Effects of Different Pesticides on the Brewing of Wine Investigated by GC-MS-Based Metabolomics
Abstract
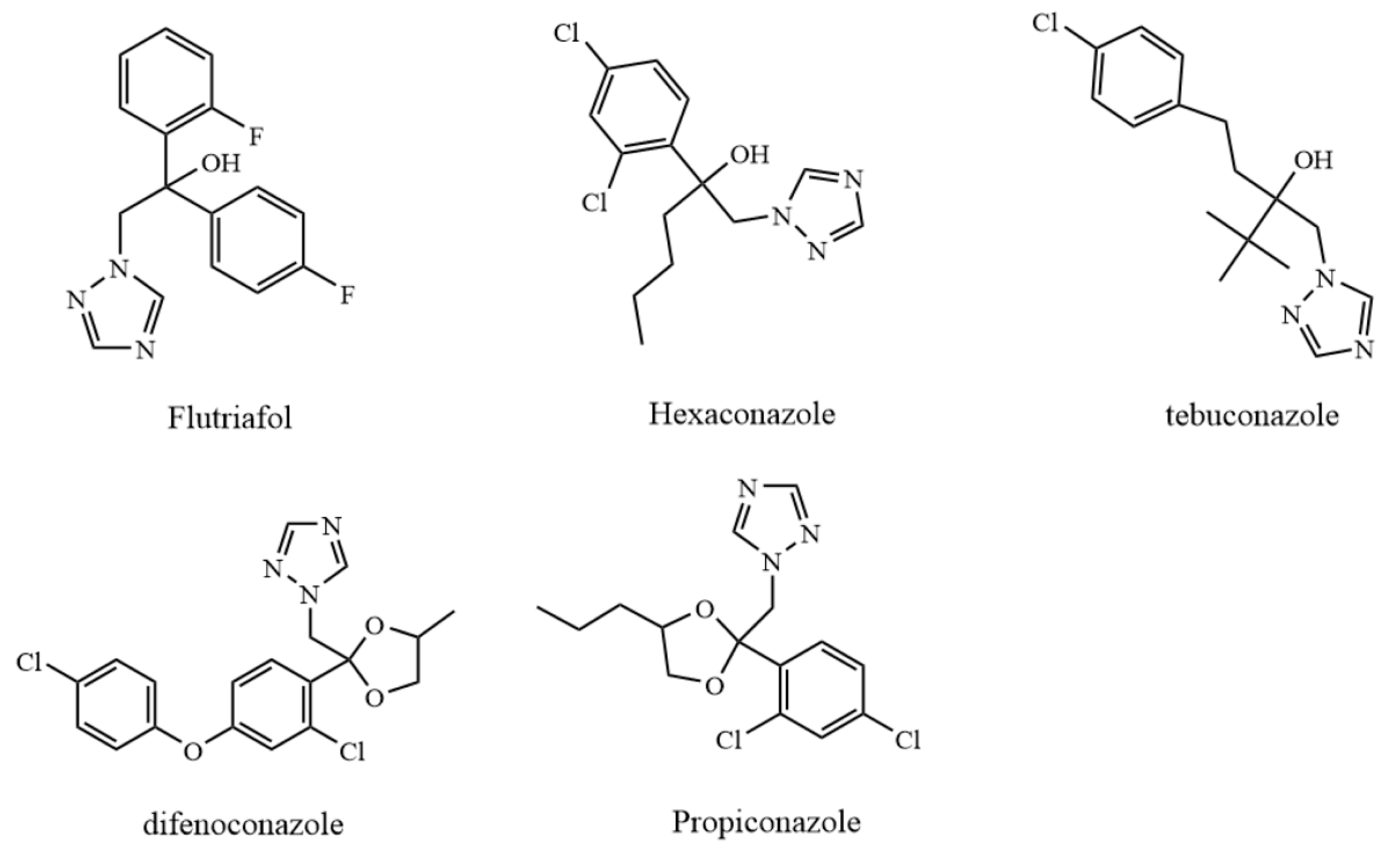
:1. Introduction
2. Result and Discussion
2.1. Effects of F, H, T, D, and P on the Growth of Saccharomyces cerevisiae
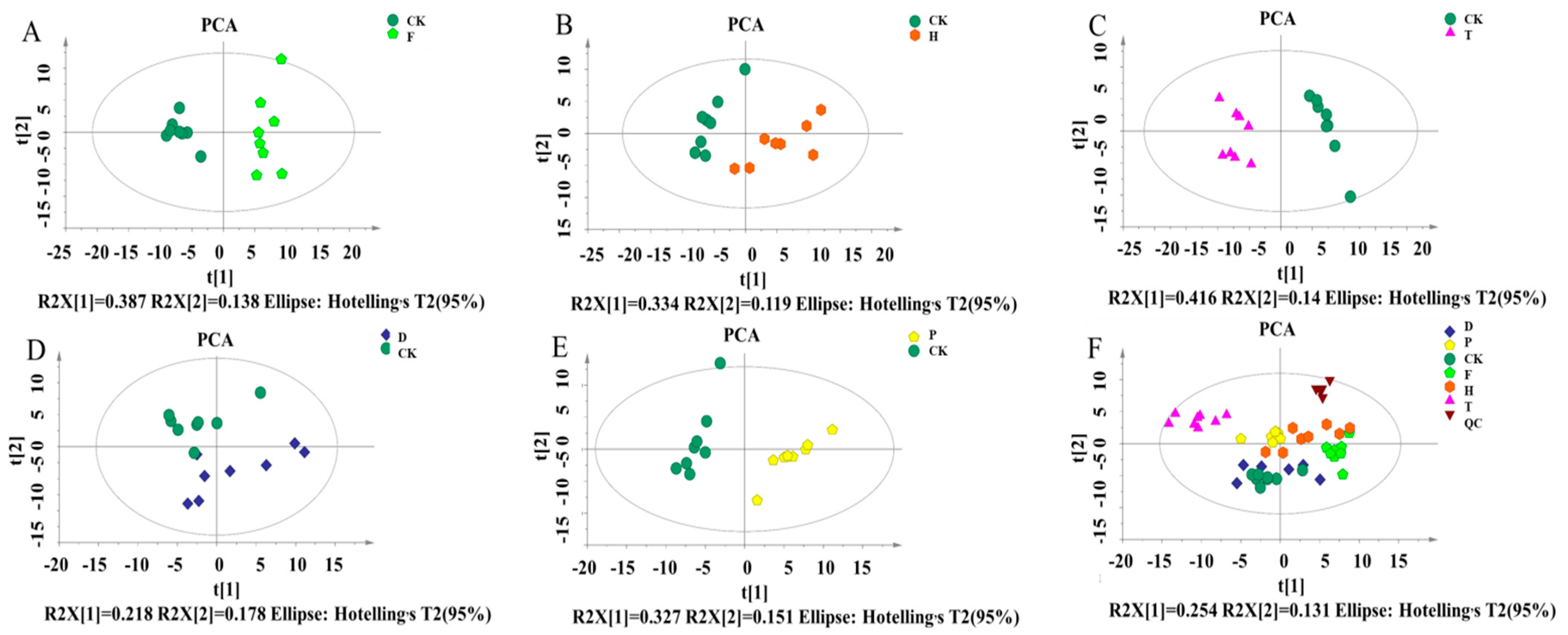
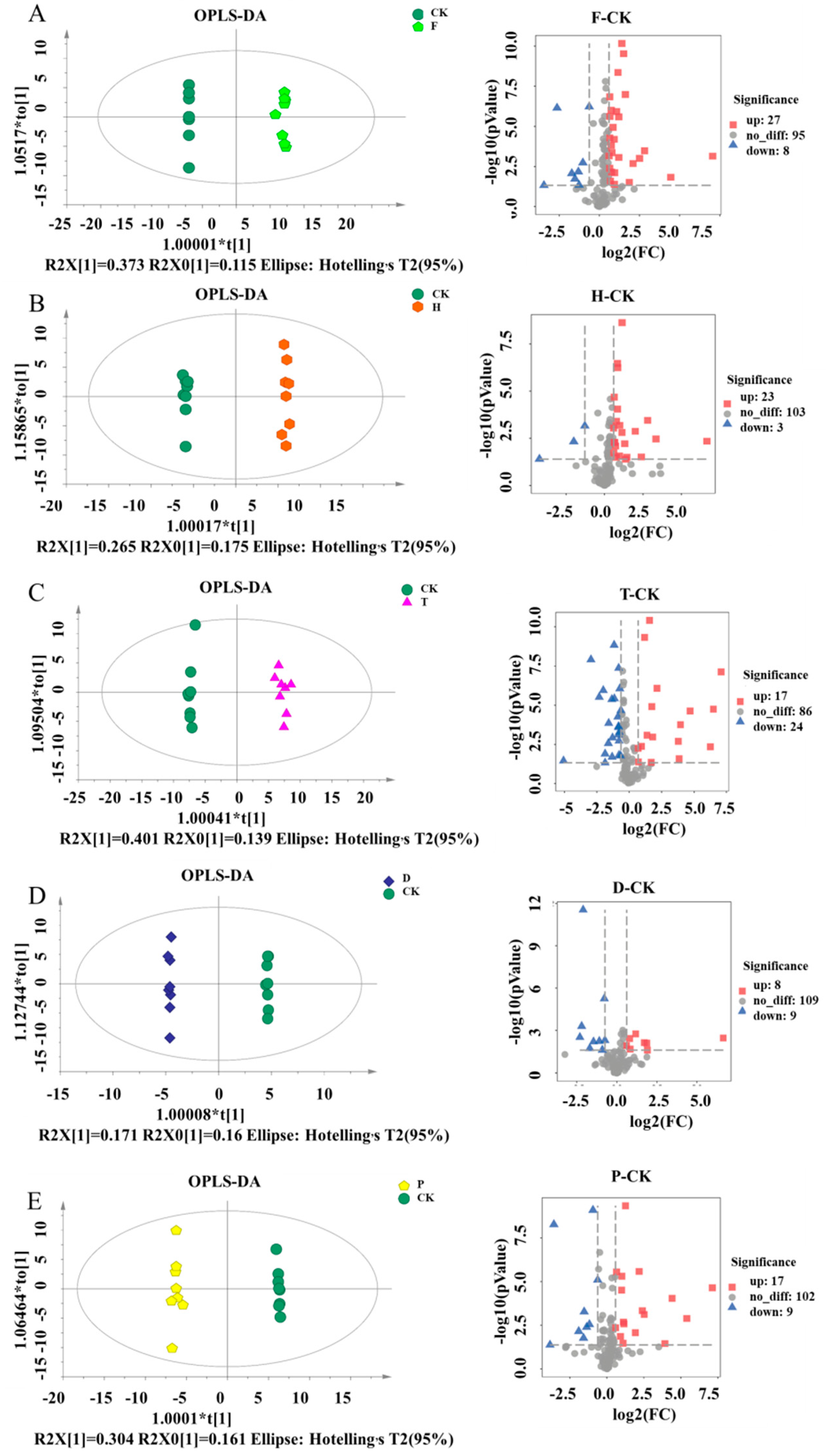
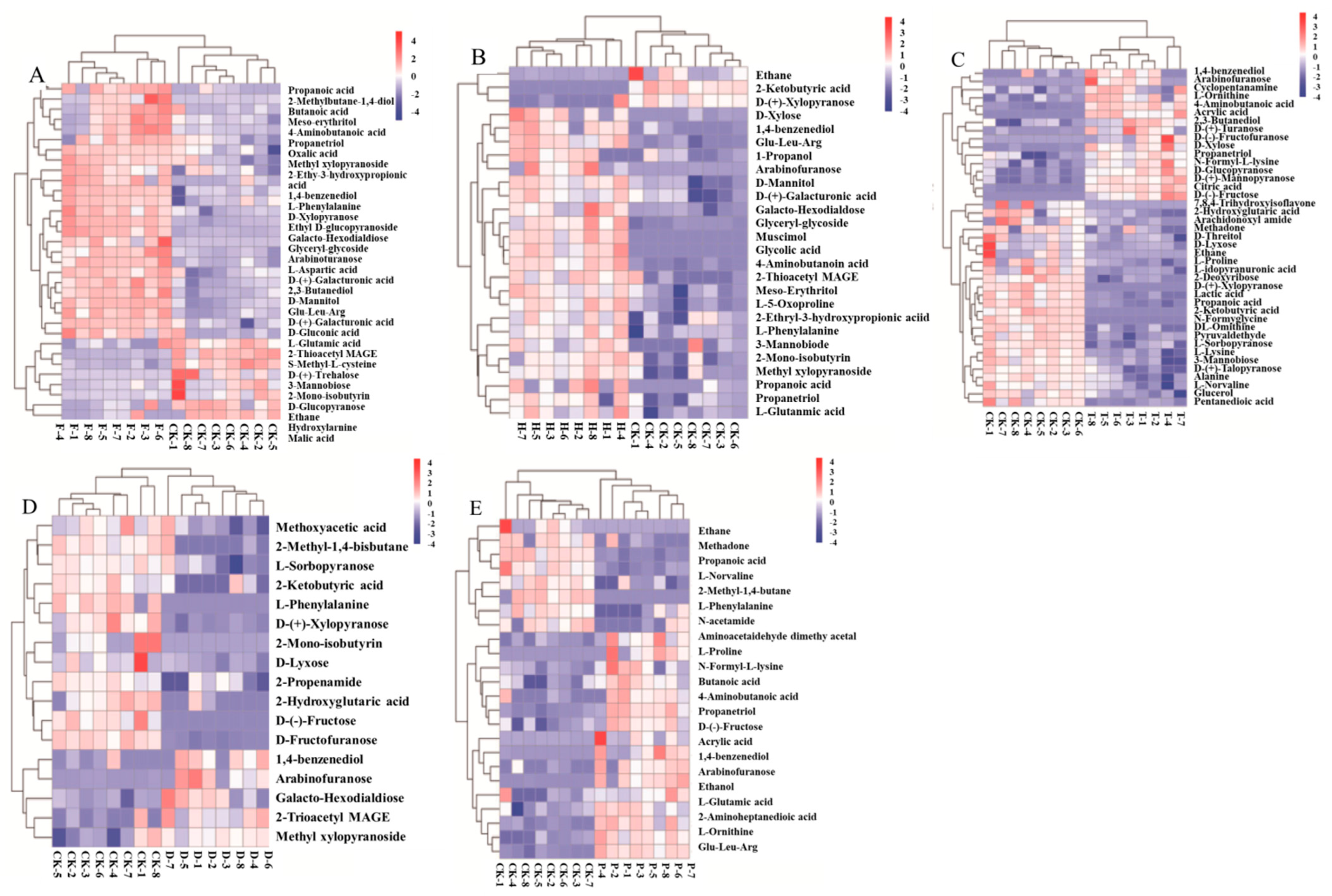
2.2. Effects of F, H, T, D, and P on Metabolites Produced in Wine Brewing
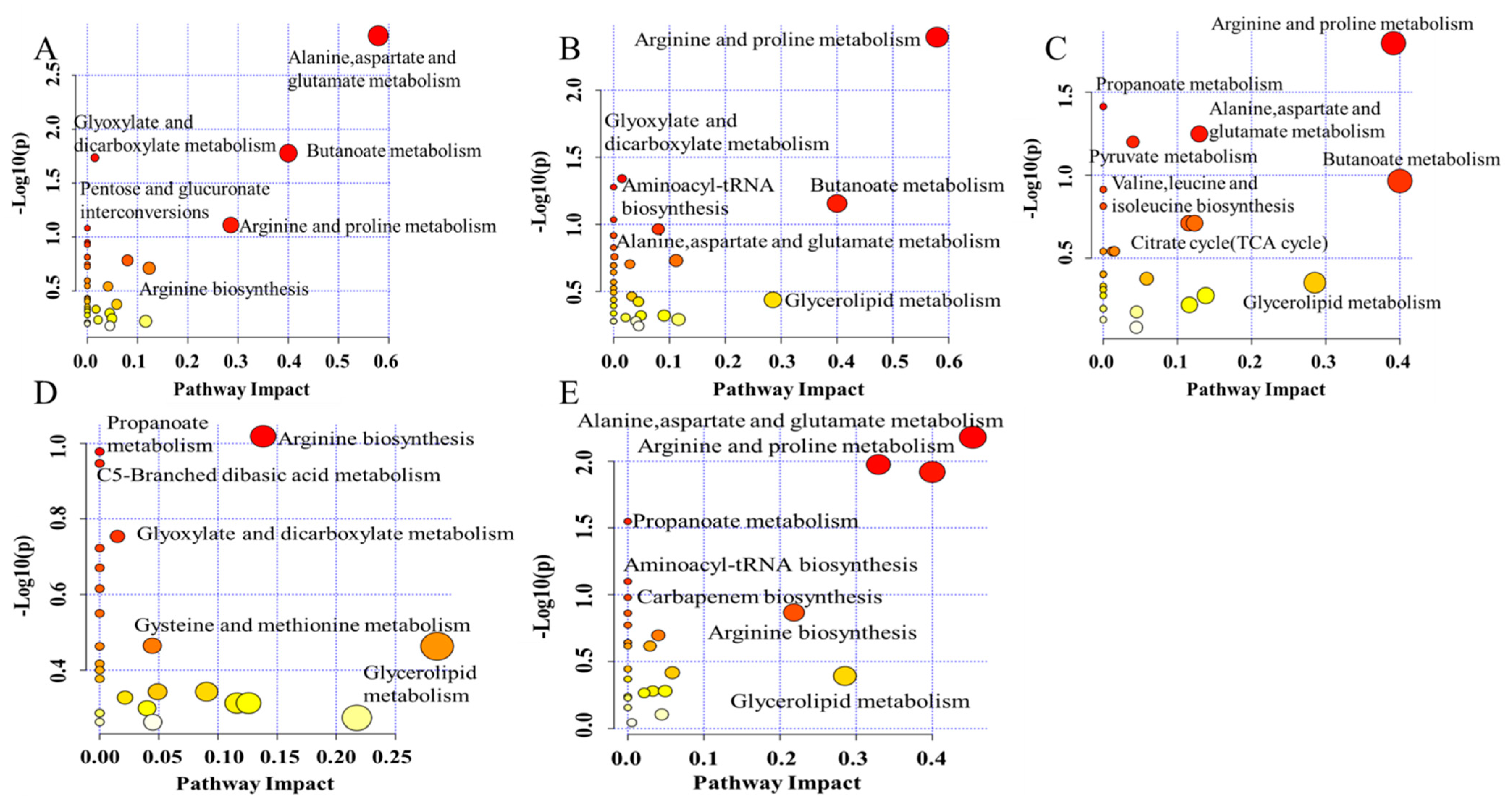
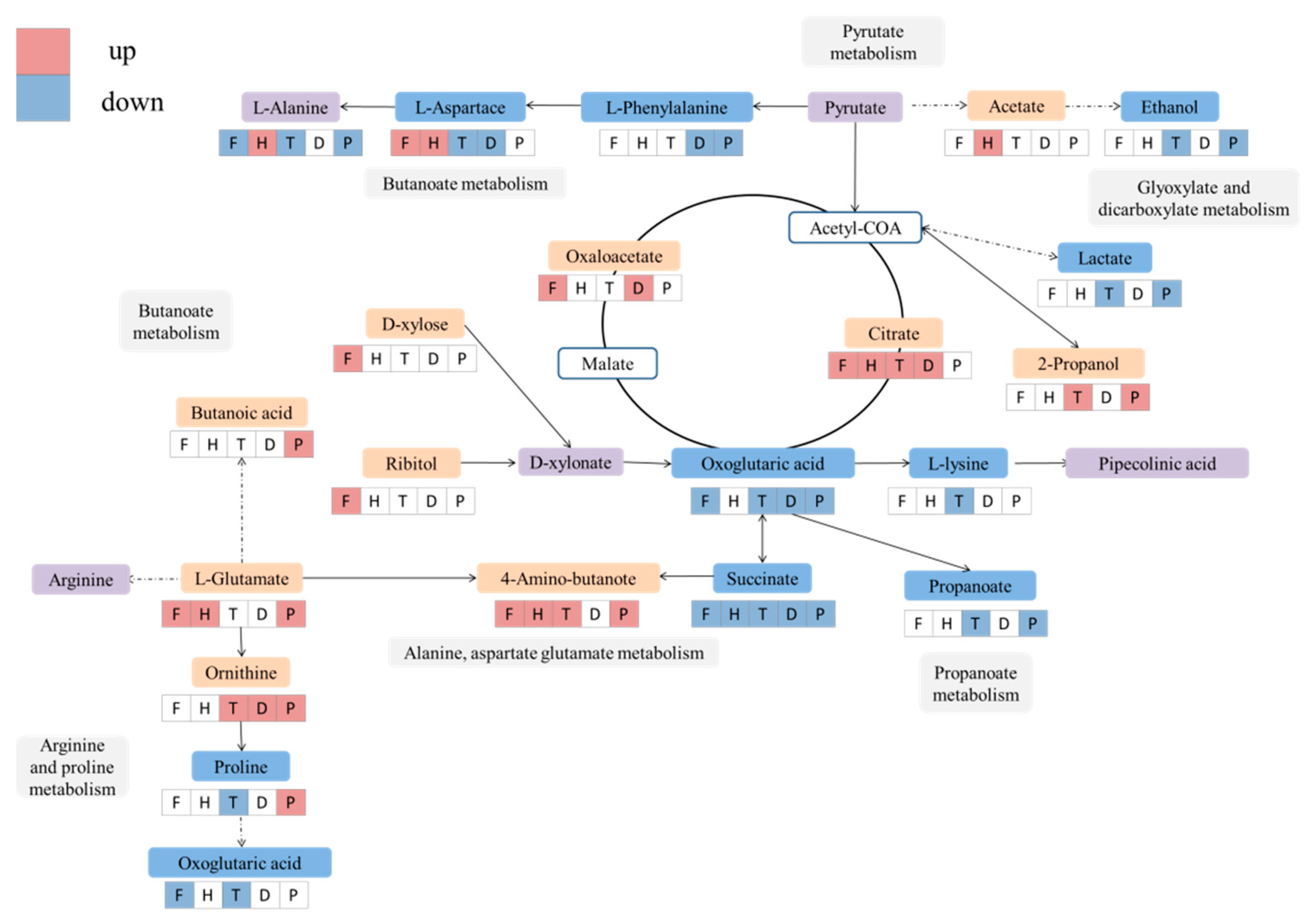
2.3. Altered Metabolic Pathway by F, H, T, D and P Treatment during Wine Brewing
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Experiment on the Effect of Pesticides on the Growth of Saccharomyces cerevisiae
3.3. Preparation of Wine Samples
3.4. Metabolite Extraction from Wine Samples
3.5. Metabolomics Analysis by GC-MS
3.6. Data Processing and Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, O.; Li, M.; Chen, J.; Li, R.; Quan, R.; Zhang, Z.; Kong, Z.; Dai, X. Influence of Triazole Pesticides on Wine Flavor and Quality Based on Multidimensional Analysis Technology. Molecules 2020, 25, 5596. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Muller, M.D.; Poiger, T. Azole Fungicides: Occurrence and Fate in Wastewater and Surface Waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalapathy, R.; Packirisamy, A.S.B.; Ramachandran, A.C.I.; Udhyasooriyan, L.P.; Peter, M.J.; Senthilnathan, K.; Basheer, V.A.; Muthusamy, S. Assessing the Effect of Chitosan on Pesticide Removal in Grape Juice during Clarification by Gas Chromatography with Tandem Mass Spectrometry. Process Biochem. 2020, 94, 305–312. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.l.