboxylation) [294,400]. This process supports the pathogenesis of PCa and hypoxia-inducible factor 1 (HIF-1) regulatory pathway because the glucose is rechanneled to the acetyl-coA pathway by the influx of glutamine [294]. α-ketoglutarate transformation (with CO2) essentially redirects the TCA cycle by producing isocitrate and citrate. The resulting citrate is transported into the cytosol, part of which is converted to acetyl-coA to support lipogenesis in PCa. The other part is then recycled as isocitrate in the TCA cycle [400]. The lactate (and some pyruvate) generated from reductive decarboxylation are consumed by PCa cells for their proliferation and anabolism [109].
Other crucial amino acids in the pathogenesis of PCa are serine (2-amino-3-hydroxypropanoic acid), glycine (aminoethanoic acid), proline (pyrrolidine-2-carboxylic acid), arginine (2-amino-5-guanidinopentanoic acid), leucine (2-amino-4-methylpentanoic acid), and sarcosine [(2-methylamino)acetic acid], among others [300]. Similarly, glutamine and proline are produced by PCa cells from arginine. While arginine is attributed to nitric oxide (NO) production, PCa cells appear to have lost their ability to synthesize arginine due to a deficiency in arginine synthetase [401,402]. Proline is also an important amino acid in that it maintains the level of pyridine nucleotides. Proline biosynthesis and its accompanying enzyme levels promote cancer cell growth, plasticity, and heterogeneity [403]. Another crucial amino acid in PCa is sarcosine, which was previously reported to be elevated in urine samples of PCa patients [121]. Sarcosine is an intermediate in glycine synthesis, produced from choline and methionine metabolism, and an essential component of glutathione, creatine, purines, and serine [404]. The sarcosine-glycine-methionine pathways promote purines and thymidylates synthesis, molecules that are essential in DNA synthesis and repair [405]. Amino acid synthesis in PCa tumor TME is also regulated by the AKT/mTORC1/4EBP1 signal transduction axis, which simultaneously loses PTEN and p53, resulting in HK2-mediated aerobic glycolysis—an event favorable to PCa proliferation, as seen in mouse models [113].
PCa cells are shown to increase the uptake of amino acids [160]. These amino acids are transported across cell membranes using mostly non-specific hydrophilic transporters. The most recognizable neutral and cationic amino acid transporter is the Na+- and Cl−-dependent SLC6A14. The L-type amino acid transporter 1 (LAT1, encoded by the SLC7A5 gene) is an antiporter, which imports branched-chain/high-molecular-weight amino acids (e.g., histidine, methionine, and phenylalanine) and thyroid hormones into the cells and exports glutamine and other essential amino acids [160]. SLC7A5 was shown in studies to be overexpressed in PCa cells [406,407]. LAT1 in PCa has a high affinity to leucine and it activates the mTOR signaling pathway [408,409]; thus, its inhibition results in tumor suppression. The dynamics between LAT1 and ASCT2 in PCa enable glutamine to enter the cytoplasm via ASCT2, glutamine to activate tumor-inducing pathways (i.e., glycolysis, TCA cycle), glutamine to leave the cytoplasm via LAT1, and leucine to enter the cytoplasm via LAT1 [160]. To summarize, Figure 4 presents an overview of the four dysregulated canonical pathways in PCa: glycolysis (Figure 4a), TCA cycle (Figure 4b), de novo lipogenesis (Figure 4c), and glycogenesis/glycogenolysis (Figure 4d).
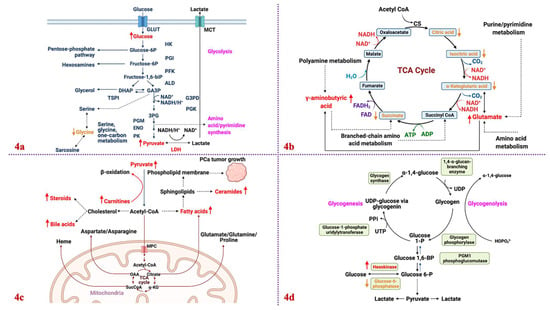
Figure 4.
Schematic overview of the four canonical pathways dysregulated in human PCa tumorigenesis. Biofluid samples are extracted and analyzed to reflect changes in metabolites and enzymes for PCa biomarker discovery: Glycolysis (a), TCA cycle (b), de novo lipogenesis (c), and glycogenesis/glycogenolysis (d). Red font = increased metabolites/upregulated enzymes; Orange = decreased metabolites/downregulated enzymes. MCT = monocarboxylate transporter; HK = hexokinase; PGI = phosphoglucose isomerase; PFK = phosphofructokinase; ALD = aldolase; DHAP = dihydroxyacetone phosphate; GA3P = gyceraldehyde-3-phosphate; GA3PD = gyceraldehyde-3-phosphate dehydrogenase; PGK = phosphoglycerate kinase; 3PG = 3-phosphoglycerate; PGM = phosphoglyceromutase; ENO = enolase; PK = pyruvate kinase; LDH = lactate dehydrogenase; CS = citrate synthase; OAA = oxaloacetate; UDP = uridine diphosphate; UTP = uridine triphosphate. Figure drawn using BioRender [26].
13. Conclusions and Future Perspectives
The benefit of integrating metabolomics with other omics is possible with the advancement in metabolite quantification and imaging, allowing the discovery of clinically relevant biomarkers for precision medicine. The elegance of a multi-omic approach is its ability to elucidate multi-level real-time molecular interactions that reflect complex biochemical pathways and potential dysregulations. The approach is practical and has generated a tremendous amount of information within the last decade crucial to understanding PCa pathology. However, considerations must be made to effectively adapt an integrated metabolomics technique in a POC setting. First, while PCa genotyping and metabolic measurements are sufficiently robust to be translated into health care facilities, transcriptomics and proteomics still require solid quantification assays. Second, because the different omics develop at different rates, there needs to be data integration and harmonization from various domains. The use of a uniform ontology allows for a streamlined integration and interpretation of PCa omics data where they can be used for validation studies. Such integrated data must be high-quality with a high level of granularity and stored in a publicly available repository/databases. Third, one of the challenges in the PCa community, including other cancers, is the challenge of risk stratification based on survival results and clinicopathological indicators during PCa’s onset. This can be addressed by developing effective and precise therapeutic targets and biomarkers, which can only be achieved via an integrated omics analysis with metabolomics as its core. We are assured that this review provides comprehensive information on a metabolomics/multi-omics approach and its role in PCa.
Author Contributions
E.P.R. and K.-w.F. conceptualized the paper’s theme and contents. E.P.R. wrote the paper and drew the figures. K.-w.F. edited the draft and provided inputs to the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by NIH NIGMS P20GM121327 and NIH NCI R03 R03CA256230 (K. Fong) This research is also supported by the start-up fund from the University of Kentucky Markey Cancer Center (K. Fong).
Conflicts of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Trock, B.J. Application of metabolomics to prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Nagana, G.G.; Raftery, D. Biomarker discovery and translation in metabolomics. Curr. Metab. 2013, 1, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Aderemi, A.V.; Ayeleso, A.O.; Oyedapo, O.O.; Mukwevho, E. Metabolomics: A Scoping Review of Its Role as a Tool for Disease Biomarker Discovery in Selected Non-Communicable Diseases. Metabolites 2021, 11, 418. [Google Scholar] [CrossRef]
- Aboud, O.A.; Weiss, R.H. New opportunities from the cancer metabolome. Clin. Chem. 2013, 59, 138–146. [Google Scholar] [CrossRef]
- European Bioinformatics Institute (EMBL-EBI). The Metabolome and Metabolic Reactions. Available online: https://www.ebi.ac.uk/training/online/courses/metabolomics-introduction/the-metabolome-and-metabolic-reactions/ (accessed on 12 January 2022).
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2012, 8, 22–33. [Google Scholar] [CrossRef]
- Lai, Z.; Kind, T.; Fiehn, O. Using accurate mass gas chromatography–mass spectrometry with the MINE database for epi-metabolite annotation. Anal. Chem. 2017, 89, 10171–10180. [Google Scholar] [CrossRef]
- Showalter, M.R.; Cajka, T.; Fiehn, O. Epimetabolites: Discovering metabolism beyond building and burning. Curr. Opin. Chem. Biol. 2017, 36, 70–76. [Google Scholar] [CrossRef]
- Mamani-Huanca, M.; Gradillas, A.; Gil de la Fuente, A.; López-Gonzálvez, Á.; Barbas, C. Unveiling the fragmentation mechanisms of modified amino acids as the key for their targeted identification. Anal. Chem. 2020, 92, 4848–4857. [Google Scholar] [CrossRef]
- Prodhan, M.A.I.; McClain, C.; Zhang, X. Comprehensive two-dimensional gas chromatography mass spectrometry-based metabolomics. In Cancer Metabolomics: Methods and Applications; Hu, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1280, pp. 57–67. [Google Scholar]
- Dueñas, M.E.; Lee, Y.J. Single-Cell Metabolomics by Mass Spectrometry Imaging. In Cancer Metabolomics: Methods and Applications; Hu, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1280, pp. 69–82. [Google Scholar]
- Liu, R.; Yang, Z. Single cell metabolomics using mass spectrometry: Techniques and data analysis. Anal. Chim. Acta 2020, 1143, 124–134. [Google Scholar] [CrossRef]
- Lin, X.; Lécuyer, L.; Liu, X.; Triba, M.; Deschasaux-Tanguy, M.; Demidem, A.; Liu, Z.; Palama, T.; Rossary, A.; Vasson, M.-P.; et al. Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Cancers 2021, 13, 3140. [Google Scholar] [CrossRef]
- Xu, X. Capillary electrophoresis-mass spectrometry for cancer metabolomics. In Cancer Metabolomics: Methods and Applications; Hu, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1280, pp. 189–200. [Google Scholar]
- Zhang, X.; Wei, D.; Yap, Y.; Li, L.; Guo, S.; Chen, F. Mass spectrometry-based “omics” technologies in cancer diagnostics. Mass Spectrom. Rev. 2007, 26, 403–431. [Google Scholar] [CrossRef]
- Ganti, S.; Taylor, S.; Abu Aboud, O.; Yang, J.; Evans, C.; Osier, M.V.; Alexander, D.C.; Kim, K.; Weiss, R.H. Kidney Tumor Biomarkers Revealed by Simultaneous Multiple Matrix Metabolomics Analysis. Cancer Res. 2012, 72, 3471–3479. [Google Scholar] [CrossRef]
- López-López, Á.; López-Gonzálvez, Á.; Barker-Tejeda, T.C.; Barbas, C. A review of validated biomarkers obtained through metabolomics. Expert Rev. Mol. Diagn. 2018, 18, 557–575. [Google Scholar] [CrossRef]
- Kuehnbaum, N.L.; Gillen, J.B.; Kormendi, A.; Lam, K.P.; Dibattista, A.; Gibala, M.J.; Britz-McKibbin, P. Multiplexed separations for biomarker discovery in metabolomics: Elucidating adaptive responses to exercise training. Electrophoresis 2015, 36, 2226–2236. [Google Scholar] [CrossRef]
- Wellington, N.; Shanmuganathan, M.; de Souza, R.J.; Zulyniak, M.A.; Azab, S.; Bloomfield, J.; Mell, A.; Ly, R.; Desai, D.; Anand, S.S.; et al. Metabolic Trajectories Following Contrasting Prudent and Western Diets from Food Provisions: Identifying Robust Biomarkers of Short-Term Changes in Habitual Diet. Nutrients 2019, 11, 2407. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; DeZiel, N.C.; Wallach, J.D.; Khan, S.A.; Vasiliou, V.; Ioannidis, J.P.A.; Johnson, C.H. Beyond genomics: Understanding exposotypes through metabolomics. Hum. Genom. 2018, 12, 4. [Google Scholar] [CrossRef]
- Gao, W.; Sun, H.-X.; Xiao, H.; Cui, G.; Hillwig, M.L.; Jackson, A.; Wang, X.; Shen, Y.; Zhao, N.; Zhang, L.; et al. Combining metabolomics and transcriptomics to characterize tanshinone biosynthesis in Salvia miltiorrhiza. BMC Genom. 2014, 15, 73. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K.; Castro-Perez, J.; Granger, J.H.; Johnson, K.A.; Smith, A.B.W.; Plumb, R.S. High Resolution “Ultra Performance” Liquid Chromatography Coupled to oa-TOF Mass Spectrometry as a Tool for Differential Metabolic Pathway Profiling in Functional Genomic Studies. J. Proteome Res. 2005, 4, 591–598. [Google Scholar] [CrossRef]
- BioRender.com. Available online: https://biorender.com/ (accessed on 12 January 2022).
