Identification and Quantification of Flavonoids in Okra (Abelmoschus esculentus L. Moench) and Antiproliferative Activity In Vitro of Four Main Components Identified
Abstract
:1. Introduction
2. Results
2.1. Identification of Flavonoids in Okra Pod
2.1.1. Six Individual Flavonoids Were Identified
2.1.2. Method Verification
2.2. Antiproliferative Activity In Vitro of the Four Main Individual Flavonoids in Okra Pod
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Identification and Determination of Flavonoids
4.2.1. Sample Solution
4.2.2. Identification of Flavonoids in Okra
4.2.3. Quantitative Analysis of Flavonoids in Okra
4.3. Determination of Antiproliferative Activity
4.3.1. Cell Culture
4.3.2. Antiproliferative Activity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bondonno, N.P.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.P.; Shin, J.H.; Croft, K.D.; Woodman, R.J.; Wong, G.; Lim, W.H.; Gopinath, B.; et al. Association of flavonoids and flavonoid-rich foods with all-cause mortality: The Blue Mountains Eye Study. Clin. Nutr. 2020, 39, 141–150. [Google Scholar] [CrossRef]
- De Araújo, F.F.; De Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharm. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Yan, T.; Liu, B.; Wang, N.; Liao, Z.; Wu, B.; He, B.; Jia, Y. The flavonoids of okra insulates against oxidative stress, neuroinflammation and restores BDNF levels in Aβ1-42 induced mouse model of Alzheimer’s disease. Exp. Gerontol. 2021, 147, 111263. [Google Scholar] [CrossRef]
- Camciuc, M.; Deplagne, M.; Vilarem, G.; Gaset, A. Okra—Abelmoschus esculentus L. (Moench.) a crop with economic potential for set aside acreage in France. Ind. Crops Prod. 1998, 7, 257–264. [Google Scholar] [CrossRef]
- Lamont, W.J. Okra—A versatile vegetable crop. HortTechnology 1999, 9, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Liu, Q.; Yu, J.; Wang, M.; Chen, M.; Wang, R.; He, X.; Gao, M.; Chen, X. Separation of α-amylase inhibitors from Abelmoschus esculentus (L). Moench by on-line two-dimensional high-speed counter-current chromatography target-guided by ultrafiltration-HPLC. 2015, 38, 3897–3904. J. Sep. Sci. 2015, 38, 3897–3904. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Jamaly, S.; Farjana, F.; Begum, N.; Zenat, E.A. A review on ethnomedicinal, pharmacological, phytochemical and pharmaceutical profile of lady’s Finger (Abelmoschus esculentus L.). Plant. Pharmacol. Pharm. 