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- He, Q.; Huang, J.; Yang, X.; Yan, X.; He, J.; Li, S.; Jiang, J. Effect of Pesticide Residues in Grapes on Alcoholic Fermentation and Elimination of Chlorothalonil Inhibition by Chlorothalonil Hydrolytic Dehalogenase. Food Control 2016, 64, 70–76. [Google Scholar] [CrossRef]
- He, J.; Zhang, B.; Zhang, H.; Hao, L.L.; Ma, T.Z.; Wang, J.; Han, S.Y. Monitoring of 49 Pesticides and 17 Mycotoxins in Wine by QuEChERS and UHPLC–MS/MS Analysis. J. Food Sci. 2019, 84, 2688–2697. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Dias, T.; Garcia, R.; da Silva, M.G.; Cabrita, M.J. Revealing the Yeast Modulation Potential on Amino Acid Composition and Volatile Profile of Arinto White Wines by a Combined Chromatographic-Based Approach. J. Chromatogr. A 2021, 1641, 461991–462001. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.M.; Farris, G.A.; Madau, G.; Emonti, G. Pesticides in Fermentative Processes of Wine. J. Agric. Food Chem. 1999, 47, 3854–3857. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.M.; Cabitza, F.; Pala, M.; Farris, G.A. Fate of Quinoxyfen Residues in Grapes, Wine, and Their Processing Products. J. Agric. Food Chem. 2000, 48, 6128–6131. [Google Scholar] [CrossRef]
- Navarro, S.; Pérez, G.; Navarro, G.; Mena, L.; Vela, N. Variability in the Fermentation Rate and Colour of Young Lager Beer as Influenced by Insecticide and Herbicide Residues. Food Chem. 2007, 105, 1495–1503. [Google Scholar] [CrossRef]
- González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Impact of Phytosanitary Treatments with Fungicides (Cyazofamid, Famoxadone, Mandipropamid and Valifenalate) on Aroma Compounds of Godello White Wines. Food Chem. 2012, 131, 826–836. [Google Scholar] [CrossRef]