- Griffin, J.L.; Shockcor, J.P. Metabolic profiles of cancer cells. Nat. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef]
- Cheung, P.K.; Ma, M.H.; Tse, H.F.; Yeung, K.F.; Tsang, H.F.; Chu, M.K.M.; Kan, C.M.; Cho, W.C.S.; Ng, L.B.W.; Chan, L.W.C.; et al. The applications of metabolomics in the molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 2019, 19, 785–793. [Google Scholar] [CrossRef]
- Pinto, R.C. Chemometrics methods and strategies in metabolomics. In Metabolomics: From Fundamentals to Clinical Applications; Sussulini, A., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 965, pp. 163–190. [Google Scholar]
- Vermeersch, K.A.; Styczynski, M.P. Applications of metabolomics in cancer research. J. Carcinog. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Jia, W.; Hu, Z. Emerging Applications of Metabolomics in Clinical Pharmacology. Clin. Pharmacol. Ther. 2019, 106, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Kyriakides, M.; Scott, F.; Shephard, E.; Varshavi, D.; Veselkov, K.; Everett, J.R. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 2016, 14, 135–153. [Google Scholar] [CrossRef]
- Lu, X.; Solmonson, A.; Lodi, A.; Nowinski, S.M.; Sentandreu, E.; Riley, C.L.; Mills, E.M.; Tiziani, S. The early metabolomic response of adipose tissue during acute cold exposure in mice. Sci. Rep. 2017, 7, 3455. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Hammock, B.D.; Watkins, S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics 2005, 1, 3–9. [Google Scholar] [CrossRef]
- Wishart, D.S. Current Progress in computational metabolomics. Brief. Bioinform. 2007, 8, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An intro-ductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Mandal, R.; Stanislaus, A.; Ramirez-Gaona, M. Cancer Metabolomics and the Human Metabolome Database. Metabolites 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health-National Human Genome Research Institute (NIH-NHGRI). The Human Genome Project. 2021. Available online: https://www.genome.gov/human-genome-project (accessed on 12 January 2022).
- Willyard, C. New human gene tally reignites debate. Nature 2018, 558, 354–355. [Google Scholar] [CrossRef]
- Yu, L.; Li, K.; Zhang, X. Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: Mini review. Oncotarget 2017, 8, 115774–115786. [Google Scholar] [CrossRef]
- Milo, R.; Jorgensen, P.; Moran, U.; Weber, G.; Springer, M. BioNumbers—the database of key numbers in molecular and cell biology. Nucleic Acids Res. 2009, 38, D750–D753. [Google Scholar] [CrossRef]
- Zhang, H.; Egger, R.L.; Kelliher, T.; Morrow, D.; Fernandes, J.; Nan, G.-L.; Walbot, V. Transcriptomes and Proteomes Define Gene Expression Progression in Pre-meiotic Maize Anthers. G3 Genes Genomes Genet. 2014, 4, 993–1010. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; White, N.M.; Schmidt, H.K.; Fulton, R.S.; Tomlinson, C.; Warren, W.C.; Wilson, R.K.; Maher, C.A. INTEGRATE: Gene fusion discovery using whole genome and transcriptome data. Genome Res. 2015, 26, 108–118. [Google Scholar] [CrossRef]
- Mertins, P.; Cptac, N.; Mani, D.R.; Ruggles, K.V.; Gillette, M.A.; Clauser, K.R.; Wang, P.; Wang, X.; Qiao, J.W.; Cao, S.; et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016, 534, 55–62. [Google Scholar] [CrossRef]
- Dang, L.; Yen, K.; Attar, E.C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. 2016, 27, 599–608. [Google Scholar] [CrossRef]
- Pirozzi, C.J.; Yan, H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Bedia, C.; Capriotti, A.L.; Cavaliere, C.; Gentile, V.; Maggi, M.; Montone, C.M.; Piovesana, S.; Sciarra, A.; Tauler, R.; et al. Untargeted metabolomics of prostate cancer zwitterionic and positively charged compounds in urine. Anal. Chim. Acta 2021, 1158, 338381. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-A.; Wu, Q.; Chen, Z.; Zhang, W.; Zhou, Y.; Mao, K.; Li, J.; Li, Y.; Chen, J.; Huang, Y.; et al. High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci. Rep. 2021, 11, 11805. [Google Scholar] [CrossRef]
- Jobard, E.; Dossus, L.; Baglietto, L.; Fornili, M.; Lécuyer, L.; Mancini, F.R.; Gunter, M.J.; Trédan, O.; Boutron-Ruault, M.-C.; Elena-Herrmann, B.; et al. Investigation of circulating metabolites associated with breast cancer risk by untargeted metabolomics: A case–control study nested within the French E3N cohort. Br. J. Cancer 2021, 124, 1734–1743. [Google Scholar] [CrossRef]
- Huan, T.; Forsberg, E.M.; Rinehart, D.; Johnson, C.H.; Ivanisevic, J.; Benton, H.P.; Fang, M.; Aisporna, A.; Hilmers, B.; Poole, F.L.; et al. Systems biology guided by XCMS Online metabolomics. Nat. Methods 2017, 14, 461–462. [Google Scholar] [CrossRef]
- Giera, M.; dos Santos, F.B.; Siuzdak, G. Metabolite-Induced Protein Expression Guided by Metabolomics and Systems Biology. Cell Metab. 2018, 27, 270–272. [Google Scholar] [CrossRef]
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; Dos Santos, V.A.P.M.; Saccenti, E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics 2018, 14, 37. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Kell, D.B. Metabolomics and systems biology: Making sense of the soup. Curr. Opin. Microbiol. 2004, 7, 296–307. [Google Scholar] [CrossRef]
- Ruepp, S.U.; Tonge, R.P.; Shaw, J.; Wallis, N.; Pognan, F. Genomics and Proteomics Analysis of Acetaminophen Toxicity in Mouse Liver. Toxicol. Sci. 2002, 65, 135–150. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D.; Lindon, J.C. Pharmacometabonomics as an effector for personalized medicine. Pharmacogenomics 2011, 12, 103–111. [Google Scholar] [CrossRef]
- Dumas, M.-E.; Wilder, S.P.; Bihoreau, M.-T.; Barton, R.H.; Fearnside, J.F.; Argoud, K.; D’Amato, L.; Wallis, R.H.; Blancher, C.; Keun, H.C.; et al. Direct quantitative trait locus mapping of mammalian metabolic phenotypes in diabetic and normoglycemic rat models. Nat. Genet. 2007, 39, 666–672. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Costigliola, V.; EPMA. General Report & Recommendations in Predictive, Preventive and Personalised Medicine 2012: White Paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012, 3, 14–53. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation-EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Filep, N.; Yeghiazaryan, K.; Blom, H.J.; Hofmann-Apitius, M.; Kuhn, W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: Lessons for predictive, preventive and personalised medicine. Amino Acids 2017, 50, 383–395. [Google Scholar] [CrossRef]
- Ibrahim, R.; Pasic, M.; Yousef, G.M. Omics for personalized medicine: Defining the current we swim in. Expert Rev. Mol. Diagn. 2016, 16, 719–722. [Google Scholar] [CrossRef]
- Chen, R.; Snyder, M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 73–82. [Google Scholar] [CrossRef]
- Turanli, B.; Yildirim, E.; Gulfidan, G.; Arga, K.Y.; Sinha, R. Current State of “Omics” Biomarkers in Pancreatic Cancer. J. Pers. Med. 2021, 11, 127. [Google Scholar] [CrossRef]
- Subbannayya, Y.; Di Fiore, R.; Urru, S.A.M.; Calleja-Agius, J. The Role of Omics Approaches to Characterize Molecular Mechanisms of Rare Ovarian Cancers: Recent Advances and Future Perspectives. Biomedicines 2021, 9, 1481. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Lindon, J.; Wilson, I.D. The challenges of modeling mammalian biocomplexity. Nat. Biotechnol. 2004, 22, 1268–1274. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wagele, B.; Altmaier, E.; Gram, C.; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Moskowitz, J.E.; Doran, A.G.; Lei, Z.; Busi, S.B.; Hart, M.L.; Franklin, C.L.; Sumner, L.W.; Keane, T.M.; Amos-Landgraf, J.M. Integration of genomics, metagenomics, and metabolomics to identify interplay between susceptibility alleles and microbiota in adenoma initiation. BMC Cancer 2020, 20, 600. [Google Scholar] [CrossRef]
- Quanbeck, S.M.M.; Brachova, L.; Campbell, A.A.; Guan, X.; Perera, A.; He, K.; Rhee, S.Y.; Bais, P.; Dickerson, J.A.; Dixon, P.; et al. Metabolomics as a Hypothesis-Generating Functional Genomics Tool for the Annotation of Arabidopsis thaliana Genes of “Unknown Function”. Front. Plant Sci. 2012, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; de Angelis, M.H.; Kronenberg, F.; Meitinger, T.; Mewes, H.-W.; Wichmann, H.-E.; Weinberger, K.; Adamski, J.; et al. Genetics Meets Metabolomics: A Genome-Wide Association Study of Metabolite Profiles in Human Serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.; Rantalainen, M.; Li, J.; Maher, A.D.; Malmodin, D.; Ahmadi, K.R.; Faber, J.H.; Barrett, A.; Min, J.L.; Rayner, N.W.; et al. A Genome-Wide Metabolic QTL Analysis in Europeans Implicates Two Loci Shaped by Recent Positive Selection. PLoS Genet. 2011, 7, e1002270. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Schänzer, W. Synthetic Anabolic Agents: Steroids and Nonsteroidal Selective Androgen Receptor Modulators. In Doping in Sports: Biochemical Principles, Effects and Analysis. Handbook of Experimental Pharmacology; Thieme, D., Hemmersbach, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 195, pp. 99–126. [Google Scholar]
- Rading, A.; Anielski, P.; Thieme, D.; Keiler, A.M. Detection of the selective androgen receptor modulator GSK2881078 and metabolites in urine and hair after single oral administration. Drug Test. Anal. 2020, 13, 217–222. [Google Scholar] [CrossRef]
- Le Bail, J.-C.; Aubourg, L.; Habrioux, G. Effects of pinostrobin on estrogen metabolism and estrogen receptor transactivation. Cancer Lett. 2000, 156, 37–44. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chiao, C.-C.; Phan, N.N.; Li, C.-Y.; Sun, Z.-D.; Jiang, J.-Z.; Hung, J.-H.; Chen, Y.-L.; Yen, M.-C.; Weng, T.-Y.; et al. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am. J. Cancer Res. 2020, 10, 95–113. [Google Scholar]
- Layton, A.C.; Sanseverino, J.; Gregory, B.W.; Easter, J.P.; Sayler, G.S.; Schultz, T.W. In Vitro estrogen receptor binding of PCBs: Measured Aativity and detection of hydroxylated metabolites in a recombinant yeast assay. Toxicol. Appl. Pharmacol. 2002, 180, 157–163. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Q.S.; Sun, Z.; Xu, H.; Zhou, Q.; Jiang, G. Perfluorinated iodine alkanes induce tissue-specific expression of estrogen receptor and its phosphorylation. Sci. Total Environ. 2021, 787, 147722. [Google Scholar] [CrossRef]
- Raut, S.; Kumar, A.V.; Deshpande, S.; Khambata, K.; Balasinor, N.H. Sex hormones regulate lipid metabolism in adult Sertoli cells: A genome-wide study of estrogen and androgen receptor binding sites. J. Steroid Biochem. Mol. Biol. 2021, 211, 105898. [Google Scholar] [CrossRef]
- Eidelman, E.; Twum-Ampofo, J.; Ansari, J.; Siddiqui, M.M. The Metabolic Phenotype of Prostate Cancer. Front. Oncol. 2017, 7, 131. [Google Scholar] [CrossRef]
- Ladurner, A.G. Rheostat Control of Gene Expression by Metabolites. Mol. Cell 2006, 24, 1–11. [Google Scholar] [CrossRef]
- Lempp, M.; Farke, N.; Kuntz, M.; Freibert, S.A.; Lill, R.; Link, H. Systematic identification of metabolites controlling gene expression in E. coli. Nat. Commun. 2019, 10, 4463. [Google Scholar] [CrossRef]
- Van der Knaap, J.A.; Verrijzer, C.P. Undercover: Gene control by metabolites and metabolic enzymes. Genes Dev. 2016, 30, 2345–2369. [Google Scholar] [CrossRef]
- Commichau, F.; Gunka, K.; Landmann, J.J.; Stülke, J. Glutamate Metabolism in Bacillus subtilis: Gene Expression and Enzyme Activities Evolved to Avoid Futile Cycles and To Allow Rapid Responses to Perturbations of the System. J. Bacteriol. 2008, 190, 3557–3564. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Bultman, S.J. Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell. Physiol. 2012, 227, 3169–3177. [Google Scholar] [CrossRef]