2019, 10, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Doreddula, S.K.; Bonam, S.R.; Gaddam, D.P.; Desu, B.S.; Ramarao, N.; Pandy, V. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci. World J. 2014, 2014, 519848. [Google Scholar] [CrossRef] [Green Version]
- Ortaç, D.; Cemek, M.; Karaca, T.; Büyükokuroğlu, M.E.; Özdemir, Z.Ö.; Kocaman, A.T.; Göneş, S. In vivo anti-ulcerogenic effect of okra (Abelmoschus esculentus) on ethanol-induced acute gastric mucosal lesions. Pharm. Biol. 2018, 56, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.M.; Ibrahim, R.K. Tricin—A potential multifunctional nutraceutical. Phytochem. Rev. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Arapitsas, P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Jiang, B.; Song, J.; Jin, Y. A flavonoid monomer tricin in Gramineous plants: Metabolism, bio/chemosynthesis, biological properties, and toxicology. Food Chem. 2020, 320, 126617. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2008. [Google Scholar] [CrossRef]
- De La Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-Garcia, J. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Carrilo-López, A., Eds.; Woodhead Publishing: London, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- Manach, C.; Regerat, F.; Texier, O.; Agullo, G.; Demigne, C.; Remesy, C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr. Res. 1996, 16, 517–544. [Google Scholar] [CrossRef]
- Yin, H.; Ma, J.; Han, J.; Li, M.; Shang, J. Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide in rats by HPLC-MS. PeerJ 2019, 7, 6665. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.Y.; Gong, C.; Zhang, L.Y.; Yu, G.; Gong, W. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Li, J.; Liu, X.; Feng, Y.; Gui, Y.; Yang, J.; He, W.; Dai, C. Quercetin inhibits fibroblast activation and kidney fibrosis involving the suppression of mammalian target of rapamycin and β-catenin signaling. Sci. Rep. 2016, 6, 23968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2015, 33, 840–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.R.; Jin, Q.; Jin, H.G.; Ko, H.J.; Woo, E.R. Phenolic compounds with IL-6 inhibitory activity from Aster yomena. Arch. Pharm. Res. 2014, 37, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Wang, M.; Chen, X.; Tian, L.; Wang, L.; Yang, W.; Chen, L.; He, F.; Yin, W. Flavonoid-rich extracts from okra flowers exert antitumor activity in colorectal cancer through induction of mitochondrial dysfunction-associated apoptosis, senescence and autophagy. Food Funct. 