- Cabras, P.; Meloni, M.; Melis, M.; Farris, G.A.; Budroni, M.; Satta, T. Interactions between Lactic Bacteria and Fungicides during Lactic Fermentation. J. Wine Res. 1994, 5, 53–59. [Google Scholar] [CrossRef]
- Sieiro-Sampedro, T.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gandara, J.; Cancho-Grande, B.; Rial-Otero, R. Impact of Mepanipyrim and Tetraconazole in Mencía Wines on the Biosynthesis of Volatile Compounds during the Winemaking Process. Food Chem. 2019, 300, 125223. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mazaira-Pérez, J. Evolution of Tebuconazole Residues through the Winemaking Process of Mencía Grapes. Food Chem. 2009, 117, 529–537. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, L.; Wang, J. Organophosphorus Pesticide Residues in Market Foods in Shaanxi Area, China. Food Chem. 2006, 98, 240–242. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Khoshmaram, L. Air-Assisted Liquid–Liquid Microextraction-Gas Chromatography-Flame Ionisation Detection: A Fast and Simple Method for the Assessment of Triazole Pesticides Residues in Surface Water, Cucumber, Tomato and Grape Juices Samples. Food Chem. 2013, 141, 1881–1887. [Google Scholar] [CrossRef]
- Dos Anjos, J.P.; de Andrade, J.B. Simultaneous Determination of Pesticide Multiresidues in White Wine and Rosé Wine by SDME/GC-MS. Microchem. J. 2015, 120, 69–76. [Google Scholar] [CrossRef]
- Moeder, M.; Bauer, C.; Popp, P.; van Pinxteren, M.; Reemtsma, T. Determination of Pesticide Residues in Wine by Membrane-Assisted Solvent Extraction and High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2012, 403, 1731–1741. [Google Scholar] [CrossRef]
- Christodoulou, D.L.; Kanari, P.; Hadjiloizou, P.; Constantinou, P. Pesticide Residues Analysis in Wine by Liquid Chromatography-Tandem Mass Spectrometry and Using Ethyl Acetate Extraction Method: Validation and Pilot Survey in Real Samples. J. Wine Res. 2015, 26, 81–98. [Google Scholar] [CrossRef]