- Winkler, W.C.; Nahvi, A.; Roth, A.; Collins, J.A.; Breaker, R.R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 2004, 428, 281–286. [Google Scholar] [CrossRef]
- Diskin, C.; Ryan, T.A.J.; O’Neill, L.A.J. Modification of Proteins by Metabolites in Immunity. Immunity 2020, 54, 19–31. [Google Scholar] [CrossRef]
- Sovova, Z.; Suttnar, J.; Dyr, J.E. Molecular dynamic simulations suggest that metabolite-induced post-translational modifi-cations alter the behavior of the fibrinogen coiled-coil domain. Metabolites 2021, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P.; Sánchez, S. Modulation of gene expression in actinobacteria by translational modification of tran-scriptional factors and secondary metabolite biosynthetic enzymes. Front. Microbiol. 2021, 12, 630694. [Google Scholar] [CrossRef] [PubMed]
- Harachi, M.; Masui, K.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. Protein Acetylation at the Interface of Genetics, Epigenetics and Environment in Cancer. Metabolites 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yang, F.; Wang, C. Chemoproteomic profiling of protein–metabolite interactions. Curr. Opin. Chem. Biol. 2020, 54, 28–36. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Atherton, P.J. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, J.; Zeng, X.; Wang, W.; Li, S.; Zang, T.; Peng, J.; Yang, Y. Prediction and collection of protein–metabolite interactions. Brief. Bioinform. 2021, 22. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Menzies, K.J.; Zhang, H.; Katsyuba, E.; Auwerx, J. Protein acetylation in metabolism—Metabolites and cofactors. Nat. Rev. Endocrinol. 2015, 12, 43–60. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef]
- Yamanaka, S.; Murai, H.; Saito, D.; Abe, G.; Tokunaga, E.; Iwasaki, T.; Takahashi, H.; Takeda, H.; Suzuki, T.; Shibata, N.; et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 2021, 40, e105375. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, S.; Zhang, K.; Qing, Y.; Xu, T. A comprehensive overview of exosomes in ovarian cancer: Emerging biomarkers and therapeutic strategies. J. Ovarian Res. 2017, 10, 73. [Google Scholar] [CrossRef]
- Kiebish, M.A.; Cullen, J.; Mishra, P.; Ali, A.; Milliman, E.; Rodrigues, L.O.; Chen, E.Y.; Tolstikov, V.; Zhang, L.; Panagopoulos, K.; et al. Multi-omic serum biomarkers for prognosis of disease progression in prostate cancer. J. Transl. Med. 2020, 18, 10. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Kane, L.E.; Mellotte, G.S.; Conlon, K.C.; Ryan, B.M.; Maher, S.G. Multi-omic biomarkers as potential tools for the char-acterisation of pancreatic cystic lesions and cancer: Innovative patient data integration. Cancers 2021, 13, 769. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2014, 15, 25–41. [Google Scholar] [CrossRef]
- Sehgal, V.; Seviour, E.; Moss, T.J.; Mills, G.B.; Azencott, R.; Ram, P.T. Robust Selection Algorithm (RSA) for Multi-Omic Biomarker Discovery; Integration with Functional Network Analysis to Identify miRNA Regulated Pathways in Multiple Cancers. PLoS ONE 2015, 10, e0140072. [Google Scholar] [CrossRef]
- Chu, S.H.; Huang, M.; Kelly, R.S.; Benedetti, E.; Siddiqui, J.K.; Zeleznik, O.A. Integration of metabolomic and other omics data in population-based study designs: An epidemiological per-spective. Metabolites 2019, 9, 117. [Google Scholar] [CrossRef]
- Stäubert, C.; Bhuiyan, H.; Lindahl, A.; Broom, O.J.; Zhu, Y.; Islam, S.; Linnarsson, S.; Lehtiö, J.; Nordström, A. Rewired Metabolism in Drug-resistant Leukemia Cells. J. Biol. Chem. 2015, 290, 8348–8359. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Saoi, M.; Britz-McKibbin, P. New Advances in Tissue Metabolomics: A Review. Metabolites 2021, 11, 672. [Google Scholar] [CrossRef]
- Lima, A.R.; De Bastos, M.L.; Carvalho, M.; de Pinho, P.G. Biomarker Discovery in Human Prostate Cancer: An Update in Metabolomics Studies. Transl. Oncol. 2016, 9, 357–370. [Google Scholar] [CrossRef]
- Lima, A.; Pinto, J.; Amaro, F.; Bastos, M.; Carvalho, M.; de Pinho, P.G. Advances and Perspectives in Prostate Cancer Biomarker Discovery in the Last 5 Years through Tissue and Urine Metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; Rojas-Benedicto, A.; Albors-Vaquer, A.; López-Guerrero, J.A.; Pineda-Lucena, A.; Puchades-Carrasco, L. Metabolomics Contributions to the Discovery of Prostate Cancer Biomarkers. Metabolites 2019, 9, 48. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.; Di Pierro, G.; Ricciuti, G.; Del Giudice, F.; et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Galleggiante, V.; Giglio, A.; Palazzo, S.; Ferro, M.; Simone, C.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev. Mol. Diagn. 2015, 15, 1211–1224. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Kdadra, M.; Höckner, S.; Leung, H.; Kremer, W.; Schiffer, E. Metabolomics Biomarkers of Prostate Cancer: A Systematic Review. Diagnostics 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Heiden, M.G.V.; Giovannucci, E.L.; Mucci, L.A. Metabolomic Biomarkers of Prostate Cancer: Prediction, Diagnosis, Progression, Prognosis, and Recurrence. Cancer Epidemiol. Biomark. Prev. 2016, 25, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Beyoğlu, D.; Idle, J. Metabolic Rewiring and the Characterization of Oncometabolites. Cancers 2021, 13, 2900. [Google Scholar] [CrossRef] [PubMed]
- Franko, A.; Shao, Y.; Heni, M.; Hennenlotter, J.; Hoene, M.; Hu, C.; Liu, X.; Zhao, X.; Wang, Q.; Birkenfeld, A.L.; et al. Human Prostate Cancer is Characterized by an Increase in Urea Cycle Metabolites. Cancers 2020, 12, 1814. [Google Scholar] [CrossRef]
- Vykoukal, J.; Fahrmann, J.F.; Gregg, J.R.; Tang, Z.; Basourakos, S.; Irajizad, E.; Park, S.; Yang, G.; Creighton, C.J.; Fleury, A.; et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat. Commun. 2020, 11, 4279. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Jentzmik, F. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identi-fication of aggressive tumours. Eur. Urol. 2010, 58, 12–18. [Google Scholar] [CrossRef]
- Cao, D.-L.; Ye, D.-W.; Zhu, Y.; Zhang, H.-L.; Wang, Y.-X.; Yao, X.-D. Efforts to resolve the contradictions in early diagnosis of prostate cancer: A comparison of different algorithms of sarcosine in urine. Prostate Cancer Prostatic Dis. 2011, 14, 166–172. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, C.; Cheng, S.; Li, G.; Griebel, J.; Neuhaus, J. Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine. Diagnostics 2021, 11, 149. [Google Scholar] [CrossRef]
- Song, Y.H.; Shiota, M.; Kuroiwa, K.; Naito, S.; Oda, Y. The important role of glycine N-methyltransferase in the carcino-genesis and progression of prostate cancer. Mod. Pathol. 2011, 24, 1272–1280. [Google Scholar] [CrossRef]
- Ottaviani, S.; Brooke, G.N.; O’Hanlon-Brown, C.; Waxman, J.; Ali, S.; Buluwela, L. Characterisation of the androgen regulation of glycine N-methyltransferase in prostate cancer cells. J. Mol. Endocrinol. 2013, 51, 301–312. [Google Scholar] [CrossRef]
- Huang, Y.-C. Haplotypes, loss of heterozygosity, and expression levels of glycine N-methyltransferase in prostate cancer. Clin. Cancer Res. 2007, 13, 1412–1420. [Google Scholar] [CrossRef]
- Heger, Z.; Rodrigo, M.A.M.; Michalek, P.; Polanska, H.; Masarik, M.; Vit, V.; Plevova, M.; Pacik, D.; Eckschlager, T.; Stiborova, M.; et al. Sarcosine Up-Regulates Expression of Genes Involved in Cell Cycle Progression of Metastatic Models of Prostate Cancer. PLoS ONE 2016, 11, e0165830. [Google Scholar] [CrossRef]
- De Vogel, S. Sarcosine and other metabolites along the choline oxidation pathway in relation to prostate cancer-A large nested case-control study within the JANUS cohort in Norway. Int. J. Cancer 2014, 134, 197–206. [Google Scholar] [CrossRef]
- Yousefi, M.; Qujeq, D.; Shafi, H.; Tilaki, K.H. Serum and Urine Levels of Sarcosine in Benign Prostatic Hyperplasia and Newly Diagnosed Prostate Cancer Patients. J. Kermanshah Univ. Med Sci. 2020, 24, e97000. [Google Scholar] [CrossRef]
- Dehghani, N.; Salehipour, M.; Javanmard, B. Evaluation of GNMT Gene Expression in Prostate Cancer Tissues using Real-Time PCR. J. Tolooebehdasht 2021, 19, 44–54. [Google Scholar] [CrossRef]
- Jendoubi, T. Approaches to Integrating Metabolomics and Multi-Omics Data: A Primer. Metabolites 2021, 11, 184. [Google Scholar] [CrossRef]
- Haukaas, T.H.; Euceda, L.R.; Giskeødegård, G.F.; Bathen, T.F. Metabolic Portraits of Breast Cancer by HR MAS MR Spectroscopy of Intact Tissue Samples. Metabolites 2017, 7, 18. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Metabolomics: The greatest omics of them all? Anal. Chem. 2006, 78, 7954–7958. [Google Scholar] [CrossRef]
- Buescher, J.M.; Driggers, E.M. Integration of omics: More than the sum of its parts. Cancer Metab. 2016, 4, 4. [Google Scholar] [CrossRef]
- Moez, E.K.; Pyne, S.; Dinu, I. Association between bivariate expression of key oncogenes and metabolic phenotypes of patients with prostate cancer. Comput. Biol. Med. 2018, 103, 55–63. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Liu, Y.; Wang, W.; Han, J.; Wang, Q.; Xu, Y.; Zhang, C.; Zhang, S.; Li, X.; et al. Topologically inferring pathway activity toward precise cancer classification via integrating genomic and metabolomic data: Prostate cancer as a case. Sci. Rep. 2015, 5, 13192. [Google Scholar] [CrossRef] [PubMed]
- Del Carratore, F. Integrated probabilistic annotation: A Bayesian-based annotation method for metabolomic profiles in-tegrating biochemical connections, isotope patterns, and adduct relationships. Anal. Chem. 2019, 91, 12799–12807. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, A.D.; Bredeweg, E.L.; Manzer, J.; Zucker, J.; Munoz, N.M.; Burnet, M.C.; Nakayasu, E.S.; Pomraning, K.R.; Merkley, E.D.; Dai, Z.; et al. Bayesian Inference for Integrating Yarrowia lipolytica Multiomics Datasets with Metabolic Modeling. ACS Synth. Biol. 2021, 10, 2968–2981. [Google Scholar] [CrossRef]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Orešič, M. Deep learning meets metabolomics: A methodological perspective. Briefings Bioinform. 2020, 22, 1531–1542. [Google Scholar] [CrossRef]
- Kang, M.; Ko, E.; Mersha, T.B. A roadmap for multi-omics data integration using deep learning. Brief. Bioinform. 2021, 23, bbab454. [Google Scholar] [CrossRef]
- Zheng, H.; Hu, Y.; Dong, L.; Shu, Q.; Zhu, M.; Li, Y.; Chen, C.; Gao, H.; Yang, L. Predictive diagnosis of chronic obstructive pulmonary disease using serum metabolic biomarkers and least-squares support vector machine. J. Clin. Lab. Anal. 2020, 35, e23641. [Google Scholar] [CrossRef]
- Gagnebin, Y.; Pezzatti, J.; Lescuyer, P.; Boccard, J.; Ponte, B.; Rudaz, S. Combining the advantages of multilevel and orthogonal partial least squares data analysis for longitudinal metabolomics: Application to kidney transplantation. Anal. Chim. Acta 2019, 1099, 26–38. [Google Scholar] [CrossRef]
- Hsu, S.-C. Arginine starvation elicits chromatin leakage and cGAS-STING activation via epigenetic silencing of metabolic and DNA-repair genes. Theranostics 2021, 11, 7527–7545. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hahm, E.-R.; Singh, K.B.; Shiva, S.; Stewart-Ornstein, J.; Singh, S.V. RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis 2020, 41, 778–789. [Google Scholar] [CrossRef]
- Adams, C.D.; Richmond, R.; Ferreira, D.L.S.; Spiller, W.; Tan, V.; Zheng, J.; Würtz, P.; Donovan, J.; Hamdy, F.; Neal, D.; et al. Circulating Metabolic Biomarkers of Screen-Detected Prostate Cancer in the ProtecT Study. Cancer Epidemiol. Biomark. Prev. 2018, 28, 208–216. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.-Y.; Liu, Y.-J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.-Y.; Prins, G.S.; Erdogan, Z.M. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef]