2020, 11, 10448–10466. [Google Scholar] [CrossRef]
- Chaemsawang, W.; Prasongchean, W.; Papadopoulos, K.I.; Ritthidej, G.; Sukrong, S.; Wattanaarsakit, P. The Effect of Okra (Abelmoschus esculentus (L.) Moench) Seed Extract on Human Cancer Cell Lines Delivered in Its Native Form and Loaded in Polymeric Micelles. Int. J. Biomater. 2019, 2019, 9404383. [Google Scholar] [CrossRef] [Green Version]
- Ping, M.-H. Hyperin Controls the Development and Therapy of Gastric Cancer via Regulating Wnt/β-Catenin Signaling. Cancer Manag. Res. 2020, 12, 11773–11782. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Busi, M.; Arredondo, F.; González, D.; Echeverry, C.; Vega-Teijido, M.A.; Carvalho, D.; Rodríguez-Haralambides, A.; Rivera, F.; Dajas, F.; Abin-Carriquiry, J.A. Purification, structural elucidation, antioxidant capacity and neuroprotective potential of the main polyphenolic compounds contained in Achyrocline satureioides (Lam) D.C. (Compositae). Bioorg. Med. Chem. 2019, 27, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Napolitano, A.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Okra fruit: LC-ESI/LTQOrbitrap/MS/MnS based deep insight on polar lipids and specialized metabolites with evaluation of anti-oxidant and anti-hyperglycemic activity. Food Funct. 2020, 11, 7856–7865. [Google Scholar] [CrossRef]
- Wu, D.T.; Nie, X.R.; Shen, D.D.; Li, H.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; Qin, W. Phenolic compounds, antioxidant activities, and inhibitory effects on digestive enzymes of different cultivars of okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef] [Green Version]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Carvalho, G.C.R.; Morales, P.; Ciudad-Mulero, M.F.H.; Flamini, G.C.F.R.; Majdoub, H.; Ferreira, I.C.F.R. Chemical composition, nutritional value, and biological evaluation of tunisian okra pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Q.S.; Wang, G.S. Flavonoid glycosides and their derivatives from the herbs of Scorzonera austriaca wild. Molecules 2016, 21, 803. [Google Scholar] [CrossRef]