- Lloyd, N.; Johnson, D.L.; Herderich, M.J. Metabolomics Approaches for Resolving and Harnessing Chemical Diversity in Grapes, Yeast and Wine. Aust. J. Grape Wine Res. 2015, 21, 723–740. [Google Scholar] [CrossRef]
- Lee, J.E.; Hwang, G.S.; Lee, C.H.; Hong, Y.S. Metabolomics Reveals Alterations in Both Primary and Secondary Metabolites by Wine Bacteria. J. Agric. Food Chem. 2009, 57, 10772–10783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Hwang, G.S.; Van Den Berg, F.; Lee, C.H.; Hong, Y.S. Evidence of Vintage Effects on Grape Wines Using 1H NMR-Based Metabolomic Study. Anal. Chim. Acta 2009, 648, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Yoo, S.A.; Seo, S.H.; Lee, K.I.; Na, C.S.; Son, H.S. GC-MS Based Metabolomics Approach of Kimchi for the Understanding of Lactobacillus plantarum Fermentation Characteristics. LWT 2016, 68, 313–321. [Google Scholar] [CrossRef]
- Long, P.; Wen, M.; Granato, D.; Zhou, J.; Wu, Y.; Hou, Y.; Zhang, L. Untargeted and Targeted Metabolomics Reveal the Chemical Characteristic of Pu-Erh Tea (Camellia assamica) during Pile-Fermentation. Food Chem. 2020, 311, 125895–125902. [Google Scholar] [CrossRef]
- Wang, L.; Hong, K.; Agbaka, J.I.; Zhu, G.; Lv, C.; Ma, C. Application of UHPLC-Q/TOF-MS-Based Metabolomics Analysis for the Evaluation of Bitter-Tasting Krausen Metabolites during Beer Fermentation. J. Food Compos. Anal. 2021, 99, 103850–103857. [Google Scholar] [CrossRef]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-Based Metabolic Phenotyping (Metabonomics/Metabolomics): The State of the Art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar] [CrossRef]