- Chen, Y. Decreased glucose bioavailability and elevated aspartate metabolism in prostate cancer cells undergoing epi-thelial-mesenchymal transition. J. Cell. Physiol. 2020, 235, 5602–5612. [Google Scholar] [CrossRef]
- Joshi, M.; Kim, J.; D’Alessandro, A.; Monk, E.; Bruce, K.; Elajaili, H.; Nozik-Grayck, E.; Goodspeed, A.; Costello, J.C.; Schlaepfer, I.R. CPT1A Over-Expression Increases Reactive Oxygen Species in the Mitochondria and Promotes Antioxidant Defenses in Prostate Cancer. Cancers 2020, 12, 3431. [Google Scholar] [CrossRef]
- De Mas, I.M.; Torrents, L.; Bedia, C.; Nielsen, L.K.; Cascante, M.; Tauler, R. Stoichiometric gene-to-reaction associations enhance model-driven analysis performance: Metabolic response to chronic exposure to Aldrin in prostate cancer. BMC Genom. 2019, 20, 652. [Google Scholar] [CrossRef]
- Teng, L.K.H.; Pereira, B.; Keerthikumar, S.; Huang, C.; Niranjan, B.; Lee, S.; Richards, M.; Schittenhelm, R.; Furic, L.; Goode, D.; et al. Mast Cell-Derived SAMD14 Is a Novel Regulator of the Human Prostate Tumor Microenvironment. Cancers 2021, 13, 1237. [Google Scholar] [CrossRef]
- Blomme, A. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat. Commun. 2020, 11, 2508. [Google Scholar] [CrossRef]
- Li, J.; Agarwal, E.; Bertolini, I.; Seo, J.H.; Caino, M.C.; Ghosh, J.C.; Kossenkov, A.V.; Liu, Q.; Tang, H.-Y.; Goldman, A.R.; et al. The mitophagy effector FUNDC1 controls mitochondrial reprogramming and cellular plasticity in cancer cells. Sci. Signal. 2020, 13, eaaz8240. [Google Scholar] [CrossRef]
- Dougan, J.; Hawsawi, O.; Burton, L.J.; Edwards, G.; Jones, K.; Zou, J.; Odero-Marah, V.A. Proteomics-metabolomics combined approach identifies peroxidasin as a protector against metabolic and oxidative stress in prostate cancer. Int. J. Mol. Sci. 2019, 20, 3046. [Google Scholar] [CrossRef]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: A continuing debate. Theranostics 2020, 10, 2944–2948. [Google Scholar] [CrossRef]
- Haj-Ahmad, T.A.; Abdalla, M.A.; Haj-Ahmad, Y. Potential Urinary miRNA Biomarker Candidates for the Accurate Detection of Prostate Cancer among Benign Prostatic Hyperplasia Patients. J. Cancer 2014, 5, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.S.; Sakellis, C.G.; Hyun, H.; Jacene, H.A. Extraprostatic Uptake of 18F-Fluciclovine: Differentiation of Nonprostatic Neoplasms From Metastatic Prostate Cancer. Am. J. Roentgenol. 2020, 214, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Rasulova, N.; Partanen, K.; Joensuu, T. Preliminary clinical experience of trans-1-amino-3-(18)F-fluorocyclobutanecarboxylic acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. BioMed Res. Int. 2014, 2014, 305182. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, I. Correlation between 18F-1-amino-3-fluorocyclobutane-1-carboxylic acid (18F-fluciclovine) uptake and ex-pression of alanine-serine-cysteine-transporter 2 (ASCT2) and L-type amino acid transporter 1 (LAT1) in primary prostate cancer. EJNMMI Res. 2019, 9, 50. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar]
- Dereziński, P.; Klupczynska, A.; Sawicki, W.; Pałka, J.A.; Kokot, Z.J. Amino Acid Profiles of Serum and Urine in Search for Prostate Cancer Biomarkers: A Pilot Study. Int. J. Med. Sci. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Lee, B.; Mahmud, I.; Marchica, J.; Dereziński, P.; Qi, F.; Wang, F.; Joshi, P.; Valerio, F.; Rivera, I.; Patel, V.; et al. Integrated RNA and metabolite profiling of urine liquid biopsies for prostate cancer biomarker discovery. Sci. Rep. 2020, 10, 3716. [Google Scholar] [CrossRef]
- Taavitsainen, S.; Engedal, N.; Cao, S.; Handle, F.; Erickson, A.; Prekovic, S.; Wetterskog, D.; Tolonen, T.; Vuorinen, E.M.; Kiviaho, A.; et al. Single-cell ATAC and RNA sequencing reveal pre-existing and persistent cells associated with prostate cancer relapse. Nat. Commun. 2021, 12, 5307. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 2015, 260, 144–160. [Google Scholar] [CrossRef]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef]
- Tikunov, Y.M.; Verstappen, F.W.A.; Hall, R.D. Metabolomic Profiling of Natural Volatiles: Headspace Trapping: GC-MS. In Metabolomics: Methods and Protocols; Weckwerth, W., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2007; Volume 358, pp. 39–56. [Google Scholar]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. BioSyst. 2012, 8, 470–481. [Google Scholar] [CrossRef]
- Sands, C.J.; Gómez-Romero, M.; Correia, G.; Chekmeneva, E.; Camuzeaux, S.; Izzi-Engbeaya, C.; Dhillo, W.S.; Takats, Z.; Lewis, M.R. Representing the Metabolome with High Fidelity: Range and Response as Quality Control Factors in LC-MS-Based Global Profiling. Anal. Chem. 2021, 93, 1924–1933. [Google Scholar] [CrossRef]
- Lima, C.; Muhamadali, H.; Goodacre, R. The Role of Raman Spectroscopy Within Quantitative Metabolomics. Annu. Rev. Anal. Chem. 2021, 14, 323–345. [Google Scholar] [CrossRef]
- Su, K.-Y.; Lee, W.-L. Fourier transform infrared spectroscopy as a cancer screening and diagnostic tool: A review and pro-spects. Cancers 2020, 12, 115. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, M.; Salomé-Abarca, L.F.; Wang, M.; Choi, Y.H. Investigation of species and environmental effects on rhubarb roots metabolome using 1H NMR combined with high performance thin layer chromatography. Metabolomics 2018, 14, 137. [Google Scholar] [CrossRef]
- Xie, G.; Wang, L.; Chen, T.; Zhou, K.; Zhang, Z.; Li, J.; Sun, B.; Guo, Y.; Wang, X.; Wang, Y.; et al. A Metabolite Array Technology for Precision Medicine. Anal. Chem. 2021, 93, 5709–5717. [Google Scholar] [CrossRef]
- Shi, Y.; Han, J.J.; Tennakoon, J.B.; Mehta, F.F.; Merchant, F.; Burns, A.R.; Howe, M.K.; McDonnell, D.P.; Frigo, D.E. Androgens Promote Prostate Cancer Cell Growth through Induction of Autophagy. Mol. Endocrinol. 2013, 27, 280–295. [Google Scholar] [CrossRef]
- Singh, R.; Mills, I.G. The Interplay Between Prostate Cancer Genomics, Metabolism, and the Epigenome: Perspectives and Future Prospects. Front. Oncol. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Demichelis, F. The Genomics of Prostate Cancer: A Historic Perspective. Cold Spring Harb. Perspect. Med. 2019, 9, a034942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhang, M.; Shi, C.; Sun, L.; Shan, L.; Zhang, H.; Song, Y. An overview of advances in multi-omics analysis in prostate cancer. Life Sci. 2020, 260, 118376. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Song, X.-L.; Wang, C.; Yu, Y.-Z.; Wang, J.-Q.; Chen, Z.-S.; Zhao, S.-C. The role of androgen therapy in prostate cancer: From testosterone replacement therapy to bipolar androgen therapy. Drug Discov. Today 2021, 26, 1293–1301. [Google Scholar] [CrossRef]
- Jääskeläinen, J.; Mongan, N.P.; Harland, S.; Hughes, I.A. Five novel androgen receptor gene mutations associated with complete androgen insensitivity syndrome. Hum. Mutat. 2006, 27, 291. [Google Scholar] [CrossRef]
- Hornig, N.C.; Holterhus, P.-M. Molecular basis of androgen insensitivity syndromes. Mol. Cell. Endocrinol. 2021, 523, 111146. [Google Scholar] [CrossRef]
- Helsen, C.; Dubois, V.; Verfaillie, A.; Young, J.; Trekels, M.; Vancraenenbroeck, R.; De Maeyer, M.; Claessens, F. Evidence for DNA-Binding Domain–Ligand-Binding Domain Communications in the Androgen Receptor. Mol. Cell. Biol. 2012, 32, 3033–3043. [Google Scholar] [CrossRef]
- Xia, F.; Xu, X.; Zhai, H.; Meng, Y.; Zhang, H.; Du, S.; Xu, H.; Wu, H.; Lu, Y. Castration-induced testosterone deficiency increases fasting glucose associated with hepatic and extra-hepatic insulin resistance in adult male rats. Reprod. Biol. Endocrinol. 2013, 11, 106. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.M.; Mourtzakis, M. Glucose impairments and insulin resistance in prostate cancer: The role of obesity, nutrition and exercise. Obes. Rev. 2018, 19, 1008–1016. [Google Scholar] [CrossRef]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401. [Google Scholar] [CrossRef]
- Shafi, A.A.; Putluri, V.; Arnold, J.; Tsouko, E.; Maity, S.; Roberts, J.M.; Coarfa, C.; Frigo, D.; Putluri, N.; Sreekumar, A.; et al. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget 2015, 6, 31997–32012. [Google Scholar] [CrossRef]
- Audet-Walsh, É.; Dufour, C.R.; Yee, T.; Zouanat, F.Z.; Yan, M.; Kalloghlian, G.; Giguère, V. Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer. Genes Dev. 2017, 31, 1228–1242. [Google Scholar] [CrossRef]
- Twum-Ampofo, J.; Fu, D.-X.; Passaniti, A.; Hussain, A.; Siddiqui, M.M. Metabolic targets for potential prostate cancer therapeutics. Curr. Opin. Oncol. 2016, 28, 241–247. [Google Scholar] [CrossRef]
- Bader, D.A.; Hartig, S.M.; Putluri, V.; Foley, C.; Hamilton, M.P.; Smith, E.A.; Saha, P.K.; Panigrahi, A.; Walker, C.; Zong, L.; et al. Mitochondrial pyruvate import is a metabolic vulnerability in androgen receptor-driven prostate cancer. Nat. Metab. 2019, 1, 70–85. [Google Scholar] [CrossRef]
- Flaig, T.W.; Salzmann-Sullivan, M.; Su, L.-J.; Zhang, Z.; Joshi, M.; Gijón, M.A.; Kim, J.; Arcaroli, J.J.; Van Bokhoven, A.; Lucia, M.S.; et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 2017, 8, 56051–56065. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Smith, R. Enzalutamide response in a panel of prostate cancer cell lines reveals a role for glucocorticoid receptor in en-zalutamide resistant disease. Sci. Rep. 2020, 10, 21750. [Google Scholar] [CrossRef] [PubMed]
- VanDeusen, H.R.; Ramroop, J.R.; Morel, K.L.; Bae, S.Y.; Sheahan, A.V.; Sychev, Z.; Lau, N.A.; Cheng, L.C.; Tan, V.M.; Li, Z.; et al. Targeting RET Kinase in Neuroendocrine Prostate Cancer. Mol. Cancer Res. 2020, 18, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, S.; Wang, F.; Fan, C.; Wang, J. Identification of key pathways and genes in PTEN mutation prostate cancer by bioinformatics analysis. BMC Med. Genet. 2019, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Bastos, D.C. Genetic ablation of FASN attenuates the invasive potential of prostate cancer driven by Pten loss. J. Pathol. 2021, 253, 292–303. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.-S.; Lee, Y.-R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef]
- Maughan, B.L. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castra-tion-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 260–268. [Google Scholar] [CrossRef]
- Chappell, W.H. Roles of p53, NF-κB and the androgen receptor in controlling NGAL expression in prostate cancer cell lines. Adv. Biol. Regul. 2018, 69, 43–62. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lou, W.; Nadiminty, N.; Chen, X.; Zhou, Q.; Shi, X.B.; White, R.W.D.; Gao, A.C. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate 2013, 73, 418–427. [Google Scholar] [CrossRef]
- Dong, J.-T. Prevalent mutations in prostate cancer. J. Cell. Biochem. 2006, 97, 433–447. [Google Scholar] [CrossRef]
- Aggarwal, M.; Saxena, R.; Sinclair, E.; Fu, Y.; Jacobs, A.; Dyba, M.; Wang, X.; Cruz, I.; Berry, D.; Kallakury, B.; et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016, 23, 1615–1627. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Linares, J.F.; Duran, A.; Cordes, T.; L’Hermitte, A.; Badur, M.G.; Bhangoo, M.S.; Thorson, P.K.; Richards, A.; Rooslid, T.; et al. Increased Serine and One-Carbon Pathway Metabolism by PKCλ/ι Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell 2019, 35, 385–400.e9. [Google Scholar] [CrossRef]
- Ben-Salem, S.; Venkadakrishnan, V.B. Novel insights in cell cycle dysregulation during prostate cancer progression. Endocr.-Relat. Cancer 2021, 28, R141–R155. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Zhang, J.; Gu, Y. Targeting “undruggable” c-Myc protein by synthetic lethality. Front. Med. 2021, 15, 541–550. [Google Scholar] [CrossRef]