- Cao, H.H.; Tse, K.W.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant Phenolics—Secondary Metabolites with Diverse Functions. In Recent Advances in Polyphenol Research; Daayf, F., Lattanzio, V., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 1–35. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high-performance liquid chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, J.; Chang, Q.; Hu, Z.; Shen, X.; Feng, J.; Zhang, Z.; Wu, X. Total flavonoid aglycones extract in Radix Scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J. Ethnopharmacol. 2019, 235, 133–140. [Google Scholar] [CrossRef]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Le, M.L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Nat. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304S–312S. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Pan, J.; Yang, A.; Li, W.; Chen, Y. Synergistic sensitization of apatinib and trastuzumab on gastric cancer cells. Chin. Clin. Oncol. 2020, 25, 205–210. [Google Scholar] [CrossRef]
- Yakhni, M.; Briat, A.; Guerrab, A.E.; Furtado, L.; Kwiatkowski, F.; Miot-Noirault, E.; Cachin, F.; Penault-Llorca, F.; Radosevic-Robin, N. Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance. Am. J. Cancer Res. 2019, 9, 1043–1060. [Google Scholar]
- Chen, J.P.; Li, L.; Su, J.Y. Antioxidant and antitumor activities of curcumin. Mod. Food Sci. Technol. 2014, 30, 11–15+6. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-β-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]

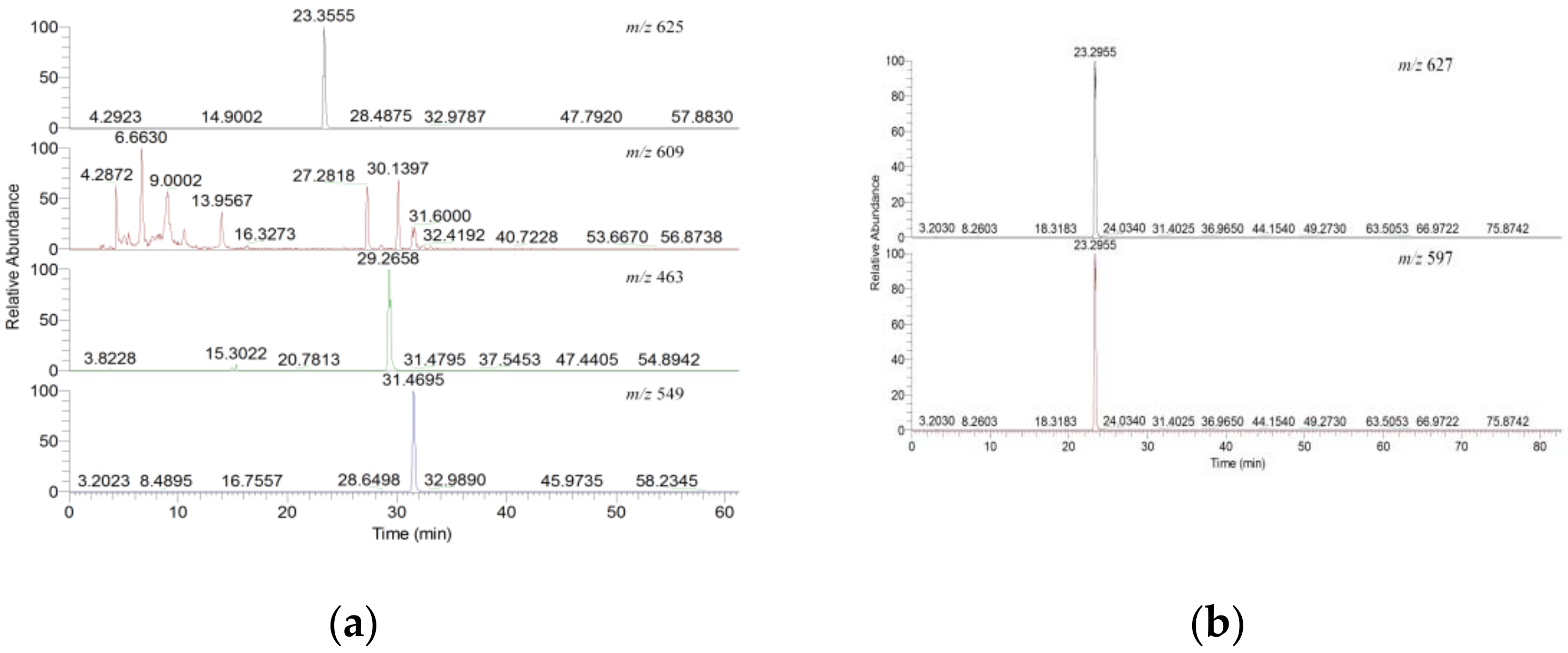
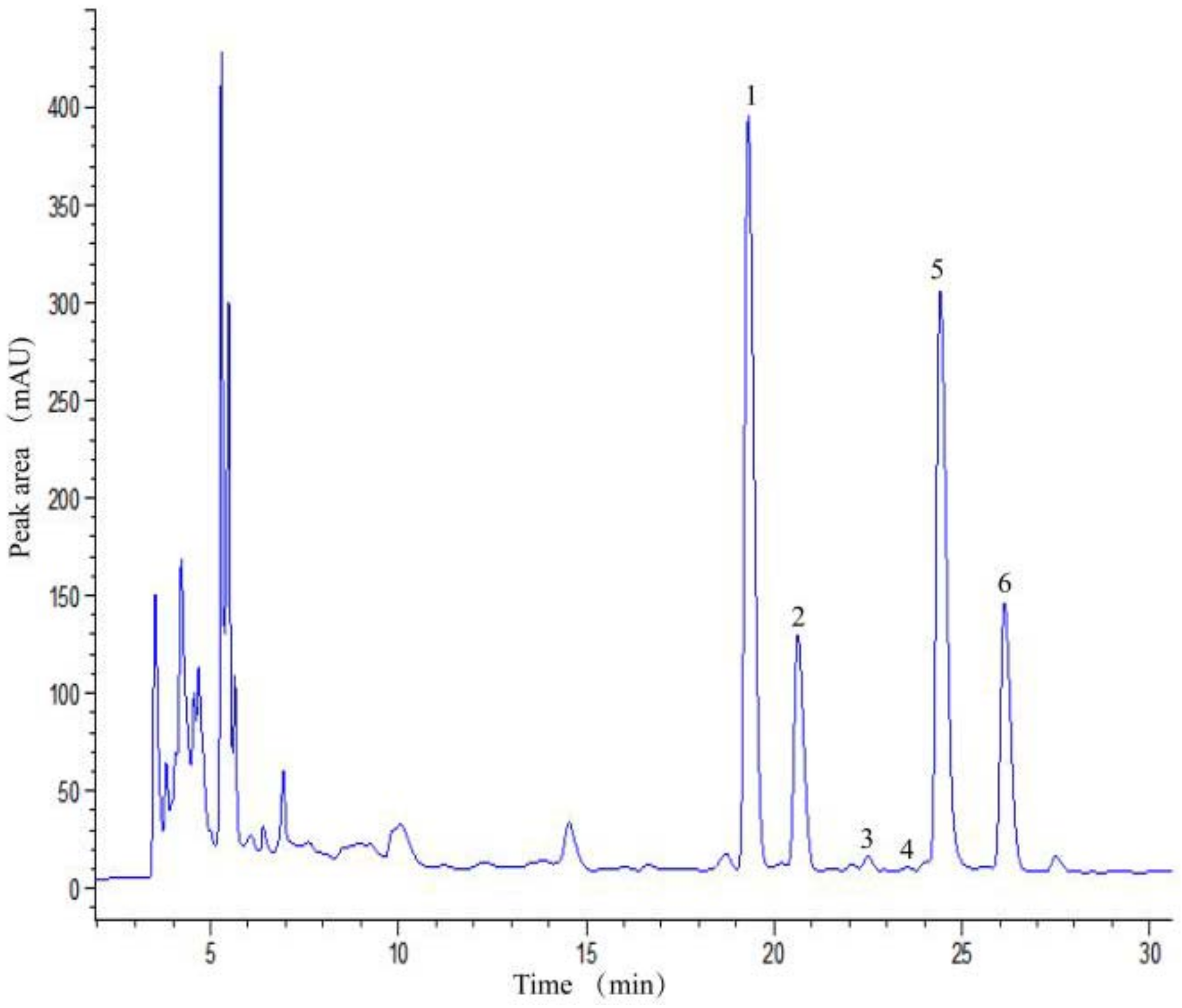

| Peak | Tentative Assignment | tR (min.) | [M+H]+ (m/z) | [M H]− (m/z) | Molecular Formula | MS/MS Fragment Ions | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | Quercetin-3-gentiobioside | 18.185 | 626.7747 | - | C27H30O17 | 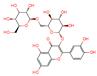 | 303.1241 | - |
| 2 | Quercetin-3-sambubioside | 19.874 | 596.7314 | - | C26H28O16 | 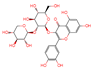 | 303.1179 | [14] |
| 3 | Rutin | 21.484 | 610.7375 | 609.1411 | C27H30O16 | 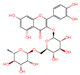 | 301.1387 | [14] |
| 4 | Quercetin-7-glucoside | 22.248 | - | 463.0782 | C21H20O12 |  | - | - |