- Van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast During Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- Varela, C.; Siebert, T.; Cozzolino, D.; Rose, L.; McLean, H.; Henschke, P.A. Discovering a Chemical Basis for Differentiating Wines Made by Fermentation with ‘Wild’ Indigenous and Inoculated Yeasts: Role of Yeast Volatile Compounds. Aust. J. Grape Wine Res. 2009, 15, 238–248. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, Ecology and Industrial Applications of Aroma Formation in Yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. South Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Scariot, F.J.; Delamare, A.P.L.; Echeverrigaray, S. The Effect of Chlorothalonil on Saccharomyces cerevisiae under Alcoholic Fermentation. Pestic. Biochem. Physiol. 2022, 182, 105032–105039. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Si, H.Y.; Yu, Y.; Duan, H.; Liang, D.M.; Jiang, W.G.; Li, X.H. Effects of the Residues of Agricultural Chemicals in Grapes on Winemaking. Sci. Agric. Sin. 2012, 45, 743–751. [Google Scholar]
- Gava, A.; Emer, C.D.; Ficagna, E.; Fernandes de Andrade, S.; Fuentefria, A.M. Occurrence and Impact of Fungicides Residues on Fermentation during Wine Production-A Review. Food Addit. Contam. Part A 2021, 38, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Li, M.; An, J.; Chen, J.; Bao, Y.; Francis, F.; Dai, X. The Fungicide Triadimefon Affects Beer Flavor and Composition by Influencing Saccharomyces cerevisiae Metabolism. Sci. Rep. 2016, 6, 33552–33561. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; James, A.; Shen, X.; Wang, Y. Roles of Microbiota in the Formation of Botrytized Grapes and Wines. CyTA-J. Food 2021, 19, 656–667. [Google Scholar] [CrossRef]