- Miller, D.R.; Ingersoll, M.A.; Teply, B.A.; Lin, M.-F. Targeting treatment options for castration-resistant prostate cancer. Am. J. Clin. Exp. Urol. 2021, 9, 101–120. [Google Scholar]
- Dey, P.; Kimmelman, A.C.; DePinho, R.A. Metabolic Codependencies in the Tumor Microenvironment. Cancer Discov. 2021, 11, 1067–1081. [Google Scholar] [CrossRef]
- Mukha, A.; Kahya, U.; Dubrovska, A. Targeting glutamine metabolism and autophagy: The combination for prostate cancer radiosensitization. Autophagy 2021, 17, 3879–3881. [Google Scholar] [CrossRef]
- Clegg, N.J.; Couto, S.S.; Wongvipat, J.; Hieronymus, H.; Carver, B.S.; Taylor, B.S.; Ellwood-Yen, K.; Gerald, W.L.; Sander, C.; Sawyers, C.L. MYC Cooperates with AKT in Prostate Tumorigenesis and Alters Sensitivity to mTOR Inhibitors. PLoS ONE 2011, 6, e17449. [Google Scholar] [CrossRef]
- Goetzman, E.S.; Prochownik, E.V. The Role for Myc in Coordinating Glycolysis, Oxidative Phosphorylation, Glutaminolysis, and Fatty Acid Metabolism in Normal and Neoplastic Tissues. Front. Endocrinol. 2018, 9, 129. [Google Scholar] [CrossRef]
- Priolo, C.; Pyne, S.; Rose, J.; Regan, E.R.; Zadra, G.; Photopoulos, C.; Cacciatore, S.; Schultz, D.; Scaglia, N.; McDunn, J.; et al. AKT1 and MYC Induce Distinctive Metabolic Fingerprints in Human Prostate Cancer. Cancer Res. 2014, 74, 7198–7204. [Google Scholar] [CrossRef]
- Bai, S.; Cao, S.; Jin, L.; Kobelski, M.; Schouest, B.; Wang, X.; Ungerleider, N.; Baddoo, M.; Zhang, W.; Corey, E.; et al. A positive role of c-Myc in regulating androgen receptor and its splice variants in prostate cancer. Oncogene 2019, 38, 4977–4989. [Google Scholar] [CrossRef]
- Bernard, D.; Pourtier-Manzanedo, A.; Gil, J.; Beach, D.H. Myc confers androgen-independent prostate cancer cell growth. J. Clin. Investig. 2003, 112, 1724–1731. [Google Scholar] [CrossRef]
- Gao, L. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLoS ONE 2013, 8, e63563. [Google Scholar]
- Barfeld, S.J.; Urbanucci, A.; Itkonen, H.M.; Fazli, L.; Hicks, J.L.; Thiede, B.; Rennie, P.S.; Yegnasubramanian, S.; DeMarzo, A.M.; Mills, I.G. c-Myc Antagonises the Transcriptional Activity of the Androgen Receptor in Prostate Cancer Affecting Key Gene Networks. EBioMedicine 2017, 18, 83–93. [Google Scholar] [CrossRef]
- Long, T.; Hicks, M.; Yu, H.-C.; Biggs, W.H.; Kirkness, E.F.; Menni, C.; Zierer, J.; Small, K.S.; Mangino, M.; Messier, H.; et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017, 49, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Deng, Y.; Ye, J.; Zhuo, Y.; Liu, Z.; Liang, Y.; Zhang, H.; Zhu, X.; Luo, Y.; Feng, Y.; et al. Aberrant Expression of Citrate Synthase is Linked to Disease Progression and Clinical Outcome in Prostate Cancer. Cancer Manag. Res. 2020, 12, 6149–6163. [Google Scholar] [CrossRef]
- Gilbert, R.; Bonilla, C.; Metcalfe, C.; Lewis, S.; Evans, D.M.; Fraser, W.D.; Kemp, J.P.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: A nested case–control study. Cancer Causes Control. 2014, 26, 205–218. [Google Scholar] [CrossRef]
- Zecchini, V. Nuclear ARRB1 induces pseudohypoxia and cellular metabolism reprogramming in prostate cancer. EMBO J. 2014, 33, 1365–1382. [Google Scholar] [CrossRef]
- Hong, M.-G.; Karlsson, R.; Magnusson, P.K.E.; Lewis, M.R.; Isaacs, W.; Zheng, L.S.; Xu, J.; Grönberg, H.; Ingelsson, E.; Pawitan, Y.; et al. A Genome-Wide Assessment of Variability in Human Serum Metabolism. Hum. Mutat. 2013, 34, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.M.; Sreekumar, A.; Chinnaiyan, A.M.; Ghosh, D. Pathway-directed weighted testing procedures for the inte-grative analysis of gene expression and metabolomic data. Genomics 2012, 99, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Asara, J.M.; Sanda, M.G.; Arredouani, M.S. The Role of the Transcription Factor SIM2 in Prostate Cancer. PLoS ONE 2011, 6, e28837. [Google Scholar] [CrossRef] [PubMed]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef]
- Van Tilborg, D.; Saccenti, E. Cancers in agreement? Exploring the cross-talk of cancer metabolomic and transcriptomic landscapes using publicly available data. Cancers 2021, 13, 393. [Google Scholar] [CrossRef]
- Wang, W.; He, Z.; Kong, Y.; Liu, Z.; Gong, L. GC-MS-based metabolomics reveals new biomarkers to assist the differentiation of prostate cancer and benign prostatic hyperplasia. Clin. Chim. Acta 2021, 519, 10–17. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; García-Flores, M.; Rubio-Briones, J.; López-Guerrero, J.A.; Pineda-Lucena, A.; Puchades-Carrasco, L. Targeted Metabolomics Analyses Reveal Specific Metabolic Alterations in High-Grade Prostate Cancer Patients. J. Proteome Res. 2020, 19, 4082–4092. [Google Scholar] [CrossRef]
- Andersen, M.K.; Rise, K.; Giskeødegård, G.F.; Richardsen, E.; Bertilsson, H.; Størkersen, Ø.; Bathen, T.F.; Rye, M.; Tessem, M.-B. Integrative metabolic and transcriptomic profiling of prostate cancer tissue containing reactive stroma. Sci. Rep. 2018, 8, 14269. [Google Scholar] [CrossRef]
- Shao, Y.; Ye, G.; Ren, S.; Piao, H.-L.; Zhao, X.; Lu, X.; Wang, F.; Ma, W.; Li, J.; Yin, P.; et al. Metabolomics and transcriptomics profiles reveal the dysregulation of the tricarboxylic acid cycle and related mechanisms in prostate cancer. Int. J. Cancer 2018, 143, 396–407. [Google Scholar] [CrossRef]
- Al Kadhi, O. Increased transcriptional and metabolic capacity for lipid metabolism in the peripheral zone of the prostate may underpin its increased susceptibility to cancer. Oncotarget 2017, 8, 84902–84916. [Google Scholar] [CrossRef]
- Sandsmark, E.; Hansen, A.F.; Selnæs, K.M.; Bertilsson, H.; Bofin, A.M.; Wright, A.J.; Viset, T.; Richardsen, E.; Drabløs, F.; Bathen, T.F.; et al. A novel non-canonical Wnt signature for prostate cancer aggressiveness. Oncotarget 2017, 8, 9572–9586. [Google Scholar] [CrossRef]
- Ren, S.; Shao, Y.; Zhao, X.; Hong, C.S.; Wang, F.; Lu, X.; Li, J.; Ye, G.; Yan, M.; Zhuang, Z.; et al. Integration of Metabolomics and Transcriptomics Reveals Major Metabolic Pathways and Potential Biomarker Involved in Prostate Cancer. Mol. Cell. Proteom. 2016, 15, 154–163. [Google Scholar] [CrossRef]
- Torrano, V.; Valcarcel-Jimenez, L.; Cortazar, A.R.; Liu, X.; Urosevic, J.; Castillo-Martin, M.; Fernández-Ruiz, S.; Morciano, G.; Caro-Maldonado, A.; Guiu, M.; et al. The metabolic co-regulator PGC1α suppresses prostate cancer metastasis. Nature 2016, 18, 645–656. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Tang, S.; Zhang, Y.; Markiewski, M.; Xing, C.; Jiang, C.; Lu, J. Pyranocoumarin Tissue Distribution, Plasma Metabolome and Prostate Transcriptome Impacts of Sub-Chronic Exposure to Korean Angelica Supplement in Mice. Am. J. Chin. Med. 2016, 44, 321–353. [Google Scholar] [CrossRef]
- Cerasuolo, M.; Paris, D.; Iannotti, F.; Melck, D.; Verde, R.; Mazzarella, E.; Motta, A.; Ligresti, A. Neuroendocrine Transdifferentiation in Human Prostate Cancer Cells: An Integrated Approach. Cancer Res. 2015, 75, 2975–2986. [Google Scholar] [CrossRef]
- Meller, S. Integration of tissue metabolomics, transcriptomics and immunohistochemistry reveals ERG- and gleason score-specific metabolomic alterations in prostate cancer. Oncotarget 2016, 7, 1421–1438. [Google Scholar] [CrossRef]
- Kim, S.; Pevzner, P.A. MS-GF+ makes progress towards a universal database search tool for proteomics. Nat. Commun. 2014, 5, 5277. [Google Scholar] [CrossRef]
- Schroeder, M.; Jakovcevski, M.; Polacheck, T.; Lebow, M.; Drori, Y.; Engel, M.; Ben-Dor, S.; Chen, A. A Methyl-Balanced Diet Prevents CRF-Induced Prenatal Stress-Triggered Predisposition to Binge Eating-like Phenotype. Cell Metab. 2017, 25, 1269–1281.e6. [Google Scholar] [CrossRef]
- Felgueiras, J.; Silva, J.; Nunes, A.; Fernandes, I.; Patrício, A.; Maia, N.; Pelech, S.; Fardilha, M. Investigation of spectroscopic and proteomic alterations underlying prostate carcinogenesis. J. Proteom. 2020, 226, 103888. [Google Scholar] [CrossRef]
- Kopylov, A.T.; Petrovsky, D.V.; Stepanov, A.A.; Rudnev, V.R.; Malsagova, K.A.; Butkova, T.V.; Zakharova, N.V.; Kostyuk, G.P.; Kulikova, L.I.; Enikeev, D.V.; et al. Convolutional neural network in proteomics and metabolomics for determination of comorbidity between cancer and schizophrenia. J. Biomed. Inform. 2021, 122, 103890. [Google Scholar] [CrossRef]
- Shen, S.; Li, J.; Huo, S.; Ma, M.; Zhu, X.; Rasam, S.; Duan, X.; Qu, M.; Titus, M.A.; Qu, J. Parallel, High-Quality Proteomic and Targeted Metabolomic Quantification Using Laser Capture Microdissected Tissues. Anal. Chem. 2021, 93, 8711–8718. [Google Scholar] [CrossRef]
- Oberhuber, M.; Pecoraro, M.; Rusz, M.; Oberhuber, G.; Wieselberg, M.; Haslinger, P.; Gurnhofer, E.; Schlederer, M.; Limberger, T.; Lagger, S.; et al. STAT 3-dependent analysis reveals PDK 4 as independent predictor of recurrence in prostate cancer. Mol. Syst. Biol. 2020, 16, e9247. [Google Scholar] [CrossRef]
- Itkonen, H.M.; Poulose, N.; Walker, S.; Mills, I.G. CDK9 Inhibition Induces a Metabolic Switch that Renders Prostate Cancer Cells Dependent on Fatty Acid Oxidation. Neoplasia 2019, 21, 713–720. [Google Scholar] [CrossRef]
- Gao, B.; Lue, H.-W.; Podolak, J.; Fan, S.; Zhang, Y.; Serawat, A.; Alumkal, J.J.; Fiehn, O.; Thomas, G.V. Multi-Omics Analyses Detail Metabolic Reprogramming in Lipids, Carnitines, and Use of Glycolytic Intermediates between Prostate Small Cell Neuroendocrine Carcinoma and Prostate Adenocarcinoma. Metabolites 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kregel, S. Functional and mechanistic interrogation of BET bromodomain degraders for the treatment of metastatic cas-tration-resistant prostate cancer. Clin. Cancer Res. 2019, 25, 4038–4048. [Google Scholar] [CrossRef]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 631–640. [Google Scholar] [CrossRef]
- Murphy, K.; Murphy, B.T.; Boyce, S.; Flynn, L.; Gilgunn, S.; O’Rourke, C.J.; Rooney, C.; Stöckmann, H.; Walsh, A.L.; Finn, S.; et al. Integrating biomarkers across omic platforms: An approach to improve stratification of patients with indolent and aggressive prostate cancer. Mol. Oncol. 2018, 12, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.F.; Sandsmark, E.; Rye, M.B.; Wright, A.J.; Bertilsson, H.; Richardsen, E.; Viset, T.; Bofin, A.M.; Angelsen, A.; Selnæs, K.M.; et al. Presence of TMPRSS2-ERG is associated with alterations of the metabolic profile in human prostate cancer. Oncotarget 2016, 7, 42071–42085. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (accessed on 28 December 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Xu, H.; Qiu, S.; Wang, X. Cell metabolomics. OMICS A J. Integr. Biol. 2013, 17, 495–501. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Concepts of citrate production and secretion by prostate 1. Metabolic relationships. Prostate 1991, 18, 25–46. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Loizaga-Iriarte, A.; Zuñiga-Garcia, P.; Sánchez-Mosquera, P.; Cortazar, A.R.; González, E.; Torrano, V.; Alonso, C.; Pérez-Cormenzana, M.; Ugalde-Olano, A.; et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J. Extracell. Vesicles 2018, 7, 1470442. [Google Scholar] [CrossRef]
- Puhka, M. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metab-olites and strategies to study prostate cancer-related changes. Theranostics 2017, 7, 3824–3841. [Google Scholar] [CrossRef]
- Davalieva, K.; Kostovska, I.M.; Kiprijanovska, S.; Markoska, K.; Kubelka-Sabit, K.; Filipovski, V.; Stavridis, S.; Stankov, O.; Komina, S.; Petrusevska, G.; et al. Proteomics analysis of malignant and benign prostate tissue by 2D DIGE/MS reveals new insights into proteins involved in prostate cancer. Prostate 2015, 75, 1586–1600. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Hansen, A.F.; Bertilsson, H.; Gonzalez, S.V.; Kristiansen, K.A.; Bruheim, P.; Mjøs, S.A.; Angelsen, A.; Bathen, T.F.; Tessem, M.-B. Metabolic markers in blood can separate prostate cancer from benign prostatic hyperplasia. Br. J. Cancer 2015, 113, 1712–1719. [Google Scholar] [CrossRef]