| 5 | Isoquercitrin | 23.815 | - | 463.0837 | C21H20O12 | 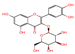 | 301.1503 | [33,34] |
| 6 | Quercetin-3-malonylglucoside | 25.194 | - | 548.6969 | C24H22O15 |  | 504.9665 | [14] |
| No. | Flavonoids Standards | Calibration Equations | Correlation Coefficient (R2) | LOD (μg/mL) | LOQ (μg/mL) | Linear Range (μg/mL) | RSD% 1 (Intra-Day) | RSD% 2 (Inter-Day) | The Recovery of Spiked Sample REC% 3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Quercetin-3-gentiobioside | y = 14.66x −1 0.596 4 | 1 | 0.204 | 0.680 | 0–800 | 0.72 | 0.95 | 96.83 |
| 2 | Quercetin-3-sambubioside | y = 20.461x − 24.366 | 0.9999 | 0.120 | 0.400 | 0–400 | 1.88 | 2.33 | 102.54 |
| 3 | Rutin | y = 10.342x + 4.8446 | 0.9999 | 0.209 | 0.696 | 0–100 | 1.75 | 3.46 | 96.60 |
| 4 | Quercetin-7-glucoside | y = 5.8708x − 1.3649 | 1 | 0.106 | 0.355 | 0–100 | 2.04 | 3.72 | 98.93 |
| 5 | Isoquercitrin | y = 34.06x + 57.405 | 0.9995 | 0.045 | 0.152 | 0–1000 | 1.60 | 2.93 | 96.53 |
| 6 | Quercetin-3-malonylglucoside | y = 27.904x + 149.3 | 0.9997 | 0.760 | 2.535 | 0–1000 | 2.26 | 2.38 | 101.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chen, X.; Rao, S.; Li, Y.; Zang, Y.; Zhu, B. Identification and Quantification of Flavonoids in Okra (Abelmoschus esculentus L. Moench) and Antiproliferative Activity In Vitro of Four Main Components Identified. Metabolites 2022, 12, 483. https://doi.org/10.3390/metabo12060483
Yang J, Chen X, Rao S, Li Y, Zang Y, Zhu B. Identification and Quantification of Flavonoids in Okra (Abelmoschus esculentus L. Moench) and Antiproliferative Activity In Vitro of Four Main Components Identified. Metabolites. 2022; 12(6):483. https://doi.org/10.3390/metabo12060483
Chicago/Turabian StyleYang, Jing, Xiaoqi Chen, Shuaiqi Rao, Yaochen Li, Yunxiang Zang, and Biao Zhu. 2022. "Identification and Quantification of Flavonoids in Okra (Abelmoschus esculentus L. Moench) and Antiproliferative Activity In Vitro of Four Main Components Identified" Metabolites 12, no. 6: 483. https://doi.org/10.3390/metabo12060483
APA StyleYang, J., Chen, X., Rao, S., Li, Y., Zang, Y., & Zhu, B. (2022). Identification and Quantification of Flavonoids in Okra (Abelmoschus esculentus L. Moench) and Antiproliferative Activity In Vitro of Four Main Components Identified. Metabolites, 12(6), 483. https://doi.org/10.3390/metabo12060483