- Oliva, J.; Girón, F.; Cayuela, J.M.; Mulero, J.; Zafrilla, P.; Cámara, M.Á. Effect of Fungicides on the Yeast Population during Spontaneous Fermentation in the Vinification of Monastrell Grapes. LWT 2020, 131, 109816–109822. [Google Scholar] [CrossRef]
- Kosel, J.; Raspor, P.; Čadež, N. Maximum Residue Limit of Fungicides Inhibits the Viability and Growth of Desirable Non-Saccharomyces Wine Yeasts. Aust. J. Grape Wine Res. 2019, 25, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xu, E.; Long, J.; Zhang, Y.; Wang, F.; Xu, X.; Jin, Z.; Jiao, A. Monitoring of Fermentation Process Parameters of Chinese Rice Wine Using Attenuated Total Reflectance Mid-Infrared Spectroscopy. Food Control 2015, 50, 405–412. [Google Scholar] [CrossRef]
- Ravanal, M.C.; Rosa, L.; Eyzaguirre, J. Alpha-L-Arabinofuranosidase 3 from Penicillium purpurogenum (ABF3): Potential Application in the Enhancement of Wine Flavour and Heterologous Expression of the Enzyme. Food Chem. 2012, 134, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of Ethanol, Tannin and Fructose on the Headspace Concentration and Potential Sensory Significance of Odorants in a Model Wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Minebois, R.; Pérez-Torrado, R.; Querol, A. A Time Course Metabolism Comparison among Saccharomyces cerevisiae, S. uvarum and S. kudriavzevii species in Wine Fermentation. Food Microbiol. 2020, 90, 103484–103497. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Sun, J.; Wu, D.; Xiao, J.; Lu, J. Objective Measures of Greengage Wine Quality: From Taste-Active Compound and Aroma-Active Compound to Sensory Profiles. Food Chem. 2021, 340, 128179–128188. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Jiang, W.W.; Niimi, J.; Ristic, R.; Bastian, S.E.P. Effects of Immersive Context and Wine Flavor on Consumer Wine Flavor Perception and Elicited Emotions. Am. J. Enol. Vitic. 2017, 68, 1–10. [Google Scholar] [CrossRef]