- Lin, H.-M.; Mahon, K.; Weir, J.M.; Mundra, P.A.; Spielman, C.; Briscoe, K.; Gurney, H.; Mallesara, G.; Marx, G.; Stockler, M.R.; et al. A distinct plasma lipid signature associated with poor prognosis in castration-resistant prostate cancer. Int. J. Cancer 2017, 141, 2112–2120. [Google Scholar] [CrossRef]
- Albanes, D.; Weinstein, S.J.; Mondul, A.M. Abstract 3987: Prospective serum metabolomic profiles of prostate cancer by size and extent of primary tumor. In Clinical Research (Excluding Clinical Trials); American Association for Cancer Research: Philadelphia, PA, USA, 2016; p. 3981. [Google Scholar] [CrossRef]
- Roberts, M.J.; Richards, R.S.; Chow, C.W.; Buck, M.; Yaxley, J.; Lavin, M.; Schirra, H.J.; Gardiner, R.A. Seminal plasma enables selection and monitoring of active surveillance candidates using nuclear magnetic resonance-based metabolomics: A preliminary investigation. Prostate Int. 2017, 5, 149–157. [Google Scholar] [CrossRef]
- Andras, I.; Crisan, N.; Vesa, S.; Rahota, R.; Romanciuc, F.; Lazar, A.; Socaciu, C.; Matei, D.-V.; De Cobelli, O.; Bocsan, I.-S.; et al. Serum metabolomics can predict the outcome of first systematic transrectal prostate biopsy in patients with PSA <10 ng/ml. Futur. Oncol. 2017, 13, 1793–1800. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Prospective serum metabolomic profile of prostate cancer by size and extent of primary tumor. Oncotarget 2017, 8, 45190–45199. [Google Scholar] [CrossRef]
- Kühn, T. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a pro-spective metabolomics study. BMC Med. 2016, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Averna, T.A.; Kline, E.E.; Smith, A.Y.; Sillerud, L.O. A decrease in 1H nuclear magnetic resonance spectroscopically determined citrate in human seminal fluid accompanies the development of prostate adenocarcinoma. J. Urol. 2005, 173, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gupta, A.; Mandhani, A.; Sankhwar, S.N. NMR spectroscopy of filtered serum of prostate cancer: A new frontier in metabolomics. Prostate 2016, 76, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Van der Mijn, J.C. Lactic acidosis in prostate cancer: Consider the Warburg effect. Case Rep. Oncol. 2017, 10, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.N.; Karami-Tehrani, F.; Salami, S. Targeting prostate cancer cell metabolism: Impact of hexokinase and CPT-1 enzymes. Tumor Biol. 2014, 36, 2893–2905. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Kwon, H.; Oh, S.; Jin, X.; An, Y.J.; Park, S. Cancer metabolomics in basic science perspective. Arch. Pharmacal Res. 2015, 38, 372–380. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Ni, Y.; Shen, P.; Han, X. Lactate shuttle: From substance exchange to regulatory mechanism. Hum. Cell 2022, 35, 1–14. [Google Scholar] [CrossRef]
- Tessem, M.-B. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1 H HR-MAS spec-troscopy of biopsy tissues. Magn. Reson. Med. 2008, 60, 510–516. [Google Scholar] [CrossRef]
- Lima, A.R.; Araújo, A.M.; Pinto, J.; Jerónimo, C.; Henrique, R.; Bastos, M.D.L.; Carvalho, M.; de Pinho, P.G. GC-MS-Based Endometabolome Analysis Differentiates Prostate Cancer from Normal Prostate Cells. Metabolites 2018, 8, 23. [Google Scholar] [CrossRef]
- Andersen, M.K.; Giskeødegård, G.F.; Tessem, M.-B. Metabolic alterations in tissues and biofluids of patients with prostate cancer. Curr. Opin. Endocr. Metab. Res. 2020, 10, 23–28. [Google Scholar] [CrossRef]
- Cascardo, F.; Anselmino, N.; Páez, A.; Labanca, E.; Sanchis, P.; Antico-Arciuch, V.; Navone, N.; Gueron, G.; Vázquez, E.; Cotignola, J. HO-1 Modulates Aerobic Glycolysis through LDH in Prostate Cancer Cells. Antioxidants 2021, 10, 966. [Google Scholar] [CrossRef]
- Baron, A.; Migita, T.; Tang, D.; Loda, M. Fatty acid synthase: A metabolic oncogene in prostate cancer? J. Cell. Biochem. 2004, 91, 47–53. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.-R.; Kim, S.-H.; Wendell, S.G.; Singh, S.V. A novel metabolic function of Myc in regulation of fatty acid synthesis in prostate cancer. Oncogene 2021, 40, 592–602. [Google Scholar] [CrossRef]
- Suburu, J.; Chen, Y.Q. Lipids and prostate cancer. Prostaglandins Other Lipid Mediat. 2012, 98, 1–10. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Bertilsson, H.; Selnæs, K.M.; Wright, A.J.; Bathen, T.F.; Viset, T.; Halgunset, J.; Angelsen, A.; Gribbestad, I.S.; Tessem, M.-B. Spermine and Citrate as Metabolic Biomarkers for Assessing Prostate Cancer Aggressiveness. PLoS ONE 2013, 8, e62375. [Google Scholar] [CrossRef]
- Zabala-Letona, A.; Arruabarrena-Aristorena, A.; Martín-Martín, N.; Fernandez-Ruiz, S.; Sutherland, J.D.; Clasquin, M.; Tomas-Cortazar, J.; Jimenez, J.; Torres, I.; Quang, P.; et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 2017, 547, 109–113. [Google Scholar] [CrossRef]
- Lloyd, S.M.; Arnold, J.; Sreekumar, A. Metabolomic profiling of hormone-dependent cancers: A bird’s eye view. Trends Endocrinol. Metab. 2015, 26, 477–485. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Jadallah, S.; Toubaji, A.; Lecksell, K.; Hicks, J.L.; Kowalski, J.; Bova, G.S.; De Marzo, A.M.; Netto, G.J.; Casero, R.A., Jr. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 2008, 68, 766–772. [Google Scholar] [CrossRef]
- Sharma, U.; Jagannathan, N.R. Metabolism of prostate cancer by magnetic resonance spectroscopy (MRS). Biophys. Rev. 2020, 12, 1163–1173. [Google Scholar] [CrossRef]
- Madhu, B.; Shaw, G.L.; Warren, A.Y.; Neal, D.E.; Griffiths, J.R. Response of Degarelix treatment in human prostate cancer monitored by HR-MAS 1H NMR spectroscopy. Metabolomics 2016, 12, 120. [Google Scholar] [CrossRef]
- Awwad, H.M.; Geisel, J.; Obeid, R. The role of choline in prostate cancer. Clin. Biochem. 2012, 45, 1548–1553. [Google Scholar] [CrossRef]
- Wen, S.; He, Y.; Wang, L.; Zhang, J.; Quan, C.; Niu, Y.; Huang, H. Aberrant activation of super enhancer and choline metabolism drive antiandrogen therapy resistance in prostate cancer. Oncogene 2020, 39, 6556–6571. [Google Scholar] [CrossRef] [PubMed]
- Tayari, N.; Wright, A.J.; Heerschap, A. Absolute choline tissue concentration mapping for prostate cancer localization and characterization using 3D 1 H MRSI without water-signal suppression. Magn. Reson. Med. 2022, 87, 561–573. [Google Scholar] [CrossRef]
- Wang, M.; Zou, L.; Liang, J.; Wang, X.; Zhang, D.; Fang, Y.; Zhang, J.; Xiao, F.; Liu, M. The Urinary Sarcosine/Creatinine Ratio is a Potential Diagnostic and Prognostic Marker in Prostate Cancer. Med Sci. Monit. 2018, 24, 3034–3041. [Google Scholar] [CrossRef]
- Corbin, J.M.; Ruiz-Echevarría, M.J. One-Carbon Metabolism in Prostate Cancer: The Role of Androgen Signaling. Int. J. Mol. Sci. 2016, 17, 1208. [Google Scholar] [CrossRef]
- Valle, S.; Sharifi, N. Targeting Glucocorticoid Metabolism in Prostate Cancer. Endocrinology 2021, 162. [Google Scholar] [CrossRef] [PubMed]
- Puhr, M. The glucocorticoid receptor Is a key player for prostate cancer cell survival and a target for improved anti-androgen therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Q. The role of glucocorticoid receptor in prostate cancer progression: From bench to bedside. Int. Urol. Nephrol. 2017, 49, 369–380. [Google Scholar] [CrossRef]
- Moon, J.-S.; Hisata, S.; Park, M.-A.; DeNicola, G.M.; Ryter, S.W.; Nakahira, K.; Choi, A.M.K. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015, 12, 102–115. [Google Scholar] [CrossRef]
- Mamouni, K.; Kallifatidis, G.; Lokeshwar, B. Targeting Mitochondrial Metabolism in Prostate Cancer with Triterpenoids. Int. J. Mol. Sci. 2021, 22, 2466. [Google Scholar] [CrossRef]
- Singh, K.K.; Desouki, M.M.; Franklin, R.B.; Costello, L.C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer 2006, 5, 14. [Google Scholar] [CrossRef]
- Barron, E.G.; Huggins, C. The Metabolism of the Prostate: Transamination and Citric Acid. J. Urol. 1946, 55, 385–390. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, J.; Li, B.; Ding, Z.; Cheng, W.; Gao, F.; Zhang, Y.; Xu, Y.; Zhang, S. Cornin protects SH-SY5Y cells against oxygen and glucose deprivation-induced autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2017, 17, 87–92. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Zinc is decreased in prostate cancer: An established relationship of prostate cancer! JBIC J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef]
- Flavin, R.; Zadra, G.; Loda, M. Metabolic alterations and targeted therapies in prostate cancer. J. Pathol. 2010, 223, 284–295. [Google Scholar] [CrossRef]
- Strmiska, V.; Michalek, P.; Eckschlager, T.; Stiborova, M.; Adam, V.; Krizkova, S.; Heger, Z. Prostate cancer-specific hallmarks of amino acids metabolism: Towards a paradigm of precision medicine. Biochim. Biophys. Acta 2019, 1871, 248–258. [Google Scholar] [CrossRef]
- Soga, T. Cancer metabolism: Key players in metabolic reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Zheng, Y.; Cho, S.; Jang, C.; England, C.; Dempsey, J.M.; Yu, Y.; Liu, X.; He, L.; Cavaliere, P.M.; et al. Post-transcriptional Regulation of De Novo Lipogenesis by mTORC1-S6K1-SRPK2 Signaling. Cell 2017, 171, 1545–1558.e18. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef]
- Hsieh, A.; Edlind, M. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy re-sistance. Asian J. Androl. 2014, 16, 378. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Ni, J.; Hao, J.; Bucci, J.; Cozzi, P.J.; Li, Y. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit. Rev. Oncol. 2015, 96, 507–517. [Google Scholar] [CrossRef]
- Fang, M.; Shen, Z.; Huang, S.; Zhao, L.; Chen, S.; Mak, T.W.; Wang, X. The ER UDPase ENTPD5 Promotes Protein N-Glycosylation, the Warburg Effect, and Proliferation in the PTEN Pathway. Cell 2010, 143, 711–724. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Tan, M.-H.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene 2011, 30, 4327–4338. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Sun, X.; Xu, X.; Li, X.; Guo, Y.; Wang, J.; Yao, L.; Wang, H.; Shen, L. Effect of PTEN loss on metabolic reprogramming in prostate cancer cells. Oncol. Lett. 2019, 17, 2856–2866. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, J. Targeting hexokinase 2 in castration-resistant prostate cancer. Mol. Cell. Oncol. 2015, 2, e974465. [Google Scholar] [CrossRef]
- Clapé, C.; Fritz, V.; Henriquet, C.; Apparailly, F.; Fernandez, P.L.; Iborra, F.; Avancès, C.; Villalba, M.; Culine, S.; Fajas, L. miR-143 Interferes with ERK5 Signaling, and Abrogates Prostate Cancer Progression in Mice. PLoS ONE 2009, 4, e7542. [Google Scholar] [CrossRef]
- Xu, B.; Niu, X.; Zhang, X.; Tao, J.; Wu, D.; Wang, Z.; Li, P.; Zhang, W.; Wu, H.; Feng, N.; et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol. Cell. Biochem. 2011, 350, 207–213. [Google Scholar] [CrossRef]
- Chu, H.; Zhong, D.; Tang, J.; Li, J.; Xue, Y.; Tong, N.; Qin, C.; Yin, C.; Zhang, Z.; Wang, M. A functional variant in miR-143 promoter contributes to prostate cancer risk. Arch. Toxicol. 2014, 90, 403–414. [Google Scholar] [CrossRef]
- Wach, S.; Brandl, M.; Borchardt, H.; Weigelt, K.; Lukat, S.; Nolte, E.; Al-Janabi, O.; Hart, M.; Grässer, F.; Giedl, J.; et al. Exploring the MIR143-UPAR Axis for the Inhibition of Human Prostate Cancer Cells In Vitro and In Vivo. Mol. Ther.-Nucleic Acids 2019, 16, 272–283. [Google Scholar] [CrossRef]
- Ros, S.; Santos, C.R.; Moco, S.; Baenke, F.; Kelly, G.; Howell, M.; Zamboni, N.; Schulze, A. Functional Metabolic Screen Identifies 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 4 as an Important Regulator of Prostate Cancer Cell Survival. Cancer Discov. 2012, 2, 328–343. [Google Scholar] [CrossRef]