- Liu, A.; Yang, X.; Guo, Q.; Li, B.; Zheng, Y.; Shi, Y.; Zhu, L. Microbial Communities and Flavor Compounds during the Fermentation of Traditional Hong Qu Glutinous Rice Wine. Foods 2022, 11, 1097. [Google Scholar] [CrossRef]
- Peris, D.; Torrado, R.; Hittinger, C.T.; Barrio, E.; Querol, A. On the Origins and Industrial Applications of Saccharomyces cerevisiae × Saccharomyces kudriavzevii Hybrids. Yeast 2018, 35, 51–69. [Google Scholar] [CrossRef] [Green Version]
- Panovská, Z.; Šedivá, A.; Jedelská, M.; Pokorný, J. Effect of Ethanol on Interactions of Bitter and Sweet Tastes in Aqueous Solutions. Czech J. Food Sci. 2008, 26, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Møretrø, T.; Daeschel, M.A. Wine is Bactericidal to Foodborne Pathogens. J. Food Sci. 2004, 69, M251–M257. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Clark, A.C.; Grant-Preece, P. Wine Metabolomics: Objective Measures of Sensory Properties of Semillon from GC-MS Profiles. J. Agric. Food Chem. 2013, 61, 11957–11967. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Granchi, L.; Caruso, M.; Borra, G.; Palla, G.; Fiore, C.; Ganucci, D.; Caligiani, A.; Brandolini, V. The Species-Specific Ratios of 2,3-Butanediol and Acetoin Isomers as a Tool to Evaluate Wine Yeast Performance. Int. J. Food Microbiol. 2003, 86, 163–168. [Google Scholar] [CrossRef]
- Ueno, H. Enzymatic and Structural Aspects on Glutamate Decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Q.; Zou, H.; Yu, Y.; Zhou, Z.; Mao, J.; Zhang, S. A Metagenomic Analysis of the Relationship between Microorganisms and Flavor Development in Shaoxing Mechanized Huangjiu Fermentation Mashes. Int. J. Food Microbiol. 