- Chiacchiera, F.; Simone, C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle 2010, 9, 1091–1096. [Google Scholar] [CrossRef]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.-Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Abrate, A.; Lughezzani, G.; Gadda, G.M.; Lista, G.; Kinzikeeva, E.; Fossati, N.; Larcher, A.; Dell’Oglio, P.; Mistretta, F.; Buffi, N.; et al. Clinical Use of [-2]proPSA (p2PSA) and Its Derivatives (% p2PSA and Prostate Health Index) for the Detection of Prostate Cancer: A Review of the Literature. Korean J. Urol. 2014, 55, 436–445. [Google Scholar] [CrossRef]
- Beattie, D.S. Bioenergetics and oxidative metabolism. In Textbook of Biochemistry with Clinical Correlations; Devlin, T.M., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2006; pp. 529–580. [Google Scholar]
- Costello, L.C.; Franklin, R.B. Prostatic fluid electrolyte composition for the screening of prostate cancer: A potential solution to a major problem. Prostate Cancer Prostatic Dis. 2008, 12, 17–24. [Google Scholar] [CrossRef][Green Version]
- Pértega-Gomes, N.; Baltazar, F. Lactate transporters in the context of prostate cancer metabolism: What do we know? Int. J. Mol. Sci. 2014, 15, 18333–18348. [Google Scholar] [CrossRef]
- Dakubo, G.D.; Parr, R.L.; Costello, L.C.; Franklin, R.B.; Thayer, R.E. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 2006, 59, 10–16. [Google Scholar] [CrossRef]
- Kratochvilova, M.; Raudenska, M.; Heger, Z.; Richtera, L.; Cernei, N.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M.; Gumulec, J. Amino Acid Profiling of Zinc Resistant Prostate Cancer Cell Lines: Associations with Cancer Progression. Prostate 2017, 77, 604–616. [Google Scholar] [CrossRef]
- Franz, M.-C.; Anderle, P.; Bürzle, M.; Suzuki, Y.; Freeman, M.; Hediger, M.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef]
- Eide, D.J. The SLC39 family of metal ion transporters. Pflug. Arch. Eur. J. Physiol. 2004, 447, 796–800. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflug. Arch. Eur. J. Physiol. 2004, 447, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional Expression of the Human hZIP2 Zinc Transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Chhabra, G.; Patel, A.; Chang, H.; Ahmad, N. Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management. Nutrients 2021, 13, 1867. [Google Scholar] [CrossRef] [PubMed]
- Desouki, M.M.; Geradts, J.; Milon, B.; Franklin, R.B.; Costello, L.C. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 2007, 6, 37. [Google Scholar] [CrossRef]
- Rishi, I.; Baidouri, H.; Abbasi, J.A.; Bullard-Dillard, R.; Kajdacsy-Balla, A.; Pestaner, J.P.; Skacel, M.; Tubbs, R.; Bagasra, O. Prostate Cancer in African American Men Is Associated With Downregulation of Zinc Transporters. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 253–260. [Google Scholar] [CrossRef]
- Chen, Q. The role of zinc transporter ZIP4 in prostate carcinoma. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 906–911. [Google Scholar] [CrossRef]
- Kuliyev, E.; Zhang, C.; Sui, D.; Hu, J. Zinc transporter mutations linked to acrodermatitis enteropathica disrupt function and cause mistrafficking. J. Biol. Chem. 2021, 296, 100269. [Google Scholar] [CrossRef]
- Golovine, K.; Uzzo, R.G.; Makhov, P.; Crispen, P.L.; Kunkle, D.; Kolenko, V.M. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-κB-dependent pathway. Prostate 2008, 68, 1443–1449. [Google Scholar] [CrossRef]
- Golovine, K. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-κB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.-L.; Guan, Z.-X.; Franklin, R.B.; Costello, L.C. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002, 52, 311–318. [Google Scholar] [CrossRef]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Crispen, P.L.; Golovine, K.; Makhov, P.; Horwitz, E.M.; Kolenko, V.M. Diverse effects of zinc on NF- B and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis 2006, 27, 1980–1990. [Google Scholar] [CrossRef]
- Latonen, L.; Afyounian, E.; Jylhä, A.; Nättinen, J.; Aapola, U.; Annala, M.; Kivinummi, K.K.; Tammela, T.T.L.; Beuerman, R.W.; Uusitalo, H.; et al. Integrative proteomics in prostate cancer uncovers robustness against genomic and transcriptomic aberrations during disease progression. Nat. Commun. 2018, 9, 1176. [Google Scholar] [CrossRef]
- Zadra, G.; Loda, M. Metabolic Vulnerabilities of Prostate Cancer: Diagnostic and Therapeutic Opportunities. Cold Spring Harb. Perspect. Med. 2018, 8, a030569. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.D. α-Methylacyl-CoA racemase (AMACR): Metabolic enzyme, drug metabolizer and cancer marker P504S. Prog. Lipid Res. 2013, 52, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Roskams, T.; Joniau, S.; Van Poppel, H.; Oyen, R.; Baert, L.; Heyns, W.; Verhoeven, G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 2002, 98, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.G.; Keshari, K.R.; Tabatabai, Z.L.; Simko, J.P.; Shinohara, K.; Carroll, P.R.; Zektzer, A.S.; Kurhanewicz, J. Quantification of choline- and ethanolamine-containing metabolites in human prostate tissues using1H HR-MAS total correlation spectroscopy. Magn. Reson. Med. 2008, 60, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, T. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J. Pathol. 2005, 206, 214–219. [Google Scholar] [CrossRef]
- Swinnen, J.V. Androgens, lipogenesis and prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 273–279. [Google Scholar] [CrossRef]
- Ettinger, S.L.; Sobel, R.; Whitmore, T.G.; Akbari, M.; Bradley, D.R.; Gleave, M.E.; Nelson, C.C. Dysregulation of Sterol Response Element-Binding Proteins and Downstream Effectors in Prostate Cancer during Progression to Androgen Independence. Cancer Res. 2004, 64, 2212–2221. [Google Scholar] [CrossRef]
- Guo, D.; Bell, E.; Mischel, P.; Chakravarti, A. Targeting SREBP-1-driven Lipid Metabolism to Treat Cancer. Curr. Pharm. Des. 2014, 20, 2619–2626. [Google Scholar] [CrossRef]
- Huang, W.-C.; Li, X.; Liu, J.; Lin, J.; Chung, L.W. Activation of Androgen Receptor, Lipogenesis, and Oxidative Stress Converged by SREBP-1 Is Responsible for Regulating Growth and Progression of Prostate Cancer Cells. Mol. Cancer Res. 2012, 10, 133–142. [Google Scholar] [CrossRef]
- De Piano, M. Exploring a role for fatty acid synthase in prostate cancer cell migration. Small GTPases 2021, 12, 265–272. [Google Scholar] [CrossRef]
- Roberts, M.J.; Schirra, H.J.; Lavin, M.; Gardiner, R.A. Metabolomics: A Novel Approach to Early and Noninvasive Prostate Cancer Detection. Korean J. Urol. 2011, 52, 79–89. [Google Scholar] [CrossRef]
- Jamnagerwalla, J.; Howard, L.E.; Allott, E.H.; Vidal, A.C.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freeman, M.R.; Freedland, S.J. Serum cholesterol and risk of high-grade prostate cancer: Results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Platz, E.A.; Till, C.; Goodman, P.J.; Parnes, H.L.; Figg, W.D.; Albanes, D.; Neuhouser, M.L.; Klein, E.A.; Thompson, I.M.; Kristal, A. Men with Low Serum Cholesterol Have a Lower Risk of High-Grade Prostate Cancer in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2807–2813. [Google Scholar] [CrossRef]
- Pelton, K.; Freeman, M.R.; Solomon, K.R. Cholesterol and prostate cancer. Curr. Opin. Pharmacol. 2012, 12, 751–759. [Google Scholar] [CrossRef]
- Wang, X.; Sun, B.; Wei, L.; Jian, X.; Shan, K.; He, Q.; Huang, F.; Ge, X.; Gao, X.; Feng, N.; et al. Cholesterol and saturated fatty acids synergistically promote the malignant progression of prostate cancer. Neoplasia 2022, 24, 86–97. [Google Scholar] [CrossRef]
- Cheng, Y.; Meng, Y.; Li, S.; Cao, D.; Ben, S.; Qin, C.; Hua, L.; Cheng, G. Genetic variants in the cholesterol biosynthesis pathway genes and risk of prostate cancer. Gene 2021, 774, 145432. [Google Scholar] [CrossRef]
- Garrido, M.M.; Marta, J.C.; Ribeiro, R.M.; Pinheiro, L.C.; Guimarães, J.T. Serum lipids and prostate cancer. J. Clin. Lab. Anal. 2021, 35, e23705. [Google Scholar] [CrossRef]
- Xiong, K. The cholesterol esterification inhibitor avasimibe suppresses tumour proliferation and metastasis via the E2F-1 signalling pathway in prostate cancer. Cancer Cell Int. 2021, 21, 461. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.-H. Modulation of Cholesterol Metabolism Improves Response to Enzalutamide Treatment in Prostate Cancer. Curr. Dev. Nutr. 2021, 5, 269. [Google Scholar] [CrossRef]
- Pan, T.; Lin, S.-C.; Lee, Y.-C.; Yu, G.; Song, J.H.; Pan, J.; Titus, M.; Satcher, R.L.; Panaretakis, T.; Logothetis, C.; et al. Statins reduce castration-induced bone marrow adiposity and prostate cancer progression in bone. Oncogene 2021, 40, 4592–4603. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, C.; Linxweiler, J.; Schmucker, P.; Snaebjornsson, M.T.; Schmitz, W.; Wach, S.; Krebs, M.; Hartmann, E.; Puhr, M.; Müller, A.; et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat. Commun. 2021, 12, 5066. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, C.; Sbiera, I.; Krebs, M.; Sbiera, S.; Spahn, M.; Kneitz, B.; Joniau, S.; Fassnacht, M.; Kübler, H.; Weigand, I.; et al. High expression of Sterol-O-Acyl transferase 1 (SOAT1), an enzyme involved in cholesterol metabolism, is associated with earlier biochemical recurrence in high risk prostate cancer. Prostate Cancer Prostatic Dis. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zabielska, J.; Sledzinski, T.; Stelmanska, E. Acyl-Coenzyme A: Cholesterol Acyltransferase Inhibition in Cancer Treatment. Anticancer Res. 2019, 39, 3385–3394. [Google Scholar] [CrossRef]
- Rogers, M.A. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J. Steroid Biochem. Mol. Biol. 2015, 151, 102–107. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Jia, R. Role of de novo cholesterol synthesis enzymes in cancer. J. Cancer 2020, 11, 1761–1767. [Google Scholar] [CrossRef]
- Krycer, J.R.; Brown, A.J. Cholesterol accumulation in prostate cancer: A classic observation from a modern perspective. Biochim. Biophys. Acta 2013, 1835, 219–229. [Google Scholar] [CrossRef]
- Krycer, J.R.; Phan, L.; Brown, A.J. A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem. J. 2012, 446, 191–201. [Google Scholar] [CrossRef]
- Ayyagari, V.N.; Wang, X.; Diaz-Sylvester, P.L.; Groesch, K.; Brard, L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression—An in vitro study. PLoS ONE 2020, 15, e0228024. [Google Scholar] [CrossRef]
- Patel, D.; Ahmad, F.; Kambach, D.M.; Sun, Q.; Halim, A.S.; Kramp, T.; Camphausen, K.A.; Stommel, J.M. LXRβ controls glioblastoma cell growth, lipid balance, and immune modulation independently of ABCA1. Sci. Rep. 2019, 9, 15458. [Google Scholar] [CrossRef]
- Yi, X.; Li, Y.; Hu, X.; Wang, F.; Liu, T. Changes in phospholipid metabolism in exosomes of hormone-sensitive and hor-mone-resistant prostate cancer cells. J. Cancer 2021, 12, 2893–2902. [Google Scholar] [CrossRef]
- Chen, J.; Guccini, I.; Di Mitri, D.; Brina, D.; Revandkar, A.; Sarti, M.; Pasquini, E.; Alajati, A.; Pinton, S.; Losa, M.; et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat. Genet. 2018, 50, 219–228. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, X.; Shao, C.; Li, J.; Cheng, J.-X.; Kuang, S.; Ahmad, N.; Ratliff, T.; Liu, X. Plk1 Inhibition Enhances the Efficacy of Androgen Signaling Blockade in Castration-Resistant Prostate Cancer. Cancer Res. 2014, 74, 6635–6647. [Google Scholar] [CrossRef]
- Creative Diagnostics (CD). PI3K-AKT Signaling Pathway. 2022. Available online: https://www.creative-diagnostics.com/PI3K-AKT-Signaling-Pathway.htm (accessed on 14 January 2022).