2019, 303, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huang, J.; Xiao, G.; You, Y.; Yuan, H.; Chen, F.; Liu, S.; Mao, J.; Li, B. Determination of γ-Aminobutyric Acid in Chinese Rice Wines and Its Evolution during Fermentation. J. Inst. Brew. 2017, 123, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, A.; Tanikawa, T.; Takagi, H. Inhibitory Effect of Arginine on Proline Utilization in Saccharomyces cerevisiae. Yeast 2020, 37, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Chitarrini, G.; Di Gangi, I.M.; Masuero, D.; Soini, E.; Mattivi, F.; Vrhovsek, U. A Rapid LC-MS/MS Method for Quantitative Profiling of Fatty Acids, Sterols, Glycerolipids, Glycerophospholipids and Sphingolipids in Grapes. Talanta 2015, 140, 52–61. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Zhou, Y.; Zhan, R.; Zhu, L.; Chen, H.; Ma, Z.; Chen, X.; Lu, Y. Effects of Different Pesticides on the Brewing of Wine Investigated by GC-MS-Based Metabolomics. Metabolites 2022, 12, 485. https://doi.org/10.3390/metabo12060485
Song B, Zhou Y, Zhan R, Zhu L, Chen H, Ma Z, Chen X, Lu Y. Effects of Different Pesticides on the Brewing of Wine Investigated by GC-MS-Based Metabolomics. Metabolites. 2022; 12(6):485. https://doi.org/10.3390/metabo12060485
Chicago/Turabian StyleSong, Beibei, Yaoyao Zhou, Rong Zhan, Linjiang Zhu, Hanchi Chen, Zhi Ma, Xiaolong Chen, and Yuele Lu. 2022. "Effects of Different Pesticides on the Brewing of Wine Investigated by GC-MS-Based Metabolomics" Metabolites 12, no. 6: 485. https://doi.org/10.3390/metabo12060485
APA StyleSong, B., Zhou, Y., Zhan, R., Zhu, L., Chen, H., Ma, Z., Chen, X., & Lu, Y. (2022). Effects of Different Pesticides on the Brewing of Wine Investigated by GC-MS-Based Metabolomics. Metabolites, 12(6), 485. https://doi.org/10.3390/metabo12060485