- Li, X.; Chen, Y.-T.; Hu, P.; Huang, W.-C. Fatostatin Displays High Antitumor Activity in Prostate Cancer by Blocking SREBP-Regulated Metabolic Pathways and Androgen Receptor Signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef]
- Wei, S.; Machamer, C.E.; Espenshade, P.J. Fatostatin blocks ER exit of SCAP but inhibits cell growth in a SCAP-independent manner. J. Lipid Res. 2016, 57, 1564–1573. [Google Scholar] [CrossRef]
- Vettenranta, A.; Murtola, T.J.; Raitanen, J.; Raittinen, P.; Talala, K.; Taari, K.; Stenman, U.-H.; Tammela, T.L.J.; Auvinen, A. Outcomes of Screening for Prostate Cancer Among Men Who Use Statins. JAMA Oncol. 2021, 8, 61. [Google Scholar] [CrossRef]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Sáez-Martínez, P.; Gómez-Gómez, E.; León-González, A.J.; Fuentes-Fayos, A.C.; Yubero-Serrano, E.M.; Requena-Tapia, M.J.; López, M.; et al. Clinical, Cellular, and Molecular Evidence of the Additive Antitumor Effects of Biguanides and Statins in Prostate Cancer. J. Clin. Endocrinol. Metab. 2021, 106, e696–e710. [Google Scholar] [CrossRef]
- Jeong, I.G.; Lim, B.; Yun, S.-C.; Lim, J.H.; Hong, J.H.; Kim, C.-S. Adjuvant Low-dose Statin Use after Radical Prostatectomy: The PRO-STAT Randomized Clinical Trial. Clin. Cancer Res. 2021, 27, 5004–5011. [Google Scholar] [CrossRef]
- Prabhu, N.; Kapur, N.; Catalona, W.; Leikin, R.; Helenowski, I.; Jovanovich, B.; Gurley, M.; Okwuosa, T.M.; Kuzel, T.M. Statin use and risk of prostate cancer biochemical recurrence after radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 130.e9–130.e15. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Ding, K.; Crook, J.M.; O’Callaghan, C.J.; Higano, C.S.; Dearnaley, D.P.; Horwitz, E.M.; Goldenberg, S.L.; Gospodarowicz, M.K.; Klotz, L. The Association Between Statin Use and Outcomes in Patients Initiating Androgen Deprivation Therapy. Eur. Urol. 2021, 79, 446–452. [Google Scholar] [CrossRef]
- Schnier, J.B.; Nishi, K.; Gumerlock, P.H.; Gorin, F.A.; Bradbury, E.M. Glycogen synthesis correlates with andro-gen-dependent growth arrest in prostate cancer. BMC Urol. 2005, 5, 6. [Google Scholar] [CrossRef]
- Pelletier, J.; Bellot, G.; Gounon, P.; Lacas-Gervais, S.; Pouysségur, J.; Mazure, N.M. Glycogen Synthesis is Induced in Hypoxia by the Hypoxia-Inducible Factor and Promotes Cancer Cell Survival. Front. Oncol. 2012, 2, 18. [Google Scholar] [CrossRef]
- Schwartz, N.B. Carbohydrates metabolism II: Special pathways and glycoconjugates. In Textbook of Biochemistry with Clinical Applications; Devlin, T.M., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2006; pp. 637–660. [Google Scholar]
- Yang, H.C.; Wu, Y.H.; Yen, W.C.; Liu, H.Y.; Hwang, T.L.; Stern, A.; Chiu, D.T.Y. The redox role of G6PD in cell growth, cell death, and cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef]
- Tsouko, E. Regulation of the pentose phosphate pathway by an androgen receptor–mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis 2014, 3, e103. [Google Scholar] [CrossRef]
- Liu, X.; Olszewski, K.; Zhang, Y.; Lim, E.W.; Shi, J.; Zhang, X.; Zhang, J.; Lee, H.; Koppula, P.; Lei, G.; et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020, 22, 476–486. [Google Scholar] [CrossRef]
- Gillis, J.L.; Hinneh, J.A.; Ryan, N.K.; Irani, S.; Moldovan, M.; Quek, L.-E.; Shrestha, R.K.; Hanson, A.R.; Xie, J.; Hoy, A.J.; et al. A feedback loop between the androgen receptor and 6-phosphogluoconate dehydrogenase (6PGD) drives prostate cancer growth. ELife 2021, 10, e62592. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Tedeschi, P.M.; Markert, E.K.; Gounder, M.; Lin, H.; Dvorzhinski, D.; Dolfi, S.C.; Chan, L.L.-Y.; Qiu, J.; DiPaola, R.S.; Hirshfield, K.M.; et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013, 4, e877. [Google Scholar] [CrossRef]
- Chaneton, B.; Hillmann, P.; Zheng, L.; Martin, A.C.L.; Maddocks, O.D.K.; Chokkathukalam, A.; Coyle, J.E.; Jankevics, A.; Holding, F.P.; Vousden, K.H.; et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 2012, 491, 458–462. [Google Scholar] [CrossRef]
- Cardoso, H.; Figueira, M.; Madureira, P.; Socorro, S. PO-255 The pivotal role of glutaminolysis in prostate cancer cells and its regulation by androgens. ESMO Open 2018, 3, A120. [Google Scholar] [CrossRef]
- Cardoso, H.J.; Figueira, M.I.; Vaz, C.V.; Carvalho, T.M.A.; Brás, L.A.; Madureira, P.A.; Oliveira, P.J.; Sardão, V.A.; Socorro, S. Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5α-dihydrotestosterone regulation. Cell. Oncol. 2021, 44, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Koochekpour, S.; Majumdar, S.; Azabdaftari, G.; Attwood, K.; Scioneaux, R.; Subramani, D.; Manhardt, C.; Lorusso, G.D.; Willard, S.S.; Thompson, H.; et al. Serum Glutamate Levels Correlate with Gleason Score and Glutamate Blockade Decreases Proliferation, Migration, and Invasion and Induces Apoptosis in Prostate Cancer Cells. Clin. Cancer Res. 2012, 18, 5888–5901. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Dorai, B.; Pinto, J.T.; Grasso, M.; Cooper, A.J.L. High Levels of Glutaminase II Pathway Enzymes in Normal and Cancerous Prostate Suggest a Role in ‘Glutamine Addiction’. Biomolecules 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, N.M.; McCullough, C.; Shanmugavelandy, S.; Lee, J.; Lee, Y.; Dutta, P.; McHenry, J.; Nguyen, L.; Norton, W.; Jones, L.W.; et al. Metabolic Differences in Glutamine Utilization Lead to Metabolic Vulnerabilities in Prostate Cancer. Sci. Rep. 2017, 7, 16159. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, V.; Infantino, V. Citrate–new functions for an old metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Panara, K.; Kuchakulla, M.; Kulandavelu, S.; Burnstein, K.L.; Schally, A.V.; Hare, J.M.; Ramasamy, R. Alterations of tumor microenvironment by nitric oxide impedes castration-resistant prostate cancer growth. Proc. Natl. Acad. Sci. USA 2018, 115, 11298–11303. [Google Scholar] [CrossRef]
- Bhowmick, R.; Girotti, A.W. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photo-dynamic therapy model. Cancer Lett. 2014, 343, 115–122. [Google Scholar] [CrossRef]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front. Oncol. 2020, 10, 776. [Google Scholar] [CrossRef]
- Coomes, M.W. Amino acid metabolism. In Textbook of Biochemistry with Clinical Applications; Devlin, T.M., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2006; pp. 743–787. [Google Scholar]
- Donkena, K.V.; Yuan, H.; Young, C.Y. Vitamin Bs, one carbon metabolism and prostate cancer. Mini-Rev. Med. Chem. 2010, 10, 1385–1392. [Google Scholar] [CrossRef]
- Malviya, G.; Patel, R.; Salji, M.; Martinez, R.S.; Repiscak, P.; Mui, E.; Champion, S.; Mrowinska, A.; Johnson, E.; AlRasheedi, M.; et al. 18F-Fluciclovine PET metabolic imaging reveals prostate cancer tumour heterogeneity associated with disease resistance to androgen deprivation therapy. EJNMMI Res. 2020, 10, 143. [Google Scholar] [CrossRef]
- Saito, Y.; Soga, T. Amino acid transporters as emerging therapeutic targets in cancer. Cancer Sci. 2021, 112, 2958–2965. [Google Scholar] [CrossRef]
- Xu, M.; Sakamoto, S.; Matsushima, J.; Kimura, T.; Ueda, T.; Mizokami, A.; Kanai, Y.; Ichikawa, T. Up-Regulation of LAT1 during Antiandrogen Therapy Contributes to Progression in Prostate Cancer Cells. J. Urol. 2016, 195, 1588–1597. [Google Scholar] [CrossRef]
- Martinez, R.S.; Salji, M.J.; Rushworth, L.; Ntala, C.; Blanco, G.R.; Hedley, A.; Clark, W.; Peixoto, P.; Hervouet, E.; Renaude, E.; et al. SLFN5 Regulates LAT1-Mediated mTOR Activation in Castration-Resistant Prostate Cancer. Cancer Res. 2021, 81, 3664–3678. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).